Abstract
Weight-loss independent mechanisms may play an important role in the improvement of glucose homeostasis after Roux-en-Y gastric bypass (RYGB). The objective of this analysis was to determine whether RYGB causes greater improvement in glucostatic parameters as compared with laparoscopic adjustable gastric banding (LAGB) or low calorie diet (LCD) after equivalent weight loss and independent of enteral nutrient passage. Study 1 recruited participants without type 2 diabetes mellitus (T2DM) who underwent LAGB (n = 8) or RYGB (n = 9). Study 2 recruited subjects with T2DM who underwent LCD (n = 7) or RYGB (n = 7). Insulin-supplemented frequently-sampled intravenous glucose tolerance test (fsIVGTT) was performed before and after equivalent weight reduction. MINMOD analysis of insulin sensitivity (Si), acute insulin response to glucose (AIRg) and C-peptide (ACPRg) response to glucose, and insulin secretion normalized to the degree of insulin resistance (disposition index (DI)) were analyzed. Weight loss was comparable in all groups (7.8 ± 0.4%). In Study 1, significant improvement of Si, ACPRg, and DI were observed only after LAGB. In Study 2, Si, ACPRg, and plasma adiponectin increased significantly in the RYGB-DM group but not in LCD. DI improved in both T2DM groups, but the absolute increase was greater after RYGB (258.2 ± 86.6 vs. 55.9 ± 19.9; P < 0.05). Antidiabetic medications were discontinued after RYGB contrasting with 55% reduction in the number of medications after LCD. No intervention affected fasting glucagon-like peptide (GLP)-1, peptide YY (PYY) or ghrelin levels. In conclusion, RYGB produced greater improvement in Si and DI compared with diet at equivalent weight loss in T2DM subjects. Such a beneficial effect was not observed in nondiabetic subjects at this early time-point.
INTRODUCTION
Obesity and type 2 diabetes mellitus (T2DM) pose a growing threat to public health (1). Weight loss is an effective modality to improve glycemic control in patients with T2DM. Unfortunately, weight loss achieved by low calorie diet (LCD) has a high degree of recidivism (2). In contrast, bariatric surgery is an effective method to obtain significant sustained weight loss as well as augmented glycemic control (3). Roux-en-Y gastric bypass (RYGB) has been reported to produce remission of T2DM in over 80% of patients and nearly 100% resolution of impaired glucose tolerance (4). Laparoscopic adjustable gastric banding (LAGB) is another bariatric procedure that produces remission of T2DM in ~50% of patients (4). Evaluation of the different mechanisms by which weight loss is achieved with RYGB, LAGB, and LCD has led to the generation of interesting hypotheses regarding glycemic control. The greater reduction in body weight with RYGB may contribute to the higher rate of diabetes remission compared with LAGB (5). However, glucose control in diabetic patients often improves drastically within the first 2 weeks after surgery, at a time when weight loss is similar between procedures. In addition, both animal studies and clinical observations show that gastrointestinal bypass surgery improves T2DM in obese as well as nonobese subjects (6). Taken together, these findings suggest that factors in addition to the degree of weight loss and decreased calorie intake are likely to contribute to the greater efficacy of RYGB.
Since the gut is a source of numerous hormones that respond to nutrients and affect glucoregulation, it has been proposed that RYGB modulates glucose control through neurohormonal mechanisms achieved by alteration of nutrient flow in addition to causing weight reduction (7). Glucagon-like peptide (GLP)-1 is an incretin secreted by endocrine cells of the intestinal mucosa to increase insulin secretion in response to food and also exerts an inhibitory effect on glucagon secretion (8). GLP-1 analogs as well as inhibitors of the GLP-1 degrading enzyme, dipeptidyl peptidase-IV, are novel antidiabetic medications that have been shown to offer benefits for patients with T2DM (9). Gut hormones may also affect insulin action through central mechanisms (10). Peptide YY (PYY), which is also secreted in response to luminal nutrients, acts in the hypothalamus to activate melanocortin neurons that affect insulin sensitivity (Si) (11). Ghrelin, another peptide that is synthesized primarily in the stomach, has the opposite effect of PYY on insulin action since this peptide hormone antagonizes the central melanocortin system (12,13); ghrelin increases cortisol and growth hormone levels, reduces insulin secretion (14,15), and decreases the expression of adiponectin, an adipokine that mediates insulin sensitivity (16). We and others have previously shown that circulating levels of several gut hormones significantly differ in patients after RYGB as compared to LAGB or LCD, with higher postprandial levels of PYY and GLP-1 and lower fasting ghrelin levels after RYGB; a hormonal profile that likely favors increased weight loss and better improvement in insulin secretion and Si in RYGB patients (17). Importantly, the incretin effect on insulin secretion has been shown to be significantly increased in T2DM patients after RYGB, but not after equivalent LCD-induced weight loss (18). However, knowledge about the nature of physiologic processes mediating chronic, food intake-independent changes following RYGB is still scarce (3,6).
The present prospective intervention study investigates whether there are effects unique to RYGB with regard to Si and β-cell function (independent of simultaneous enteral nutrient passage and the changes in gut hormones associated therewith) in subjects with and without T2DM. The first study was a comparison between nondiabetic subjects before and after an equivalent amount of weight loss induced by either LAGB or RYGB. The second study was a comparison between subjects with T2DM before and after an equivalent amount of weight loss induced by either LCD or RYGB. LAGB was not used as a comparator group for the second study as this procedure is performed infrequently on patients with T2DM at our institution. Insulin sensitivity and secretion were assessed with frequently-sampled intravenous glucose tolerance tests (fsIVGTT).
METHODS AND PROCEDURES
Study participants
Four groups (two nondiabetic, two T2DM) of subjects were recruited: LAGB (n = 8), RYGB (n = 9), LCD-DM (n = 7), and RYGB-DM (n = 7). The decision to undergo surgery and the choice of procedure was made between patient and physician and was independent of this research protocol. Individuals with fasting blood glucose ≥126 mg/dl or on antidiabetic medications with a self-reported history of T2DM were included into the T2DM group. Number of medications was recorded independent of dosage for pre vs. post-intervention comparisons. Major exclusion criteria were use of thiozolidinedione or insulin at a dose >50 IU/day, weight change >5% during previous 3 months, or significant illness. All applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed. The study was approved by the Columbia University institutional review board and written informed consent was obtained from all subjects.
