Abstract
Objectives
Delayed onset of lactogenesis II (initiation of copious milk production) is frequently been utilized as an outcome but only twice as a hypothesis-testing exposure variable. It remains unclear if previous results are replicable in a large nationally representative population. We evaluated the association between delayed lactogenesis II (>3 days postpartum; DLII) and the cessation of any and exclusive breastfeeding at 4-weeks postpartum.
Study Design
We constructed multivariable logistic regression models using data from 2491 mothers enrolled in the prospective Infant Feeding Practice Study II cohort. Models included DLII, known risk factors for breastfeeding cessation (age, education, race, parity/previous breastfeeding and exclusive breastfeeding plan) and potential confounders identified in bivariate analyses (p≤ 0.1). Backward selection processes (p≤ 0.1) determined risk factor retention in the final model.
Results
DLII was associated with cessation of any and exclusive breastfeeding at 4-weeks postpartum (OR: 1.62; CI: 1.14–2.31; OR: 1.62; CI 1.18–2.22 respectively); numerous independent risk factors qualified for inclusion in the multivariable model(s) and were associated with the outcome(s) of interest (e.g. WIC enrollment, onset of prenatal care, feeding on-demand, time initiated first breastfeed, hospital rooming-in, obstetric provider preference for exclusive breastfeeding and maternal tobacco use).
Conclusions
Women experiencing DLII may be less able to sustain any and/or exclusive breastfeeding in the early postpartum period. These findings have significant clinical and programmatic implications. Routine assessment of DLII in postpartum breastfeeding follow-up is warranted, and women with DLII may benefit from additional early postpartum interventions to support favorable breastfeeding outcomes.
Keywords: lactation, infant feeding, lactogenesis, maternal child nursing
Introduction
Lactogenesis II represents the onset of copious milk secretion, and due to maternal reports of breast fullness and increased volume, lactogenesis II is anecdotally referred to as “milk coming in”.1 Through detailed longitudinal measurement of breast milk volume and composition, Neville and colleagues observed that milk volume increased steadily between 36 and 96 hours post partum and subsequently plateaued (n=13).2
Delayed lactogenesis II (DLII) is defined as lactogenesis II onset more than 72 hours post partum.3, 4 DLII can contribute to early breastfeeding cessation.5–7 In various populations and non-emergent cesarean births, incidence rates of DLII range from 17% to 44%.3, 4, 8, 9 Numerous factors associated with DLII have been extensively reviewed elsewhere.7 Evidence suggests the following factors are independent predictors of DLII: age9, parity4, 10–13, maternal obesity3, 4, 9, 14, prenatal care provider15, delivery mode3, 4, 11, 15, prolonged second stage labor3, 4, labor pain medication4, exogenous oxytocin use4, stress during delivery4, 10, 16, infant birthweight9, infant Apgar score <88, flat/inverted nipples4, supplementation within 48 hours post partum3, 4, 6, infant excess weight loss4 and nipple pain when breastfeeding9. Of note, many of these predictors are also associated with poor breastfeeding outcomes (e.g. lower initiation rates or increased cessation of any and/or exclusive breastfeeding).5, 17, 18
To our knowledge, DLII, most commonly used as an outcome variable3, 8, 13, 15, has only been used twice as a formal exposure variable5, 6 (in one US and one non-US population). It remains unclear if previous results are replicable in a large nationally representative population of women, and whether DLII (as an exposure variable) affects early breastfeeding cessation in the presence of other risk factors. We conducted a secondary analysis to evaluate the following research question: among women initiating breastfeeding, is there an association between DLII and the cessation of any and exclusive breastfeeding at 4 weeks postpartum?
Patients and Methods
These analyses used the US Food and Drug Administration and the Centers for Disease Control and Prevention prospective cohort study, the Infant Feeding Practice Study II.19 Conducted to better understand the infant feeding practices used by mothers in the United States, this study provided detailed information regarding: infant feeding patterns during the first year, various aspects of infant health (specifically factors that may affect infant feeding), and information regarding mothers’ health and diet.19 Participants were drawn from a nationally distributed large consumer opinion panel comprised of almost 5000 women.19 Data, collected from May 2005 through June 2007, included 1 prenatal and 10 post partum surveys spanning one year postpartum.19
Our analysis included 2491 mothers who initiated breastfeeding and met the following criteria: aged ≥ 18 with healthy singleton infants of at least 35 weeks gestational age, ≥ 5 lbs and no NICU admission. Additionally mothers had to have returned, at minimum, the neonatal survey distributed at about 3 weeks postpartum.
