Abstract
Persistent pain is a common reason for reduced quality of life after a spinal cord injury (SCI). Biomarkers of neuropathic pain may facilitate translational research and the understanding of underlying mechanisms. Research suggests that pain and affective distress are anatomically and functionally integrated in the anterior cingulate cortex and can modulate sensory and affective aspects of pain. We hypothesized that severe neuropathic pain with a significant psychosocial impact would be associated with metabolite concentrations (obtained by magnetic resonance spectroscopy) in the anterior cingulate cortex, indicating neuronal and/or glial dysfunction. Participants with SCI and severe, high-impact neuropathic pain (SCI-HPI; n = 16), SCI and moderate, low-impact neuropathic pain (SCI-LPI; n = 24), SCI without neuropathic pain (SCI-noNP; n = 14), and able-bodied, pain-free control subjects (A-B; n = 22) underwent a 3-T magnetic resonance imaging brain scan. Analyses revealed that the SCI-HPI group had significantly higher levels of myoinositol (Ins) (P < .000), creatine (P = .007), and choline (P = .014), and significantly lower levels of N-acetyl aspartate/Ins (P = .024) and glutamate-glutamine (Glx)/Ins (P = .003) ratios than the SCI-LPI group. The lower Glx/Ins ratio significantly discriminated between SCI-HPI and the A-B (P = .006) and SCI-noNP (P = .026) groups, displayed excellent test-retest reliability, and was significantly related to greater pain severity, interference, and affective distress. This suggests that the combination of lower glutamatergic metabolism and proliferation of glia and glial activation are underlying mechanisms contributing to the maintenance of severe neuropathic pain with significant psychosocial impact in chronic SCI. These findings indicate that the Glx/Ins ratio may be a useful biomarker for severe SCI-related neuropathic pain with significant psychosocial impact.
Keywords: Cingulate cortex, Magnetic resonance spectroscopy, Neuropathic pain, Psychosocial impact, Spinal cord injury
1. Introduction
Persistent pain is one of the most common reasons for reduced quality of life after spinal cord injury (SCI) [8,59]. The most rational approach for managing these refractory pain conditions is to identify the primary underlying mechanisms in each person and tailor treatment to these [5,60]. Unfortunately, the identification of clinical subgroups, i.e., clinical phenotypes or other surrogate biomarkers of specific spinal cord, thalamic, and cortical mechanisms [24,61], has been difficult. There is a need to further examine potential biomarkers of neuropathic pain that may facilitate translational research and increase the understanding of underlying mechanisms.
The biopsychosocial model of pain suggests a dynamic interaction among biological, psychological, and social factors. Although biological mechanisms may initiate, maintain, and modulate pain, psychological factors influence the perception of pain and behavior in response to pain [50]. Therefore, an integrated assessment, including measures of pain severity and psychosocial impact, may be particularly relevant for complex persistent neuropathic pain phenotypes. An area of the brain pain network [2] suggested to be involved in the attentional and affective processing of pain is the anterior cingulate cortex (ACC; for this and other abbreviations please see Table 1) [3,10,13,38]. Research indicates that the ACC can exert powerful modulation of both sensory and affective aspects of pain via activation of a variety of receptor systems including μ-opioid [62] and gamma-amminobutyric-acid (GABA) [30] receptors, and via activation of the periaqueductal grey (PAG) [2].
Table 1.
Acronyms and abbreviations.
| A-B | Able-bodied pain-free control subjects |
| ACC | Anterior cingulate cortex |
| AD | Affective Distress subscale of the MPI-SCI |
| AIS | ASIA Impairment Scale |
| ATP | Adenosine triphosphate |
| BDI | Beck Depression Inventory |
| Cho | Choline |
| Cr | Creatine |
| DSM | Diagnostic and Statistical Manual of Mental Disorders |
| GABA | Gamma-aminobutyric acid |
| Glu | Glutamate |
| Glx | Compound of glutamate and glutamine MRS signals |
| ICC | Intraclass correlations |
| Ins | Myoinositol |
| ISNCSCI | International Standards for Neurological Classification of Spinal Cord Injury |
| LI | Life Interference subscale of the MPI-SCI |
| MMSE | Folstein Mini-Mental State Examination |
| MPI | Multidimensional Pain Inventory |
| MPI-SCI | Multidimensional Pain Inventory–SCI version |
| NAA | N-acetyl aspartate |
| MRS | Magnetic resonance spectroscopy |
| PAG | Periaqueductal grey |
| PS | Pain Severity subscale of the MPI-SCI |
| SCI-HPI | SCI high psychosocial impact |
| SCI-LPI | SCI low psychosocial impact |
| SCI-noNP | SCI no neuropathic pain |
| STAI | Spielberger State-Trait Anxiety Inventory |
| SWLS | Satisfaction with Life Scale |
SCI = spinal cord injury.
Magnetic resonance spectroscopy (MRS) research has demonstrated abnormal thalamic concentrations of common metabolites such as N-acetyl aspartate (NAA) and myoinositol (Ins) in chronic pain conditions [3,16,34]. NAA is localized predominantly in neurons and their processes [44], and a decrease in concentration may reflect loss of neurons or neuronal dysfunction [15]. In contrast, a higher concentration of Ins, a glial marker with a major role in the volume and osmoregulation of astrocytes [26], may indicate gliosis or glial activation. In the thalamus, lower NAA concentrations have been associated with neuropathic pain in diabetes [45] and after SCI [34]. Basic research suggests that glial activation is an important mechanism underlying neuropathic pain after SCI [22,23]. Increased brain concentrations of Ins have previously been found in people with neuropathic pain after SCI [34]. Other brain metabolites also suggesting higher levels of glia are choline (Cho), which is involved in membrane synthesis and degradation [14], and creatine (Cr), which is involved in energy metabolism via the creatine kinase reaction generating adenosine triphosphate [15]. The latter has been found in 2- to 4-fold higher concentrations in glial cells than in neurons [36,52]. Reduced glutamatergic metabolism, including lower concentrations of brain glutamate (Glu) or glutamate-glutamine (Glx) has also been observed in mood disorders[27,29], and specifically in the ACC in people with major depressive disorders [31] and chronic low back pain [17].
Research suggests that the experience of pain, affective distress, and associated cognitive control is anatomically and functionally integrated in the ACC [41]. Therefore, we hypothesized that severe neuropathic pain with a significant psychosocial impact would be associated with metabolic concentrations in the ACC indicating neuronal and/or glial dysfunction.
2. Methods
2.1. General study design
The present study included data from 50 subjects with SCI and chronic neuropathic pain, 18 subjects with SCI and no neuropathic pain, and 24 able-bodied pain-free individuals. Each subject completed a screening session to determine study eligibility, which included a neurological examination, a cognitive screening assessment (Folstein Mini-Mental State Examination), a brief pain evaluation, and a psychological evaluation performed by a licensed psychologist with extensive experience with SCI (S. Perez). During a second session, each subject completed a structured pain history interview, a battery of psychosocial measures, and a 3-T MRS session performed at the Applebaum Diagnostic Center at the University of Miami, Miller School of Medicine. Able-bodied control participants completed a subset of these evaluations appropriate for nondisabled control subjects. Subjects with SCI and neuropathic pain repeated the components of the second session during a third session, 2 to 4 weeks after the second session, to examine the test-retest reliability of study measures. The data presented in this article are part of a larger study approved by the Miami Veterans Affairs Medical Center Human Subjects subcommittee and the Institutional Review Board of the University of Miami, Miller School of Medicine, in Miami, Florida.
