Abstract
Trimetazidine (TMZ) is used successfully for treatment of ischemic cardiomyopathy, however its therapeutic potential in heart failure (HF) remains to be established. While the cardioprotective action of TMZ has been linked to inhibition of free fatty acid oxidation (FAO) via 3-ketoacyl CoA thiolase (3-KAT), additional mechanisms have been suggested. The aim of this study was to evaluate systematically the effects of TMZ on calcium signaling and mitochondrial function in a rabbit model of non-ischemic HF and to determine the cellular mechanisms of the cardioprotective action of TMZ. TMZ protected HF ventricular myocytes from cytosolic Ca2+ overload and subsequent hypercontracture, induced by electrical and β-adrenergic (isoproterenol) stimulation. This effect was mediated by the ability of TMZ to protect HF myocytes against mitochondrial permeability transition pore (mPTP) opening via attenuation of reactive oxygen species (ROS) generation by the mitochondrial electron transport chain (ETC) and uncoupled mitochondrial nitric oxide synthase (mtNOS). The majority of ROS generated by the ETC in HF arose from enhanced complex II-mediated electron leak. TMZ inhibited the elevated electron leak at the level of mitochondrial ETC complex II and improved impaired activity of mitochondrial complex I, thereby restoring redox balance and mitochondrial membrane potential in HF. While TMZ decreased FAO by ~15%, the 3-KAT inhibitor 4-bromotiglic acid did not provide protection against palmitic acid-induced mPTP opening, indicating that TMZ effects were 3-KAT independent. Thus, the beneficial effect of TMZ in rabbit HF was not linked to FAO inhibition, but rather associated with reduced complex II- and uncoupled mtNOS-mediated oxidative stress and decreased propensity for mPTP opening.
Keywords: trimetazidine, heart failure, permeability transition pore, oxidative stress, mitochondrial nitric oxide synthase, reactive oxygen species, redox balance, β-adrenergic signaling, mitochondrial complex II-mediated ROS generation
1. Introduction
Heart failure (HF) is a hyperadrenergic state associated with high plasma levels of free fatty acids (FFA), that leads to energy depletion and decreased contractility [1, 2]. Current medical therapies for HF act via suppression of neurohormonal activation (e.g. β–adrenergic antagonists, angiotensin converting enzyme inhibitors), reducing volume overload (diuretics), or improving hemodynamic symptoms (inotropic agents). Despite optimal treatment with current drugs, most patients continue to deteriorate and prognosis remains poor. Recent studies suggest that new therapies designed to decrease FFA metabolism may be particularly attractive because they could support current treatment strategies without producing negative hemodynamic effects [3–5]. Indeed, myocardial function was generally improved in HF patients treated with trimetazidine (TMZ) [6–11], an agent that optimizes energy metabolism presumably via inhibition of long-chain 3-ketoacyl CoA thiolase (3-KAT), with subsequent decrease in fatty acid oxidation (FAO) and stimulation of glucose oxidation [12]. However, direct measurement of cardiac FAO in patients with chronic non-ischemic HF revealed no changes in FFA uptake and only a 10% decrease in FAO by TMZ [8]. This finding challenges the concept that the beneficial effect of TMZ is mediated primarily by FAO inhibition [2]. Other studies [13–19] have also suggested that the cardioprotective effect of TMZ may occur via different mechanisms possibly involving regulation of mitochondrial function. The comprehensive analysis of TMZ effects on mitochondrial function in HF conditions, however, has not been performed. Therefore, the overall goal of this study was to characterize mitochondrial function and mitochondria-dependent changes in cardiac excitation-contraction coupling (ecc) in non-ischemic HF and determine the mechanisms underlying the cardioprotective effects of TMZ.
2. Materials and methods
2.1. Rabbit HF model and cardiac myocyte isolation
Single myocytes were isolated enzymatically from control and HF New Zealand white rabbits of either sex [20, 21]. HF was induced by combined volume- and pressure-overload resulting from aortic valve insufficiency and aortic constriction [20]. Myocytes were also isolated from cyclophilin D (CypD) knockout (KO) mice (mice with genetically deleted Ppif gene (Ppif−/−) [22]) and their age-matched wild type C57BL/6 controls. CypD-KO mice were a kind gift from Dr. Shey-Shing Sheu, Thomas Jefferson University, Philadelphia, PA. Mouse ventricular myocytes were isolated as described before [23]. The animal sources and procedures for HF induction and cell isolation were fully approved by the Institutional Animal Care and Use Committee.
2.2. Solutions and Chemicals
Normal Tyrode solution contained (in mM): 135 NaCl, 4 KCl, 2 CaCl2, 1 MgCl2, 10 D-glucose, and 10 HEPES; pH 7.4. For experiments with permeabilized myocytes cells were treated with 10 μM digitonin for 1–2 min [24–27]. Digitonin was added to the “internal” solution containing (in mM): 135 KCl, 10 NaCl, 20 HEPES, 5 pyruvate, 2 glutamate, 2 malate, 0.5 KH2PO4, 0.5 MgCl2, 15 2,3-butanedione monoxime (BDM), 5 EGTA, and 1.86 CaCl2 to yield a free [Ca2+] of ~100 nM. In some experiments, the CaCl2 content of the internal solution was adjusted to achieve a final free [Ca2+] of 2 μM or 50 μM. Trimetazidine (1-[2,3,4-trimethoxybenzyl]piperazine dihydrochloride) was obtained from Sigma-Aldrich (St. Louis, MO) and dissolved in water as a 1 mM stock solution. Fresh TMZ stock solution was prepared on each experimental day. Unless noted otherwise, all other chemicals were purchased from Sigma-Aldrich. Spermine NONOate (Sper/NO), (4S)-N-(4-Amino-5[aminoethyl]aminopentyl)-N′-nitroguanidine (nNOS blocker I, nNOS I), and Mn(III)tetrakis(4-benzoic acid)porphyrin chloride (MnTBAP) were from Calbiochem-EMD Millipore (San Diego, CA).
2.3. Serum concentration of total FFA ([FFAtot]) and FFA unbound to albumin ([FFAu])
[FFAtot] (FFA bound and unbound to albumin) was measured by an enzymatic, colorimetric method (assay obtained from Wako Chemicals, Richmond, VA) [28]. [FFAu] was determined with the acrylodated intestinal fatty-acid-binding (ADIFAB) protein method (assay obtained from FFA Sciences, San Diego, CA) [29].
2.4. Measurements of cytosolic [Ca2+] ([Ca2+]i) and cell shortening
[Ca2+]i measurements were performed simultaneously with cell shortening measurements in intact Indo-1 (Molecular Probes-Life Technologies, Grand Island, NY) loaded cells using an epi-fluorescence microscopy set-up (Ionoptix, Milton, MA) [30]. Indo-1 was excited at 340 nm, with emission signals simultaneously recorded at 405 nm (F405) and 485 nm (F485). Changes in [Ca2+]i are expressed as changes of the ratio R = F405/F485. Action potentials were triggered at 1 Hz by electrical field stimulation (FS) with a pair of platinum electrodes. The electrical stimulus was set at a voltage ~50% greater than the threshold to induce myocyte contraction. Diastolic cell length (L) and myocyte shortening (decrease in L during FS, ΔL) were measured by a video-edge detection system (IonOptix) and expressed in micrometers (μm). Fluorescent and contractile signals were analyzed with IonWizard analysis software.
2.5. Parameters of mitochondrial function
Mitochondrial NO production was measured in permeabilized cells with the fluorescent NO-sensitive dye 4,5-diaminofluorescein diacetate (DAF-2 DA, λexc=488 nm, λem=510 nm) loaded for 40 min at 37°C [24, 31, 32]. Mitochondrial DAF-2 fluorescence intensity (F) in each experiment was normalized to the level of fluorescence recorded prior to stimulation (F0) but after cell permeabilization. Changes in [NO]mt are expressed as ΔF/F0 (where ΔF=F-F0). Regions of interest (ROIs; ≤40 μm2) were positioned over mitochondria thus representing [NO]mt measurements from a small number of mitochondria (≤40).
ROS production was detected in intact myocytes loaded with 10 μM 5-(6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (CM-H2DCF DA; λexc=488 nm, λem=510 nm) [24, 32] or 0.5 μM MitoSox Red (λex=543 nm, λem=555–617 nm) for 30 min at 37°C. Changes in CM-H2DCF (DCF) fluorescence intensity (F) were normalized to the level of fluorescence recorded prior to stimulation (F0), and expressed as ΔF/F0. The rate of ROS production (d(ΔF/F0)/dt) was estimated from the initial linear phase of the DCF or MitoSox Red fluorescence increase in order to minimize potential problems arising from mitochondrial dye saturation and leakage.
mPTP activity was monitored in permeabilized cells loaded with 5 μM calcein/AM (λex=488 nm, λem=510 nm) for 40 min at 37°C [24, 25, 32]. Opening of mPTP resulted in the loss of mitochondria-trapped calcein (620 Da) and a decrease of fluorescence. At the end of each recording 10 μg/ml of the pore-forming antibiotic alamethicin [33] was applied to provide a control measure for maximum calcein release from mitochondria. Loss of mitochondrial calcein induced by elevating extramitochondrial [Ca2+] ([Ca2+]em) was quantified as the rate of decline of fluorescence (d(ΔF)/dt) calculated from the linear fit to the initial decrease of calcein fluorescence. The rate of decline was normalized (%) to the basal decline of calcein fluorescence addition (taken as 100%) before [Ca2+]em elevation or exposure to mPTP inducers palmitic acid (PA) or carboxyatractyloside (CAT).
Calcium retention capacity (CRC) was measured in permeabilized myocytes placed in a medium containing (in mM): 150 sucrose, 50 KCl, 2 KH2PO4, 5 glutamate, 2 malate and 20 Tris/HCl pH 7.4. Cells were treated with 1 μM TMZ, 240 nM nNOS I, or 1 μM CsA and compared to control. After 15 min of incubation, CRC was measured by adding 1 μM Calcium Green-5N followed by repetitive 10 μM Ca2+ pulses applied at 1 min interval until massive release of accumulated Ca2+ was observed (i.e., mPTP opening) [34–36]. Measurements of [Ca2+]em were performed confocally (λexc=488 nm, λem=530 nm) from ROIs outside cells. Reported results are the means of four to five independent experiments for each condition normalized to 106 cells.
Changes in mitochondrial membrane potential (ΔΨm) were followed using the potential-sensitive dye tetramethylrodamine methyl ester (TMRM; λex=514 nm, λem=590 nm) [25]. Cells were exposed to 20 nM TMRM for 15 min at 37°C prior to experiments, and then permeabilized with digitonin [24]. All solutions contained 20 nM TMRM during recordings. The rate of the mitochondrial complex I (i.e., in the presence of 5 mM malate and 2 mM glutamate)-dependent ΔΨm changes was calculated from a linear fit to TMRM fluorescence increase. The rate constant k (s−1) for the mitochondrial complex II (i.e., in the presence of 5 mM succinate plus 1 μM rotenone)-dependent ΔΨm recovery was calculated from the time required to reach 63% of the maximal TMRM fluorescence signal upon succinate addition.
