Abstract
Background/Rationale
: Currently, we cannot reliably differentiate individuals at risk of cognitive decline, for example, mild cognitive impairment (MCI), Alzheimer’s disease (AD), from those individuals who are not at risk.
Methods
: A total of 32 participants with MCI and 60 control (CON) participants were tested on an innovative, sensitive behavioral assay, the visual paired comparison (VPC) task using infrared eye tracking. The participants were followed for 3 years after testing.
Results
: Scores on the VPC task predicted, up to 3 years prior to a change in clinical diagnosis, those patients with MCI who would and who would not progress to AD and CON participants who would and would not progress to MCI.
Conclusions
: The present findings show that the VPC task can predict impending cognitive decline. To our knowledge, this is the first behavioral task that can identify CON participants who will develop MCI or patients with MCI who will develop AD within the next few years.
Keywords: memory, mild cognitive impairment, Alzheimer’s disease, prognosis, memory impairment, cognitive impairment
Introduction
The diagnosis of amnestic mild cognitive impairment (aMCI) refers to the individuals who have memory loss with relatively preserved cognitive and daily living abilities (single domain aMCI), or memory loss together with other impaired cognitive abilities and preserved daily living abilities (multidomain aMCI).1–3 Individuals diagnosed with aMCI, whether single- or multidomain, are at higher than normal risk of developing Alzheimer’s disease (AD).4–6 Although many individuals diagnosed with MCI do not convert to AD, the risk of conversion to AD can range between 6% and 25% per year. 4 Currently, we cannot reliably differentiate between patients with MCI at risk of further cognitive decline from those patients with MCI who are not at risk. Moreover, we are unable to differentiate between matched control (CON) participants who are at risk of cognitive decline and those who are not.
Although there has been considerable progress in the development of genetic, imaging, and cerebrospinal fluid biomarkers for AD,7,8 much of this work is aimed at detecting the presence of the disease. The present study, in contrast, is aimed at predicting whether and when the disease will occur. The visual paired comparison (VPC) task assesses memory function by determining whether the participants exhibit a preference for a novel picture compared to a previously viewed picture, measured by viewing time. The measure of interest is the percentage of time viewing the novel picture. Individuals with intact memory typically view the novel picture about two-third of the viewing time relative to the familiar picture. The task has been useful in detecting memory impairment both in humans and in nonhuman primates with damage to the medial temporal lobe memory system.9–12 For example, the task successfully differentiated patients with MCI from both CON participants and participants diagnosed with Parkinson’s disease. 9
Although these findings have pointed to the value of the VPC task in detecting memory impairment, an interesting and important question remains. Could the VPC task be useful in predicting the onset of aMCI and/or AD by reliably distinguishing individuals at risk from those not at risk for memory decline?
The current study directly addresses this question. Our findings demonstrate that the VPC task performance scores are a sensitive early predictor of which patients diagnosed with aMCI will progress to AD during the subsequent 3 years and which patients are not at such risk. Additionally, the VPC task is also a sensitive early predictor of which CON participants will progress to aMCI and which are not at such risk. Accordingly, regardless of diagnostic status at the time of testing, the VPC task accurately predicts cognitive decline.