We derived the exposure (DLII) from the following neonatal survey item: “How long did it take your milk to come in…1 day or less, 2 days, 3 days, 4 days or more than 4 days?” (http://www.cdc.gov/ifps/questionnaires.htm). Adapting the standard 72 hour postpartum cutoff, we collapsed and dichotomized responses to create the exposure variable (≤3 days, DLII=no; ≥4 days DLII=yes).3 The primary outcome was cessation of any breastfeeding at 4 weeks; the secondary outcomes of interest was cessation of exclusive breastfeeding at 4 weeks. Due to the nature of available data, we used actual maternal return to work time as a proxy for planned return to work and tobacco use at three months as a surrogate for tobacco use in the early post partum period. All data were self-reported.
Statistical Methods
Bivariate analyses compared mothers who had and had not experienced DLII (the exposure) across identified maternal demographics, exclusive breastfeeding plan, obstetric factors, hospital practices and various lifestyle factors. To describe differences in the distribution of these factors across exposure groups, chi-square tests detected covariates independently associated with DLII.
Odds ratios estimated the crude effect of DLII on the cessation of any and exclusive breastfeeding at 4 weeks. Multivariable logistic regression models were developed and adjusted for known risk factors including maternal age, race, education, parity/previous breastfeeding experience and exclusive breastfeeding plan. Additional potential confounders, identified in the bivariate analyses (p≤0.10), were retained in the model as determined by a backward selection process (retention cut off p≤0.10).
For a comparison of two independent binomial proportions using Pearson’s Chi-square statistic with a Chi-square approximation and a two-sided alpha of 0.05, we had over 80% power to detect a 5% difference in cessation of any breastfeeding within 4 weeks post partum between DLII groups. Analyses were conducted in Statistical Analysis Systems, version 9.2 (SAS Institute, Cary, NC). All p-values were two-sided, and significance was set at alpha <0.05.
Results
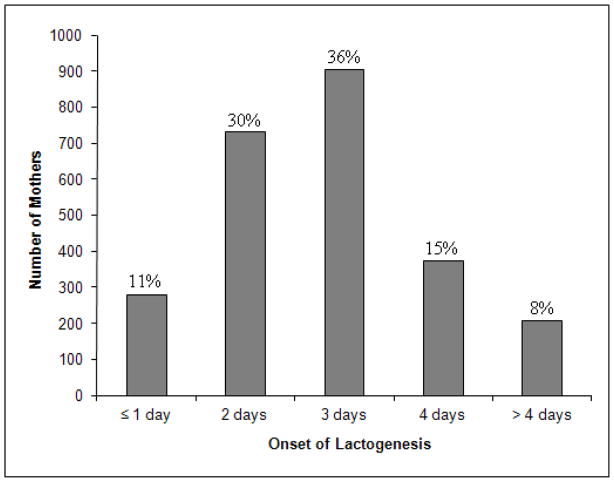
For the participants meeting our study inclusion criteria, the neonatal survey response time ranged from < 1 week up to 24 weeks post partum (mean=6.1; SD=2.5; median=5.6; mode=4.9). This range reflects both the timing of survey mailings (earlier outliers) and the lag in response time (later outliers). Among the above measures of central tendency, we selected the highest and determined that mean response time (< 6.1 and ≥ 6.1 weeks) did not differ by DLII status (chi-square p-value=0.44). Thus, we included all participants in our analyses. The distribution of days of self report onset of lactation are presented in Figure 1 (mean=2.8 days; SD=1.1). Mean duration of any breastfeeding was 26.0 weeks (SD=19.86; median=21.5 weeks; mode=51.6 weeks; range: 0.1–64.7 weeks). The incidence of DLII in the study population was 23.2%. Regardless of DLII status, the pattern of breastfeeding outcomes was similar (Figure 2).
Figure 1.
Distribution of Maternal Self Report Onset of Lactogenesis (n=2491)
Figure 2.
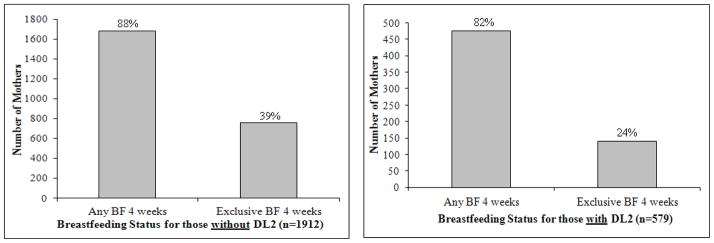
Breastfeeding outcomes at 4 weeks for women experiencing delayed lactogenesis II.