All fluorescent indicators (DCF, MitoSox Red, calcein, Calcium Green-5N and TMRM) were obtained from Molecular Probes-Life Technologies, except for DAF-2 which was obtained from Calbiochem-EMD Millipore.
Flavin adenine dinucleotide (FAD)-linked protein autofluorescence (λex=488 nm, λem=510 nm) was measured to evaluate mitochondrial redox state [32, 37, 38]. Data are presented as the ratio of oxidized FAD to reduced FADH2 (FAD/FADH2) calculated as (F-Fmin)/(Fmax-Fmin) where F is the fluorescence intensity, and Fmin is the fluorescence obtained after addition of 4 mM NaCN (inhibits respiration and promotes maximal FAD reduction, i.e. FADH2 formation), taken as 0%. Fmax is the fluorescence obtained after addition of 1 μM FCCP (stimulates maximal respiration, completely oxidizing the mitochondrial FADH2 pool), taken as 100%.
Oxygen consumption was measured in permeabilized myocytes polarographically at 37°C with a Clark-type oxygen electrode (Model 1302; Strathkelvin Instruments; Glasgow, Scotland) in 300 μl of MiRO5 respiration buffer containing (in mM): 110 Sucrose, 60 K-lactobionate, 20 HEPES, 3 MgCl2, 20 taurine, 10 KH2PO4, 0.5 EGTA, and 1 g/l BSA, pH =7.1. Complex I (NADH dehydrogenase) activity was measured using 5 mM malate plus 2 mM glutamate as substrates. Complex II (succinate dehydrogenase) activity was measured using 5 mM succinate as a substrate in the presence of 1 μM rotenone. For each complex, the ADP-stimulated respiration rate (state 3) was measured after addition of 1 mM ADP. The ADP-independent respiration rate was measured after substrate addition, but before ADP application (state 2). Rates were calculated as nmol O2/min and normalized to 105 permeabilized cells. The respiratory control ratio (RCR) was calculated as state 3 divided by state 2 respiration rate to estimate mitochondrial integrity.
2.6. Statistical analysis
Statistical differences of the data were determined with the Student’s t test for unpaired and paired data and considered significant at P<0.05. Results are reported as means ± standard error of the mean (S.E.M.) for the indicated number (n) of cells, experiments, or blood samples.
3. Results
3.1. FFA serum levels in rabbit heart failure
Determination of FFA serum levels revealed that in HF rabbits both [FFAtot] and [FFAu] were significantly elevated. [FFAu] increased ~4 times in HF (from 13±4 to 53±7 nM, n=16 animals) while the [FFAtot] increased two-fold (from 58±16 to 121±29 μM, n=16 animals).
3.2. TMZ prevents isoproterenol-induced Ca2+-overload and cell hypercontracture in ventricular myocytes from HF rabbits
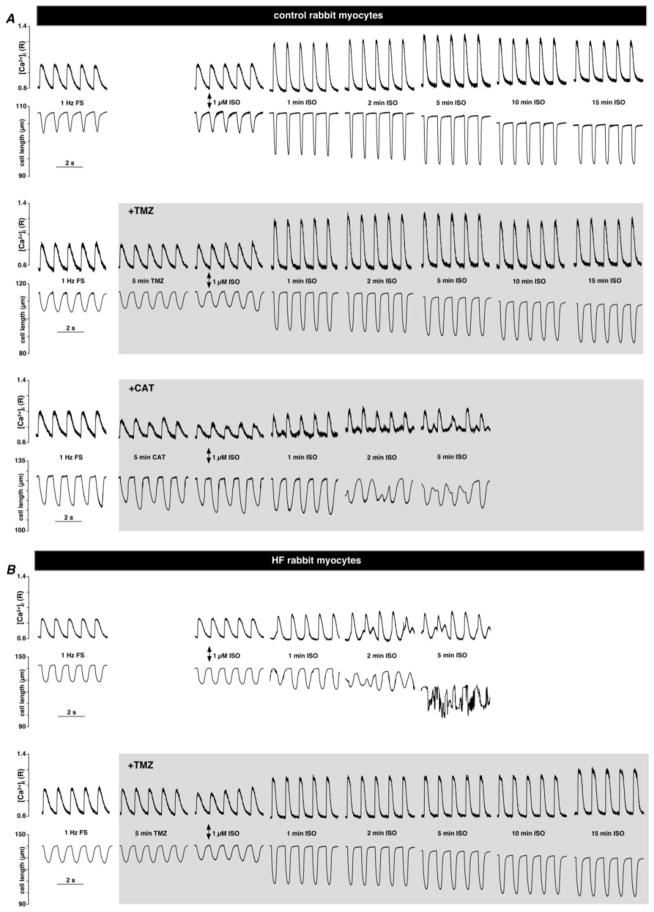
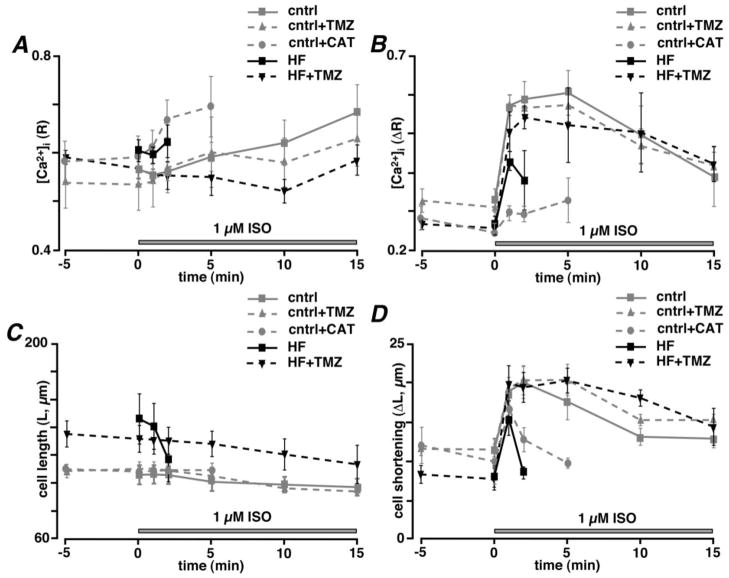
To determine the effect of TMZ on Ca2+ handling during excitation-contraction coupling (ecc) in control and HF myocytes, electrical field stimulation (FS, 1 Hz) was applied to elicit [Ca2+]i transients (Fig. 1), followed by β–adrenergic receptor stimulation with 1 μM isoproterenol (ISO). ISO application led to the expected increase in [Ca2+]i transient amplitude (ΔR) measured at 1 min (ΔR=0.57±0.03 versus 0.34±0.01, P<0.01; n=5 cells) in control myocytes which remained relatively steady for 15 min of stimulation. These [Ca2+]i transients were accompanied by enhanced contractions during ISO stimulation. Cell shortening (ΔL) increased from 11.4±0.8 μm to 18.9±1.8 μm (n=5; P<0.01) with β–adrenergic receptor stimulation. TMZ treatment did not affect [Ca2+]i transient amplitude or cell shortening significantly in control myocytes (Figs. 1A and 2). Field stimulation of HF myocytes, however, revealed signs of impaired ecc in form of elevated diastolic [Ca2+]i (R=0.55±0.02, n=5 in control versus 0.61±0.02 in HF; n=6; P<0.05), diminished [Ca2+]i transient amplitude (ΔR=0.34±0.01 in control versus 0.26±0.02 in HF; n=6; P<0.01) and cell shortening (ΔL=11±0.8 μm in control versus 8±1.0 μm in HF; n=6; P<0.05) despite the fact that the average cell length was increased (L=106±7 μm in control versus 146±18 μm in HF; P<0.05; n=6) (Figs. 1B and 2). β–adrenergic receptor stimulation also failed to increase [Ca2+]i transient amplitude and cell shortening significantly after 1 min of ISO application in HF myocytes compared to healthy control myocytes (Figs. 1A and B). Moreover, continuous β–adrenergic receptor stimulation of HF myocytes resulted in irreversible cell hypercontracture between 2 and 5 min of ISO application due to Ca2+ overload characterized by a significant increase in diastolic [Ca2+]i (R increased from 0.61±0.02 to 0.64±0.03; n=6; P<0.05) and the appearance of irregular spontaneous Ca2+ release events and a decline in [Ca2+]i transient amplitude (ΔR) from 0.42±0.07 to 0.36±0.08 (n=6; P<0.05) after 2 min of ISO application compared to 1 min of ISO exposure (Fig. 1B). Treatment with 1 μM TMZ by itself did not affect [Ca2+]i transient amplitude or cell shortening in HF cells (n=7; Fig. 1B and 2). However, it prevented Ca2+ overload and spontaneous Ca2+ release for at least 15 min of ISO application and [Ca2+]i transient amplitude remained stable over this time (n=7; Figs. 1B and 2). Also, diastolic [Ca2+]i levels and cell length remained relatively stable after ISO application in TMZ-treated HF myocytes. These data demonstrate that TMZ normalized Ca2+ cycling in HF myocytes and protected against irreversible cell hypercontracture induced by Ca2+ overload.
Fig. 1.
β–adrenergic receptor stimulation with 1 μM isoproterenol (ISO) in field-stimulated (1 Hz) leads to excitation-contraction coupling impairment in ventricular myocytes from HF rabbits and control myocytes treated with carboxyatractyloside (CAT). (A) [Ca2+]i transients and cell length changes observed in field-stimulated (1 Hz) control myocytes before and after 1 μM ISO application in the absence (top panel) or presence (middle panel) of 1 μM TMZ or 25 μM CAT (lower panel) applied for 5 min before ISO stimulation. (B) [Ca2+]i transients and cell length changes observed in field-stimulated (1 Hz) HF myocytes before and after 1 μM ISO application in the absence (top panel) or presence of 1 μM TMZ (lower panel) applied for 5 min before ISO stimulation.
Fig. 2.
(A) Changes in diastolic [Ca2+]i levels (R) and (B) [Ca2+]i transient amplitudes (ΔR) induced by 1 μM ISO in control and HF myocytes in the absence or presence of 1 μM TMZ or 25 μM CAT during 1 Hz FS. (C) Diastolic cell length (L) and (D) cell shortening (ΔL) during the same protocol as in panels A and B.
Irreversible hypercontracture and subsequent necrotic cell death have been linked to opening of the mitochondrial permeability transition pore (mPTP) during cytosolic calcium overload in cardiac myocytes [39]. We therefore tested whether the ISO-dependent effects in HF myocytes (Fig. 1B) could be mimicked in control cells under conditions of enhanced mPTP activity. For this purpose we treated control rabbit myocytes with 25 μM carboxyatractyloside (CAT), a known mPTP activator, followed by application of 1 μM of ISO. CAT binds to adenine nucleotide binding site of the adenine nucleotide translocase (ANT), stabilizes the nucleoside binding site of ANT on the cytoplasmic side of the inner mitochondrial membrane and blocks the exchange of matrix ATP and cytoplasmic ADP therefore promoting mPTP opening independently of ROS generation [40]. As shown in Figs. 1A and 2, CAT application by itself led to the development of Ca2+ and mechanical alternans in electrically-stimulated cardiac myocytes, however no hypercontracture was observed (L was 108±4 μm after 5 min of CAT treatment versus 109±3 μm in control; P=0.17; n=4). However, subsequent β–adrenergic receptor activation with ISO failed to produce a positive inotropic effect in CAT-treated cells (Fig. 1) but rather led to the increase in the diastolic [Ca2+]i levels (R increased from 0.58±0.04 to 0.69±0.06; n=4; P<0.05), appearance of irregular Ca2+ and mechanical events similar to those observed in HF (Fig. 1B). These data suggest enhanced mPTP activity as a possible cause underlying the observed severe impairment of ecc in HF myocytes during β–adrenergic stimulation (Fig. 1B). This hypothesis was further tested in the following experiments.