2.2. Subjects
The participants of the present study consisted of men and women with a traumatic SCI of at least 1 year duration with and without neuropathic pain at or below the level of injury and able-bodied control subjects. The criteria for neuropathic pain included pain in an area with neurological deficits and described as sharp, shooting, burning, and electric [43] for a minimum of 6 months, with an average pain intensity of at least 4 on a numerical rating scale with anchors of 0 = no pain and 10 = the most intense pain imaginable. The 2 other groups consisted of: (1) subjects with SCI and no neuropathic pain (SCI-noNP), who did not experience pain, or had only mild (numerical rating scale (NRS) < 4) nonneuropathic pain described as dull, aching, and cramping, in areas of sensory preservation [43]; and (2) able-bodied pain-free people (A-B) with no history of chronic pain or other major chronic health conditions. Exclusion criteria included present or past alcohol or other drug abuse, intracerebral pathology or epilepsy, cognitive impairment (Folstein Mini-Mental State Examination [12] < 25), diagnosis of a DSM-IV Axis I (clinical syndromes) disorder including major depression and drug or alcohol abuse, and general magnetic resonance imaging (MRI) contraindications (e.g., metal implants, claustrophobia). Demographic factors, including age, age at injury, time since injury, sex, level of education, and use of prescription of pain medications during the past 3 months were recorded for each participant.
2.3. The Folstein Mini-Mental State Examination
The Folstein Mini-Mental State Examination [12] is a brief screening test that assesses the following cognitive abilities: orientation, registration, attention and calculation, recall, naming, repetition, comprehension, reading, writing, and drawing. It is a widely used, valid, and reliable method of cognitive assessment in medical settings. Items involving drawing and writing (constructional praxis and dysgraphia) were omitted because some participants with SCI had significantly reduced ability to draw secondary to their physical impairment. These items are frequently omitted in rehabilitation settings when patients are unable to utilize their hands to complete necessary tasks. A score above 25 (out of 30) is considered normal, and the final score was prorated for subjects with SCI who were unable to draw or write because of upper limb motor impairment. Only subjects with scores indicating normal cognitive function were included as subjects in the study.
2.4. Neurological examination
Neurological examinations were conducted according to the International Standards for Neurological Classification of Spinal Cord Injury by 2 physicians with extensive experience in SCI (A. Martinez-Arizala and D.D. Cardenas) to assess neurological status and to determine severity of the SCI. The grading of the severity of the SCI was based on the ASIA Impairment Scale (AIS), from AIS A (no motor or sensory function in the sacral segments S4-S5) to AIS E (motor and sensory function are normal) [1]. All AIS grades represent incomplete neurological injuries, with the exception of AIS A, which represents a complete injury. If more than one level of injury was defined in the examination for motor or sensory function for either side of the body, the neurologic level of injury used in the analyses was the most rostral segment with normal findings as defined by the International Standards for Neurological Classification of Spinal Cord Injury. For purposes of analyses in the present study, the level of injury was divided into 2 categories: tetraplegia (cervical neurologic level of injury) and paraplegia (all those below cervical).
2.5. MRI and MRS
MRS is a noninvasive method to assess brain chemistry. The signal of the hydrogen atom attached to a certain molecule or metabolite has a particular frequency associated with a given metabolite. The utility of MRS is based on the fact that the nuclei of atoms have magnetic properties that can be used to obtain chemical information regarding both the type of molecule and its concentration. The advantage of MRS for utility in clinical research is the stability of the metabolite signals analyzed [3]. Therefore, when changes are detected, they are presumed to reflect long-term plasticity.
The subjects were scanned on a 3-T Siemens TIM TRIO MRI System (Erlangen, Germany), and an 8-channel phased-array coil was used to provide an optimum signal-to-noise ratio for MRI images and spectroscopy data. Axial, sagittal, and coronal localizer images were acquired using true fast imaging with steady state precession (FISP) localizer, sagittal images were acquired using spin-echo sequence [400 ms, 9.5 ms, and 1 (Repetation time (TR), Echo time (TE), signal averages)], with in-plane resolution of 256 × 256 and a slice thickness of 2 mm. The sagittal images were used to align the axial images for the study along the anterior commissure– posterior commissure line. Axial T1-weighted images were acquired using 3-dimensional magnetization prepared rapid acquisition gradient echo (MP_RAGE) sequence [2150 ms, 1100 ms, 4.38 ms, and 1 (TR, inversion time (TI), TE, and signal averages)], with in-plane resolution of 256 × 256, slice thickness of 1 mm, field of view of 256 mm, and 162 slices, this provided isotropic voxel resolution of 1 mm3. The axial images were used to generate sagittal and coronal images using a multiplanar reconstruction (MPR) routine provided by the manufacturer. These images had the same resolution as the axial images. Axial Turbo fluid attenuated inversion recovery (FLAIR) with fat saturation images were acquired [9000 ms, 2500 ms, 128, and 1 (TR, TI, effective TE, and signal averages)], with turbo factor of 11, in-plane resolution of 256 × 256, slice thickness of 5 mm, field of view of 220 mm, and 22 slices. These images used the same slice orientation and offsets as the axial MP_RAGE sequences. Similarly, axial T2-weighted turbo spin-echo images were acquired [8000 ms, 107, and 2 (TR, effective TE, signal averages)], with turbo factor of 15, and using similar image matrix and slices as turbo FLAIR sequence.
A single voxel Point RESolved Spectroscopy (PRESS) sequence with and without water suppression pulse [2000 ms, 30 ms (TR, TE)], with voxel size of 2.5 cm (left-right), 3.5 cm (anterior-posterior), 1.0 cm (head-foot), 512 sampling points, and 2 KHz bandwidth, was used to acquire data from the ACC (Fig. 1). For water-suppressed data, 256 signal averages were acquired, and for water-unsuppressed data, 4 signal averages were acquired.
Fig. 1.
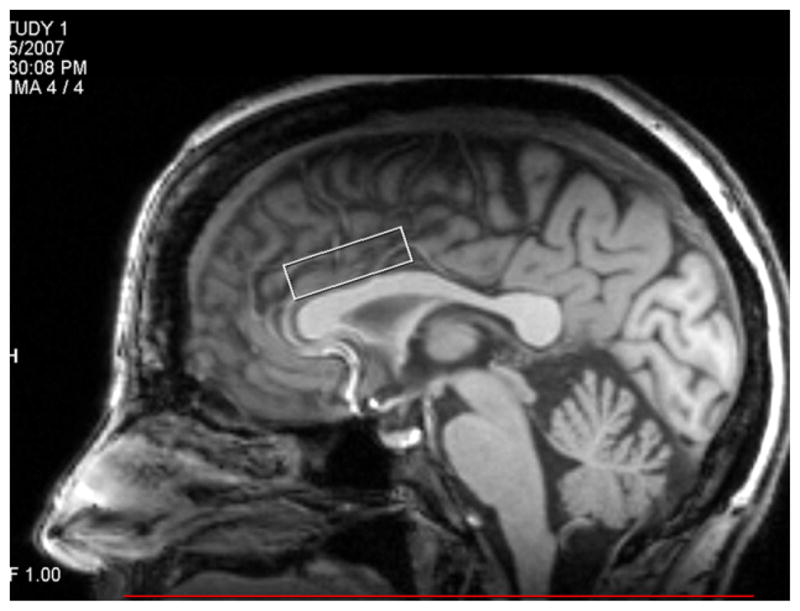
Voxel placement over the anterior cingulate cortex.
Spectral analysis was performed by using the linear combination model (LC Model) software [35], a user-independent time-domain fitting routine that uses a basis set of concentration-calibrated model spectra of individual metabolites to estimate the absolute concentrations of similar brain metabolites from in vivo spectral data. This method exploits the full spectroscopic information of each metabolite and not just isolated resonances. In the present study we determined the concentrations for NAA, total Cr, Cho, Glx, and Ins. Consistent with our previous observations of thalamic NAA and Ins in individuals with SCI and neuropathic pain [34], we created a NAA/Ins ratio because we expected NAA and Ins concentrations to be inversely related to levels of pain and psychosocial impact. Similarly, a Glx/Ins ratio was created based on the literature [17,31], which suggested that Glx and Ins concentrations would be inversely related to pain and psychosocial impact. The analysis of the spectral data was performed from 4.0 ppm to 0.2 ppm, and eddy current correction was also performed using an unsuppressed water signal. To account for subject-to-subject variability in coil loading, the unsuppressed water signal for each subject was used while processing the data to normalize the concentration of metabolites to the water signal of each individual subject.