Methods
Participants
Two participant groups were assessed. Group aMCI included 32 participants diagnosed with aMCI (mean age = 70.2; 11 single-domain patients with aMCI and 21 patients with multidomain aMCI; 56% male), and group CON included 60 elderly CON participants (mean age = 69.7; 33% male; see Table 1). All participants were recruited from the Alzheimer’s Disease Research Center at Emory University, Atlanta, Georgia. The study protocol and consent forms were approved by the Emory University institutional review board. Written informed consent was obtained from each participant. Clinical diagnoses of aMCI or CON were established following a standardized assessment which includes a uniform data set including neuropsychological measures described elsewhere13–15 and consensus conference involving at least 3 clinicians, expert in evaluation and management of dementia. As described in Crutcher et al, 9 clinical diagnosis of MCI required evidence of a decline in baseline memory function and possibly additional cognitive domains, with the severity of symptoms or consequent functional limitations insufficient to meet Diagnostic and Statistical Manual of Mental Disorder (Third Edition Revised) criteria for dementia. Participants were classified as CON if they demonstrated no evidence of cognitive decline from baseline functioning based on their clinical interview and assessment. Exclusion criteria included a history of substance abuse or learning disability, dementia, neurological (eg, stroke, tumor), or psychiatric illness. Because the VPC task involves visual memory, participants were also excluded if (1) they had significant ophthalmological or visual problems (eg, detached retinas or glaucoma); (2) the eye-tracking equipment could not achieve proper pupil and corneal reflection due to physiological constraints (eg, droopy eyelid, cataracts, and pupils too small); and/or (3) poor calibration and/or they could not complete the calibration procedure. Overall, these criteria resulted in the exclusion of 14 initially recruited participants. It is important to note that the clinicians who provided diagnoses were “blind” to the VPC performance scores, and the technicians who carried out the VPC testing were “blind” to any changes in diagnoses until the completion of the study.
Table 1.
Demographic Information/Neuropsychological Performance Scores by Group.a
| Measure | CON | aMCI | AD | Tukey-Kramerb |
|---|---|---|---|---|
| Total N | 60 [20/40] | 32 [18/14] | 20 [10/10] | |
| Age | 69.7 (7.2) | 70.2 (8.0) | 72.2 (10.2) | NS |
| Education | 15.8 (2.6) | 15.3 (3.3) | 13.7 (3.0) | CON vs AD, P < .05 |
| CERAD | ||||
| Animal Fluencyc | 19.9 (5.0) | 16.2 (5.3) | 11.6 (4.6) | aMCI vs CON, P < .01, aMCI vs AD, P < .001, CON vs AD, P < .001 |
| Boston Naming Testd | 27.6 (4.6) | 24.4 (4.1) | 17.9 (8.4) | aMCI vs CON, P < .01, aMCI vs AD, P < .001, CON vs AD, P < .001 |
| Mini-Mental State Examinationd | 29.2 (1.1) | 27.3 (1.8) | 22.2 (5.0) | aMCI vs CON, P < .001, aMCI vs AD, P < .001, CON vs AD, P < .001 |
| Word List Memory (WLM)e | ||||
| WLM total | 22.4 (3.5) | 15.3 (4.8) | 14.4 (4.0) | aMCI vs CON, P < .001, CON vs AD, P < .001 |
| WLM delayed recall | 7.4 (1.7) | 3.1 (2.2) | 2.1 (1.5) | aMCI vs CON, P < .001, CON vs AD, P < .001 |
| Trail Making Test (TMT) | ||||
| TMT-Af | 33.9 (11.0) | 48.5 (21.4) | 89.3 (57.9) | aMCI vs CON, P < .05, aMCI vs AD, P < .001, CON vs AD, P < .001 |
| TMT-Bd,f | 86.1 (36.6) | 139.7 (66.9) | 173.4 (98.9) | aMCI vs CON, P < .001, CON vs AD, P < .001 |
| Digit Span Forward | 8.8 (1.8) | 8.4 (1.6) | 7.7 (2.5) | NS |
| Digit Span Backward | 6.9 (2.0) | 5.7 (2.0) | 5.1 (2.1) | aMCI vs CON, P < .05, CON vs AD, P < .01 |
| Clock Drawing Test | 12.3 (0.9) | 12.2 (0.8) | 7.7 (3.9) | aMCI vs AD, P < .001, CON vs AD, P < .001 |
| Geriatric Depression Scaleg | 1.6 (1.9) | 2.4 (2.5) | 2.0 (2.3) | NS |
Abbreviations: AD, Alzheimer’s disease; aMCI, amnestic mild cognitive impairment; CERAD, Consortium to Establish a Registry for Alzheimer’s disease; CON, control; NS, not significant.