Study design
Target weight loss was 7–10% of total body weight. Pre and postintervention subjects underwent fsIVGTT and nuclear magnetic resonance imaging (MRI) for analysis of body composition. LAGB and RYGB were performed as previously described (17). Subjects were instructed to avoid strenuous exercise for 2 days prior to tests and fast (except for water) for a minimum of 10 hours. All subjects were given a menu from which they could choose 1 of 4 dinner options and 1 of 4 after-dinner snack options to be consumed before 10 PM the evening before the first IVGTT. Dinner and snack consisted of ~60 and 15 g carbohydrate, respectively. Postintervention, subjects followed dietary instructions provided by the surgical team. Meal composition of the LCD was designed to be similar to that consumed by bypass patients. The LCD (Optifast, Novartis, MN) consisted of 50% carbohydrate, 35% protein, and 15% fat, divided in five servings of 160 kcal (800 kcal/day) in 237 ml per serving.
fsIVGTT
Subjects were asked to hold oral diabetes medications and/or exenatide for 3 days prior to testing and insulin or pramlintide for 24 h. Fasting blood samples were obtained at −15 and −5 min. Glucose (0.3 g/kg as dextrose 50 g/dl) was administered intravenously within 2 min at t = 0, and subsequent samples were obtained at 2, 3, 4, 5, 6, 8, 10, 12, 14, 16, 19, 22, 24, 25, 27, 30, 40, 50, 60, 70, 90, 100, 120, 140, 160, and 180 min. At 20 min, an intravenous injection of regular insulin (0.03 U/kg body weight for nondiabetic, and 0.05 U/kg for diabetic subjects) was administered to increase the accuracy of the modeling analyses (19,20). The Bergman minimal model (MINMOD Millennium 6.02 software) used for analysis correlates highly with measures from insulin clamps (21,22). The following parameters were quantified: glucose-dependent glucose elimination (Sg), sensitivity of glucose elimination to insulin (Si), acute insulin response to glucose (AIRg), and the disposition index (DI), a measurement of β-cell function (insulin secretion) in relation to Si. The relative mean increase of C-peptide 3 to 5 min after glucose injection (ACPRg) was also determined. Glucose disappearance rate (Kg) was determined as the natural logarithm of the glucose concentrations vs. time from samples drawn between 2 and 19 min following glucose injection (and prior to injection of exogenous insulin) as previously described (23). All data from the MINMOD analysis had a fractional s.d. <0.5. Glucose values from time 0–7 min were weighted 0 as per Boston et al. (24). In eleven out of the 62 fsIVGTT analyses, weighing of the data points was set to 1 at 5 and/or 6 min for better fit to model. All other data points were set to a weight of 1, except a few spurious glucose values were weighed with 0 to avoid miss-modeling based on outlier data.
MRI
Intermuscular, subcutaneous, and visceral adipose tissue (IMAT, SAT, and VAT, respectively) as well as skeletal muscle tissue were quantified from 1 axial image, 10 mm thickness, between the L2–L3 intervertebral space (25) using MRI (1.5T GE Twin Speed scanner, General Electric, Milwaukee, WI) (26,27).
Analytic assays
Leptin, total ghrelin, insulin, and glucose were measured as described (28). Total PYY was measured by ELISA (Millipore, St Charles, MO) with a sensitivity of 10 pg/ml and 2.3% intra-assay and 7.2% inter-assay coefficients of variation. Total GLP-1 was measured by RIA after alcohol extraction according to manufacturer’s protocol (Millipore). Sensitivity of the assay is 3 pmol/l and recovery in each assay was tested by parallel extraction of standards. High molecular weight adiponectin was quantified by ELISA (Millipore) with an assay sensitivity of 0.5 ng/ml and 2.4% intra-assay and 5.5% inter-assay coefficients of variation. Serum C-peptide and high sensitivity C-reactive protein (CRP) were measured with the Immulite Analyzer (Siemens, Los Angeles, CA). Serum free fatty acids were measured by an enzymatic colorimetric assay (Wako Diagnostics, Richmond, VA).
Statistical analyses
SAS version 9.1 software (Cary, NC) was used for statistical analysis. Differences in the distribution of continuous variables at baseline were tested with Student’s independent t-test. Longitudinal changes from baseline were tested with linear mixed models with fixed effects for surgical group, week and group by week interaction with an autoregressive covariance structure for the repeated measures (29). The association between variables was estimated using Pearson’s correlations. All tests were two-tailed, with P values <0.05 considered statistically significant. No adjustment of the critical value of the test statistic was made for the separate tests of different hormones or for homeostasis model assessment of insulin resistance (HOMAIR) (30), although a significant F-test was required for the fixed effect for post hoc comparisons of between group differences at specific times, or within-group differences between times using 95% confidence intervals and the model estimated means and standard errors. Statistical model estimated means and standard errors are presented.
RESULTS
Study 1: Nondiabetic obese subjects before and after LAGB or RYGB