2.6. Assessment of pain and psychosocial factors
2.6.1. The Multidimensional Pain Inventory–Spinal Cord Injury version (MPI-SCI)
The MPI is a psychometric instrument designed to assess pain and a range of psychosocial factors associated with chronic pain [28]. Based on exploratory and confirmatory factor analyses, Widerström-Noga et al. [55] revised the MPI for use in the SCI chronic pain population. The internal consistency, stability, and validity of the MPI-SCI have been demonstrated in the SCI chronic pain population [54]. The MPI-SCI [55] can be used to classify individuals with SCI and pain into 3 different psychosocial profiles: (1) “Dysfunctional” with higher pain severity (PS) and life interference (LI); (2) “Interpersonally Supported” with moderately high PS, high levels of social support and life control, and less LI; or (3) “Adaptive Copers” with lower levels of PS and LI and higher levels of life control [57]. The profile with the least favorable characteristics is the Dysfunctional, which shows more neuropathic pain symptoms, including frequent exacerbation of pain, electric quality of the pain, and continuous pain, than the other subgroups [53,56]. Because of the multimodal nature of the MPI, individual subscales can be used to assess specific domains of interest.
In the present study, we used 3 of the MPI-SCI Pain Impact subscales (i.e., Pain Severity [PS], Life Interference [LI], and Affective Distress [AD]) to identify an SCI neuropathic pain subgroup with high levels of pain and psychosocial impact. These specific subscales were selected to reflect the key domains of pain severity, pain interference, and emotional function recommended by the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) group [51].
Three psychometric instruments were used for the purpose of external validation of the SCI neuropathic subgroups: (1) Spielberger State-Trait Anxiety Inventory (STAI): The STAI [46] is a self-report questionnaire designed to assess both state and trait anxiety. It has documented reliability and validity for use in research on adults [33]. Both state and trait anxiety have been linked to many aspects of the pain experience, and to activation within areas of the ACC [39]. (2) Beck Depression Inventory (BDI): The BDI [7] assesses symptoms associated with depressed patients described in the psychiatric literature. The BDI has been recommended specifically for use in assessing emotional function in neuropathic pain [9]. (3) Satisfaction with Life Scale (SWLS): The SWLS [11] is a measure designed to assess global satisfaction with life that allows people to indicate how satisfied they are with their lives based on their own values. It has previously been strongly linked to severity and impact of persistent pain associated with SCI [54].
2.7. Statistical analysis
2.7.1. Reliability analysis
Stability of a measurement should be established before it can be considered valid for the assessment of clinical outcomes in a specific population [32]. Test-retest reliability or stability of a measure is the degree to which an instrument yields stable scores over time among respondents who are assumed not to have changed on the variables that are assessed. Variations in health, learning, reaction to the test, or regression to the mean (tendency to return to normal values) may yield test-retest data underestimating the reliability of a test. A common metric used for this purpose is the intraclass correlation (ICC). One-way random effect coefficients were calculated for each of the measures [42] and used to determine test-rest reliability of measures in the SCI neuropathic pain group, which completed 2 identical study sessions. ICC coefficients ranging from 0.41 to 0.60 were considered fair, 0.61 to 0.80 moderate, and 0.81 to 1.0 substantial according to the Shrout criteria [42].
2.7.2. Cluster analysis
We determined the number of clusters by using the automatic PASW Statistics 18 for Windows default criterion (Schwarz criterion) [40]. Once the number of clusters was determined, we examined the characteristics of the MPI-SCI scores to determine the appropriateness of the clusters. After this step, the clusters were statistically compared with regard to ACC metabolite concentrations and ratios of interest, psychosocial factors, severity of injury, and demographic factors using 1-way analysis of variance (ANOVA) with Dunnett post-hoc adjustments [37]. The nonparametric Kruskal-Wallis and Mann-Whitney tests were used to determine statistically significant differences between the clusters when analyzing dichotomous variables. Independent t test, χ2, and Pearson correlations were used to determine pairwise differences and correlations. Statistical analyses were performed with PASW Statistics 18 for Windows [47]. A probability of <.05 was considered statistically significant.
3. Results
3.1. Reliability
Intraclass correlations coefficients (ICC) were calculated to determine test-retest reliability of all metabolite concentrations and ratios of interest obtained by the MRS (Table 2) and the psychometric instruments (Table 3) over a 2- to 4-week period in subjects with SCI and neuropathic pain. As shown in Table 2, all metabolites displayed ICC coefficients in the moderate range, with the exception of Glx, which showed fair test-retest reliability. ICC coefficients in the moderate range support the stability and utility of these measures in people with SCI and neuropathic pain.
Table 2.
Intraclass correlation analyses (1-way random effects) for the ACC metabolite concentrations in people with SCI and neuropathic pain (n = 38).
| Metabolite | Visit 1 Mean ± SD | Visit 2 Mean ± SD | ICC | F, P value |
|---|---|---|---|---|
| NAA | 6.08 ± 0.68 | 6.08 ± 0.60 | 0.63 | 4.37, P < .000 |
| Cho | 1.47 ± 0.19 | 1.47 ± 0.18 | 0.69 | 5.46, P < .000 |
| Cr | 5.12 ± 0.45 | 5.09 ± 0.34 | 0.65 | 4.69, P < .000 |
| Ins | 5.39 ± 0.57 | 5.33 ± 0.56 | 0.70 | 5.62, P < .000 |
| Glx | 7.46 ± 1.06 | 7.49 ± 1.34 | 0.41 | 2.38, P = .004 |
| Glx/Ins | 1.41 ± 0.23 | 1.42 ± 0.26 | 0.74 | 3.78, P < .000 |
| NAA/Ins | 1.13 ± 0.13 | 1.15 ± 0.14 | 0.62 | 4.27, P < .000 |
ACC = xxx; Cho = choline; Cr = creatine; Glx = glutamate and glutamine; ICC = intraclass correlation coefficient; Ins = myoinositol; NAA = N-acetyl aspartate; SCI = xxx.
Table 3.
Intraclass correlation analyses (1-way random effects) for pain and psychosocial measures in people with SCI and neuropathic pain (n = 48).
| Measure | Visit 1 Mean ± SD | Visit 2 Mean ± SD | ICC | F, P value |
|---|---|---|---|---|
| PS | 3.45 ± 1.06 | 3.22 ± 1.14 | 0.71 | 3.45, P < .000 |
| LI | 1.72 ± 1.37 | 1.53 ± 1.43 | 0.94 | 16.26, P < .000 |
| AD | 1.71 ± 1.42 | 1.67 ± 1.39 | 0.83 | 6.01, P < .000 |
| STAI | 32.5 ± 10.11 | 32.98 ± 11.36 | 0.92 | 12.41, P < .000 |
| BDI | 8.31 ± 8.14 | 8.41 ± 7.98 | 0.95 | 18.50, P < .000 |
| SWLS | 19.5 ± 7.53 | 20.8 ± 7.74 | 0.87 | 7.68, P < .000 |
AD = MPI-SCI Affective Distress subscale; BDI = Beck Depression Inventory; ICC = intraclass correlation coefficient; LI = MPI-SCI Life Interference subscale; PS = MPI-SCI Pain Severity subscale; SCI = spinal cord injury; STAI = Spielberger State-Trait Anxiety Inventory; SWLS = Satisfaction with Life Scale.
As shown in Table 3, all psychometric measures displayed ICC coefficients in the substantial range, with the exception of PS, which showed moderate test-retest reliability. These results support the stability and utility of these measures in the neuropathic pain SCI population.