3.3. TMZ prevents opening of the mPTP
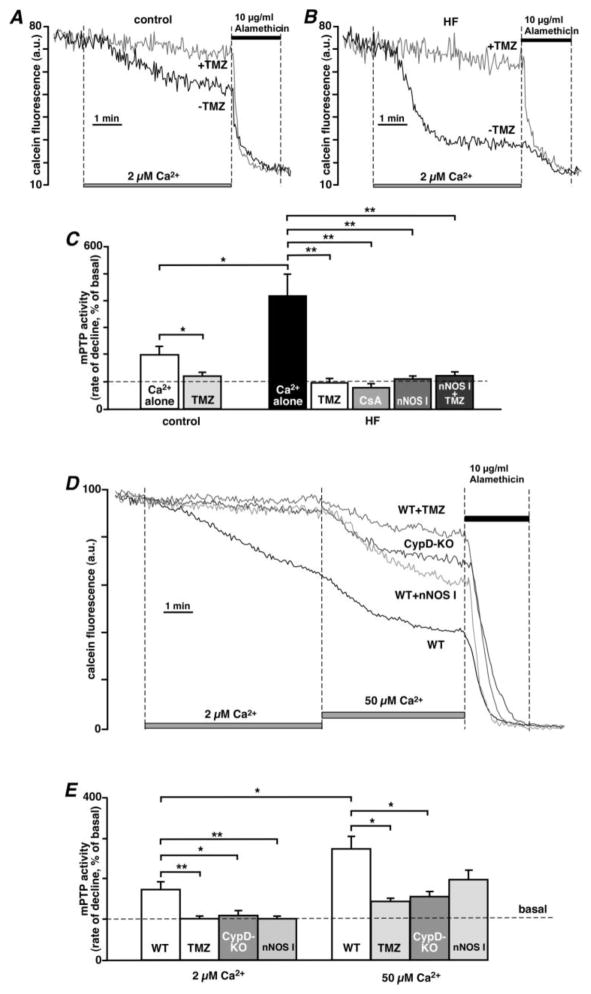
A critical event leading to cell death is the opening of the mPTP in the inner mitochondrial membrane [41]. We therefore tested the resistance of cardiac mitochondria from control and HF hearts to Ca2+-dependent mPTP opening detected by calcein release from mitochondria [24, 25]. Mitochondrial Ca2+ uptake was stimulated in permeabilized cells by increasing [Ca2+]em from 0.1 to 2 μM (Fig. 3) [27]. In these experiments (Figs. 3 and 4) mPTP activity was quantified as % change in the rate of calcein fluorescence decline compared to basal conditions (defined as 100% in [Ca2+]em=0.1 μM). Elevation of [Ca2+]em to 2 μM led to a small decrease in calcein fluorescence in control cells (rate of fluorescence decline was 200±32%; n=12), while in HF cells the rate of calcein release was ~2 times higher (420±81%; n=8). The Ca2+-induced decrease in calcein fluorescence was abolished by 10 μM cyclosporine A (80±15%; n=6, P<0.01 compared to control; Fig. 3C), confirming that this decrease in fluorescence was due to mPTP opening. TMZ prevented Ca2+-induced opening of the mitochondrial mPTP both in control (121±15%; n=5, P<0.05; Figs. 3A and C) and HF (98±16%; n=8, P<0.01; Figs. 3B and C) myocytes. Moreover, when permeabilized HF cells were treated with a specific inhibitor of neuronal NOS (nNOS I, 240 nM, 15 min pre-treatment), which we demonstrated to decrease mitochondrial nitric oxide synthase (mtNOS) activity [24], the opening of the mPTP was significantly decreased (112±11%; n=6, P< 0.01, Fig. 3C) compared to untreated HF myocytes. Combined treatment with nNOS I and TMZ did not have an additive effect (125±15%; n=6, P< 0.01; Fig. 3C), indicating that these agents were acting on the same target and were fully effective individually, possibly by preventing mitochondrial nNOS uncoupling and subsequent ROS production. To verify the fidelity of the calcein assay to detect mPTP activity, we performed the calcein assay in cardiomyocytes isolated from control mice and mice with genetically inactivated Ppif (Ppif−/−) gene. The Ppif gene encodes cyclophilin D (CypD), a major regulatory protein of the mPTP [22]. As shown in Figure 3D, an elevation of [Ca2+]em from 0.1 to 2 μM induced calcein release from control mouse myocytes, but not from CypD-KO myocytes and control mouse myocytes pre-treated with TMZ or nNOS I. The subsequent elevation in [Ca2+]em from 2 to 50 μM, induced further release of calcein from mitochondria of control myocytes (from 184±26 to 282±53%; n=9; P<0.05; Figs. 3D and E). However, calcein release was significantly decreased in TMZ-treated myocytes (144±16%; n=14; P<0.05) and CypD-ablated myocytes (156±11%; n=9; P<0.05). nNOS I was not able to prevent mPTP opening induced by 50 μM Ca2+ addition (197.6±24%; n=5; P=0.08) to the same degree as TMZ or CypD ablation.
Fig. 3.
TMZ prevents Ca2+-induced opening of the mPTP. Effects of TMZ on the mPTP activity in permeabilized (A) control and (B) HF rabbit myocytes upon activation of mitochondrial Ca2+ uptake by increasing [Ca2+]em from 0.1 to 2 μM. (C) Summary of Ca2+-induced mitochondrial mPTP activity in control and HF rabbit myocytes in the absence or presence of 1 μM TMZ, 10 μM cyclosporine A (CsA), 240 nM nNOS I, and 1 μM TMZ applied together with 240 nM nNOS I. (D) Effects of TMZ, nNOS I and CypD ablation on Ca2+-dependent mPTP opening in mouse cardiomyocytes. [Ca2+]em was step-wise increased from 0.1 to 2 and to 50 μM. WT, wild type. (E) Summary of Ca2+-induced mitochondrial mPTP activity in control mouse myocytes (WT), control mouse myocytes treated with 1 μM TMZ (TMZ) or 240 nM nNOS I, and myocytes from CypD-KO mice. The pore-forming antibiotic alamethicin (10 μg/ml) was added at the end of each experiment to provide a positive control for maximal calcein release from mitochondrial matrix. **P<0.01, *P<0.05.
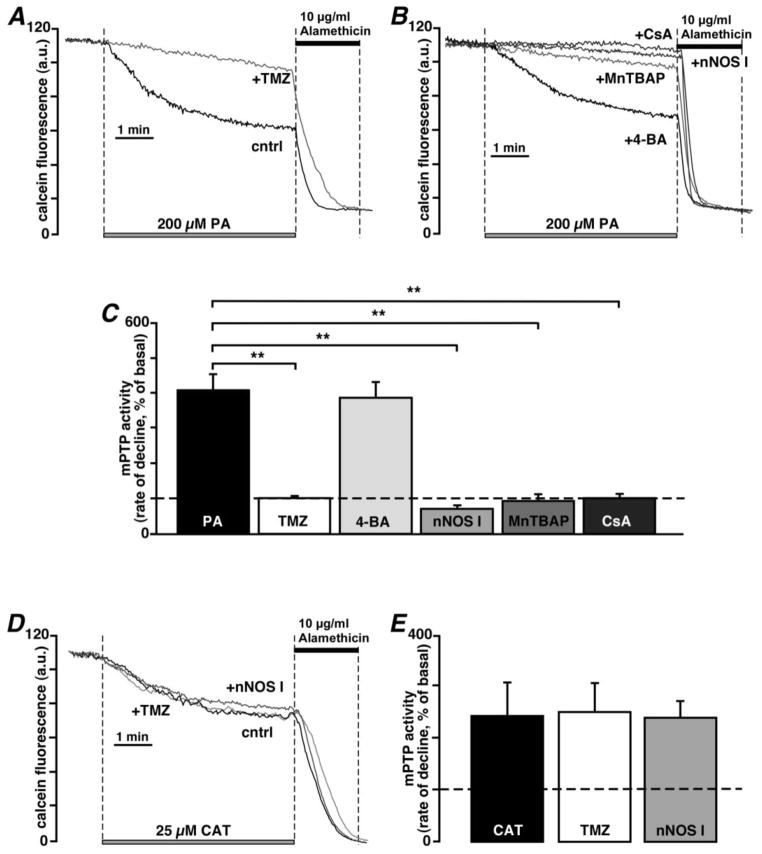
Fig. 4.
TMZ prevents palmitic acid-induced opening of the mPTP. (A) Cell treatment with 1 μM TMZ prevented mPTP opening induced by palmitic acid (PA, 200 μM) in permeabilized myocytes from control rabbits. (B) Representative traces of mPTP activity in permeabilized control myocytes exposed to 200 μM PA in the presence of 20 μM 4-bromotiglic acid (4-BA), 50 μM MnTBAP, 240 nM nNOS I, or 5 μM cyclosporine A (CsA). (C) Summary of the TMZ, 4-BA, MnTBAP, nNOS I and CsA effects on PA-induced mPTP opening in control myocytes. (D) Representative traces of mPTP activity in permeabilized control myocytes exposed to 25 μM CAT in the absence and presence of 1 μM TMZ or 240 nM nNOS I. (E) Summary of the TMZ and nNOS I effects on CAT-induced mPTP opening in control myocytes.**P<0.01.
In agreement with the calcein release experiments (Fig. 3), calcium retention capacity (CRC) measurements indicated that the ability of permeabilized rabbit myocytes to resist progressive Ca2+ overload achieved by sequential 10 μM Ca2+ pulses was significantly decreased in HF myocytes (from 73±5 μM Ca2+/106 cells in control (n=4) versus 28±2 μM Ca2+/106 cells in HF (n=5) myocytes; P<0.001). TMZ (1 μM) significantly increased CRC in control cells to 238±69 μM Ca2+/106 cells (n=4) and partially restored CRC to 52±10 μM Ca2+/106 cells (n=5) in HF cells. CsA (1 μM) treatment had similar effects on CRC in both control (173±25 μM Ca2+/106 cells; n=5) and HF myocytes (54±8 μM Ca2+/106 cells; n=5). nNOS I increased CRC in control cells (158±54 μM Ca2+/106 cells; n=5), however the effect was not significant (P=0.1). In contrast, nNOS I was effective in increasing CRC (60±9 μM Ca2+/106 cells; n=5; P<0.05) in HF cells. Altogether, these data confirm that TMZ prevented Ca2+ overload-induced mPTP opening both in mouse and rabbit myocytes, and the degree of protection was similar to that obtained in CypD-KO, or CsA-treated, and to a lesser degree in nNOS I-treated myocytes.