a Brackets indicate [male/female]. The mean for each variable is given with standard deviations in parentheses.
b If the analysis of variance F was significant (P < .05), then the Tukey-Kramer post hoc pair-wise comparisons were performed and P values are given.
c Animal Fluency: 9 of the AD participants did not complete or were not administered the test.
d Variances are not equal and differ significantly.
e WLM: 32 participants (aMCI = 8; CON = 15; AD = 9) did not complete or were not administered the test.
f TMT-B: 14 participants (aMCI = 3; CON = 2; AD = 9) did not complete the test in the allotted time frame. Scores for these participants are not included in the TMT-B score.
g Geriatric Depression Scale: 4 participants were not administered the GDS.
Equipment and Stimuli
During the task, the participants’ eye movements were recorded using an Applied Science Laboratories (ASL) Model 6000 chinrest-mounted camera system (Applied Science Laboratories, Bedford, MA). The system sampled at 120 Hz and the gaze angle was determined by the relative positions of corneal and pupil centers. Participants were seated approximately 27 in from a 19-in flat panel monitor that displayed the stimuli. Eye data were recorded with ASL EYEPOS software. Stimuli consisted of black and white, high-contrast clipart images measuring 4.4 in wide by 6.5 in high. Unique images were used for each trial.
Procedure
Participants were seated comfortably in front of the monitor and their heads positioned within a chin rest to maintain their head/viewing position. Eye position was calibrated for each participant using a 9-point array. System parameters were adjusted until the participant’s fixations accurately mapped onto the calibration points. Participants were told that images would begin to appear on the computer screen and were instructed to look at the images “as if watching television.” During testing, the participants eye fixations and eye movements were recorded and stored for later analyses. The entire testing procedure lasted approximately 25 to 30 minutes, including calibration.
For the VPC task, each trial consisted of 2 phases; a familiarization phase followed by a test phase. During the familiarization phase, 2 identical images were presented side by side on the monitor for 5 seconds. The monitor then went dark for a delay interval of either 2 seconds or 2 minutes. In the test phase, 2 images were again presented side by side for 5 seconds. One of the images was identical to the image presented during the familiarization phase and the other was a novel image. Presentation of the novel image on the left or right side was selected pseudorandomly and distributed equally. Following the test phase of the trial, the monitor was darkened for 10 seconds until the start of the next trial. To ensure participant attention for test trials that had 2-minute delays, the experimenter verbally alerted all participants that there was “approximately 10 seconds before the next pair of images.”
Data Analysis
Eye fixation and movement data for each participant were extracted and analyzed offline using ASL EYENAL software. A fixation was defined as a point of gaze continually remaining within 1° visual angle for a period of 100 milliseconds or more. Fixations used for data analysis could occur within 2 designated areas of interest, the area of the novel image and the area of the familiar image. Eye data were characterized using percent looking time on the novel image. For this measure, the median of the 10 trials at the delay interval (2 minutes) was selected as the representative value for each participant in order to accommodate for outliers.
Results
As shown in Table 1, there were no significant differences between the CON and the aMCI groups in age or education. Additional analyses showed no significant differences between the CON and the aMCI groups on scores in Digit Span Forward, Clock Drawing Test, and the Geriatric Depression Scale (all P’s > .05). As might be expected, the aMCI group was significantly impaired relative to the CON group in other measures including the Word List Memory Test and the Mini-Mental State Examination. Demographic and neuropsychological performance data are also included for a group consisting of 20 patients with AD used in 1 of the analyses.
During the course of the study, 13 patients with aMCI had a change in diagnosis to AD and 4 CON participants had a change in diagnosis to aMCI (converter [CONV] total N = 17, Table 2, upper panel). On the VPC task, the 17 participants in the CONV group had significantly lower scores in the measure of percentage looking time on Novel during the test phase than the nonconverter (NONCONV) group (Table 2, lower panel). Within the CONV group, the scores for the CON and the aMCI groups on percentage looking time on Novel did not differ (52.3% vs 53.7%, P > .05). In additional analyses, we compared the scores of the CONV group (separated into aMCI and CON) to those of 20 patients with AD (Table 1) using the same testing paradigm. The scores on percentage looking time on Novel for the aMCI and CON groups did not differ from the score of the AD group (53.1%; P’s > .05). Finally, in separate comparisons for both the CON and the aMCI in the CONV group, their mean scores were significantly lower than the mean scores for the NONCONV (all P’s < .01).