3.2. Neuropathic pain subgroups
The cluster analysis resulted in 2 distinctly different clusters. The comparison of the MPI-SCI PS, LI, and AD subscales showed that the scores were highly and significantly different between the 2 clusters on all subscales: PS (t = 3.663; P = .001), LI (t = 5.552; P < .000), and AD (t = 4.922; P < .000). Cluster 1 scored significantly higher on PS (4.05 ± 0.75), LI (2.66 ± 1.39), and AD (2.95 ± 1.48) compared with the other cluster. Therefore, this cluster was labeled SCI High Pain Impact (SCI-HPI). The PS, LI, and AD scores of the SCI-HPI group were similar to the Dysfunctional cluster in a previous study [58]. The SCI-HPI group consisted of 19 subjects (38.0%) of the neuropathic pain sample. This proportion was also similar to our previous study, in which 34.6% of SCI pain subjects were classified as Dysfunctional [58]. The second cluster (n = 31) was characterized by significantly lower levels of PS (3.06 ± 1.03), LI (0.91 ± 0.81), and AD (1.05 ± 1.00) compared with the SCI-HPI group, and was therefore labeled SCI Low Pain Impact (SCI-LPI).
3.3. Use of prescription pain medication
The SCI-HPI and SCI-LPI neuropathic pain subgroups were compared with respect to current use of prescription pain medication. Overall, 23 (out of 50) subjects, or 47.9%, were taking prescription medication for their pain. The 3 most common prescription pain medications used by our participants were opioids (14 of 50; 28.0%), antidepressants (9 of 50; 18.0%), and anticonvulsants (13 of 50; 26.0%). Although the overall use of prescription pain medication was somewhat higher in the SCI-HPI group (n = 10; 52.6%) compared with the SCI-LPI group (n = 13; 41.9%), the frequency was not significantly different between groups (χ2 = 0.543, P = .461). There were no significant differences between the groups regarding the use of: (1) opioids (SCI-HPI group [n = 5; 26.3%] vs the SCI-LPI group [n = 8; 25.8%], χ2 = 0.195, P = .659); and (2) anticonvulsants (SCI-HPI group [n = 5; 26.3%] vs the SCI-LPI group [n = 8; 25.8%], χ2 = 0.002, P = .968). Although there was a small, significant difference regarding the use of antidepressants (HPI group [n = 6; 31.6%] and LPI group [n = 3; 9.7%], χ2 = 3.828, P = .050), this was not significant when the Bonferroni correction was used to adjust for multiple comparisons.
3.4. Demographics and injury characteristics
Demographic factors and injury characteristics were compared between the 4 groups, i.e., the A-B and the SCI-noNP and the SCI-HPI and SCI-LPI groups (Table 4). There were no significant differences between groups on any of these characteristics.
Table 4.
Demographics and injury characteristics of study sample.
| Variables | A-B (n = 24) | SCI-noNP (n = 18) | SCI-LPI (n = 31) | SCI-HPI (n = 19) | ANOVA F or t; P value |
|---|---|---|---|---|---|
| Age | 34.4 ± 8.61 | 36.8 ± 11.03 | 37.5 ± 13.4 | 40.4 ± 11.8 | 0.979; .407 |
| Age at injury | NA | 20.3 ± 7.05 | 26.7 ± 11.8 | 28.2 ± 12.7 | 2.735; .072 |
| Time since injury | NA | 16.2 ± 9.48 | 10.6 ± 9.07 | 12.0 ± 9.85 | 2.056; .136 |
| Sex | Kruskal-Wallis; .855 | ||||
| Female | 5 | 4 | 5 | 5 | |
| Male | 19 | 14 | 26 | 14 | |
| Level of education | χ2 25.336; .116 | ||||
| Less than high school | 0 | 0 | 0 | 1 | |
| High school | 1 | 5 | 10 | 5 | |
| Trade school | 0 | 0 | 1 | 1 | |
| Associate degree or some college | 7 | 7 | 9 | 7 | |
| Bachelor’s degree | 7 | 4 | 8 | 2 | |
| Advanced degree | 9 | 1 | 3 | 2 | |
| Missing data | 0 | 0 | 0 | 1 | |
| Level of injury | NA | Kruskal-Wallis; .216 | |||
| Cervical | 8 | 21 | 13 | ||
| Below cervical | 10 | 10 | 6 | ||
| Completeness of SCI* | NA | Kruskal-Wallis; .080 | |||
| Incomplete | 3 | 14 | 10 | ||
| Complete | 14 | 17 | 9 | ||
| Missing | 1 |
A-B = able-bodied control subjects; NA = not applicable; SCI-HPI = spinal cord injury with high pain impact; SCI-LPI = spinal cord injury with low pain impact; SCI-noN-P = spinal cord injury without neuropathic pain.
Neurological completeness as defined by the International Standards for Neurological Classification of Spinal Cord Injury [1].
3.5. Psychosocial factors
Psychosocial pain-relevant factors (depression [BDI], anxiety [STAI], and satisfaction with life [SWLS]) were compared among control subjects (A-B and SCI-noNP) and SCI neuropathic pain subjects (SCI-HPI and SCI-LPI) using an ANOVA to externally validate the SCI neuropathic subgroups produced by the cluster analysis (Fig. 2). Post hoc analyses examined the differences between the SCI-HPI group and the 3 other subgroups. Specifically, the SCI-HPI group scored significantly higher on the BDI and STAI compared with the A-B group (P < .000), the SCI-noNP group (P < .000), and the SCI-LPI group (P < .000). The SCI-HPI group also scored significantly lower on the SWLS than the A-B group (P = .001), the SCI-noNP group (P = .001), and the SCI-LPI group (P = .044).
Fig. 2.
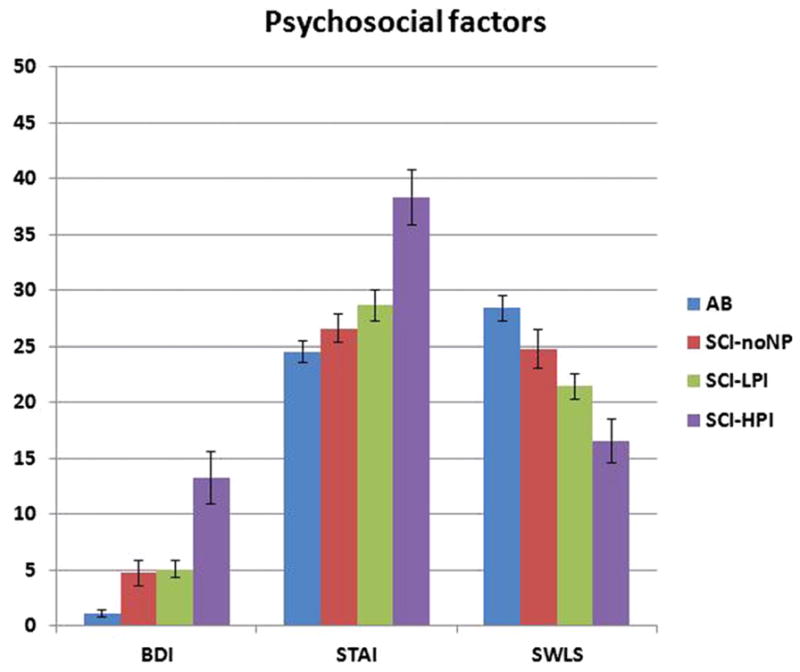
Comparisons between the A-B, SCI-noNP, SCI-LPI, and SCI-HPI groups with regard to psychosocial factors: BDI, STAI, and SWLS. Analysis of variance with post hoc Dunnett adjustment comparing the SCI-HPI with the A-B, SCI-noNP, and SCI-LPI showed that the SCI-HPI group had significantly higher BDI and STAI scores than the A-B (P < .000), SCI-noNP (P < .000), and SCI-LPI (P < .000); and that the SCI-HPI group had significantly lower SWLS scores than the A-B (P = .001), SCI-noNP (P = .001), and SCI-LPI (P = .044). A-B = able-bodied control subject; BDI = Beck Depression Inventory; SCI-HPI = spinal cord injury with high pain impact; SCI-LPI = spinal cord injury with low pain impact; SCI-noNP = spinal cord injury without neuropathic pain; STAI = Spielberger Trait Anxiety Inventory; SWLS = Satisfaction with Life Scale.