Next, we tested whether the observed effects of TMZ were mediated by the activity of 3-KAT, the enzyme which catalyses the terminal reaction of β–oxidation of fatty acids. If TMZ selectively inhibits 3-KAT, it would be expected to block the oxidation of palmitoylcarnitine (an activated fatty acid and upstream substrate of FFA β–oxidation). TMZ (1 μM) caused only minor inhibition of palmitoylcarnitine oxidation (state 2 of mitochondrial respiration was inhibited by 14.5±4.9%, while state 3 was inhibited by 21.5±2.8%; n=4; Table 1). We compared the effect of TMZ with a second inhibitor of 3-KAT, 4-bromotiglic acid (4-BA) [42], and found that 20 μM 4-BA had a similar inhibitory effect on palmitoylcarnitine oxidation (state 2 was inhibited by 16.7±4.1%, while state 3 of respiration was inhibited by 24.9±8.1%; n=4; Table 1). Furthermore, we tested the effect of 4-BA on mPTP activity, using the calcein assay (Fig. 4). In contrast to TMZ (Fig. 4A, 101±6%; n=9; P<0.01), however, 4-BA (387±45%; n=5) failed to protect against mPTP opening induced by exposure to palmitic acid (PA, 200 μM; 408±46%; n=13) in control rabbit myocytes (Fig. 4B). Similar to TMZ (Figs. 4B and C), inhibition of mtNOS with nNOS I (71±8%; n=5; P<0.01 compared to PA alone), and exposure to the superoxide dismutase mimetic and peroxynitrite scavenger MnTBAP (94±19%; n=5; P<0.01) or CsA (102±13%; n=6; P<0.01) prevented PA-induced opening of the mPTP. Taken together, these data indicate that TMZ has a cardioprotective effect that was not related to inhibition of FFA oxidation, but was mediated by modulation of mitochondrial ROS and NO production.
Table 1.
Effects of TMZ and nNOS I on mitochondrial respiration
| Substrates | Experiments | State 2 | State 3 | RCR |
|---|---|---|---|---|
|
| ||||
| Complex I | cntrl (n=6) | 78.3±8.9 | 493.8±0.03 | 6.62±0.78 |
| cntrl + TMZ (n=6) | 77.3±7.5 | 409.7±0.04 | 5.61±0.92 | |
| cntrl + nNOS I (n=6) | 60.5±7.4# | 449.9±4.94 | 7.86±0.58# | |
|
| ||||
| HF (n=8) | 56.0±7.8* | 136.5±26.6* | 2.43±0.21* | |
| HF + TMZ (n=9) | 93.7±7.3# | 243.1±17.3# | 2.59±0.32 | |
| HF + nNOS I (n=8) | 75.2±3.9# | 189.7±9.4# | 2.52±0.18 | |
|
| ||||
| Complex II | cntrl (n=6) | 230.2±32.1 | 548.5±27.5 | 2.53±0.22 |
| cntrl + TMZ (n=6) | 275.7±31.2 | 558.7±43.6 | 2.13±0.19 | |
| cntrl + nNOS I (n=6) | 235.1±21.6 | 500.3±26.6 | 2.13±0.18 | |
|
| ||||
| HF (n=5) | 428.6±51.7* | 584.0±34.3 | 1.50±0.28* | |
| HF + TMZ (n=5) | 329.4±65.9# | 356.4±62.6# | 1.54±0.58 | |
| HF + nNOS I (n=5) | 424.1±49.3 | 552.4±46.5 | 1.30±0.59 | |
|
| ||||
| Palmitoyl-carnitine | cntrl (n=4) | 124±6.3 | 522.0±52.0 | 4.19±0.38 |
| cntrl + TMZ (n=4) | 106±2.5# | 405.7±27.3# | 3.84±0.25 | |
| cntrl + 4-BA (n=4) | 102.9±6.9# | 391.5±58.4# | 3.80±0.21 | |
State 2 and state 3: rates of O2 consumption, nmol O2/min per 105 cells
RCR: state 3 respiration/state 2 respiration
P<0.05, HF compared to control cells
P<0.05, TMZ or nNOS I treated cells compared to cells in the absence of the drug
To exclude the possibility of direct mPTP inhibition by TMZ and nNOS I, we stimulated mPTP opening by application of 25 μM CAT and monitored CAT-induced calcein release in the absence and presence of 1 μM TMZ or 240 nM nNOS I. We found that neither TMZ (251±54%; n=6; P=0.45 compared to CAT alone) nor nNOS I (239±30%; n=7; P=0.46 compared to CAT alone) were able to block CAT-induced pore opening (243±63%; n=7) (Figs. 4D and E) ruling out the possibility of direct inhibition of mPTP by TMZ or nNOS I.
3.4. TMZ prevents field stimulation- and ISO-induced ROS generation
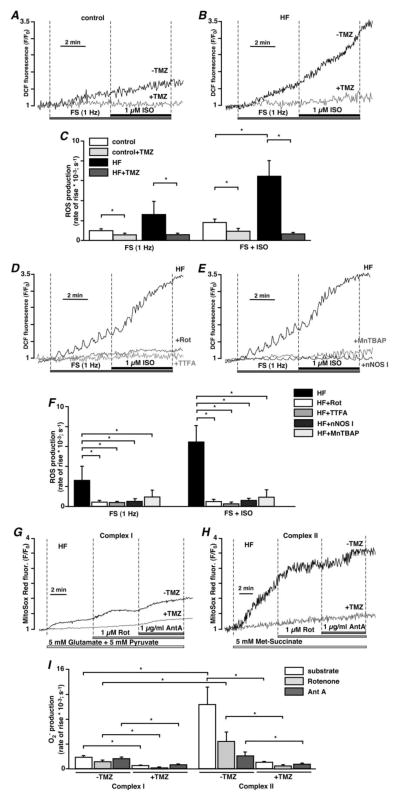
We determined whether the observed TMZ effects involved modulation of mitochondrial oxidative stress. Field stimulation of intact cardiac myocytes was accompanied by a small amount of ROS generation in control myocytes (rate of change of mitochondrial DCF fluorescence: d(ΔF/F0)/dt =0.00098±0.0002 s−1; n=6; Figs. 5A and C), however it led to a significant increase in the rate of ROS generation in HF rabbit myocytes (0.00274±0.0017 s−1; n=6; P<0.05; Figs. 5B and C). Addition of ISO (1 μM) further augmented ROS generation in HF myocytes (0.00559±0.0022 s−1; n=5; P<0.05; Figs. 5B and C), while it increased the rate of ROS generation to a lesser extent in control cells (0.00181±0.0003 s−1; n=6; Figs. 5A and C). Pretreatment with TMZ (1 μM, 15 min) prevented both FS- and ISO-induced ROS generation in control (FS: 0.00056±0.0002 s−1; n=5; FS+ISO: 0.00093±0.0003 s−1; n=5; P<0.05; Figs. 5A and C) and HF (FS: 0.00057±0.0002 s−1; n=5; FS+ISO: 0.00069±0.0002 s−1; n=5; P<0.05; Figs. 5B and C) myocytes. These data demonstrate that β–adrenergic stimulation of paced HF myocytes induced a significant burst of ROS generation, which was prevented by TMZ treatment.
Fig. 5.
TMZ prevents mitochondrial ROS production. (A) TMZ (1 μM) prevents increase in ROS generation induced by FS (1 Hz) and subsequent addition of 1 μM ISO in control myocytes. (B) Significant increase in ROS generation observed in HF cells during FS and ISO application was prevented by TMZ. (C) Summary of TMZ effects on FS- and ISO-induced ROS production. (D) Inhibition of the ETC at the level of mitochondrial complex I with 1 μM rotenone (Rot) or complex II with 100 μM TTFA abolished FS- and ISO-induced ROS production in HF myocytes. (E) Exposure to nNOS I (240 nM) or scavenging ROS with 50 μM MnTBAP abolished FS- and ISO-induced ROS production in HF myocytes. (F) Summary of the effects of mitochondrial ETC inhibitors, nNOS I and MnTBAP on FS- and ISO-induced ROS production in HF myocytes. (G) Superoxide (O2−) generation induced in intact HF myocytes by stimulation of the mitochondrial complex I with 5 mM glutamate and 5 mM pyruvate, and subsequent application of 1 μM rotenone and 1 μg/ml antimycin A in the presence and absence of TMZ. (H) Superoxide generation induced in intact HF myocytes by stimulation of mitochondrial complex II with 5 mM methyl succinate, and subsequent application of 1 μM rotenone and 1 μg/ml antimycin A in the presence and absence of TMZ. (I) Summary of mitochondrial superoxide production induced by activation of mitochondrial complex I and II in HF myocytes in the presence and absence of TMZ. *P<0.05.
To provide experimental evidence that ISO-induced ROS generation was mediated by mitochondria, HF cells were treated with either 1 μM rotenone (Rot) or 100 μM 2-thenoyltrifluoroacetone (TTFA), inhibitors of the mitochondrial electron transport chain (ETC) complex I and II, respectively. As shown in Figure 5D, these treatments decreased both FS- and ISO-induced ROS generation indicating that ROS production was mediated by increased electron leak of the mitochondrial ETC. Moreover, cell treatment with nNOS I (240 nM, 15 min pre-treatment) also prevented FS- (0.00051±0.0001 s−1; n=6; P<0.05) and ISO-induced (0.00060±0.0001 s−1; n=6; P<0.05; Figs. 5E and F) ROS generation in HF cells suggesting that uncoupled mtNOS contributes to ISO-induced ROS generation in HF myocytes. In addition, cell treatment with superoxide dismutase (SOD) mimetic MnTBAP (50 μM) also abolished the increase in ROS elicited by FS (0.00093±0.0006 s−1; n=4; P<0.05) and subsequent ISO application (0.00092±0.0006 s−1; n=4; P<0.05; Figs. 5E and F) confirming the fidelity of ROS measurement with DCF.
3.5. TMZ prevents mitochondrial ETC complex I- and complex II-mediated ROS generation
Next, we supplied intact HF myocytes with substrates for mitochondrial ETC complex I (5 mM glutamate and 5 mM pyruvate) or complex II (5 mM methyl succinate, a cell membrane permeable analog of succinate) and monitored the rate of superoxide (O2−) generation with the fluorescent indicator Mito-Sox Red. Cells were electrically field-stimulated at 0.5 Hz. This stimulation frequency does not lead to O2− generation by itself. Activation of mitochondrial complex II (Fig. 5H) led to a dramatic increase in O2− production (Fig. 5I: 0.01025±0.0028 s−1; n=10; P<0.05) compared to (Fig. 5G) cells supplemented with complex I substrates (Fig. 5I: 0.00183±0.0003 s−1; n=9). After 5 minutes of incubation with substrates, the electron transfer between mitochondrial complex I and ubiquinone was inhibited by application of 1 μM rotenone. Rotenone decreased the rate of O2− production, however the effect was more pronounced in complex II substrate-supplemented cells (0.00436±0.0015 s−1; n=10; P<0.05 compared to methyl succinate alone) compared to complex I substrate-supplemented cells (0.00112±0.0003 s−1; n=9; P<0.05 compared to glutamate/pyruvate). Next, the electron transfer was blocked at the level of the mitochondrial complex III by addition of 1 μg/ml antimycin A (Ant A). Ant A led to a slight increase in O2− generation (0.00159±0.0003 s−1; n=9) in myocytes supplemented with complex I substrates, but it decreased the rate of O2− production even further in complex II substrate-supplemented myocytes (0.00203±0.0006 s−1; n=10; P<0.05 compared to methyl succinate alone). Most importantly, cell pre-treatment with 1 μM TMZ for 15 min, completely prevented complex I- and complex II-mediated O2− generation (summarized in Fig. 5I), thus underpinning the notion that cardioprotective effects of TMZ involve protection against ROS effects.