Characteristics of the sample were stratified by DLII status and presented in Table 1. Bivariate analyses identified the following potential confounders eligible for inclusion in the multivariable models (p-value ≤ 0.10): maternal characteristics (geographic residential location, federal poverty level, postnatal WIC enrollment, household size), obstetric/hospital characteristics (timing of first prenatal care visit, low intervention vaginal delivery, epidural/spinal use, hospital rooming-in, timing of initiation of first breastfeed, feeding on demand, in-hospital breastfeeding support and obstetric provider prefers exclusive breastfeeding) and postpartum/lifestyle characteristics (pediatric provider favors exclusive breastfeeding, maternal return to work and tobacco use).
Table 1.
Demographics and Confounders by DLII Status.
| Variables | DLII- Yes n=579 n (%) | DLII- No n=1912 n (%) | p-valuea |
|---|---|---|---|
| Demographics | |||
| Ageb | 0.29 | ||
| 18–24 | 116 (20.07) | 438 (22.94) | |
| 25–34 | 372 (64.36) | 1165 (61.03) | |
| > 34 | 90 (15.57) | 306 (16.03) | |
| Race/Ethnicityb | 0.29 | ||
| White | 468 (83.72) | 1556 (83.39) | |
| Black | 18 (3.22) | 97 (5.20) | |
| Hispanic | 43 (7.69) | 118 (6.32) | |
| Other | 30 (5.37) | 95 (5.09) | |
| Educationb | 0.61 | ||
| ≤ High School | 93 (17.35) | 328 (18.43) | |
| Some College | 230 (42.91) | 722 (40.56) | |
| ≥ College Completion | 213 (39.74) | 730 (41.01) | |
| Federal Poverty Level | < 0.01 | ||
| < 185 % | 213 (36.79) | 791 (41.37) | |
| 185–350 % | 204 (35.23) | 701 (36.66) | |
| > 350 % | 162 (27.98) | 420 (21.97) | |
| Postnatal WIC Enrollmentb | 0.03 | ||
| Yes | 140 (24.18) | 549 (28.77) | |
| No | 439 (75.82) | 1359 (71.23) | |
| Geographic Residential Location | 0.04 | ||
| Northeast | 89 (15.37) | 304 (15.90) | |
| Midwest | 150 (25.91) | 584 (30.54) | |
| South | 214 (36.96) | 595 (31.12) | |
| West | 126 (21.76) | 429 (22.44) | |
| Household Size | < 0.01 | ||
| ≤ 4 Members | 522 (90.16) | 1589 (83.11) | |
| > 4 Members | 57 (9.84) | 323 (16.89) | |
| Infant Feeding Factors | |||
| Parityb | < 0.01 | ||
| Primiparous | 255 (44.97) | 481 (25.83) | |
| Multiparous | 312 (55.03) | 1381 (74.17) | |
| Previous BF Experienceb | < 0.01 | ||
| Yes | 272 (48.31) | 1289 (69.68) | |
| No | 291 (51.69) | 561 (30.32) | |
| Parity/Previous BF Experienceb, c | < 0.01 | ||
| Primparous | 255 (45.29) | 481 (26.00) | |
| Multiparous with BF experience | 272 (48.31) | 1289 (69.68) | |
| Multiparous without BF experience | 36 (6.39) | 80 (4.32) | |
| Exclusive BF Planb | 0.10 | ||
| Exclusively BF | 387 (66.96) | 1350 (70.90) | |
| Not Exclusively BF | 167 (28.89) | 500 (26.26) | |
| Doesn’t Know | 24 (4.15) | 54 (2.84) | |
| Mother Believes Infant Formula is as Good as Breast Milk | 0.16 | ||
| Yes or Neutral | 198 (34.20) | 594 (31.07) | |
| No | 381 (65.80) | 1318 (68.93) | |
| Feeding on Demandb | 0.02 | ||
| Whenever Baby Cried or Felt Hungry | 278 (48.10) | 1040 (54.59) | |
| On a Schedule or Routine | 46 (7.96) | 129 (6.77) | |
| Both | 254 (43.94) | 736 (38.64) | |
| Initiation of First BFb | < 0.01 | ||
| 0–30 Minutes Post Partum | 125 (21.74) | 653 (34.48) | |
| 31–60 Minutes Post Partum | 166 (28.87) | 481 (25.40) | |
| > 1 Hour Post Partum | 284 (49.39) | 760 (40.12) | |
| Health Care Provider Factors | |||
| Timing of First Prenatal Care Visitb | 0.10 | ||
| < 13 weeks | 524 (91.13) | 1678 (88.74) | |
| ≥ 13 weeks | 51 (8.87) | 213 (11.26) | |