3.6. ACC metabolites and ratios
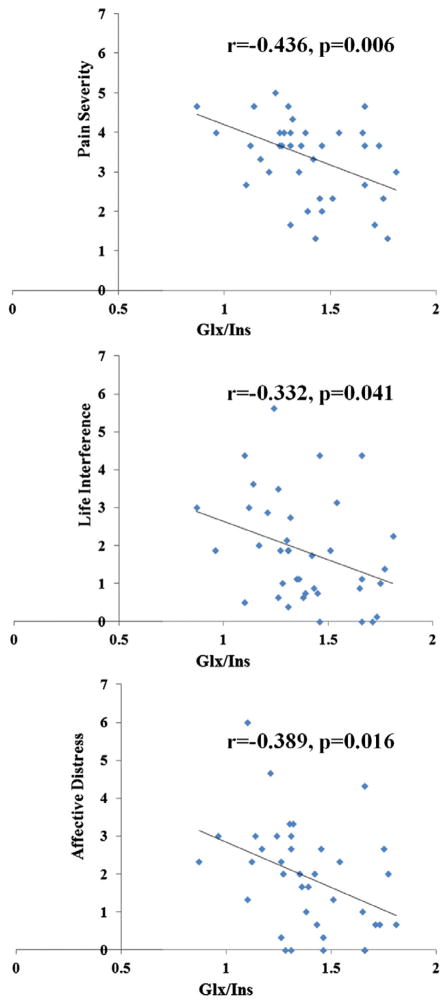
The ANOVA comparing the metabolite levels and ratios between the A-B, SCI-noNP, SCI-LPI, and SCI-HPI groups indicated differences among the groups in the concentrations of Ins (P < .000), Cr (P = .009), Cho (P = .039), NAA/Ins (P = .038), and Glx/Ins (P = .005) (Table 5). Post hoc comparisons revealed that the SCI-HPI group had significantly higher ACC concentrations of Ins (P < .000), Cr (P = .007), Cho (P = .014), and significantly lower NAA/Ins (P = .024) ratio compared with the SCI-LPI group. Only the Glx/Ins ratio significantly discriminated between the SCI-HPI and the SCI-LPI (P = .003), the A-B (P = .006), and SCI-noNP (P = .026) groups. To confirm this a priori relationship, the correlation between the Glx/Ins ratio and each of the PS, LI, and AD subscales was examined for all individuals with SCI and neuropathic pain. Pearson correlation analyses (Fig. 3) showed significant relationships between the Glx/Ins ratio and PS (r = −0.436; P = .006), LI (r = −0.332; P = .041), and AD (r = −0.389; P = .016), indicating that a lower Glx/Ins ratio was independently related to greater pain severity, life interference, and affective distress.
Table 5.
ACC metabolite concentrations and ratios in A-B and SCI-noNP, SCI-HPI, and SCI-LPI groups.
| Metabolites | A-B (n = 22) Mean ± SEM | SCI-noNP (n = 14) Mean ± SEM | SCI-LPI (n = 24) Mean ± SEM | SCI-HPI (n = 16) Mean ± SEM | ANOVA F; P value |
|---|---|---|---|---|---|
| NAA | 6.21 ± 0.12 | 6.22 ± 0.14 | 6.04 ± 0.16 | 6.18 ± 0.10 | 0.422; .738 |
| Ins | 5.37 ± 0.09 | 5.65 ± 0.15 | 5.12 ± 0.11 | 5.75 ± 0.10 | 6.700; .000* |
| Glx | 7.95 ± 0.22 | 8.28 ± 0.46 | 7.59 ± 0.22 | 7.22 ± 0.24 | 2.258; .089† |
| Cr | 5.07 ± 0.39 | 5.29 ± 0.41 | 4.95 ± 0.48 | 5.35 ± 0.25 | 4.139; .009‡ |
| Cho | 1.45 ± 0.17 | 1.46 ± 0.09 | 1.42 ± 0.20 | 1.56 ± 0.12 | 2.945; .039§ |
| NAA/Ins | 1.16 ± 0.02 | 1.11 ± 0.03 | 1.18 ± 0.03 | 1.08 ± 0.02 | 2.964; .038|| |
| GLX/Ins | 1.48 ± 0.04 | 1.46 ± 0.07 | 1.49 ± 0.04 | 1.26 ± 0.05 | 4.712; .005¶ |
A-B = able-bodied control subjects; Cho = choline; Cr = creatine; Glx = glutamate and glutamine; Ins = myoinositol; NAA = N-acetyl aspartate; SCI-HPI = spinal cord injury with high pain impact; SCI-LPI = spinal cord injury with low pain impact; SCI-noNP = spinal cord injury without neuropathic pain.
Post hoc Dunnett test comparing the SCI-HPI group with AB or SCI-noNP, and SCI-LPI:
the SCI-HPI group had significantly higher Ins concentrations than SCI-LPI (P < .000) and higher although not significantly (P = .056) than the A-B group;
the SCI-HPI group had significantly lower concentrations of Glx (P = .050) than the SCI-noNP group;
the SCI-HPI group had significantly higher concentrations of Cr (P = .007) than SCI-LPI, and higher although not significantly (P = .084) than the A-B group;
the SCI-HPI group had significantly higher concentrations of Cho (P = .014) than SCI-LPI, and lower although not significantly (P = .070) than the A-B group;
the SCI-HPI group had significantly (P = .024) lower NAA/Ins ratio than the SCI-LPI group;
the SCI-HPI group had significantly lower Glx/Ins ratio than SCI-LPI (P = .003), A-B (P = .006), and SCI-noNP groups (P = .026).
Fig. 3.
Three scatter plot diagrams that illustrate the relationship between the Multidimensional Pain Inventory–Spinal Cord Injury subscales Pain Severity, Life Interference, and Affective Distress, and the Glx/Ins ratio. Glx/Ins = glutamate-glutamine/myoinositol.
Because the use of antidepressant medication for relieving pain was slightly more common (although not significantly so after adjustment for multiple comparisons) in the SCI-HPI group than in the SCI-LPI group, we compared the Glx/Ins ratios between those who reported current use of antidepressant medication (n = 8) with those who did not use this medication (n = 32) to determine whether the difference in the Glx/Ins ratio between the HPI and LPI groups was significantly influenced by antidepressant medication use. An independent t test showed no significant differences (t = −0.895; P = .377) in Glx/Ins ratios between people who were taking antidepressant medication and those who did not use this medication.
The high-resolution T1-weighted structural images obtained using the MPRAGE method were used to visually compare whether there were any signs of atrophy among the 3 groups of subjects. We did not visually detect any changes in brain volume or gray/white matter in the region of ACC where the voxel was placed. Quantitative analysis of percent gray matter, white matter, and cerebro spinal fluid (CSF) high resolution based on segmentation of MPRAGE images encompassing the spectroscopy voxel in the ACC was not performed in this study.
4. Discussion
The results of the present study support the hypothesis that severe neuropathic pain with a significant psychosocial impact is associated with a combination of neuronal and glial dysfunction. The comparisons between the SCI-HPI group and the other subgroups showed a significantly lower Glx/Ins ratio in the SCI-HPI group than in all other subgroups. The Glx/Ins ratio was also significantly related to the severity of neuropathic pain and psychosocial impact. The SCI-HPI group had a significantly lower NAA/Ins ratio and significantly higher concentrations of Ins, Cr, and Cho than the SCI-LPI group. Collectively, these results suggest that mechanisms including lower glutamatergic metabolism, glial proliferation, glial hypertrophy, or activation [14,15,52] are involved in the development and maintenance of severe neuropathic pain with high psychosocial impact.