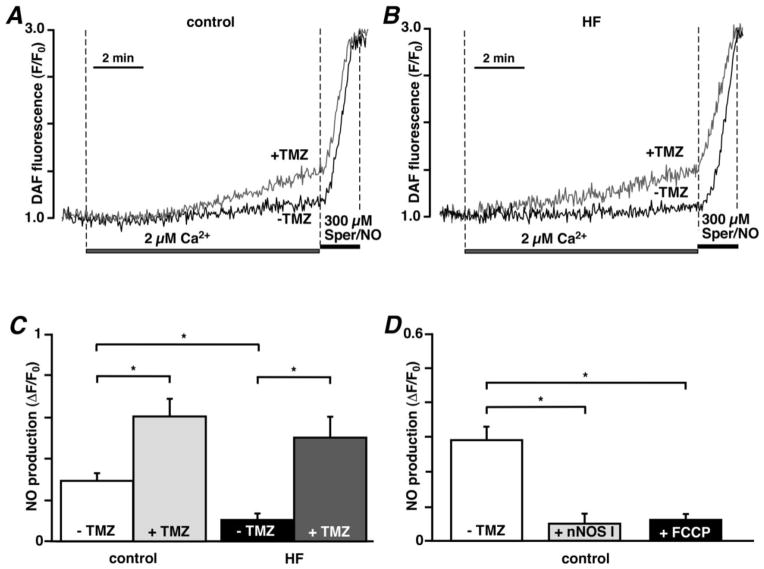
3.6. TMZ increases mitochondrial NO production
Because nNOS I prevented mPTP opening, but also blocked ROS production by uncoupled mtNOS [24], we determined the effect of TMZ on mitochondrial NO production directly. Elevation of [Ca2+]em from 0.1 to 2 μM in permeabilized control myocytes stimulated mitochondrial NO production (ΔF/F0=0.2919±0.0365 measured over a period of 10 minutes; n=15; Figs. 6A and C) which was significantly lower in HF cells (0.1034±0.0305; n=4, P<0.05; Figs. 6B and C), indicative of the fact that mtNOS activity had shifted from NO to ROS production in HF myocytes. TMZ (1 μM) however, enhanced NO production significantly in both control (0.6022±0.0844; n=14; P<0.05; Figs. 6A and C) and HF cells (0.4994±0.1005; n=6; P<0.05; Figs. 6B and C). Application of 300 μM Spermine NONOate (Sper/NO, exogenous NO donor) to control and HF cells led to a similar increase in DAF-2 fluorescence that was not affected by TMZ treatment, indicating that observed effects were not related to differences in dye loading or non-specific effects of TMZ (Figs. 6A and B). Furthermore, we verified that NO production was mediated by mtNOS since Ca2+-induced NO generation was prevented by cell treatment with 240 nM nNOS I (0.0493±0.0254; n=6, P<0.05; Fig. 6D) and mitochondrial uncoupling with 10 μM FCCP (0.0602±0.0119; n=3, P<0.05; Figs. 6D) in control TMZ-untreated myocytes (traces not shown). These data suggest that mtNOS uncoupling occurred in rabbit HF myocytes, and TMZ treatment was able to re-couple the enzyme, diminish detrimental ROS production and enhance NO production.
Fig. 6.
TMZ increases mitochondrial Ca2+-induced NO production. Representative traces of changes in DAF-2 fluorescence during elevation of [Ca2+]em from 0.1 to 2 μM and subsequent application of the NO donor Sper/NO (300 μM) in the absence and presence of 1 μM TMZ in control (A) and HF (B) myocytes. (C) Summary of TMZ effects on Ca2+-induced NO production in control and HF myocytes measured 10 minutes after exposure to [Ca2+]em = 2 μM. (D) Summary of the effects of mtNOS inhibition with nNOS I (240 nM) and mitochondrial uncoupling with the protonophore FCCP (1 μM) on Ca2+-induced mitochondrial NO production in control myocytes.*P<0.05.
3.7. Effects of TMZ on the activity of the mitochondrial ETC complexes I and II
Since the uncoupling of mtNOS is associated with decreased activity of mitochondrial ETC complex I [43], we evaluated the effect of TMZ on the activity of ETC complexes in control and HF myocytes. Mitochondrial respiration from permeabilized control and HF cells was monitored in the presence of the appropriate substrates (complex I: 5 mM malate + 2 mM glutamate; complex II: 5 mM succinate + 1 μM rotenone to inhibit complex I). As summarized in Table 1, in HF myocytes the complex I-mediated state 3 respiration rate (coupled to oxidative phosphorylation) was significantly decreased (−72%) and state 2 respiration rate (reflects substrate oxidation rate by the mitochondrial complex) was decreased by 29% indicating an uncoupling of the respiratory electron flux from ATP production in HF. TMZ (1 μM) increased both state 2 respiration (+67%) and state 3 (+78%) in HF myocytes respiring on complex I substrates. No significant effects of TMZ were found in state 3 respiration in control cells respiring on complex I substrates (−17%) and complex II substrates (+2%). Moreover, ADP-linked state 3 respiration rate in HF myocytes respiring on complex II substrate was similar to the rates observed in control myocytes (+7% in HF myocytes). However, state 2 respiration was significantly increased in HF myocytes respiring on complex II substrate (~2-fold compared to control myocytes), which led to a decreased respiratory control ratio indicating that there was uncoupling between increased activity of complex II and ATP generation (Table 1). TMZ treatment decreased both complex II-mediated state 2 (−23%) and state 3 (−39%) respiration in HF cells. Taken together, TMZ caused a mild inhibition of the elevated activity of mitochondrial complex II, and increased state 2 respiration of mitochondrial complex I in HF myocytes. Furthermore, we compared the effects of TMZ and nNOS I on mitochondrial respiration in both control and HF myocytes. We found that in control myocytes nNOS I decreased state 2 respiration by 23%, while it did not affect state 3 respiration of complex I (−9% decrease in state 3) and complex II-mediated (+2% increase in state 2 and −9% decrease in state 3) respiration in control myocytes (Table 1). In HF myocytes however nNOS I increased the overall diminished complex I-mediated respiration (+34% increase in state 2 and +39% increase in state 3) but it did not affect the elevated electron leak at the level of mitochondrial complex II in HF myocytes (−1% decrease in state 2 and −5% decrease in state 3). To conclude, in HF myocytes both TMZ and nNOS I improved the diminished activity of mitochondrial complex I, but only TMZ decreased the complex II-mediated electron leak.
3.8. Effects of TMZ on ΔΨm
To evaluate effects of TMZ on ΔΨm, TMRM loaded cells were permeabilized with digitonin in the absence of substrates for the ETC. Subsequently mitochondrial complex I (5 mM malate + 2 mM glutamate) or complex II (5 mM succinate + 1 μM rotenone) substrates were added. Figures 7A and C demonstrate that addition of complex I substrates led to an increase in TMRM fluorescence (corresponding to an increase of ΔΨm or further polarization of the inner mitochondrial membrane) which was significantly smaller in HF myocytes (linear rate of increase was 0.0127±0.0039 s−1 in HF (n=5) versus 0.0323±0.0066 in control (n=6); P<0.05), indicating that the activity of complex I was significantly impaired in HF (~60% inhibition). In contrast, complex II-mediated rate of ΔΨm recovery was significantly increased in HF (~64% increase; rate constant k=0.0041±0.0003 s−1, n=8, P<0.05) compared to control (k=0.0025±0.0001 s−1, n=6) cells (Figs. 7B and D). The complex II activity was determined by the ability of mitochondria to restore ΔΨm during 5 mM succinate supplementation when complex I was blocked by 1 μM rotenone. TMZ (1 μM, 15 min) had no effect on complex I activity in control cells (linear rate of increase=0.0312±0.0043, n=5, P=0.84), while it increased the activity of complex I (linear rate of TMRM fluorescence increase=0.0229±0.0036, n=5, P<0.05) by +79% in HF myocytes. However, TMZ significantly decreased (~30% inhibition) the elevated activity of complex II in HF cells to restore ΔΨm (k=0.0029±0.0001, n=8, P<0.01) while it had no effect on complex II activity in control cells (k=0.0026±0.0001, n=5, P=0.3). Despite the increased rate of ΔΨm recovery, the net TMRM fluorescence signal generated by succinate (calculated as the difference between low TMRM fluorescence levels measured after rotenone addition and highest levels obtained after succinate addition; Fig. 7B) was not different in HF compared to control (ΔF=72.9±3.4, n=8 versus 77.2±10.8, n=6 in control, P=0.36), and was not affected by TMZ treatment (ΔF=73.7±5.9, n=9 versus 72.9±3.4, n=8 in HF without TMZ, P=0.45). This points towards an electron leakage at the level of the mitochondrial complex II since the higher activity did not translate into further polarization of ΔΨm.
Fig. 7.
Effects of TMZ on ΔΨm. Effect of TMZ on the ability of permeabilized control and HF ventricular myocytes to maintain ΔΨm when respiring (A) on complex I (5 mM malate + 2 mM glutamate) substrates or (B) on complex II (5 mM succinate + 1 μM rotenone) substrates. (C–D) Summary of TMZ effects on the rate of ΔΨm changes mediated by the complex I and complex II respiration, respectively. *P<0.05.
3.9. Effect TMZ on FAD-mediated redox state
The redox state of the flavoprotein pools reflects the balance between the rate of reduction by substrate processing and rate of oxidation by mitochondrial respiration [32]. Thus, both up-regulation of substrate processing and inhibition of respiration would shift the redox balance towards a reduced state. On the other hand, an increase in respiration rate would favor net oxidation of the flavoprotein pool. The basal level of the FAD/FADH2 ratio was expressed as a “redox index”, i.e. as a fraction of the maximally oxidized minus the maximally reduced signal. These limits were determined from the response to 4 mM NaCN (maximal FAD reduction, i.e. FADH2 formation) and to 1 μM FCCP (complete oxidation of mitochondrial FADH2 pool). The basal level of FAD-mediated autofluorescence was significantly decreased in HF (9.7 ± 0.9%, n=9 versus 17.3±1.6%, n=13 in control, P<0.05) indicating an increase in the reduced state of FADH2, and an impairment of the ETC (Table 2). TMZ (1 μM, 15 min) led to ~2-fold increase in the basal FAD/FADH2 level in control (38.1±10.5%, n=8) and ~3-fold increase in HF (30.2±7.2%, n=5) cells, suggesting that TMZ helps maintain the redox balance of the cell. Moreover, cell treatment with nNOS I (240 nM, 15 min) induced a similar increase in FAD/FADH2 level in both control (27.5±3.3%, n=9) and HF myocytes (27.1±0.7%, n=6) cells. This again points towards the conclusion that TMZ acts by re-balancing NO and ROS production at the level of mitochondria.