| Obstetric Provider Favors Exclusive BFb | 0.01 | ||
| Yes | 189 (32.70) | 732 (38.61) | |
| No | 389 (67.30) | 1164 (61.39) | |
| Pediatric Provider Favors Exclusive BFb | < 0.01 | ||
| Yes | 259 (44.97) | 981 (51.74) | |
| No | 317 (55.03) | 915 (48.26) | |
| Hospital Practices | |||
| Low Intervention Vaginal Birthb, d | < 0.01 | ||
| Yes | 175 (30.33) | 800 (41.95) | |
| No | 402 (69.67) | 1107 (58.05) | |
| Epidural/Spinal Statusb | < 0.01 | ||
| Yes | 484 (83.74) | 1388 (72.78) | |
| No | 94 (16.26) | 519 (27.22) | |
| Hospital Rooming-Inb | 0.03 | ||
| All the Time | 308 (53.29) | 1132 (59.58) | |
| Sometimes | 154 (26.64) | 437 (23.00) | |
| Never | 116 (20.07) | 331 (17.42) | |
| In-Hospital BF Supportb | < 0.01 | ||
| Yes | 485 (83.91) | 1360 (71.47) | |
| No | 93 (16.09) | 543 (28.53) | |
| Lifestyle Factors | |||
| Prenatal Obesityb, e | 0.06 | ||
| Yes | 148 (25.78) | 415 (21.98) | |
| No | 426 (74.22) | 1473 (78.02) | |
| Maternal Return to Workb | 0.02 | ||
| 0–6 Months | 64 (15.65) | 190 (14.88) | |
| > 6 Months | 252 (61.61) | 706 (55.29) | |
| Did Not Return | 93 (22.74) | 381 (29.84) | |
| Post Partum Maternal Tobacco Useb | 0.06 | ||
| Any | 65 (13.98) | 162 (10.81) | |
| None | 400 (86.02) | 1337 (89.19) | |
Abbreviations: NICU, neonatal intensive care unit; DLII, delayed onset of lactogenesis II; WIC, Women Infant and Children; BF, breastfeeding; FF, formula feeding; N/A, not applicable.
Missing data were excluded when calculating global p-values
Observations<2491
Combined variables (parity and breastfeeding experience variables) used in the multivariable model, instead of 2 separate variables.
Maternal BMI cutoff of a score of 30 or higher
Vaginal birth with no induction
Cessation of Any Breastfeeding
The crude odds ratios for the effect of DLII on any breastfeeding was statistically significant (OR: 1.59; CI: 1.24–2.05) and remained so after multivariable analyses (OR: 1.62; CI: 1.14–2.31) (Table 2). Additional statistically significant risk factors were also identified in the multivariable model (Table 2).
Table 2.
Early cessation of any and exclusive breastfeeding: multivariable logistic regression.
| Variables | Any BF Cessation within 4 weeks | Exclusive BF Cessation by 4 weeks | ||
|---|---|---|---|---|
|
| ||||
| OR (95% CI) n=1754 | p-valuea | OR (95% CI) n=1372 | p-valuea | |
| DLII | < 0.01 | < 0.01 | ||
| Yes | 1.62 (1.14–2.31) | 1.62 (1.18–2.22) | ||
| No | 1 [Reference] | 1 [Reference] | ||
| Age | < 0.01 | 0.04 | ||
| 18–24 | 1.75 (1.15–2.66) | 1.58 (1.04–2.39) | ||
| 25–34 | 1 [Reference] | 1 [Reference] | ||
| > 34 | 0.62 (0.37–1.03) | 1.30 (0.94–1.80) | ||
| Race/Ethnicity | 0.05 | 0.10 | ||
| White | 1 [Reference] | 1 [Reference] | ||
| Black | 0.85 (0.41–1.75) | 2.13 (0.94–4.82) | ||
| Hispanic | 0.37 (0.16–0.83) | 1.03 (0.58–1.83) | ||
| Other | 0.47 (0.17–1.28) | 1.88 (0.94–3.75) | ||
| Education | 0.02 | 0.11 | ||
| ≤ High School | 1.81 (1.09–3.01) | 1.55 (1.00–2.40) | ||
| Some College | 1.76 (1.16–2.66) | 1.29 (0.95–1.75) | ||
| ≥ College | 1 [Reference] | 1 [Reference] | ||
| Previous BF Experience | < 0.01 | 0.03 | ||
| Primparous | 1.91 (1.29–2.81) | 1.45 (1.05–2.00) | ||
| Multiparous with previous BF | 1 [Reference] | 1 [Reference] | ||
| Multiparous without previous BF | 4.26 (2.39–7.59) | 1.95 (0.85–4.49) | ||
| Exclusive BF Plan | < 0.01 | < 0.01 | ||
| Exclusively BF | 1 [Reference] | 1 [Reference] | ||
| Not Exclusively BF | 3.68 (2.59–5.22) | 4.72 (3.26–6.83) | ||