Basic research studies strongly support glial activation as one important mechanism underlying neuropathic pain after SCI [21– 23]. Elevated brain concentrations of Ins have been found in neuropathic pain after SCI [34,48]. For example, Stanwell et al. [48] used a classification scheme based on wavelet decomposition and statistical processing of MRS data to determine which metabolites best discriminated between subjects with and without neuropathic pain after SCI. They found that the ACC Ins concentration (in combination with other metabolites) was one of the metabolites that best discriminated between the 2 groups. Elevated levels of Ins in the ACC have also been measured in medication-free subjects who previously suffered from depression [49]. This is consistent with the results of the present study, in which elevated ACC concentrations of Ins, greater scores on the AD subscale of the MPI-SCI and on the anxiety and depression scales were obtained in the SCI-HPI group compared with the SCI-LPI group. The results from the present study partly concur with previous findings in that Ins concentrations were higher in those with severe neuropathic pain with high psychosocial impact compared to those with less severe neuropathic pain. However, in our study the Ins concentrations by themselves failed to differentiate between the SCI-HPI and SCI-noNP groups. In fact, subjects with SCI but without neuropathic pain had similar Ins concentrations as the SCI-HPI group. This finding is consistent with our previous study in SCI [34], in which thalamic metabolite concentrations of Ins did not significantly differ between subjects with and without neuropathic pain. However, when combined with NAA, the NAA/Ins ratio was significantly lower in those with neuropathic pain compared to those with no neuropathic pain after their SCI.
Our finding that the ACC concentrations of NAA were not significantly different between the SCI-HPI and the other groups is consistent both with a previous study in SCI [48] and with a study in diabetes neuropathy [45] in which ACC NAA concentrations were no different in subjects with neuropathic pain compared with pain-free control subjects. However, the combination of NAA and Ins (i.e., the NAA/Ins ratio) was significantly lower in the SCI-HPI group compared with the SCI-LPI group, and lower, although not significantly, than the other groups, possibly indicating that a combination of neuronal dysfunction and gliosis is associated with severe neuropathic pain after SCI.
Glu is a major excitatory neurotransmitter in the human brain, and the conversion to Gln is an important process in the brain’s energy metabolism [25]. The composite Glx signal (Glu and Gln) is commonly used in research studies because it is difficult to reliably separate the Glx and Gln peaks at 3 T and lower field strengths [15]. In the present study, levels of Glx in ACC of the SCI-HPI group were significantly lower compared with the SCI-noNP group. A decrease in Glx concentration in the ACC may represent reduced synaptic activity or a downregulation of the Glu–Gln cycle. Indeed, data obtained in psychiatric samples suggest that the glutamate-glutamine cycle is downregulated in major depression [31]. Although the subjects of the present study did not experience major depression, the significantly greater affective distress in the SCI-HPI group may possibly explain the lower levels of Glx in this group.
A relationship between glia and glutamate neurotransmission has been suggested through the glutamate-glutamine recycling process [6]. Basic research studies also support this relationship in that the lower GABA inhibitory tone observed in neuropathic SCI-related pain is proposed to be associated with hyperexcitable neurons and glial activation interfering with dorsal horn glutamate and GABA concentrations [20,21]. Consistent with these ideas, our analyses showed opposite relationships between Glx and Ins, respectively, and high neuropathic pain impact, i.e., Glx concentrations in the ACC tended to be lower and Ins concentrations were higher in the SCI-HPI compared with the SCI-LPI group. These results concur with previous MRS literature, in which elevated Ins concentrations have primarily been associated with chronic pain [16,34], whereas reduced Glx has been more associated with affective factors [17,27,29].
MRS is considered to produce stable signals over time, and thus to be suitable for longitudinal research in chronic conditions [3]. Our analyses support this claim because all ACC metabolite concentrations and ratios had acceptable test-retest values in the moderate range, with the exception of Glx, which showed fair test-retest reliability. However, when Glx was combined with Ins, the Glx/Ins ratio showed the greatest level of stability of all measured metabolites and ratios. As far as we know, this is the first study that has examined these properties of MRS in this population.
Chronic neuropathic pain can cause dysfunction and functional reorganization in supraspinal structures such as the cortex and the thalamus. Indeed, a study by Apkarian et al. [4] showed significant atrophy of thalamic gray matter in subjects with chronic heterogeneous back pain compared with control subjects. Similarly, other human studies in SCI show that greater cortical reorganization is significantly associated with more severe neuropathic pain [58]. In an fMRI study by Gustin et al. [18], subjects with complete thoracic SCI and below-level neuropathic pain were asked to imagine foot movements. This procedure evoked pain in nonpainful areas or caused significant increases in pain ratings within painful areas. fMRI showed increases in activation of the primary motor cortex and the cerebellar cortex, and the activation of the perigenual ACC and right dorsolateral prefrontal cortex was significantly correlated with increases in pain intensity. Using diffusion tensor imaging, it has been suggested that SCI can cause reductions in gray matter volume in the brain, including the ACC, irrespective of pain [19]. Although no changes in brain volume or gray/white matter in the region of the ACC was suggested by visual inspection of MPRAGE high resolution T1-weighted structural images, no quantitative analysis of this was performed in the present study.
Limitations of this study include the complexity of pain after SCI in combination with the injury itself, which may confound the findings. In particular, the similar levels of Ins concentrations observed in the SCI-HPI and SCI-noNP groups suggest that ACC Ins concentrations by themselves are not useful in differentiating subjects with neuropathic pain from those with no pain. It is possible that ACC Ins concentrations reflect changes related to both neuropathic pain and to the SCI. The Glx/Ins ratio, however, differentiated the SCI-HPI group from the SCI-LPI, the SCI-noNP, and the A-B control groups. Future studies should examine Ins concentrations and Glx/Ins ratios in different brain areas and their ability to differentiate between neuropathic pain, nonneuropathic pain, and no pain. It should be noted that Glx did not show adequate stability in the present study. However, when combined with Ins, the Glx/Ins ratio showed excellent stability. Therefore, it seems that a metabolite ratio may be a more reliable way to assess longitudinal changes. The lack of quantitative analyses of changes in brain volume or gray/white matter is a limitation of the present study. Future studies should include high-resolution MPRAGE images segmented for percent gray matter, white matter, and CSF, and diffusion tensor imaging to conclusively rule out brain atrophy as a contributing factor to neuropathic pain. The present study excluded participants with moderate to major depression, which may limit the generalizability of our findings. However, this exclusion criterion was adopted to control for possible confounding effects of depression on ACC metabolites. Therefore, our study results may be specifically indicative of pain and pain-related affective distress rather than affective distress related to other factors.
In summary, the Glx/Ins ratio significantly discriminated the SCI-HPI group from the SCI-LPI, A-B, and SCI-noNP groups. The reduced Glx/Ins ratio in the SCI-HPI group supports the idea that the combination of lower glutamatergic metabolism and proliferation of glia and glial activation are underlying mechanisms contributing to the development and maintenance of severe neuropathic pain with a significant psychosocial impact after SCI. This is consistent with basic research suggesting that neuropathic pain behaviors after SCI are caused by astrocytic and microglial activation influencing various cellular processes, including transmitter, cytokine, and glutamatergic systems [21]. The Glx/Ins ratio was significantly correlated with greater pain severity, pain interference, and affective distress, confirming its relevance for both neuropathic pain severity and psychosocial impact. Our findings suggest that the Glx/Ins ratio may be a useful biomarker for severe SCI-related neuropathic pain with significant psychosocial impact.
Acknowledgments
The authors gratefully acknowledge excellent technical assistance by Mr. James Adcock, Ms. Letitia Fisher, and Mr. Qing He. This work was supported by the Department of Veterans Affairs Office of Rehabilitation Research and Development Service (Merit Review grant B5023R), and The Miami Project.
Footnotes
None of the authors have a conflict of interest with the present work.