Table 2.
Effect of TMZ and nNOS I on the mitochondrial redox state measured by flavoprotein-mediated autofluorescence in intact control and HF cells
| Experiments | no treatment | +TMZ | +nNOS I |
|---|---|---|---|
| Control rabbit | 17.3 ±1.56%, n=13 | 38.14 ± 10.52%#, n=8 | 27.54 ± 3.03%#, n=8 |
| HF rabbit | 9.67 ±0.91.63%*, n=9 | 30.19 ± 7.20%#, n=5 | 27.12 ± 0.77%#, n=6 |
P<0.05, HF compared to control cells
P<0.05, TMZ treated cells compared to cells in the absence of TMZ
4. Discussion
A key finding of our study is the observation that in HF myocytes TMZ prevented Ca2+ overload and cell hypercontracture induced by electrical pacing and concomitant β–adrenergic stimulation while it did not affect [Ca2+]i transients and cell contraction in control myocytes (Figs. 1 and 2). It has been shown that mPTP opening at the inner mitochondrial membrane induced ATP-dependent hypercontracture in Ca2+ overloaded myocytes [39], eventually leading to sarcolemmal disruption and necrotic cell death [44, 45]. In this study, we demonstrate that the propensity for mPTP opening was nearly 2-fold higher in HF cells compared to control as determined by calcein release from mitochondria (Fig. 3) and mitochondrial CRC (see 3.3.). The opening of the mPTP was also prevented by superoxide dismutase mimetic MnTBAP and inhibition of mtNOS, indicating that mitochondrial ROS generation was leading to mPTP opening. Remarkably, ROS-independent mPTP opening induced by CAT did not result in hypercontractrure and dramatic changes in ecc, however when β–adrenergic receptors were activated subsequently with ISO, significant impairment of ecc was observed (Figs. 1 and 2). These data further show that ROS-independent mPTP opening contributes to Ca2+ and mechanical dysregulation in cardiac myocytes, but for severe ecc impairment to develop β–adrenergic receptor activation was required. We found that ISO application to electrically-stimulated HF cells induced a dramatic increase in ROS generation compared to control cells (Fig. 5). This ROS generation was completely prevented by blocking complexes I and II of the mitochondrial ETC or inhibiting mtNOS confirming the mitochondrial origin of ROS and contribution of uncoupled mtNOS to ROS production (Fig. 5). At the same time we found that NO production was significantly decreased in HF myocytes (Fig. 6) indicating that mtNOS uncoupling shifted activity from NO production to ROS generation. Consistent with our previous findings, Ca2+-induced NO production was abolished after treatment with the mitochondrial uncoupler FCCP and a specific blocker of nNOS (nNOS I, Fig. 6D) confirming that NO was generated by an nNOS isoform localized to mitochondria and previously characterized as mtNOS [24]. Moreover, we found that TMZ protected HF cells from Ca2+-induced mPTP opening (Fig. 3) via suppression ISO-induced ROS generation (Fig. 5) and restoring impaired mitochondrial NO production (Fig. 6). While the anti-oxidant effect of TMZ was previously suggested [16, 17, 46], this is the first study to provide direct evidence that TMZ abolished ROS generation induced by combined electrical and β-adrenergic stimulation in HF (Fig. 5).
It has long been believed that the main mode of action of TMZ in the setting of myocardial ischemia occurs via “metabolic switch” [5, 7] through inhibition of fatty acid oxidation, however this paradigm has been challenged recently. FFA levels have been found to be unchanged in a rat model of HF induced by myocardial infarction [47]. Direct measurements of cardiac FAO in a clinical study [8] in patients with chronic HF caused by idiopathic cardiomyopathy revealed no changes in myocardial fatty acid uptake and only a 10% decrease in FAO by TMZ. However, since FFA serum levels were increased in our rabbit HF model, we evaluated whether TMZ had any effect on the activity of 3-KAT, the terminal enzyme in the FAO cascade. We determined that the oxidation of palmitoylcarnitine, a substrate for FFA oxidation, was decreased ~15% by TMZ and 4-BA, a different inhibitor of FAO (Table 1). In contrast, despite their similar effects on FAO, only TMZ prevented opening of the mitochondrial mPTP induced by PA (Fig. 4), suggesting that TMZ had mitochondrial effects independent of FFA metabolism. We also found that both Ca2+-induced and PA-induced mPTP opening were blocked by superoxide dismutase mimetic MnTBAP and nNOS I, suggesting that mPTP opening was induced by elevated ROS production generated by the ETC and/or uncoupled mtNOS. It has been shown previously that uncoupled NOS can be a major source of ROS generation in cardiac hypertrophy and HF [48–50], which could lead to the opening of the mPTP [24]. In the present study, we demonstrated that TMZ was able to improve the diminished NO production and inhibit the enhanced ROS generation observed during HF (Figs. 5 and 6), which indicates that the cardioprotective action of TMZ may be related to re-coupling of mtNOS. Data from our [31] and other laboratories [51, 52] strongly indicate that mtNOS enzyme activity is voltage-dependent and regulated by ΔΨm. The regulation of H+ electrochemical potential across the inner mitochondrial membrane by elevated FFA might be one of the reasons of mtNOS uncoupling during HF. We explored the effect of TMZ on the ability of mitochondria to maintain ΔΨm when supplemented with mitochondrial complex I and II substrates. TMZ treatment increased the ability of mitochondrial complex I to maintain ΔΨm in HF. Although the rate of ΔΨm recovery by succinate was almost 2 times faster in HF cells, it did not lead to a more pronounced polarization of ΔΨm (Fig. 7). Mitochondrial respiration measurements (Table 1) demonstrated that an elevated activity of complex II (i.e. increased electron flux through succinate dehydrogenase) in HF was not paralleled by an increased ATP production, suggesting an electron leakage at the level of the complex II. TMZ decreased both rates of succinate-mediated ΔΨm recovery and oxidation of succinate, but did not affect the steady-state ΔΨm level, suggesting that it prevented electron leak from a super-active mitochondrial complex II. It has been suggested previously that elevated electron flow through mitochondrial complex II at stable polarized ΔΨm allows conditions for singlet electron leak responsible for enhanced ROS formation [53]. Moreover, our data are in agreement with a recent proteomic study [54] which revealed that FAD-containing subunit A of the succinate dehydrogenase (complex II) was up-regulated 1.58 times in pressure overload-induced HF, while ten subunits of mitochondrial complex I, three subunits of complex III, and three subunits of complex IV were down-regulated to various degrees. Increased activity of the mitochondrial complex II was also reported in a rat model of right-ventricular failure induced by pulmonary arterial hypertension [55] and was associated with elevated ROS production. ROS generation was partially blocked by thenoyltrifluoroacetone (complex II inhibitor) and completely prevented by the complex III inhibitor myxothiazol, identifying mitochondrial complex III as the main source of ROS upon increased activity of complex II [55]. However, under certain conditions complex II itself can be a site of ROS production [56] where electron transfer from reduced flavins in succinate dehydrogenase can generate flavin semiquinone radicals that can reduce O2 to form O2−. Furthermore, despite the general view that complex II is not a significant contributor to ROS generation, high rates of O2− and hydrogen peroxide production were determined in skeletal muscle mitochondria when complex I and III activity was compromised [57]. Our data demonstrate that stimulation of the mitochondrial complex II with methyl succinate in HF myocytes led to a dramatic increase in O2− generation (Fig. 5H) compared to ROS-generation observed upon stimulation of mitochondrial complex I (Fig. 5G). Complex II-induced ROS generation was partially inhibited by rotenone (rotenone blocks the transfer of electrons from iron-sulfur centers in complex I to ubiquinone) and antimycin A (Ant A binds to the Qi site of the mitochondrial complex III, thereby inhibiting the oxidation of ubiquinol in the ETC), demonstrating that both reverse and forward electron fluxes were involved in this ROS generation. TMZ completely prevented ROS generation induced by methyl succinate (Fig. 5I). This data are in agreement with the notion that TMZ decreased an elevated electron leak from mitochondrial complex II (see above and Table 1). Moreover, it has been suggested that flavins of mitochondrial complex II should be reduced but not occupied in order to produce significant amount of ROS [57]. We found that in rabbit HF myocytes FAD was more reduced compared with control cells, and both TMZ and nNOS I significantly increased FAD/FADH2 ratio in HF cells (Table 2), which indicates that TMZ modifies the cellular redox potential possibly via modulation of mitochondrial ROS and NO production. FAD is also an essential co-factor for NOS activation [24, 58], and the decreased availability of FAD may also contribute to mtNOS uncoupling and ROS generation. Several reports demonstrated the dependence of mtNOS activity on the function of mitochondrial complex I [59, 60]. This fact was interpreted as an indication of mtNOS being structurally adjacent to complex I with an intermolecular mtNOS-complex I hydrophobic bonding that is stronger at high ΔΨm and weaker at low (depolarized) ΔΨm [60]. Moreover, it has been demonstrated that inactivation of mitochondrial ETC complex I (similar to what we observed in rabbit HF) was associated with uncoupling of mtNOS to produce ROS instead of NO [43]. The fact that mtNOS inhibition by nNOS I in HF improved mitochondrial respiration at the level of the mitochondrial complex I (Table 1) further support the hypothesis of a close association of mtNOS and complex I. Furthermore, electron flow through the ETC complex I has been implicated in mPTP regulation [34, 36, 61]. In addition, the increased activity of the mitochondrial complex II can stimulate electron flux in the reverse direction and initiate ROS generation at the level of the flavin mononucleotide (FMN) of mitochondrial complex I [62, 63]. By decreasing complex II activity, TMZ would diminish reverse electron flow, and therefore ROS generation at FMN of complex I. In this study, we found that inhibition of either mitochondrial complexes I or II of the ETC or mtNOS resulted in decreased ROS generation induced by β-adrenergic receptor activation in field-stimulated HF myocytes (Fig. 5 D–F). This indicates that enhanced electron leak at the level of the mitochondrial complex II can be linked to the uncoupling of mtNOS, however more studies are required to explore this intriguing possibility. In summary, our study clearly shows that mild inhibition of mitochondrial complex II by TMZ ultimately results in reduced mitochondria-dependent oxidative stress and enhancement of mitochondrial NO generation.
In conclusion, our data provide evidence that the anti-ischemic agent TMZ protects HF myocytes from Ca2+ overload upon combined electrical and β–adrenergic stimulation without having negative inotropic effects (no reduction in [Ca2+]i transient amplitude and cell shortening), i.e. TMZ protects against proarrhythmic Ca2+ overload and Ca2+ release while maintaining adequate contractility. Furthermore, HF cells revealed a reduced protection against mPTP opening induced by elevated [Ca2+]em. Opening of the mPTP was accompanied by increased ROS generation and decreased NO production in HF cells, which was prevented by TMZ. Moreover, an increased activity of mitochondrial complex II in HF did not lead to increased ATP production (estimated from the rate 3 of respiration) suggesting uncoupling of the ETC at the level of mitochondrial complex II. Treatment with TMZ partially decreased the elevated activity of mitochondrial complex II, prevented complex II-mediated ROS generation and restored the redox balance of the cell. Our data indicate that uncoupled mtNOS and respiratory complex II are likely candidates for increased ROS production in HF. We confirmed that TMZ has an inhibitory effect on FAO, however the observed novel cardioprotective action of TMZ was independent of this mechanism, and suggests altered mitochondrial function as a promising therapeutic target for the treatment of heart failure.