Table 2.
Demographic Information and Neuropsychological Performance Scores for aMCI and CON Participants Sorted by Conversion Status (CONV = Converters; NONCONV = Nonconverters).a
| Measure | CONV | NONCONV | t Tests |
|---|---|---|---|
| Total N | 17 [4/13] | 75 [56/19] | NS |
| Age | 67.4 (9.2) | 70.5 (6.9) | NS |
| Education | 15.2 (3.4) | 15.7 (2.7) | NS |
| CERAD | |||
| Animal Fluency | 15.5 (5.1) | 19.3 (5.2) | t = 2.71, P = .008 |
| Boston Naming Test | 25.7 (3.2) | 26.3 (3.7) | NS |
| Mini-Mental State Examination | 27.4 (1.8) | 28.8 (1.5) | t = 3.27, P = .002 |
| Word List Memory (WLM)b | |||
| WLM total | 14.7 (4.3) | 21.4 (4.5) | t = 5.18, P < .0001 |
| WLM delayed recall | 3.4 (2.0) | 6.6 (2.5) | t = 4.67, P < .0001 |
| Trail Making Test (TMT) | |||
| TMT-A | 51.7 (20.3) | 36.2 (14.7) | t = 3.63, P = .0005 |
| TMT-Bc,d | 140.9 (75.1) | 95.6 (45.6) | t = 3.15, P = .002 |
| Digit Span Forward | 8.3 (1.7) | 8.7 (1.8) | NS |
| Digit Span Backward | 5.9 (2.4) | 6.7 (2.0) | NS |
| Clock Drawing Test | 12.0 (0.9) | 12.3 (0.9) | NS |
| Geriatric Depression Scalee | 2.9 (2.7) | 1.6 (1.9) | t = 2.18, P = .032 |
| Eye tracking variables (2-minute delay) | |||
| Familiarization phase | |||
| Total number of fixations | 14.7 (1.9) | 14.4 (2.3) | NS |
| Total Looking Time, seconds | 3.9 (0.5) | 4.0 (0.5) | NS |
| Test phase | |||
| % Looking Time on Novel | 53.3 (7.8) | 68.9 (8.6) | t = 6.82, P < .0001 |
| Total number of fixations | 13.6 (2.5) | 13.6 (2.4) | NS |
| Total Looking Time, seconds | 3.9 (0.6) | 4.1 (0.5) | NS |
Abbreviations: AD, Alzheimer’s disease; aMCI, amnestic mild cognitive impairment; CERAD, Consortium to Establish a Registry for Alzheimer’s disease; CON, control; GDS, Geriatric Depression Scale; NS, not significant.
a Brackets indicate [CON/aMCI]; the mean for each variable is given with standard deviationsin parentheses.
b WLM: 23 participants (CONV = 2; NONCONV = 21) were not administered the WLM test.
c Indicates the variances are not equal and differ significantly.
d TMT-B: 5 participants (CONV = 1; NONCONV = 4) did not complete the test in the allotted time frame. Scores for these participants are not included in the TMT-B score.
e GDS: 4 participants (CONV = 1; NONCONV = 3) were not administered the GDS.