| Doesn’t Know | 5.65 (2.90–11.03) | 2.35 (0.96–5.76) | ||
| Geographic Residential Location | 0.02 | |||
| Northeast | 1 [Reference] | NA | ||
| Midwest | 1.45 (0.89–2.39) | NA | ||
| South | 1.22 (0.74–2.03) | NA | ||
| West | 0.63 (0.34–1.17) | NA | ||
| Household Size | 0.26 | |||
| ≤ 4 Members | 1 [Reference] | NA | ||
| > 4 Members | 1.31 (0.82–2.10) | NA | ||
| Pediatric Provider Favors Exclusive BF | < 0.01 | |||
| Yes | 1 [Reference] | NA | ||
| No | 2.12 (1.50–2.99) | NA | ||
| Hospital Support | 0.01 | |||
| Yes | 1 [Reference] | NA | ||
| No | 0.58 (0.37–0.90) | NA | ||
| Maternal Tobacco Use | < 0.01 | 0.09 | ||
| Yes | 1.82 (1.19–2.78) | 1.50 (0.94–2.39) | ||
| No | 1 [Reference] | 1 [Reference] | ||
| FPL | 0.04 | |||
| < 185% | NA | 1 [Reference] | ||
| 185–350% | NA | 1.54 (1.11–2.15) | ||
| > 350% | NA | 1.31 (0.88–1.95) | ||
| Postnatal WIC Enrollment | 0.04 | |||
| Yes | NA | 1.52 (1.05–2.21) | ||
| No | NA | 1 [Reference] | ||
| Feeding on Demand | < 0.01 | |||
| Whenever Baby Cried or Felt Hungry | NA | 1 [Reference] | ||
| On a Schedule or Routine | NA | 2.15 (1.18–3.95) | ||
| Both | NA | 1.43 (1.10–1.87) | ||
| Initiation of First BF | < 0.01 | |||
| 0–30 Min PP | NA | 1 [Reference] | ||
| 31–60 Min PP | NA | 1.06 (0.77–1.45) | ||
| > 1 Hour PP | NA | 1.93 (1.41–2.64) | ||
| Timing of First Prenatal Care Visit | 0.02 | |||
| < 13 Weeks | NA | 1 [Reference] | ||
| ≥ 13 Weeks | NA | 1.70 (1.07–2.69) | ||
| Obstetric Provider Favors Exclusive BF | < 0.01 | |||
| Yes | NA | 1 [Reference] | ||
| No | NA | 1.53 (1.18–1.98) | ||
| Low Intervention Vaginal Birthb | 0.09 | |||
| Yes | NA | 1 [Reference] | ||
| No | NA | 1.27 (0.97–1.67) | ||
| Epidural/Spinal Status | 0.38 | |||
| Yes | NA | 1.15 (0.84–1.57) | ||
| No | NA | 1 [Reference] | ||
| Prenatal Obesityc | < 0.01 | |||
| Yes | NA | 1.66 (1.22–2.27) | ||
| No | NA | 1 [Reference] | ||
| Maternal Return to Work | 0.50 | |||
| 0–6 Months | NA | 1.09 (0.73–1.64) | ||
| > 6 Months | NA | 1.19 (0.89–1.58) | ||
| Did Not Return | NA | 1 [Reference] | ||
Abbreviations: BF, breastfeeding; OR, odds ratio; CI, confidence interval; DLII, delayed onset of lactogenesis II; NA, not applicable; FPL, federal poverty level; WIC, Women Infant and Children; Min, minutes; PP Post Partum.
p-values represent the type 3 analysis of effects wald chi-square p-value.
Vaginal birth with no induction.
Maternal BMI cutoff of a score of 30 or higher.
Exclusive Breastfeeding Cessation
The crude odds ratio for the effect of DLII on exclusive breastfeeding was also statistically significant (OR: 2.04; CI: 1.66–2.52), and though slightly attenuated, this significant association remained after multivariable analysis (OR: 1.62; CI: 1.18–2.22) (Table 2). Again, numerous statistically significant risk factors were identified in the multivariable model (Table 2).
Discussion
Utilizing a large national dataset, these analyses included a wide variety of demographic, obstetric, institutional and post partum lifestyle factors to examine the effect of DLII on the cessation of any and exclusive breastfeeding in the early post partum period. Since the variable selection process did not produce identical models (due to variability in covariate retention), we cannot statistically comment on the robustness of the DLII point estimates. However, we observed a similar and statistically significant effect of DLII on the cessation of any and exclusive breastfeeding at 4 weeks postpartum.