Baseline and postintervention characteristics of nondiabetic obese subjects who elected to undergo either LAGB or RYGB are presented in Table 1. Mean age, body weight, and BMI between groups did not differ. The mean reduction in body weight/BMI was comparable in both groups (7.5 ± 0.6%), corresponding to an excess body weight loss of 13.6 ± 1.5% and 15.0 ± 2.1%, in the RYGB and the LAGB group, respectively (P = 0.58). However, the period over which weight loss was achieved was significantly longer in LAGB.
Table 1.
Study 1: Anthropometric and frequently sampled intravenous glucose tolerance test (fsIVGTT) parameters, fasting plasma hormone levels, and body composition in nondiabetic subjects
| Parameter | LAGB
|
RYGB
|
||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| N (F/M) | 8 (7/1) | 9 (8/1) | ||
| Anthropometrics | ||||
| Age (years) | 47.4 ± 4.8 | 40.3 ± 3.4 | ||
| Body weight (kg) | 123 ± 6 | 113 ± 6*** | 128 ± 11 | 119 ± 9*** |
| BMI (kg/m2) | 45.1 ± 2.0 | 41.7 ± 1.9*** | 48.0 ± 3.6 | 44.3 ± 3.1*** |
| Weight loss (%) | 7.7 ± 0.9 | 7.4 ± 0.8 | ||
| Weight loss period (week) | 4.4 ± 0.3 | 2.6 ± 0.3†† | ||
| Glucostatic measures | ||||
| Insulin (μIU/ml) | 19.5 ± 3.0 | 11.5 ± 1.7** | 17.0 ± 4.2 | 13.3 ± 3.0 |
| C-peptide (ng/ml) | 3.4 ± 0.4 | 2.5 ± 0.3* | 2.7 ± 0.5 | 2.7 ± 0.5 |
| Glucose (mg/dl) | 100 ± 5 | 93 ± 2 | 103 ± 5 | 95 ± 4 |
| HOMAIR (mU × mmol × l−2) | 4.8 ± 0.8 | 2.7 ± 0.5** | 4.6 ± 1.3 | 3.2 ± 0.7 |
| Sg (× 10−2× min−1) | 1.3 ± 0.2 | 1.7 ± 0.3 | 1.6 ± 0.4 | 1.0 ± 0.2 |
| Si (ml × μU−1× min−1) | 1.9 ± 0.3 | 3.1 ± 0.7* | 2.4 ± 0.5 | 3.3 ± 1.0 |
| AIRg (ml−1× μU × min) | 346 ± 158 | 423 ± 109 | 451 ± 187 | 380 ± 116 |
| DI | 456 ± 107 | 1,003 ± 161*** | 813 ± 234 | 956 ± 256 |
| ACPRg (%) | 123 ± 45 | 217 ± 51** | 147 ± 39.0 | 168 ± 40 |
| Kg (% × min−1) | 1.40 ± 0.24 | 1.56 ± 0.32 | 1.55 ± 0.39 | 1.00 ± 0.28 |
| Peptides | ||||
| Leptin (ng/ml) | 41 ± 6 | 24 ± 3** | 41 ± 5 | 28 ± 3* |
| GLP-1 (pmol/l) | 5.2 ± 0.8 | 6.3 ± 1.8 | 4.7 ± 1.3 | 4.5 ± 1.4 |
| PYY (pg/ml) | 56 ± 17 | 63 ± 14 | 68 ± 18 | 50 ± 20 |
| Ghrelin (pg/ml) | 231 ± 28 | 259 ± 16 | 346 ± 69 | 334 ± 84 |
| Adiponectin (μg/ml) | 2.5 ± 0.4 | 2.9 ± 0.4 | 3.4 ± 0.6 | 3.6 ± 0.5 |
| CRP (mg/l) | 6.6 ± 1.0 | 9.6 ± 2.7 | 14.4 ± 3.3 | 8.9 ± 2.8 |
| Lipids | ||||
| Total cholesterol (mg/dl) | 157 ± 13 | 137 ± 8 | 156 ± 5 | 130 ± 7** |
| HDL cholesterol (mg/dl) | 48 ± 2 | 43 ± 2** | 47 ± 3 | 37 ± 2** |
| Total/HDL cholesterol | 3.4 ± 0.4 | 3.3 ± 0.3 | 3.4 ± 0.2 | 3.6 ± 0.3 |
| LDL cholesterol (mg/dl) | 90 ± 12 | 78 ± 8 | 88 ± 5 | 77 ± 8 |
| Triglycerides (mg/dl) | 96 ± 17 | 85 ± 10 | 106 ± 13 | 82 ± 14 |
| FFA (mmol/l) | 0.6 ± 0.1 | 0.8 ± 0.1* | 0.8 ± 0.1 | 1.1 ± 0.1 |
| Body compositiona (cm2) | ||||
| SM | 158 ± 19 | 141 ± 21** | 129 ± 7 | 120 ± 7* |
| SAT | 354 ± 25 | 316 ± 31 | 573 ± 82 | 530 ± 80†,** |
| VAT | 227 ± 75 | 213 ± 67 | 159 ± 18 | 147 ± 19 |
| IMAT | 18 ± 10 | 17 ± 10 | 3 ± 2 | 7 ± 3* |
Data are presented as mean ± s.e.m.
ACRPg, acute C-peptide response to glucose; AIRg, acute insulin response to glucose; CRP, C-reactive protein; DI, disposition index; FFA, free fatty acids; GLP-1, glucagon-like peptide; HDL, high density lipoprotein; HOMAIR, homeostasis model assessment of insulin resistance; IMAT, intermuscular adipose tissue; Kg, Glucose disappearance rate; LAGB, laparoscopic adjustable gastric banding; LDL, low density lipoprotein; PYY, Peptide YY; RYGB, Roux-en-Y gastric bypass; SAT, subcutaneous adipose tissue; Sg, glucose-dependent glucose elimination; Si, insulin sensitivity; SM, skeletal muscle; VAT, visceral adipose tissue.
Magnetic resonance imaging (MRI) data are only available for four subjects in each group due to either limited field of view or inability of subject to tolerate MRI. Differences between V1 and V2 in the same group:
P < 0.05;
P < 0.01;
P < 0.001 by paired t-test.
Differences between groups at the same time-point:
P < 0.05;
P < 0.001 by mixed model analysis.
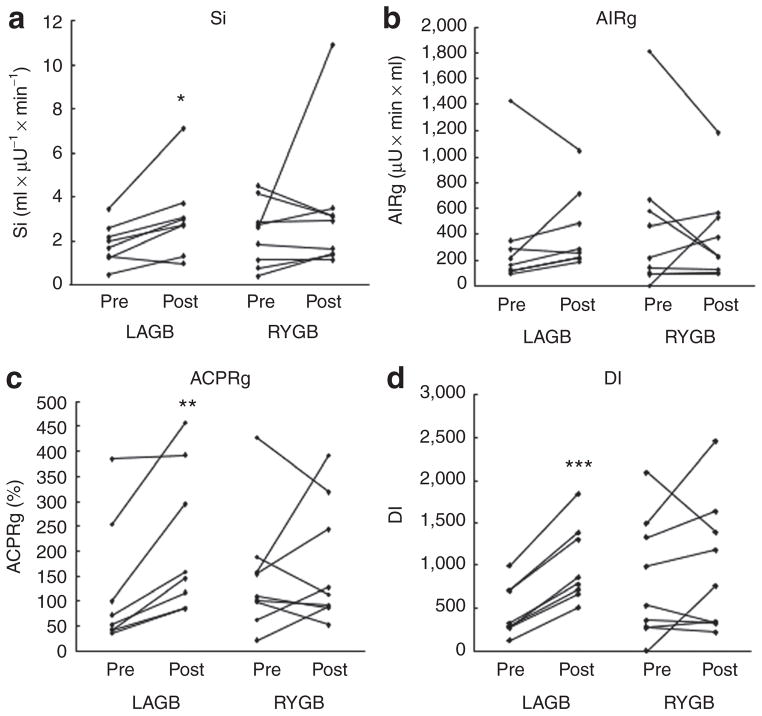
Baseline glucostatic parameters were similar between groups. Although DI tended to be lower in the LAGB compared to the RYGB group prior to intervention, the difference was not statistically significant (P = 0.20). None of the glucostatic parameters differed between groups at the second visit, however, significant improvement of Si, ACPRg, and DI were observed in the LAGB group (Figure 1).
Figure 1.
Glucostatic parameters for individual obese nondiabetic subjects pre and post laparoscopic adjustable gastric banding (LAGB) (n = 9) or Roux-en-Y gastric bypass (RYGB) (n = 8). (a) Insulin sensitivity, Si. (b) Acute insulin response to glucose, AIRg. (c) Acute C-peptide response to glucose, ACPRg. (d) Insulin secretion relative to insulin sensitivity (disposition index), DI. *P < 0.05; **P ≤ 0.01; ***P ≤ 0.001.
Fasting plasma levels of leptin, adiponectin, and gut hormones (GLP-1, PYY, ghrelin) were similar at baseline. Leptin levels decreased in both groups, however, no statistically significant changes in levels of the other hormones were observed after weight loss. Plasma levels of CRP were higher in the RYGB group prior to intervention, but were indistinguishable between RYGB and LAGB at the second visit. Decreases in low density lipoprotein, and triglycerides were noted but none reached statistical significance, whereas the decrease in high density lipoprotein was significant in both groups. An increase in free fatty acids reached statistical significance only after LAGB. There was a marked reduction in lean body mass (as measured by MRI) in both groups. In contrast to SAT, which decreased significantly after RYGB, there was a statistically significant increase in IMAT.