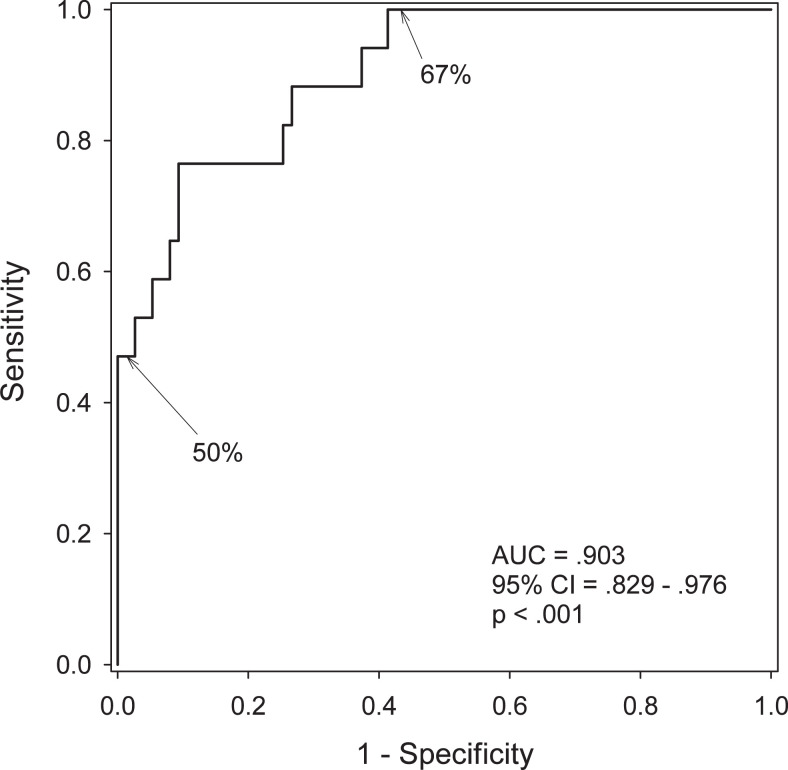
A receiver–operating characteristic curve (Figure 1) was generated using the VPC scores for all 92 participants who were classified into 2 categories (CONV vs NONCONV), based on whether or not a participant’s diagnosis had worsened during the 3 years following VPC testing. The area under the curve is 0.903, which indicates the VPC task can powerfully discriminate between participants who will convert and those who will not convert to aMCI/AD.
Figure 1.
Receiver–operating characteristic (ROC) curve for the VPC task shows that the task powerfully discriminates between participants who will convert and those who will not convert to aMCI/AD. The 2-minute delay scores from the VPC task for all participants (N = 92) were divided into 2 groups (converters = 17 and nonconverters = 75), with group designation based on diagnosis at time of writing (up to 3 years from testing). The 50% and 67% on the graph indicate the cut points in the score ranges. AD indicates Alzheimer’s disease; aMCI, amnestic mild cognitive impairment; VPC, Visual Paired Comparison.
Figure 2 illustrates the relationship between 3 ranges of scores on the VPC task and time to subsequent conversion to aMCI/AD. All but 1 of the participants who scored <50% on the VPC task converted to AD or aMCI within 3 years of testing. For participants with scores >50% but less than 67%, there was less risk of conversion to aMCI or AD. Importantly, individuals with scores >67%, regardless of whether they were categorized as CON participants or patients with aMCI, were at zero risk for further cognitive decline within 3 years of testing.
Figure 2.