To our knowledge, only two other groups previously utilized DLII as an exposure to examine breastfeeding outcomes. Chapman and Perez-Escamilla conducted a secondary analysis of a larger cohort of breastfeeding women in Hartford, CT (n=92).5 Among women planning to breastfeed, they assessed the effect of DLII (<6 months and ≥6 months) on breastfeeding duration.5 Among women planning to breastfeed <6 months, no statistically significant effect was observed regarding DLII on breastfeeding duration (OR: 0.7; CI: 0.4–1.0).5 Among women planning to breastfeed ≥6 months, women without DLII were more likely to continue breastfeeding (OR: 3.0; CI: 1.19–4.8).5 No global point estimates assessed the singular effect of DLII on breastfeeding duration (without accounting for breastfeeding plan).
In four rural Guatemalan communities, Hruschka and colleagues evaluated the effect of DLII on full breastfeeding cessation within 6 months post partum.6 In their analyses, the median duration of full breastfeeding was 5 months; immediate post partum supplementation (prior to transitioning to lactogenesis II) was associated with DLII (OR: 4.87; CI: 2.29–10.36) and also predicted full breastfeeding cessation within 6 months post partum (HR: 1.49; CI: 1.05–2.11).6 DLII significantly predicted full breastfeeding cessation in 2 of the 4 communities (HR: 2.87; CI: 1.25–6.60 and HR: 3.43; CI: 1.55–7.59).6
Our overall results and directionality of the point estimates are consistent with previous research, although the study populations, outcomes of interest and availability of relevant confounders differed.5, 6 Though the timing of the outcome is different, our results add evidence to support the robustness of the demonstrated deleterious association between DLII and breastfeeding outcomes. The incidence of DLII in our study population (23%) fell within the range of previously published incidence rates for DLII (17% – 44%).3, 4, 8, 9
These results have considerable clinical and programmatic implications. Care providers may advise mothers who report a delay in the onset of copious milk production to give infant formula supplements. This approach may ultimately shorten breastfeeding duration.6 DLII may be a useful clinical indicator to identify women at risk of early post partum breastfeeding cessation.7 In this case, encouraging effective and frequent milk removal (rather than introduction of formula supplements) to maintain lactation would be appropriate; however, we acknowledge that clinical management of women with DLII should be individualized and temporary use of donor milk or formula supplements may be necessary to prevent excessive infant dehydration or weight loss.1, 4, 20, 21, 22
These analyses have several important limitations. First, this is a secondary analysis; results are generalizable to women satisfying the primary study’s inclusion criteria. Second, we relied exclusively on self reported data (including in-hospital supplementation); while potential misclassification may occur due to recall bias, we expect this to be non-differential and therefore underestimate our demonstrated effects. Third, it is unclear if the lactogenesis onset survey question intends for mothers to count calendar or 24 hour intervals when reporting ‘days’ post partum. Collection of these data in hourly units is ideal, but given the available data, the dichotomous nature of the constructed DLII variable and the proximity of the mean to the cut point, we would again expect a non-differential misclassification bias, which in turn would attenuate our results. Fourth, if reported within 3 years post partum, maternal self-report of any breastfeeding duration is a validated measure, but this is not applicable to exclusive breastfeeding cessation.23 Of note, our breastfeeding outcome data were recalled, at most, within one year post partum. Fifth, the nature of these data did not allow for evaluation of interaction terms included in previous analyses (e.g. breastfeeding frequency and maternal obesity, intervention group and parity or intervention community and DLII).6, 24 Finally, while significant, our findings may be spurious due to unmeasured confounding. We did not have data on all previously identified predictors of both DLII and breastfeeding outcomes and specifically maternal stress, relevant in-hospital infant feeding measures (e.g. quality, frequency of feed) or additional infant factors (e.g. birth weight, Apgar Score).
Strengths of this study include the consistency with existing literature, the large sample size and corresponding sufficient power to estimate the effect, the prospective design of the primary study and the use of conservative statistical methods to minimize spurious associations (e.g. utilization of a p-value ≤0.1 cutoff for multivariable model inclusion and retention). DLII is a previously validated measure with reasonable sensitivity and specificity (71.4% and 79.3% respectively).24 Of the wide array of variables eligible for inclusion into the multivariable models, these data contained both modifiable and non-modifiable risk factors for breastfeeding cessation, spanning maternal demographics, planned infant feeding behavior, obstetric factors, hospital practices and various post partum lifestyle factors. To our knowledge, preferences of obstetrical and pediatric providers for exclusive breastfeeding have not been included in an analysis of this kind. Previous evidence from the Pregnancy Risk Assessment Monitoring System indicated that older, more educated, non-smoking women of higher socioeconomic status who did not either participate in WIC or deliver a low birth weight baby, had better breastfeeding outcomes.25 When compared to respondents of the National Survey of Family Growth (1998–2000), participants in the Infant Feeding Practice Study II were primarily older women with both higher education and incomes.19 Consequently, given the sample’s relative homogeneity, our results may underestimate the effect of DLII on the cessation of any and exclusive breastfeeding in other populations.