Study 2: Obese subjects with T2DM before and after LCD or RYGB
Baseline and postintervention characteristics of the two diabetic groups are presented in Table 2. There was a mean reduction of 8.1 ± 0.7% in total body weight. Loss of excess body weight was 17.4 ± 2.1% and 14.7 ± 1.2% in the LCD and RYGB group, respectively (P = 0.29). However, equivalent weight loss was achieved in less than half the time after RYGB as compared to LCD. There were no statistically significant differences between the two diabetic groups regarding baseline glucostatic parameters and time since diagnosis of T2DM (8 ± 2 and 5 ± 2 years, for LCD and RYGB, respectively). Members of both study groups took an average of two antidiabetic medications, with one individual in each group on insulin therapy prior to intervention (data not shown). Of note, all antidiabetic medications were discontinued in the RYGB group before the time of the second visit; in the LCD group the number of antidiabetic medications was reduced by 55%.
Table 2.
Study 2: Anthropometric and frequently sampled intravenous glucose tolerance test (fsIVGTT) parameters, fasting plasma hormone levels, and body composition in type 2 diabetes (T2DM) subjects
| Parameter | LCD-DM
|
RYGB-DM
|
||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| N (F/M) | 7 (4/3) | 7 (5/2) | ||
| Anthropometrics | ||||
| Age (years) | 51.7 ± 3.5 | 5.0 ± 3.9 | ||
| Body weight (kg) | 122 ± 7 | 111 ± 6*** | 132 ± 8 | 121 ± 7*** |
| BMI (kg/m2) | 43.3 ± 1.8 | 39.5 ± 1.5** | 46.8 ± 3.1 | 43.1 ± 2.7*** |
| Weight loss (%) | 8.5 ± 1.1 | 7.7 ± 0.8 | ||
| Weight loss period (week) | 8.1 ± 0.5 | 3.4 ± 0.3††† | ||
| Glucostatic measures | ||||
| HbA1c (%) | 7.8 ± 0.5 | — | 7.5 ± 0.4 | — |
| Insulin (μIU/ml) | 16.5 ± 3.7 | 15.1 ± 1.9 | 22.9 ± 4.9 | 15.1 ± 3.1* |
| C-peptide (ng/ml) | 4.4 ± 1.4 | 4.1 ± 0.6 | 4.0 ± 0.3 | 3.1 ± 0.4*,† |
| Glucose (mg/dl) | 195 ± 30 | 147 ± 14 | 155 ± 13 | 116 ± 8* |
| HOMAIR (mU × mmol × l−2) | 8.4 ± 2.9 | 5.6 ± 1.1 | 8.2 ± 1.5 | 4.2 ± 0.8** |
| Sg (× 10−2× min−1) | 1.2 ± 0.2 | 1.0 ± 0.2 | 1.0 ± 0.1 | 1.1 ± 0.3 |
| Si (ml× μU−1× min−1) | 1.4 ± 0.5 | 1.8 ± 0.4 | 1.2 ± 0.4 | 1.9 ± 0.3** |
| AIRg (ml−1× μU × min) | 38 ± 23 | 63 ± 27* | 29 ± 16 | 113 ± 41* |
| DI | 38 ± 26 | 94 ± 35* | 10 ± 5 | 268 ± 88* |
| ACPRg (%) | 5 ± 4 | 18 ± 8 | 8 ± 4 | 57 ± 20* |
| Kg (% × min−1) | 0.79 ± 0.11 | 0.95 ± 0.33 | 0.75 ± 0.13 | 1.13 ± 0.28 |
| Peptides | ||||
| Leptin (ng/ml) | 27.2 ± 3.8 | 26.4 ± 3.9 | 30.1 ± 2.0 | 19.7 ± 2.4* |
| GLP-1 (pmol/l) | 9.4 ± 2.0 | 8.8 ± 3.1 | 6.9 ± 1.2 | 11.2 ± 3.6 |
| PYY (pg/ml) | 68 ± 32 | 73 ± 23 | 66 ± 52 | 57 ± 21 |
| Ghrelin (pg/ml) | 328 ± 24 | 311 ± 29 | 232 ± 15†† | 268 ± 27 |
| Adiponectin (μg/ml) | 2.5 ± 0.4 | 3.1 ± 0.5 | 2.5 ± 0.3 | 3.8 ± 0.4** |
| CRP (mg/l) | 10.6 ± 3.5 | 7.1 ± 4.5 | 17.1 ± 6.3 | 14.9 ± 4.1 |
| Lipids | ||||
| Total cholesterol (mg/dl) | 184 ± 14 | 170 ± 16 | 162 ± 11 | 134 ± 17 |
| HDL cholesterol (mg/dl) | 46 ± 4 | 43 ± 3 | 43 ± 2 | 33 ± 2*,† |
| Total/HDL cholesterol | 4.0 ± 0.3 | 3.9 ± 0.4 | 3.9 ± 0.4 | 4.1 ± 0.4 |
| LDL cholesterol (mg/dl) | 107 ± 12 | 99 ± 11 | 101 ± 12 | 82 ± 17 |
| Triglycerides (mg/dl) | 156 ± 35 | 143 ± 43 | 87± 14 | 93 ± 8 |
| FFA (mmol/l) | 0.9 ± 0.1 | 0.8 ± 0.2 | 0.7 ± 0.1 | 1.0 ± 0.1 |
| Body compositiona (cm²) | ||||
| SM | 160 ± 12 | 156 ± 11 | 179 ± 6 | 165 ± 9 |
| SAT | 524 ± 84 | 414 ± 11* | 511 ± 170 | 454 ± 133 |
| VAT | 218 ± 35 | 205 ± 23 | 240 ± 88 | 198 ± 76 |
| IMAT | 10 ± 4 | 6 ± 2 | 11 ± 3 | 14 ± 2†,** |
Data are presented as mean ± s.e.m.
ACRPg, acute C-peptide response to glucose; AIRg, acute insulin response to glucose; CRP, C-reactive protein; DI, disposition index; DM, diabetes mellitus; FFA, free fatty acids; GLP-1, glucagon-like peptide; HbA1c, hemoglobin A1c; HDL, high density lipoprotein; HOMAIR, homeostasis model assessment of insulin resistance; IMAT, intermuscular adipose tissue; Kg, Glucose disappearance rate; LAGB, laparoscopic adjustable gastric banding; LCD, low calorie diet; LDL, low density lipoprotein; PYY, peptide YY; RYGB, Roux-en-Y gastric bypass; SAT, subcutaneous adipose tissue; Sg, glucose-dependent glucose elimination; Si, insulin sensitivity; SM, skeletal muscle; VAT, visceral adipose tissue.
MRI data are only available for four (RYGB) and six (LCD) subjects due to inability of subject to tolerate MRI, and SAT data are only available for three (RYGB) and four (LCD) subjects due to limited field of view. Differences between V1 and V2 in the same group:
P < 0.05;
P < 0.01;
P < 0.001 by paired t-test.
Differences between groups at the same time-point:
P < 0.05;
P < 0.01;
P < 0.001 mixed model analysis.