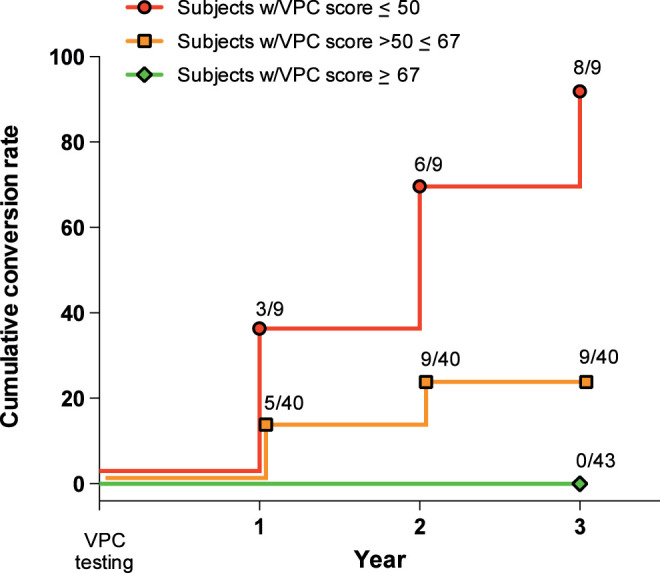
Cumulative proportion of participants whose diagnosis changed across time based on their VPC 2-minute delay score. The colors delineate 3 ranges of scores: red (<50% novelty preference); orange (novelty preference between 50% and 67%); and green (>67% novelty preference). The left axis indicates percentage of participants whose diagnosis converted from aMCI to AD (n = 13) or from CON to aMCI (n = 4; at the time of writing, 1 of these 4 participants has converted to AD). Log rank P < .001. AD indicates Alzheimer’s disease; aMCI, amnestic mild cognitive impairment; CON, control; VPC, Visual Paired Comparison.
A Cox regression model revealed that a low VPC score was a significant predictor of conversion (hazard ratio = 0.834 per percent, 95% confidence interval = 0.739-0.941, P = .003) while neither baseline diagnostic category (P = .350) nor the interaction (P = .221) predicted conversion, indicating that VPC score was not differentially predictive across the diagnostic groups.
Discussion
The present results make several points regarding the usefulness of the VPC task. First, the scores on the VPC task predicted change in diagnosis from aMCI to AD or from CON to aMCI, for some individuals up to 3 years before a change in clinical diagnosis. Thus, these new findings suggest that performance on the VPC task serves as a powerful prognostic indicator of looming cognitive decline.
Second, in the present study, the distinction between patients with single- or multidomain aMCI was irrelevant to the prognostic capability of the VPC task. That is, in the aMCI group, the percentage of single-domain participants who converted (36%), was not different than the percentage of multidomain participants who converted (43%, P = .72). Moreover, a low performance score on the VPC task was predictive of later conversion regardless of whether the participants were in the aMCI or CON groups. This is particularly relevant since 1 participant in the CON group, with a low score on the VPC task, was diagnosed with aMCI, and subsequently with AD, within the time frame of this study.
Third, normal performance on the VPC task has been shown to require the integrity of the medial temporal lobe memory system.9–12 Accordingly, the VPC task might prove useful in predicting onset and progression of memory dysfunction that is linked to several other medical conditions where disruption of the medial temporal lobe memory system could occur, for example, depression, autism spectrum disorder, and human immunodeficiency virus (HIV)/AIDS.
Fourth, no matter the disease, early detection is an important strategy for effective therapeutic intervention. Because the VPC task can detect oncoming cognitive decline up to 3 years sooner than standard clinical diagnostic approaches, intervention could both begin sooner, when the brain is less compromised, and could be more effective than it would be later in the course of the disease. This kind of information can be crucial in order to inform planning and treatment options for the individual, the family, and the clinician.
Fifth, few effective interventions are available to significantly alter the course of decline associated with AD. Nevertheless, a 3-year early warning about the potential onset of AD could give individuals and families an important window of time to prepare for the future. Although other neuropsychological tests have some predictive value,16–18 none approximates the demonstrated predictability of the VPC task.
Finally, there has been controversy regarding the nomenclature and the clinical utility of MCI as a diagnostic category.2,3 That is, whether MCI is truly an independent diagnostic category and a predictor of oncoming AD or simply an early form of AD itself.1–3 The findings here suggest that the VPC task is predictive of worsening cognition regardless of the diagnostic category at the time of testing, that is, no matter whether the diagnosis was aMCI or CON. Further research using the VPC task could help clarify the utility of MCI diagnosis for predicting the onset of cognitive decline. Additional research with the task could also help inform the selection of individuals for clinical trials as well as help identify who might benefit most from emerging treatment strategies.