Conclusion
Women experiencing DLII may be less able to sustain any and exclusive breastfeeding at 4 weeks. DLII could be used as an early clinical marker to help identify women in need of additional breastfeeding support or interventions to increase breast milk production. Additionally, these analyses identified several other independent risk factors that warrant further study including postnatal WIC enrollment and the preferences of obstetric providers for not exclusive breastfeeding.
Acknowledgments
The study was funded through an R01 1R01HD055191 (PI: Dozier).
This project would not have been possible without manuscript assistance from Alice Nelson.
Abbreviations
- BF
breastfeeding
- WIC
supplemental nutrition program for women, infants and children
- OR
odds ratio
- CI
95% confidence interval
- DLII
delayed onset lactogenesis II
Footnotes
The authors declare no conflicts of interest.
Contributor’s Statement: All authors listed on this manuscript contributed substantially to either the conception and design or analysis and interpretation of data. They were also involved in the drafting or revision of its intellectual content and approved the final version submitted for publication.
Contributor Information
Elizabeth Brownell, Email: EBrownell@CCMCKids.org, Research Associate, Connecticut Children’s Medical Center, Departments of Research and Neonatology, 80 Jefferson Street, Hartford, CT, Phone: 860-545-8749, Fax: 860-545-8945.
Cynthia R. Howard, Rochester General Hospital, and Departments of Pediatrics and Community and Preventive Medicine, University of Rochester.
Ruth A. Lawrence, Departments of Pediatrics and Obstetrics and Gynecology, University of Rochester.
Ann M. Dozier, Department of Community and Preventive Medicine, University of Rochester.
References
- 1.Neville MC, Morton J, Umemura S. Lactogenesis. The transition from pregnancy to lactation. Pediatr Clin North Am. 2001 Feb;48:35–52. doi: 10.1016/s0031-3955(05)70284-4. [DOI] [PubMed] [Google Scholar]
- 2.Neville MC, Allen JC, Archer PC, Casey CE, Seacat J, Keller RP, et al. Studies in human lactation: milk volume and nutrient composition during weaning and lactogenesis. Am J Clin Nutr. 1991 Jul;54:81–92. doi: 10.1093/ajcn/54.1.81. [DOI] [PubMed] [Google Scholar]
- 3.Chapman DJ, Perez-Escamilla R. Identification of risk factors for delayed onset of lactation. J Am Diet Assoc. 1999 Apr;99:450–454. doi: 10.1016/S0002-8223(99)00109-1. quiz 455–456. [DOI] [PubMed] [Google Scholar]
- 4.Dewey KG, Nommsen-Rivers LA, Heinig MJ, Cohen RJ. Risk factors for suboptimal infant breastfeeding behavior, delayed onset of lactation, and excess neonatal weight loss. Pediatrics. 2003 Sep;112:607–619. doi: 10.1542/peds.112.3.607. [DOI] [PubMed] [Google Scholar]
- 5.Chapman DJ, Perez-Escamilla R. Does delayed perception of the onset of lactation shorten breastfeeding duration? J Hum Lact. 1999 Jun;15:107–111. doi: 10.1177/089033449901500207. quiz 137-109. [DOI] [PubMed] [Google Scholar]
- 6.Hruschka DJ, Sellen DW, Stein AD, Martorell R. Delayed onset of lactation and risk of ending full breast-feeding early in rural Guatemala. J Nutr. 2003 Aug;133:2592–2599. doi: 10.1093/jn/133.8.2592. [DOI] [PubMed] [Google Scholar]
- 7.Hurst NM. Recognizing and treating delayed or failed lactogenesis II. J Midwifery Womens Health. 2007 Nov-Dec;52:588–594. doi: 10.1016/j.jmwh.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Matias SL, Nommsen-Rivers LA, Creed-Kanashiro H, Dewey KG. Risk factors for early lactation problems among Peruvian primiparous mothers. Matern Child Nutr. 2010 Apr;6:120–133. doi: 10.1111/j.1740-8709.2009.00195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nommsen-Rivers LA, Chantry CJ, Peerson JM, Cohen RJ, Dewey KG. Delayed onset of lactogenesis among first-time mothers is related to maternal obesity and factors associated with ineffective breastfeeding. Am J Clin Nutr. 2010 Sep;92:574–584. doi: 10.3945/ajcn.2010.29192. [DOI] [PubMed] [Google Scholar]