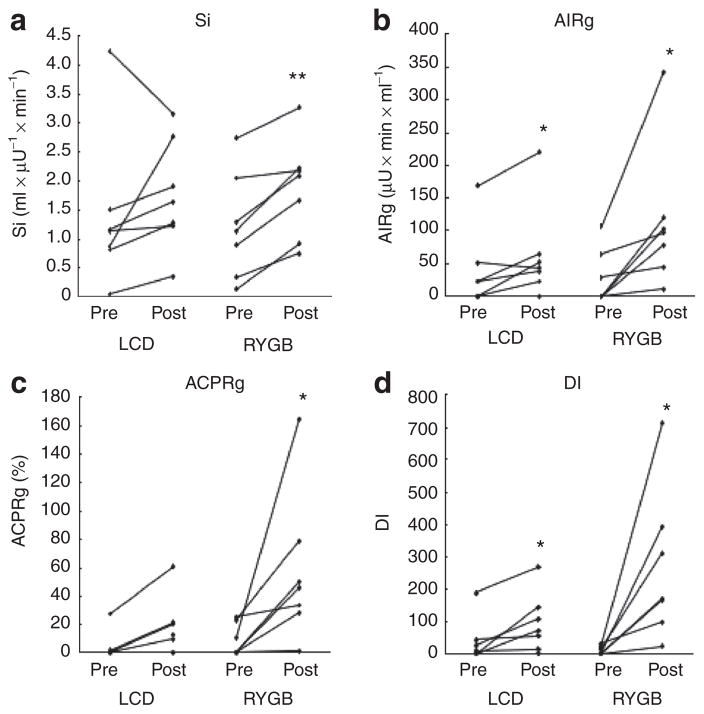
Si markedly improved only in the RYGB group (P = 0.002), but no significant change was detected in the LCD group (P = 0.30; Figure 2a). When adjusted for baseline Si, the change remained significant in the RYGB group (P = 0.02) but still did not reach statistical significance in the LCD group (P = 0.09). HOMAIR was significantly reduced only in the RYGB but not in the LCD group. AIRg significantly increased in both groups (Figure 2b), with a mean increase of 25.1 ± 9.3 (P = 0.04) and 84.7 ± 29.7 (P = 0.03) μU × min × ml−1 in the LCD and the RYGB group, respectively. RYGB subjects showed an important 6.8-fold mean increase in ACPRg (P = 0.04), while the 3.6-fold increase observed in the LCD group tended towards statistical significance (P = 0.06; Figure 2c). While a significant improvement in DI was observed in both groups (Figure 2), the mean increase was substantially greater in RYGB compared with LCD subjects (258.2 ± 86.6 vs. 55.9 ± 19.9; P = 0.04). When adjusted for baseline values, changes in AIRg, ACPRg, and DI were not statistically significant in LCD group (P = 0.40; 0.46; 0.54, respectively) but remained significant in RYGB group (P = 0.003; 0.008; 0.003, respectively). Both LCD (r = −0.52; P = 0.19) and RYGB (r = −0.67; P = 0.10) showed an inverse, but not statistically significant relationship between the change in DI and duration of T2DM.
Figure 2.
Glucostatic parameters for individual obese type 2 diabetes (T2DM) subjects. Pre and post low calorie diet (LCD) (n = 7) or Roux-en-Y gastric bypass (RYGB) (n = 7). (a) Insulin sensitivity, Si. (b) Acute insulin response to glucose, AIRg. (c) Acute C-peptide response to glucose, ACPRg. (d) Insulin secretion relative to insulin sensitivity (disposition index), DI. *P < 0.05; **P ≤ 0.01.
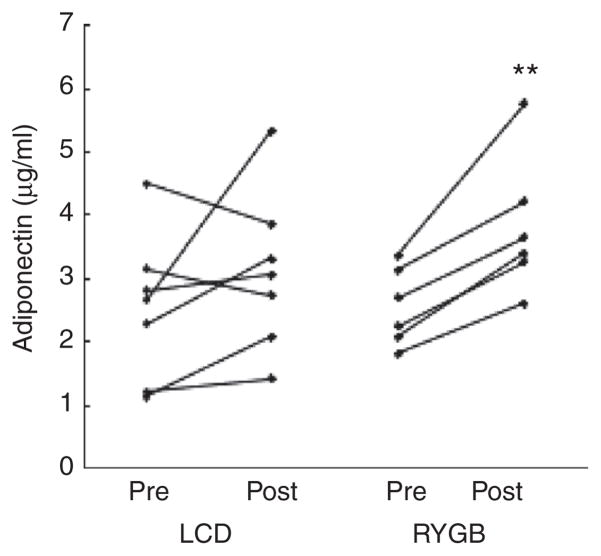
While baseline ghrelin levels were lower in the RYGB group, fasting levels of gut hormones were similar in LCD and RYGB subjects at the second visit and no significant changes were noted in gut hormones or CRP after weight loss (Table 2). Interestingly, the finding of improved Si was paralleled by a significant increase in adiponectin levels in subjects after RYGB that was not present after LCD (Figure 3). Leptin levels were decreased significantly only after RYGB. Serum lipid levels tended to decrease after weight loss but the only significant change was in high density lipoprotein after RYGB. The decreases noted in SAT, VAT, and skeletal muscle tissue reached statistical significance only in SAT after LAGB. As seen in the nondiabetic group, RYGB-induced weight loss was associated with a statistically significant increase in IMAT.
Figure 3.
Fasting serum adiponectin levels for individual obese type 2 diabetes subjects pre and post low calorie diet (LCD) or Roux-en-Y gastric bypass (RYGB). **P < 0.01.
DISCUSSION
The key finding of this investigation is the demonstration that RYGB in subjects with T2DM results in greater improvement in Si and DI as compared to equivalent weight loss achieved by simple calorie restriction. These glucostatic changes are detected as early as 3 weeks postsurgery in the absence of simultaneous enteral nutrient passage and antidiabetic medication. Improvement in the glucostatic response is characterized by increases in both insulin secretion (AIRg, ACPRg) and Si and an increase in plasma levels of adiponectin. Moreover, we demonstrate that these changes occur in diabetic patients independent of the degree of weight loss per se, as effects in LCD patients are less pronounced with equivalent weight loss. These data are consistent with studies reporting beneficial effects on glucose metabolism in nonobese T2DM subjects after surgery that functionally excludes the duodenum from nutrient passage (endoluminal sleeve, duodenal-jejunal bypass; reviewed in ref. 31). Similarly, clinical outcome was better 1 month after RYGB compared with an equivalent diet-induced weight loss in T2DM subjects (18).
We and others have previously proposed that acute changes of gut hormone levels induced by enteral nutrient passage are responsible for a considerable part of the beneficial effects of RYGB (17,18).