Highlights.
TMZ preserves cardiac function in the conditions of non-ischemic rabbit HF.
The beneficial effect of TMZ was not linked to fatty acid oxidation inhibition.
Cardioprotection by TMZ was mediated by prevention of Ca2+-induced mPTP opening.
The mechanism relies on re-coupling of the mitochondrial respiratory chain and mtNOS.
Mild inhibition of the mitochondrial complex II by TMZ was beneficial in HF.
Acknowledgments
Funding
This work was supported by the National Institutes of Health Grants R01HL062231, P01HL080101 and R01HL101235 (to LAB), the Leducq Foundation (to LAB), the American Heart Association (AHA) National Scientist Development Grant AHA 0735071N (to END) and Rush University Medical Center New Investigator Grant-in-Aid 31196 (to END).
Non-standard Abbreviations and Acronyms
- ΔΨm
mitochondrial membrane potential
- 4-BA
4-bromotiglic acid
- [Ca2+]i
cytosolic calcium concentration
- [Ca2+]em
extramitochondrial free calcium
- CAT
carboxyatractyloside
- ecc
excitation-contraction coupling
- ETC
electron transport chain
- FAO
fatty acid oxidation
- FCCP
carbonyl cyanide 4-(trifluoromethyhoxy)-phenylhydrazone
- FFA
free fatty acids
- FS
field stimulation
- HF
heart failure
- 3-KAT
3-ketoacyl CoA thiolase
- mtNOS
mitochondrial nitric oxide synthase
- NO
nitric oxide
- [NO]mt
mitochondrial nitric oxide concentration
- PA
palmitic acid
- mPTP
mitochondrial permeability transition pore
- ROS
reactive oxygen species
- Rot
rotenone
- TMRM
tetramethylrhodamine methyl ester
- TMZ
trimetazidine
- TTFA
2-thenoyltrifluoroacetone
Footnotes
Disclosure statement
There is no conflict of interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005 Jul;85(3):1093–129. doi: 10.1152/physrev.00006.2004. [DOI] [PubMed] [Google Scholar]
- 2.Opie LH, Knuuti J. The adrenergic-fatty acid load in heart failure. J Am Coll Cardiol. 2009 Oct 27;54(18):1637–46. doi: 10.1016/j.jacc.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 3.Essop MF, Opie LH. Metabolic therapy for heart failure. Eur Heart J. 2004 Oct;25(20):1765–8. doi: 10.1016/j.ehj.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 4.Morrow DA, Givertz MM. Modulation of myocardial energetics: emerging evidence for a therapeutic target in cardiovascular disease. Circulation. 2005 Nov 22;112(21):3218–21. doi: 10.1161/CIRCULATIONAHA.105.581819. [DOI] [PubMed] [Google Scholar]
- 5.Tang WH. Metabolic approach in heart failure: rethinking how we translate from theory to clinical practice. J Am Coll Cardiol. 2006 Sep 5;48(5):999–1000. doi: 10.1016/j.jacc.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 6.Vitale C, Wajngaten M, Sposato B, Gebara O, Rossini P, Fini M, et al. Trimetazidine improves left ventricular function and quality of life in elderly patients with coronary artery disease. Eur Heart J. 2004 Oct;25(20):1814–21. doi: 10.1016/j.ehj.2004.06.034. [DOI] [PubMed] [Google Scholar]
- 7.Fragasso G, Perseghin G, De Cobelli F, Esposito A, Palloshi A, Lattuada G, et al. Effects of metabolic modulation by trimetazidine on left ventricular function and phosphocreatine/adenosine triphosphate ratio in patients with heart failure. Eur Heart J. 2006 Apr;27(8):942–8. doi: 10.1093/eurheartj/ehi816. [DOI] [PubMed] [Google Scholar]
- 8.Tuunanen H, Engblom E, Naum A, Nagren K, Scheinin M, Hesse B, et al. Trimetazidine, a metabolic modulator, has cardiac and extracardiac benefits in idiopathic dilated cardiomyopathy. Circulation. 2008 Sep 16;118(12):1250–8. doi: 10.1161/CIRCULATIONAHA.108.778019. [DOI] [PubMed] [Google Scholar]
- 9.Ashrafian H, Frenneaux MP, Opie LH. Metabolic mechanisms in heart failure. Circulation. 2007 Jul 24;116(4):434–48. doi: 10.1161/CIRCULATIONAHA.107.702795. [DOI] [PubMed] [Google Scholar]
- 10.Gunes Y, Guntekin U, Tuncer M, Sahin M. Improved left and right ventricular functions with trimetazidine in patients with heart failure: a tissue Doppler study. Heart Vessels. 2009 Jul;24(4):277–82. doi: 10.1007/s00380-008-1118-x. [DOI] [PubMed] [Google Scholar]
- 11.Cera M, Salerno A, Fragasso G, Montanaro C, Gardini C, Marinosci G, et al. Beneficial electrophysiological effects of trimetazidine in patients with postischemic chronic heart failure. J Cardiovasc Pharmacol Ther. 2010 Mar;15(1):24–30. doi: 10.1177/1074248409356431. [DOI] [PubMed] [Google Scholar]
- 12.Kantor PF, Lucien A, Kozak R, Lopaschuk GD. The antianginal drug trimetazidine shifts cardiac energy metabolism from fatty acid oxidation to glucose oxidation by inhibiting mitochondrial long-chain 3-ketoacyl coenzyme A thiolase. Circ Res. 2000 Mar 17;86(5):580–8. doi: 10.1161/01.res.86.5.580. [DOI] [PubMed] [Google Scholar]
- 13.Saeedi R, Grist M, Wambolt RB, Bescond-Jacquet A, Lucien A, Allard MF. Trimetazidine normalizes postischemic function of hypertrophied rat hearts. J Pharmacol Exp Ther. 2005 Jul;314(1):446–54. doi: 10.1124/jpet.104.082636. [DOI] [PubMed] [Google Scholar]
- 14.Morgan EE, Young ME, McElfresh TA, Kung TA, Hoit BD, Chandler MP, et al. Chronic treatment with trimetazidine reduces the upregulation of atrial natriuretic peptide in heart failure. Fundam Clin Pharmacol. 2006 Oct;20(5):503–5. doi: 10.1111/j.1472-8206.2006.00424.x. [DOI] [PubMed] [Google Scholar]
- 15.MacInnes A, Fairman DA, Binding P, Rhodes J, Wyatt MJ, Phelan A, et al. The antianginal agent trimetazidine does not exert its functional benefit via inhibition of mitochondrial long-chain 3-ketoacyl coenzyme A thiolase. Circ Res. 2003 Aug 8;93(3):e26–32. doi: 10.1161/01.RES.0000086943.72932.71. [DOI] [PubMed] [Google Scholar]
- 16.Gambert S, Vergely C, Filomenko R, Moreau D, Bettaieb A, Opie LH, et al. Adverse effects of free fatty acid associated with increased oxidative stress in postischemic isolated rat hearts. Mol Cell Biochem. 2006 Feb;283(1–2):147–52. doi: 10.1007/s11010-006-2518-9. [DOI] [PubMed] [Google Scholar]
- 17.Monteiro P, Duarte AI, Goncalves LM, Moreno A, Providencia LA. Protective effect of trimetazidine on myocardial mitochondrial function in an ex-vivo model of global myocardial ischemia. Eur J Pharmacol. 2004 Oct 25;503(1–3):123–8. doi: 10.1016/j.ejphar.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Argaud L, Gomez L, Gateau-Roesch O, Couture-Lepetit E, Loufouat J, Robert D, et al. Trimetazidine inhibits mitochondrial permeability transition pore opening and prevents lethal ischemia-reperfusion injury. J Mol Cell Cardiol. 2005 Dec;39(6):893–9. doi: 10.1016/j.yjmcc.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 19.Di Napoli P, Chierchia S, Taccardi AA, Grilli A, Felaco M, Caterina RD, et al. Trimetazidine improves post-ischemic recovery by preserving endothelial nitric oxide synthase expression in isolated working rat hearts. Nitric Oxide. 2007 Mar;16(2):228–36. doi: 10.1016/j.niox.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Pogwizd SM. Nonreentrant mechanisms underlying spontaneous ventricular arrhythmias in a model of nonischemic heart failure in rabbits. Circulation. 1995 Aug 15;92(4):1034–48. doi: 10.1161/01.cir.92.4.1034. [DOI] [PubMed] [Google Scholar]
- 21.Pogwizd SM, Qi M, Yuan W, Samarel AM, Bers DM. Upregulation of Na(+)/Ca(2+) exchanger expression and function in an arrhythmogenic rabbit model of heart failure. Circ Res. 1999 Nov 26;85(11):1009–19. doi: 10.1161/01.res.85.11.1009. [DOI] [PubMed] [Google Scholar]
- 22.Basso E, Fante L, Fowlkes J, Petronilli V, Forte MA, Bernardi P. Properties of the permeability transition pore in mitochondria devoid of Cyclophilin D. J Biol Chem. 2005 May 13;280(19):18558–61. doi: 10.1074/jbc.C500089200. [DOI] [PubMed] [Google Scholar]
- 23.Santiago DJ, Curran JW, Bers DM, Lederer WJ, Stern MD, Rios E, et al. Ca sparks do not explain all ryanodine receptor-mediated SR Ca leak in mouse ventricular myocytes. Biophys J. 2010 May 19;98(10):2111–20. doi: 10.1016/j.bpj.2010.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dedkova EN, Blatter LA. Characteristics and function of cardiac mitochondrial nitric oxide synthase. J Physiol. 2009 Feb 15;587(Pt 4):851–72. doi: 10.1113/jphysiol.2008.165423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dedkova EN, Blatter LA. Modulation of mitochondrial Ca2+ by nitric oxide in cultured bovine vascular endothelial cells. Am J Physiol Cell Physiol. 2005 Oct;289(4):C836–45. doi: 10.1152/ajpcell.00011.2005. [DOI] [PubMed] [Google Scholar]
- 26.Dedkova EN, Blatter LA. Mitochondrial calcium uptake stimulates nitric oxide and ROS production by mitochondria-specific nitric oxide synthase (mtNOS) in cat ventricular myocytes. Biophysical Journal. 2006;90:521a. [Google Scholar]
- 27.Sedova M, Dedkova EN, Blatter LA. Integration of rapid cytosolic Ca2+ signals by mitochondria in cat ventricular myocytes. Am J Physiol Cell Physiol. 2006 Nov;291(5):C840–50. doi: 10.1152/ajpcell.00619.2005. [DOI] [PubMed] [Google Scholar]
- 28.Pilz S, Scharnagl H, Tiran B, Seelhorst U, Wellnitz B, Boehm BO, et al. Free fatty acids are independently associated with all-cause and cardiovascular mortality in subjects with coronary artery disease. J Clin Endocrinol Metab. 2006 Jul;91(7):2542–7. doi: 10.1210/jc.2006-0195. [DOI] [PubMed] [Google Scholar]
- 29.Richieri GV, Ogata RT, Kleinfeld AM. A fluorescently labeled intestinal fatty acid binding protein. Interactions with fatty acids and its use in monitoring free fatty acids. J Biol Chem. 1992 Nov 25;267(33):23495–501. [PubMed] [Google Scholar]