- 10.Chen DC, Nommsen-Rivers L, Dewey KG, Lonnerdal B. Stress during labor and delivery and early lactation performance. Am J Clin Nutr. 1998 Aug;68:335–344. doi: 10.1093/ajcn/68.2.335. [DOI] [PubMed] [Google Scholar]
- 11.Hildebrandt HM. Maternal perception of lactogenesis time: a clinical report. J Hum Lact. 1999 Dec;15:317–323. doi: 10.1177/089033449901500409. [DOI] [PubMed] [Google Scholar]
- 12.Hilson JA, Rasmussen KM, Kjolhede CL. High prepregnant body mass index is associated with poor lactation outcomes among white, rural women independent of psychosocial and demographic correlates. J Hum Lact. 2004 Feb;20:18–29. doi: 10.1177/0890334403261345. [DOI] [PubMed] [Google Scholar]
- 13.Scott JA, Binns CW, Oddy WH. Predictors of delayed onset of lactation. Matern Child Nutr. 2007 Jul;3:186–193. doi: 10.1111/j.1740-8709.2007.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rasmussen KM, Hilson JA, Kjolhede CL. Obesity may impair lactogenesis II. J Nutr. 2001 Nov;131:3009S–3011S. doi: 10.1093/jn/131.11.3009S. [DOI] [PubMed] [Google Scholar]
- 15.Nommsen-Rivers LA, Mastergeorge AM, Hansen RL, Cullum AS, Dewey KG. Doula care, early breastfeeding outcomes, and breastfeeding status at 6 weeks postpartum among low-income primiparae. J Obstet Gynecol Neonatal Nurs. 2009 Mar-Apr;38:157–173. doi: 10.1111/j.1552-6909.2009.01005.x. [DOI] [PubMed] [Google Scholar]
- 16.Grajeda R, Perez-Escamilla R. Stress during labor and delivery is associated with delayed onset of lactation among urban Guatemalan women. J Nutr. 2002 Oct;132:3055–3060. doi: 10.1093/jn/131.10.3055. [DOI] [PubMed] [Google Scholar]
- 17.McLeod D, Pullon S, Cookson T. Factors influencing continuation of breastfeeding in a cohort of women. J Hum Lact. 2002 Nov;18:335–343. doi: 10.1177/089033402237906. [DOI] [PubMed] [Google Scholar]
- 18.Cernadas JM, Noceda G, Barrera L, Martinez AM, Garsd A. Maternal and perinatal factors influencing the duration of exclusive breastfeeding during the first 6 months of life. J Hum Lact. 2003 May;19:136–144. doi: 10.1177/0890334403253292. [DOI] [PubMed] [Google Scholar]
- 19.Fein SB, Labiner-Wolfe J, Shealy KR, Li R, Chen J, Grummer-Strawn LM. Infant Feeding Practices Study II: study methods. Pediatrics. 2008 Oct;122 (Suppl 2):S28–35. doi: 10.1542/peds.2008-1315c. [DOI] [PubMed] [Google Scholar]
- 20.Neville MC, Morton J. Physiology and endocrine changes underlying human lactogenesis II. J Nutr. 2001 Nov;131:3005S–3008S. doi: 10.1093/jn/131.11.3005S. [DOI] [PubMed] [Google Scholar]
- 21.Flaherman VJ, Bokser S, Newman TB. First-Day newborn weight loss predicts inhospital weight nadir for breastfeeding infants. Breastfeed Med. 2010 Aug;5:165–168. doi: 10.1089/bfm.2009.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wright NE, Cordes R. ABM clinical protocol #3: hospital guidelines for the use of supplementary feedings in the healthy term breastfed neonate, revised 2009. Breastfeed Med. 2009 Sep;4:175–82. doi: 10.1089/bfm.2009.9991. [DOI] [PubMed] [Google Scholar]
- 23.Li R, Scanlon KS, Serdula MK. The validity and reliability of maternal recall of breastfeeding practice. Nutr Rev. 2005;63:103–110. doi: 10.1111/j.1753-4887.2005.tb00128.x. [DOI] [PubMed] [Google Scholar]
- 24.Chapman DJ, Perez-Escamilla R. Maternal perception of the onset of lactation is a valid, public health indicator of lactogenesis stage II. J Nutr. 2000 Dec;130:2972–2980. doi: 10.1093/jn/130.12.2972. [DOI] [PubMed] [Google Scholar]
- 25.Ahluwalia IB, Morrow B, Hsia J. Why do women stop breastfeeding? Findings from the Pregnancy Risk Assessment and Monitoring System. Pediatrics. 2005 Dec;116:1408–1412. doi: 10.1542/peds.2005-0013. [DOI] [PubMed] [Google Scholar]