Footnotes
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Zola and Ms Manzanares are inventors of Emory University's patent of the technology used in this manuscript and are entitled to licensing fee and royalties from Neurotrack Technologies, Inc, which is developing products related to the research described in this article. In addition, these authors are founders of Neurotrack Technologies, Inc and they (and Emory University) own equity in the company. A part of this article was presented at the Alzheimer's Association International Conference, Vancouver, Canada, July 2012.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: National Institute of Health Grant AG 025688, Yerkes NPRC Base Grant RR000165 (now ORIP/OD P51OD11132), Robert W. Woodruff Health Science Award from Emory University.
References
- 1.Dubois B, Albert ML. Amnestic MCI or prodromal Alzheimer’s disease? Lancet Neurol. 2004;3(4):246–248. [DOI] [PubMed] [Google Scholar]
- 2.Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment—beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256(3):240–246. [DOI] [PubMed] [Google Scholar]
- 3.Allegri RF, Glaser FB, Taragano FE, Buschke H. Mild cognitive impairment: believe it or not? Int Rev Psychiatry. 2008;20(4):357–363. [DOI] [PubMed] [Google Scholar]
- 4.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303–308. [Erratum, Arch Neurol. 1999;56:760]. [DOI] [PubMed] [Google Scholar]
- 5.Peterson RC, Roberts RO, Knopman DS, et al. Mild cognitive impairment: ten years later. Arch Neurol. 2009;66(12):1447–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Neurology. 2001;56(9):1133–1142. [DOI] [PubMed] [Google Scholar]
- 7.Riverol M, Lopez OL. Biomarkers in Alzheimer’s disease. Front Neurol. 2011;2:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Praticò D. Alzheimer's disease and the quest for its biological measures [published online June 12, 2012]. J Alzheimers Dis. [DOI] [PubMed] [Google Scholar]
- 9.Crutcher MD, Calhoun-Haney R, Manzanares CM, Lah JJ, Levey AI, Zola SM. Eye tracking during a visual paired comparison task as a predictor of early dementia. Am J Alzheimers Dis Other Demen. 2009;24(3):258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zola SM, Squire LR, Teng E, Stefanacci L, Buffalo EA, Clark RE. Impaired recognition memory in monkeys after damage limited to the hippocampal region. J Neurosci. 2000;20(1):451–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manns JR, Stark CE, Squire LR. The visual paired-comparison task as a measure of declarative memory. Proc Natl Acad Sci U S A. 2000;97(22):12375–12379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bachevalier J, Brickson M, Hagger C. Limbic-dependent recognition memory in monkeys develops early in infancy. Neuroreport. 1993;4(1):77–80. [DOI] [PubMed] [Google Scholar]
- 13.Morris JC, Weintraub S, Chui HC, et al. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord. 2006;20(4):210–216. [DOI] [PubMed] [Google Scholar]
- 14.Beekly DL, Ramos EM, Lee WW, et al. The National Alzheimer’s Coordinating Center (NACC) database: the Uniform Data Set. Alzheimer Dis Assoc Disord. 2007;21(3):249–258. [DOI] [PubMed] [Google Scholar]
- 15.Weintraub S, Salmon D, Mercaldo N, et al. The Alzheimer’s Disease Centers’ Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord. 2009;23(2):91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen P, Ratcliff G, Belle SH, Cauley JA, DeKosky ST, Ganguli M. Patterns of cognitive decline in presymptomatic Alzheimer disease: a prospective community study. Arch Gen Psychiatry. 2001:58(9):853–858. [DOI] [PubMed] [Google Scholar]
- 17.Saxton J, Lopez OL, Ratcliff G, et al. Preclinical Alzheimer disease: Neuropsychological test performance 1.5 to 8 years prior to onset. Neurology. 2004:63(12):2341–2347. [DOI] [PubMed] [Google Scholar]
- 18.Tabert MH, Manly JJ, Liu X, et al. Neuropsychological prediction of conversion to Alzheimer disease in patients with mild cognitive impairment. Arch Gen Psychiatry. 2006:63(8):916–924. [DOI] [PubMed] [Google Scholar]