However, the observation that glucose levels in patients with T2DM rapidly improve after RYGB—not only after a meal but also in the fasted state—fuels the notion of chronic glucostatic effects that occur independent of acute changes in levels of gut hormones that affect Si, insulin secretion, and gut motility. Indeed, significant changes in fasting gut hormone levels were not noted in this study. Even though gut hormone levels in this study were not measured during the fsIVGTT, previous studies have demonstrated that the increase in GLP-1 upon intravenous glucose infusion is negligible (32,33). Thus, our findings suggest that changes in glucostatic parameters reflect chronic effects of RYGB in addition to acute alterations in food intake-induced gut hormone release. It is tempting to speculate that marked caloric restriction as achieved by RYGB ipso facto represents a particular early benefit for T2DM patients with regard to insulin secretion and sensitivity (34). Further well-controlled (“pair-feeding”) studies will be warranted to explore this hypothesis.
The present study also provides a side-by-side comparison of glucose homeostatic parameters in nondiabetic subjects after equivalent weight loss achieved by LAGB and RYGB. With regard to glucose homeostasis, RYGB does not appear to be superior in the short-term to LAGB in obese nondiabetic subjects. It is conceivable that the inter-individual variability in response to surgery, and in fact decrease in Si in some individuals, may reflect residual sub-clinical inflammation in the early postoperative period not detected by measurements of CRP and/or increased fat mobilization over a shorter time period that could increase insulin resistance (35). These results are consistent with our previous long-term study showing similar changes in insulin resistance, as measured by HOMAIR, in nondiabetics 2 weeks after LAGB and RYGB (17). In a larger scale study, it was also observed that 1 month after LAGB or RYGB improvements in HOMAIR were similar (36). In both studies, over time and with further weight loss, improvement was greater in RYGB presumably due to a combination of both weight-loss dependent and independent mechanisms.
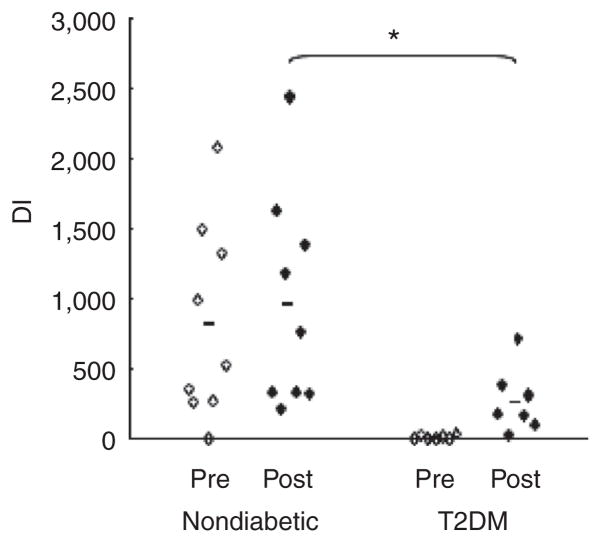
Various terms, such as remission or resolution, have been used to describe the early effect of RYGB on T2DM. In our T2DM subjects who underwent RYGB surgery, all antidiabetic medication could be discontinued within the first weeks after intervention while maintaining improved glycemia. However, assessment of fsIVGTT parameters reveals that this clinical improvement is not reflective of remaining substantial aberrations in β-cell function and/or insulin sensitivity. Comparison of DI values in nondiabetic (Study 1) and diabetic (Study 2) subjects before and after RYGB highlights the persistent impaired metabolic milieu in T2DM subjects (Figure 4). Albeit fsIVGTT values for what is considered “normal” Si or DI are quite variable (e.g., mean values of Si for nondiabetic subjects have been as varied as greater than 3.4 (19) or 7.1 (20), and normal DI values are usually greater than 2,000 (37,38)), none of the parameters analyzed in the T2DM group reverted to those consistent with normal after intervention. Our post-RYGB T2DM subjects more closely resemble our cohort of obese individuals prior to weight-loss intervention (from Study 1: Si 2.24 ± 0.32 ml × μU−1× min−1; DI 704 ± 149; n = 17) as opposed to what would be expected for normal weight healthy controls. Interestingly, these results are consistent with the improvement, but not complete restoration of glucose homeostasis, 6 weeks after RYGB reported by Morinigo et al. (39), but differ from the restoration of first-phase insulin secretion and amelioration of Si in T2DM subjects to levels of obese nondiabetic control subjects after bilio-pancreatic diversion (with degree and time of weight loss similar to our study), suggesting that malabsorptive surgery provides additional weight-independent mechanisms of glycemic control such as depletion of intramyocellular lipids and diversion of bile flow (40,41). Taken together, on the one hand, our data demonstrate that glucose metabolism significantly and rapidly improves after RYGB intervention in T2DM subjects with an average time of 6 years after established diagnosis. On the other hand, they further suggest that glycemic control may not be considered as “normalized” in the early postoperative period. Although different outcomes between studies may be due to the severity and duration of diabetes in the subject population, the notions suggested by some papers that “the manifestations of T2DM can totally clear within days after gastric bypass (42)” or that “correction of abnormal diabetic indexes occurs within days after surgery (7)” may have to be revisited.
Figure 4.
Disposition index in obese patients with and without type 2 diabetes (T2DM) pre and post Roux-en-Y gastric bypass (RYGB). *P < 0.05.
An interesting finding from the body composition analysis is that IMAT was increased in both RYGB groups despite an overall reduction in body mass. This result is unexpected given that IMAT has been reported as increased in association with greater adiposity, T2DM (43), cardiovascular disease risk (44) and with insulin resistance in patients with acromegaly (45). Having said that, limitations in the field of view of the scanner together with inability of many of our subjects to tolerate the MRI due to claustrophobia, impaired our ability to detect small changes and provide definitive conclusions regarding body composition. The implications of this finding, particularly given the small sample size, warrant further investigation.
Some of the strengths of these studies are the prospective analysis, similar degree of weight loss between groups and the separate examination of subjects with and without T2DM. Given the different glucostatic response to RYGB dependent in part on the presence/absence of diabetes, studies that combine groups may have different interpretations. Furthermore, the mean duration of diabetes in this report was 6 years, arguably making results more clinically meaningful than studies that include only individuals with relatively new onset diabetes.
The primary endpoint of this study was DI. Poststudy power calculation affirmed that the DI effect can be considered a “large effect size,” while the effect size for AIRg is conditioned by diabetes status and/or intervention and the effect size for Si is “small.” Thus, differences in AIRg or Si may only be detected in a considerably larger study population due to low statistical power for these parameters in the current study.