- 30.Wang YG, Dedkova EN, Fiening JP, Ojamaa K, Blatter LA, Lipsius SL. Acute exposure to thyroid hormone increases Na+ current and intracellular Ca2+ in cat atrial myocytes. J Physiol. 2003 Jan 15;546(Pt 2):491–9. doi: 10.1113/jphysiol.2002.032847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dedkova EN, Ji X, Lipsius SL, Blatter LA. Mitochondrial calcium uptake stimulates nitric oxide production in mitochondria of bovine vascular endothelial cells. Am J Physiol Cell Physiol. 2004 Feb;286(2):C406–15. doi: 10.1152/ajpcell.00155.2003. [DOI] [PubMed] [Google Scholar]
- 32.Dedkova EN, Blatter LA. Measuring mitochondrial function in intact cardiac myocytes. J Mol Cell Cardiol. 2012 Jan;52(1):48–61. doi: 10.1016/j.yjmcc.2011.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marsh D. Peptide models for membrane channels. Biochem J. 1996 Apr 15;315( Pt 2):345–61. doi: 10.1042/bj3150345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fontaine E, Eriksson O, Ichas F, Bernardi P. Regulation of the permeability transition pore in skeletal muscle mitochondria. Modulation By electron flow through the respiratory chain complex i. J Biol Chem. 1998 May 15;273(20):12662–8. doi: 10.1074/jbc.273.20.12662. [DOI] [PubMed] [Google Scholar]
- 35.Fontaine E, Ichas F, Bernardi P. A ubiquinone-binding site regulates the mitochondrial permeability transition pore. J Biol Chem. 1998 Oct 2;273(40):25734–40. doi: 10.1074/jbc.273.40.25734. [DOI] [PubMed] [Google Scholar]
- 36.Li B, Chauvin C, De Paulis D, De Oliveira F, Gharib A, Vial G, et al. Inhibition of complex I regulates the mitochondrial permeability transition through a phosphate-sensitive inhibitory site masked by cyclophilin D. Biochim Biophys Acta. 2012 Sep;1817(9):1628–34. doi: 10.1016/j.bbabio.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 37.Romashko DN, Marban E, O’Rourke B. Subcellular metabolic transients and mitochondrial redox waves in heart cells. Proc Natl Acad Sci U S A. 1998 Feb 17;95(4):1618–23. doi: 10.1073/pnas.95.4.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zima AV, Kockskamper J, Mejia-Alvarez R, Blatter LA. Pyruvate modulates cardiac sarcoplasmic reticulum Ca2+ release in rats via mitochondria-dependent and -independent mechanisms. J Physiol. 2003 Aug 1;550(Pt 3):765–83. doi: 10.1113/jphysiol.2003.040345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruiz-Meana M, Abellan A, Miro-Casas E, Garcia-Dorado D. Opening of mitochondrial permeability transition pore induces hypercontracture in Ca2+ overloaded cardiac myocytes. Basic Res Cardiol. 2007 Nov;102(6):542–52. doi: 10.1007/s00395-007-0675-y. [DOI] [PubMed] [Google Scholar]
- 40.Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J. 1999 Jul 15;341( Pt 2):233–49. [PMC free article] [PubMed] [Google Scholar]
- 41.Di Lisa F, Carpi A, Giorgio V, Bernardi P. The mitochondrial permeability transition pore and cyclophilin D in cardioprotection. Biochim Biophys Acta. 2011 Jul;1813(7):1316–22. doi: 10.1016/j.bbamcr.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 42.Liang X, Schulz H. 4-bromotiglic acid, a novel inhibitor of thiolases and a tool for assessing the cooperation between the membrane-bound and soluble beta-oxidation systems of rat liver mitochondria. Biochemistry. 1998 Nov 3;37(44):15548–54. doi: 10.1021/bi981613f. [DOI] [PubMed] [Google Scholar]
- 43.Parihar MS, Parihar A, Villamena FA, Vaccaro PS, Ghafourifar P. Inactivation of mitochondrial respiratory chain complex I leads mitochondrial nitric oxide synthase to become pro-oxidative. Biochem Biophys Res Commun. 2008 Mar 21;367(4):761–7. doi: 10.1016/j.bbrc.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 44.Ruiz-Meana M, Abellan A, Miro-Casas E, Agullo E, Garcia-Dorado D. Role of sarcoplasmic reticulum in mitochondrial permeability transition and cardiomyocyte death during reperfusion. Am J Physiol Heart Circ Physiol. 2009 Oct;297(4):H1281–9. doi: 10.1152/ajpheart.00435.2009. [DOI] [PubMed] [Google Scholar]
- 45.Honda HM, Ping P. Mitochondrial permeability transition in cardiac cell injury and death. Cardiovasc Drugs Ther. 2006 Dec;20(6):425–32. doi: 10.1007/s10557-006-0642-0. [DOI] [PubMed] [Google Scholar]
- 46.Iskesen I, Saribulbul O, Cerrahoglu M, Var A, Nazli Y, Sirin H. Trimetazidine reduces oxidative stress in cardiac surgery. Circ J. 2006 Sep;70(9):1169–73. doi: 10.1253/circj.70.1169. [DOI] [PubMed] [Google Scholar]
- 47.O’Shea KM, Khairallah RJ, Sparagna GC, Xu W, Hecker PA, Robillard-Frayne I, et al. Dietary omega-3 fatty acids alter cardiac mitochondrial phospholipid composition and delay Ca2+-induced permeability transition. J Mol Cell Cardiol. 2009 Dec;47(6):819–27. doi: 10.1016/j.yjmcc.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takimoto E, Champion HC, Li M, Ren S, Rodriguez ER, Tavazzi B, et al. Oxidant stress from nitric oxide synthase-3 uncoupling stimulates cardiac pathologic remodeling from chronic pressure load. J Clin Invest. 2005 May;115(5):1221–31. doi: 10.1172/JCI21968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moens AL, Takimoto E, Tocchetti CG, Chakir K, Bedja D, Cormaci G, et al. Reversal of cardiac hypertrophy and fibrosis from pressure overload by tetrahydrobiopterin: efficacy of recoupling nitric oxide synthase as a therapeutic strategy. Circulation. 2008 May 20;117(20):2626–36. doi: 10.1161/CIRCULATIONAHA.107.737031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moens AL, Leyton-Mange JS, Niu X, Yang R, Cingolani O, Arkenbout EK, et al. Adverse ventricular remodeling and exacerbated NOS uncoupling from pressure-overload in mice lacking the beta3-adrenoreceptor. J Mol Cell Cardiol. 2009 Nov;47(5):576–85. doi: 10.1016/j.yjmcc.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Valdez LB, Zaobornyj T, Boveris A. Mitochondrial metabolic states and membrane potential modulate mtNOS activity. Biochim Biophys Acta. 2006 Mar;1757(3):166–72. doi: 10.1016/j.bbabio.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 52.Kanai AJ, Pearce LL, Clemens PR, Birder LA, VanBibber MM, Choi SY, et al. Identification of a neuronal nitric oxide synthase in isolated cardiac mitochondria using electrochemical detection. Proc Natl Acad Sci U S A. 2001 Nov 20;98(24):14126–31. doi: 10.1073/pnas.241380298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heinen A, Camara AK, Aldakkak M, Rhodes SS, Riess ML, Stowe DF. Mitochondrial Ca2+-induced K+ influx increases respiration and enhances ROS production while maintaining membrane potential. Am J Physiol Cell Physiol. 2007 Jan;292(1):C148–56. doi: 10.1152/ajpcell.00215.2006. [DOI] [PubMed] [Google Scholar]
- 54.Bugger H, Schwarzer M, Chen D, Schrepper A, Amorim PA, Schoepe M, et al. Proteomic remodelling of mitochondrial oxidative pathways in pressure overload-induced heart failure. Cardiovasc Res. 2010 Jan 15;85(2):376–84. doi: 10.1093/cvr/cvp344. [DOI] [PubMed] [Google Scholar]
- 55.Redout EM, Wagner MJ, Zuidwijk MJ, Boer C, Musters RJ, van Hardeveld C, et al. Right-ventricular failure is associated with increased mitochondrial complex II activity and production of reactive oxygen species. Cardiovasc Res. 2007 Sep 1;75(4):770–81. doi: 10.1016/j.cardiores.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 56.Zhang L, Yu L, Yu CA. Generation of superoxide anion by succinate-cytochrome c reductase from bovine heart mitochondria. J Biol Chem. 1998 Dec 18;273(51):33972–6. doi: 10.1074/jbc.273.51.33972. [DOI] [PubMed] [Google Scholar]
- 57.Quinlan CL, Orr AL, Perevoshchikova IV, Treberg JR, Ackrell BA, Brand MD. Mitochondrial complex II can generate reactive oxygen species at high rates in both the forward and reverse reactions. J Biol Chem. 2012 Aug 3;287(32):27255–64. doi: 10.1074/jbc.M112.374629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–42. [PubMed] [Google Scholar]
- 59.Parihar MS, Nazarewicz RR, Kincaid E, Bringold U, Ghafourifar P. Association of mitochondrial nitric oxide synthase activity with respiratory chain complex I. Biochem Biophys Res Commun. 2008 Feb 1;366(1):23–8. doi: 10.1016/j.bbrc.2007.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Navarro A, Bandez MJ, Gomez C, Repetto MG, Boveris A. Effects of rotenone and pyridaben on complex I electron transfer and on mitochondrial nitric oxide synthase functional activity. J Bioenerg Biomembr. 2010 Oct;42(5):405–12. doi: 10.1007/s10863-010-9309-4. [DOI] [PubMed] [Google Scholar]
- 61.Guigas B, Detaille D, Chauvin C, Batandier C, De Oliveira F, Fontaine E, et al. Metformin inhibits mitochondrial permeability transition and cell death: a pharmacological in vitro study. Biochem J. 2004 Sep 15;382(Pt 3):877–84. doi: 10.1042/BJ20040885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kudin AP, Bimpong-Buta NY, Vielhaber S, Elger CE, Kunz WS. Characterization of superoxide-producing sites in isolated brain mitochondria. J Biol Chem. 2004 Feb 6;279(6):4127–35. doi: 10.1074/jbc.M310341200. [DOI] [PubMed] [Google Scholar]
- 63.Liu Y, Fiskum G, Schubert D. Generation of reactive oxygen species by the mitochondrial electron transport chain. J Neurochem. 2002 Mar;80(5):780–7. doi: 10.1046/j.0022-3042.2002.00744.x. [DOI] [PubMed] [Google Scholar]