References
- 1.Waring WP, 3rd, Biering-Sorensen F, Burns S, Donovan W, Graves D, Jha A, Jones L, Kirshblum S, Marino R, Mulcahey MJ, Reeves R, Scelza WM, Schmidt-Read M, Stein A. 2009 Review and revisions of the international standards for the neurological classification of spinal cord injury. J Spinal Cord Med. 2010;33:346–52. doi: 10.1080/10790268.2010.11689712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apkarian AV, Baliki MN, Geha PY. Towards a theory of chronic pain. Prog Neurobiol. 2009;87:81–97. doi: 10.1016/j.pneurobio.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463–84. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Apkarian AV, Sosa Y, Sonty S, Levy RM, Harden RN, Parrish TB, Gitelman DR. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci. 2004;24:10410–5. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baastrup C, Finnerup NB. Pharmacological management of neuropathic pain following spinal cord injury. CNS Drugs. 2008;22:455–75. doi: 10.2165/00023210-200822060-00002. [DOI] [PubMed] [Google Scholar]
- 6.Banasr M, Chowdhury GM, Terwilliger R, Newton SS, Duman RS, Behar KL, Sanacora G. Glial pathology in an animal model of depression: reversal of stress-induced cellular, metabolic and behavioral deficits by the glutamate-modulating drug riluzole. Mol Psychiatry. 2010;15:501–11. doi: 10.1038/mp.2008.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 8.Boschen KA, Tonack M, Gargaro J. Long-term adjustment and community reintegration following spinal cord injury. Int J Rehabil Res. 2003;26:157–64. doi: 10.1097/00004356-200309000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Cruccu G, Sommer C, Anand P, Attal N, Baron R, Garcia-Larrea L, Haanpaa M, Treede RD. EFNS guidelines on neuropathic pain assessment: revised 2009. Eur J Neurol. 2010;17:1010–8. doi: 10.1111/j.1468-1331.2010.02969.x. [DOI] [PubMed] [Google Scholar]
- 10.Davis KD, Taylor SJ, Crawley AP, Wood ML, Mikulis DJ. Functional MRI of pain- and attention-related activations in the human cingulate cortex. J Neurophysiol. 1997;77:3370–80. doi: 10.1152/jn.1997.77.6.3370. [DOI] [PubMed] [Google Scholar]
- 11.Diener E, Emmons RA, Larsen RJ, Griffin S. The satisfaction with life scale. J Pers Assess. 1985;49:71–5. doi: 10.1207/s15327752jpa4901_13. [DOI] [PubMed] [Google Scholar]
- 12.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 13.Friebel U, Eickhoff SB, Lotze M. Coordinate-based meta-analysis of experimentally induced and chronic persistent neuropathic pain. Neuroimage. 2011;58:1070–80. doi: 10.1016/j.neuroimage.2011.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gill SS, Small RK, Thomas DG, Patel P, Porteous R, Van BN, Gadian DG, Kauppinen RA, Williams SR. Brain metabolites as 1H NMR markers of neuronal and glial disorders. NMR Biomed. 1989;2:196–200. doi: 10.1002/nbm.1940020505. [DOI] [PubMed] [Google Scholar]
- 15.Govindaraju V, Young K, Maudsley AA. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000;13:129–53. doi: 10.1002/1099-1492(200005)13:3<129::aid-nbm619>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 16.Grachev ID, Fredrickson BE, Apkarian AV. Abnormal brain chemistry in chronic back pain: an in vivo proton magnetic resonance spectroscopy study. PAIN®. 2000;89:7–18. doi: 10.1016/S0304-3959(00)00340-7. [DOI] [PubMed] [Google Scholar]
- 17.Gussew A, Rzanny R, Gullmar D, Scholle HC, Reichenbach JR. 1H-MR spectroscopic detection of metabolic changes in pain processing brain regions in the presence of non-specific chronic low back pain. Neuroimage. 2011;54:1315–23. doi: 10.1016/j.neuroimage.2010.09.039. [DOI] [PubMed] [Google Scholar]
- 18.Gustin SM, Wrigley PJ, Henderson LA, Siddall PJ. Brain circuitry underlying pain in response to imagined movement in people with spinal cord injury. PAIN®. 2010;148:438–45. doi: 10.1016/j.pain.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Gustin SM, Wrigley PJ, Siddall PJ, Henderson LA. Brain anatomy changes associated with persistent neuropathic pain following spinal cord injury. Cereb Cortex. 2010;20:1409–19. doi: 10.1093/cercor/bhp205. [DOI] [PubMed] [Google Scholar]
- 20.Gwak YS, Hulsebosch CE. GABA and central neuropathic pain following spinal cord injury. Neuropharmacology. 2011;60:799–808. doi: 10.1016/j.neuropharm.2010.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gwak YS, Kang J, Unabia GC, Hulsebosch CE. Spatial and temporal activation of spinal glial cells: role of gliopathy in central neuropathic pain following spinal cord injury in rats. Exp Neurol. 2012;234:362–72. doi: 10.1016/j.expneurol.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hains BC, Waxman SG. Activated microglia contribute to the maintenance of chronic pain after spinal cord injury. J Neurosci. 2006;26:4308–17. doi: 10.1523/JNEUROSCI.0003-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hulsebosch CE. Gliopathy ensures persistent inflammation and chronic pain after spinal cord injury. Exp Neurol. 2008;214:6–9. doi: 10.1016/j.expneurol.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hulsebosch CE, Hains BC, Crown ED, Carlton SM. Mechanisms of chronic central neuropathic pain after spinal cord injury. Brain Res Rev. 2009;60:202–13. doi: 10.1016/j.brainresrev.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hyder F, Patel AB, Gjedde A, Rothman DL, Behar KL, Shulman RG. Neuronalglial glucose oxidation and glutamatergic-GABAergic function. J Cereb Blood Flow Metab. 2006;26:865–77. doi: 10.1038/sj.jcbfm.9600263. [DOI] [PubMed] [Google Scholar]
- 26.Isaacks RE, Bender AS, Kim CY, Norenberg MD. Effect of osmolality and myoinositol deprivation on the transport properties of myoinositol in primary astrocyte cultures. Neurochem Res. 1997;22:1461–9. doi: 10.1023/a:1021950311308. [DOI] [PubMed] [Google Scholar]
- 27.Javitt DC. Glutamate as a therapeutic target in psychiatric disorders. Mol Psychiatry. 2004;9:984–97. doi: 10.1038/sj.mp.4001551. [DOI] [PubMed] [Google Scholar]
- 28.Kerns RD, Turk DC, Rudy TE. The West Haven-Yale Multidimensional Pain Inventory (WHYMPI) PAIN®. 1985;23:345–56. doi: 10.1016/0304-3959(85)90004-1. [DOI] [PubMed] [Google Scholar]
- 29.Krystal JH, Sanacora G, Blumberg H, Anand A, Charney DS, Marek G, Epperson CN, Goddard A, Mason GF. Glutamate and GABA systems as targets for novel antidepressant and mood-stabilizing treatments. Mol Psychiatry. 2002;7:S71–80. doi: 10.1038/sj.mp.4001021. [DOI] [PubMed] [Google Scholar]
- 30.LaGraize SC, Fuchs PN. GABAA but not GABAB receptors in the rostral anterior cingulate cortex selectively modulate pain-induced escape/avoidance behavior. Exp Neurol. 2007;204:182–94. doi: 10.1016/j.expneurol.2006.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luykx JJ, Laban KG, van den Heuvel MP, Boks MP, Mandl RC, Kahn RS, Bakker SC. Region and state specific glutamate downregulation in major depressive disorder: a meta-analysis of (1)H-MRS findings. Neurosci Biobehav Rev. 2012;36:198–205. doi: 10.1016/j.neubiorev.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 32.Nunnally J, Bernstein I. Psychometric theory. 3. New York: McGraw Hill; 1994. [Google Scholar]