One limitation of the studies described herein is the nonrandomized intervention scheme. Furthermore, inherent to the protocol, subjects were in negative energy balance and results may not reflect outcomes once a stable weight is reached. A general problem when comparing RYGB to LAGB or LCD is differences in the rate of weight loss that would be expected to have an effect on Si independent from the amount of weight loss (34). The designated degree of weight loss was determined based on the amount of weight loss observed in RYGB patients ~3 weeks after surgery. As caloric restriction inherently differs between RYGB and LCD groups, the degree of caloric restriction as well as the duration of weight loss was different in these comparator groups. It would have been ideal to match groups for both weight loss and time. However, we felt that compliance would have been extremely poor for the degree of caloric restriction (~400–600 kcal/day) required for achieving this goal in the LCD group. The relatively slow rate of weight loss in the LCD group suggests some lack of compliance already with the 800 kcal/day dietary regimen. Other factors such as physical activity and macronutrient content were not strictly controlled, however, in the early postoperative period LAGB and RYGB are given similar instructions with regard to exercise, and the Optifast diet was chosen based on similar macronutrient content to food consumed post-RYGB. Another limitation is the small sample size. Groups were mean-matched for factors associated with remission of T2DM such as diabetes duration, severity and medication usage (46), but unaccounted for differences between groups may have affected outcomes. While the mean duration of T2DM was not significantly different between groups (P = 0.29), there seems to be a trend towards longer duration in the LCD group. It should be noted though that duration of diabetes refers to self-reported time of diagnosis, which may vary considerably from the actual time of onset.
Overall, our data suggest that RYGB is superior to LCD with regard to changes in Si and DI in obese diabetic subjects even in the absence of a simultaneous enteral nutrient stimulus. However, given the rather small sample size of the current study and the variability in β-cell function typical of T2DM, larger studies are required to determine if the difference we have observed between RYGB and LCD is reproducible. Furthermore, our results highlight the fact that marked short-term improvement in hyperglycemia does not necessarily reflect normalization of β-cell function. While incretins likely play a role in improvements unique to the bypass physiology, further study is warranted to identify non enteral mechanisms associated with the short and long-term glycemic control after gastric bypass in patients with T2DM.
Acknowledgments
This work was supported by National Institutes of Health (NIH) grant DK072011 to J.K., the National Center for Research Resources (NCRR) grant UL1 RR024156, the New York Obesity Research Center Grant P30-DK026687-299012 and the Deutsche Forschungsgemeinschaft (DFG) grant PL542/1-1 to L.P. We would like to acknowledge the cooperation of participants in this study and the expert technical support of Irene M. Conwell and research assistance of Carmen Taveras, M.D. We would also like to thank Dr Joy Hirsch and Stephen Dashnaw at the Program for Imaging and Cognitive Science, Neurological Institute, Columbia University for obtaining MRI scans and Dr Dympna Gallagher and Mark Punyanitya at St Luke’s-Roosevelt Hospital, Columbia University, for MRI image analysis and interpretation.
Footnotes
DISCLOSURE
L.P., G.F., L.A., E.K., and D.J.M. have nothing to declare. J.K. is on the Scientific Advisory Board of Nutrisystem, Inc., and has received research support from Covidien and NGM Biopharmaceuticals. M.B. has received lecture fees from Ethicon and Inamed. W.I. has received consulting fees from the Surgical Review Corporation and research support from Covidien.
References
- 1.Yach D, Stuckler D, Brownell KD. Epidemiologic and economic consequences of the global epidemics of obesity and diabetes. Nat Med. 2006;12:62–66. doi: 10.1038/nm0106-62. [DOI] [PubMed] [Google Scholar]
- 2.Wadden TA. Treatment of obesity by moderate and severe caloric restriction. Results of clinical research trials. Ann Intern Med. 1993;119:688–693. doi: 10.7326/0003-4819-119-7_part_2-199310011-00012. [DOI] [PubMed] [Google Scholar]
- 3.Cummings DE. Endocrine mechanisms mediating remission of diabetes after gastric bypass surgery. Int J Obes (Lond) 2009;33 (Suppl 1):S33–S40. doi: 10.1038/ijo.2009.15. [DOI] [PubMed] [Google Scholar]
- 4.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 5.Pories WJ, MacDonald KG. The surgical treatment of morbid obesity. Curr Opin Gen Surg. 1993:195–205. [PubMed] [Google Scholar]
- 6.Moo TA, Rubino F. Gastrointestinal surgery as treatment for type 2 diabetes. Curr Opin Endocrinol Diabetes Obes. 2008;15:153–158. doi: 10.1097/MED.0b013e3282f88a0a. [DOI] [PubMed] [Google Scholar]
- 7.Hickey MS, Pories WJ, MacDonald KG, Jr, et al. A new paradigm for type 2 diabetes mellitus: could it be a disease of the foregut? Ann Surg. 1998;227:637–643. doi: 10.1097/00000658-199805000-00004. discussion 643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holst JJ, Gromada J. Role of incretin hormones in the regulation of insulin secretion in diabetic and nondiabetic humans. Am J Physiol Endocrinol Metab. 2004;287:E199–E206. doi: 10.1152/ajpendo.00545.2003. [DOI] [PubMed] [Google Scholar]
- 9.Funnell MM. The therapeutic role of incretin mimetics and DPP-4 inhibitors. Diabetes Educ. 2009;35 (Suppl 1):12S–17S. doi: 10.1177/0145721709331521. [DOI] [PubMed] [Google Scholar]
- 10.Sandoval DA, Bagnol D, Woods SC, D’Alessio DA, Seeley RJ. Arcuate glucagon-like peptide 1 receptors regulate glucose homeostasis but not food intake. Diabetes. 2008;57:2046–2054. doi: 10.2337/db07-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Korner J, Leibel RL. To eat or not to eat - how the gut talks to the brain. N Engl J Med. 2003;349:926–928. doi: 10.1056/NEJMp038114. [DOI] [PubMed] [Google Scholar]
- 12.Cowley MA, Smith RG, Diano S, et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 13.Nakazato M, Murakami N, Date Y, et al. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 14.Broglio F, Arvat E, Benso A, et al. Ghrelin, a natural GH secretagogue produced by the stomach, induces hyperglycemia and reduces insulin secretion in humans. J Clin Endocrinol Metab. 2001;86:5083–5086. doi: 10.1210/jcem.86.10.8098. [DOI] [PubMed] [Google Scholar]
- 15.Tassone F, Broglio F, Destefanis S, et al. Neuroendocrine and metabolic effects of acute ghrelin administration in human obesity. J Clin Endocrinol Metab. 2003;88:5478–5483. doi: 10.1210/jc.2003-030564. [DOI] [PubMed] [Google Scholar]
- 16.Ott V, Fasshauer M, Dalski A, et al. Direct peripheral effects of ghrelin include suppression of adiponectin expression. Horm Metab Res. 2002;34:640–645. doi: 10.1055/s-2002-38261. [DOI] [PubMed] [Google Scholar]