- 33.Oei TP, Evans L, Crook GM. Utility and validity of the STAI with anxiety disorder patients. Br J Clin Psychol. 1990;29:429–32. doi: 10.1111/j.2044-8260.1990.tb00906.x. [DOI] [PubMed] [Google Scholar]
- 34.Pattany PM, Yezierski RP, Widerstrom-Noga EG, Bowen BC, Martinez-Arizala A, Garcia BR, Quencer RM. Proton magnetic resonance spectroscopy of the thalamus in patients with chronic neuropathic pain after spinal cord injury. AJNR Am J Neuroradiol. 2002;23:901–5. [PMC free article] [PubMed] [Google Scholar]
- 35.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–9. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 36.Ratai EM, Annamalai L, Burdo T, Joo CG, Bombardier JP, Fell R, Hakimelahi R, He J, Lentz MR, Campbell J, Curran E, Halpern EF, Masliah E, Westmoreland SV, Williams KC, Gonzalez RG. Brain creatine elevation and N-acetylaspartate reduction indicates neuronal dysfunction in the setting of enhanced glial energy metabolism in a macaque model of neuro AIDS. Magn Reson Med. 2011;66:625–34. doi: 10.1002/mrm.22821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosner B. Fundamentals of biostatistics. 3. Boston, Massachusetts: PWS-Kent; 1990. [Google Scholar]
- 38.Sawamoto N, Honda M, Okada T, Hanakawa T, Kanda M, Fukuyama H, Konishi J, Shibasaki H. Expectation of pain enhances responses to nonpainful somatosensory stimulation in the anterior cingulate cortex and parietal operculum/posterior insula: an event-related functional magnetic resonance imaging study. J Neurosci. 2000;20:7438–45. doi: 10.1523/JNEUROSCI.20-19-07438.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schunck T, Erb G, Mathis A, Jacob N, Gilles C, Namer IJ, Meier D, Luthringer R. 733 Test-retest reliability of a functional MRI anticipatory anxiety paradigm in 734 healthy volunteers. J Magn Reson Imaging. 2008;27:459–68. doi: 10.1002/jmri.21237. [DOI] [PubMed] [Google Scholar]
- 40.Schwarz GE. Estimating the dimension of a model. Annals Stats. 1978;6:461–4. [Google Scholar]
- 41.Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci. 2011;12:154–67. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86:420–7. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 43.Siddall PJ, Taylor DA, Cousins MJ. Classification of pain following spinal cord injury. Spinal Cord. 1997;35:69–75. doi: 10.1038/sj.sc.3100365. [DOI] [PubMed] [Google Scholar]
- 44.Simmons ML, Frondoza CG, Coyle JT. Immunocytochemical localization of N-acetyl-aspartate with monoclonal antibodies. Neuroscience. 1991;45:37–45. doi: 10.1016/0306-4522(91)90101-s. [DOI] [PubMed] [Google Scholar]
- 45.Sorensen L, Siddall PJ, Trenell MI, Yue DK. Differences in metabolites in pain-processing brain regions in patients with diabetes and painful neuropathy. Diabetes Care. 2008;31:980–1. doi: 10.2337/dc07-2088. [DOI] [PubMed] [Google Scholar]
- 46.Spielberger CD, Garsuch RC, Lushene RE. Manual for the state-trait anxiety inventory. Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- 47.Inc SPSS. Two step cluster analysis: technical report. Chicago: SPSS; 2004. [Google Scholar]
- 48.Stanwell P, Siddall P, Keshava N, Cocuzzo D, Ramadan S, Lin A, Herbert D, Craig A, Tran Y, Middleton J, Gautam S, Cousins M, Mountford C. Neuro magnetic resonance spectroscopy using wavelet decomposition and statistical testing identifies biochemical changes in people with spinal cord injury and pain. Neuroimage. 2010;53:544–52. doi: 10.1016/j.neuroimage.2010.06.051. [DOI] [PubMed] [Google Scholar]
- 49.Taylor MJ, Selvaraj S, Norbury R, Jezzard P, Cowen PJ. Normal glutamate but elevated myoinositol in anterior cingulate cortex in recovered depressed patients. J Affect Disord. 2009;119:186–9. doi: 10.1016/j.jad.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Turk DC. Biopsychosocial perspective on chronic pain. In: Gatchel R, Turk DC, editors. Psychological approaches to chronic pain management: a clinician’s handbook. New York: Guilford Press; 1996. pp. 3–33. [Google Scholar]
- 51.Turk DC, Dworkin RH, Allen RR, Bellamy N, Brandenburg N, Carr DB, Cleeland C, Dionne R, Farrar JT, Galer BS, Hewitt DJ, Jadad AR, Katz NP, Kramer LD, Manning DC, McCormick CG, McDermott MP, McGrath P, Quessy S, Rappaport BA, Robinson JP, Royal MA, Simon L, Stauffer JW, Stein W, Tollett J, Witter J. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. PAIN®. 2003;106:337–45. doi: 10.1016/j.pain.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 52.Urenjak J, Williams SR, Gadian DG, Noble M. Proton nuclear magnetic resonance spectroscopy unambiguously identifies different neural cell types. J Neurosci. 1993;13:981–9. doi: 10.1523/JNEUROSCI.13-03-00981.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Widerström-Noga EG, Cruz-Almeida Y, Felix ER, Adcock JP. Relationship between pain characteristics and pain adaptation type in persons with SCI. J Rehabil Res Dev. 2009;46:43–56. [PubMed] [Google Scholar]
- 54.Widerström-Noga EG, Cruz-Almeida Y, Martinez-Arizala A, Turk DC. Internal consistency, stability, and validity of the spinal cord injury version of the multidimensional pain inventory. Arch Phys Med Rehabil. 2006;87:516–23. doi: 10.1016/j.apmr.2005.12.036. [DOI] [PubMed] [Google Scholar]
- 55.Widerström-Noga EG, Duncan R, Felipe-Cuervo E, Turk DC. Assessment of the impact of pain and impairments associated with spinal cord injuries. Arch Phys Med Rehabil. 2002;83:395–404. doi: 10.1053/apmr.2002.28028. [DOI] [PubMed] [Google Scholar]
- 56.Widerström-Noga EG, Duncan R, Turk DC. Psychosocial profiles of people with pain associated with spinal cord injury: identification and comparison with other chronic pain syndromes. Clin J Pain. 2004;20:261–71. doi: 10.1097/00002508-200407000-00008. [DOI] [PubMed] [Google Scholar]
- 57.Widerström-Noga EG, Felix ER, Cruz-Almeida Y, Turk DC. Psychosocial subgroups in persons with spinal cord injuries and chronic pain. Arch Phys Med Rehabil. 2007;88:1628–35. doi: 10.1016/j.apmr.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 58.Wrigley PJ, Press SR, Gustin SM, Macefield VG, Gandevia SC, Cousins MJ, Middleton JW, Henderson LA, Siddall PJ. Neuropathic pain and primary somatosensory cortex reorganization following spinal cord injury. PAIN®. 2009;141:52–9. doi: 10.1016/j.pain.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 59.Wollaars MM, Post MW, Brand N. Spinal cord injury pain: the influence of psychologic factors and impact on quality of life. Clin J Pain. 2007;23:383–91. doi: 10.1097/AJP.0b013e31804463e5. [DOI] [PubMed] [Google Scholar]
- 60.Woolf CJ, Max MB. Mechanism-based pain diagnosis: issues for analgesic drug development. Anesthesiology. 2001;95:241–9. doi: 10.1097/00000542-200107000-00034. [DOI] [PubMed] [Google Scholar]
- 61.Yezierski RP. Spinal cord injury pain: spinal and supraspinal mechanisms. J Rehabil Res Dev. 2009;46:95–107. [PubMed] [Google Scholar]
- 62.Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, Meyer CR, Koeppe RA, Stohler CS. Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science. 2001;293:311–5. doi: 10.1126/science.1060952. [DOI] [PubMed] [Google Scholar]