- 17.Korner J, Inabnet W, Febres G, et al. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. Int J Obes (Lond) 2009;33:786–795. doi: 10.1038/ijo.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laferrère B, Teixeira J, McGinty J, et al. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab. 2008;93:2479–2485. doi: 10.1210/jc.2007-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Welch S, Gebhart SS, Bergman RN, Phillips LS. Minimal model analysis of intravenous glucose tolerance test-derived insulin sensitivity in diabetic subjects. J Clin Endocrinol Metab. 1990;71:1508–1518. doi: 10.1210/jcem-71-6-1508. [DOI] [PubMed] [Google Scholar]
- 20.Steil GM, Volund A, Kahn SE, Bergman RN. Reduced sample number for calculation of insulin sensitivity and glucose effectiveness from the minimal model. Suitability for use in population studies. Diabetes. 1993;42:250–256. doi: 10.2337/diab.42.2.250. [DOI] [PubMed] [Google Scholar]
- 21.Bergman RN, Ider YZ, Bowden CR, Cobelli C. Quantitative estimation of insulin sensitivity. Am J Physiol. 1979;236:E667–E677. doi: 10.1152/ajpendo.1979.236.6.E667. [DOI] [PubMed] [Google Scholar]
- 22.Saad MF, Anderson RL, Laws A, et al. A comparison between the minimal model and the glucose clamp in the assessment of insulin sensitivity across the spectrum of glucose tolerance. Insulin Resistance Atherosclerosis Study. Diabetes. 1994;43:1114–1121. doi: 10.2337/diab.43.9.1114. [DOI] [PubMed] [Google Scholar]
- 23.Kahn SE, Montgomery B, Howell W, et al. Importance of early phase insulin secretion to intravenous glucose tolerance in subjects with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2001;86:5824–5829. doi: 10.1210/jcem.86.12.8105. [DOI] [PubMed] [Google Scholar]
- 24.Boston RC, Stefanovski D, Moate PJ, et al. MINMOD Millennium: a computer program to calculate glucose effectiveness and insulin sensitivity from the frequently sampled intravenous glucose tolerance test. Diabetes Technol Ther. 2003;5:1003–1015. doi: 10.1089/152091503322641060. [DOI] [PubMed] [Google Scholar]
- 25.Shen W, Punyanitya M, Wang Z, et al. Visceral adipose tissue: relations between single-slice areas and total volume. Am J Clin Nutr. 2004;80:271–278. doi: 10.1093/ajcn/80.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korner J, Punyanitya M, Taveras C, et al. Sex differences in visceral adipose tissue post-bariatric surgery compared to matched non-surgical controls. Int J Body Compos Res. 2008;6:93–99. [PMC free article] [PubMed] [Google Scholar]
- 27.Gallagher D, Kuznia P, Heshka S, et al. Adipose tissue in muscle: a novel depot similar in size to visceral adipose tissue. Am J Clin Nutr. 2005;81:903–910. doi: 10.1093/ajcn/81.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Korner J, Bessler M, Cirilo LJ, et al. Effects of Roux-en-Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide YY, and insulin. J Clin Endocrinol Metab. 2005;90:359–365. doi: 10.1210/jc.2004-1076. [DOI] [PubMed] [Google Scholar]
- 29.Freedman DS, Khan LK, Serdula MK, Galuska DA, Dietz WH. Trends and correlates of class 3 obesity in the United States from 1990 through 2000. JAMA. 2002;288:1758–1761. doi: 10.1001/jama.288.14.1758. [DOI] [PubMed] [Google Scholar]
- 30.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 31.Rubino F, Schauer PR, Kaplan LM, Cummings DE. Metabolic surgery to treat type 2 diabetes: clinical outcomes and mechanisms of action. Annu Rev Med. 2010;61:393–411. doi: 10.1146/annurev.med.051308.105148. [DOI] [PubMed] [Google Scholar]
- 32.Haltia LT, Savontaus E, Vahlberg T, Rinne JO, Kaasinen V. Acute hormonal changes following intravenous glucose challenge in lean and obese human subjects. Scand J Clin Lab Invest. 2010;70:275–280. doi: 10.3109/00365511003792975. [DOI] [PubMed] [Google Scholar]
- 33.Laferrère B. Effect of gastric bypass surgery on the incretins. Diabetes Metab. 2009;35:513–517. doi: 10.1016/S1262-3636(09)73458-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wing RR, Blair EH, Bononi P, et al. Caloric restriction per se is a significant factor in improvements in glycemic control and insulin sensitivity during weight loss in obese NIDDM patients. Diabetes Care. 1994;17:30–36. doi: 10.2337/diacare.17.1.30. [DOI] [PubMed] [Google Scholar]
- 35.Boden G. Obesity, insulin resistance and free fatty acids. Curr Opin Endocrinol Diabetes Obes. 2011;18:139–143. doi: 10.1097/MED.0b013e3283444b09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee WJ, Lee YC, Ser KH, Chen JC, Chen SC. Improvement of insulin resistance after obesity surgery: a comparison of gastric banding and bypass procedures. Obes Surg. 2008;18:1119–1125. doi: 10.1007/s11695-008-9457-3. [DOI] [PubMed] [Google Scholar]
- 37.Boston RC, Moate PJ, Stefanovski D, Sumner AE, Bergman RN. AKA-Glucose: a program for kinetic and epidemiological analysis of frequently sampled intravenous glucose tolerance test data using database technology. Diabetes Technol Ther. 2005;7:298–307. doi: 10.1089/dia.2005.7.298. [DOI] [PubMed] [Google Scholar]
- 38.Clausen JO, Borch-Johnsen K, Ibsen H, et al. Insulin sensitivity index, acute insulin response, and glucose effectiveness in a population-based sample of 380 young healthy Caucasians. Analysis of the impact of gender, body fat, physical fitness, and life-style factors. J Clin Invest. 1996;98:1195–1209. doi: 10.1172/JCI118903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mor’nigo R, Lacy AM, Casamitjana R, et al. GLP-1 and changes in glucose tolerance following gastric bypass surgery in morbidly obese subjects. Obes Surg. 2006;16:1594–1601. doi: 10.1381/096089206779319338. [DOI] [PubMed] [Google Scholar]
- 40.Ferrannini E, Mingrone G. Impact of different bariatric surgical procedures on insulin action and beta-cell function in type 2 diabetes. Diabetes Care. 2009;32:514–520. doi: 10.2337/dc08-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salinari S, Bertuzzi A, Asnaghi S, et al. First-phase insulin secretion restoration and differential response to glucose load depending on the route of administration in type 2 diabetic subjects after bariatric surgery. Diabetes Care. 2009;32:375–380. doi: 10.2337/dc08-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122:248–256.e5. doi: 10.1016/j.amjmed.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 43.Gallagher D, Kelley DE, Yim JE, et al. MRI Ancillary Study Group of the Look AHEAD Research Group. Adipose tissue distribution is different in type 2 diabetes. Am J Clin Nutr. 2009;89:807–814. doi: 10.3945/ajcn.2008.26955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yim JE, Heshka S, Albu J, et al. Intermuscular adipose tissue rivals visceral adipose tissue in independent associations with cardiovascular risk. Int J Obes (Lond) 2007;31:1400–1405. doi: 10.1038/sj.ijo.0803621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Freda PU, Shen W, Heymsfield SB, et al. Lower visceral and subcutaneous but higher intermuscular adipose tissue depots in patients with growth hormone and insulin-like growth factor I excess due to acromegaly. J Clin Endocrinol Metab. 2008;93:2334–2343. doi: 10.1210/jc.2007-2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schauer PR, Burguera B, Ikramuddin S, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg. 2003;238:467–484. doi: 10.1097/01.sla.0000089851.41115.1b. discussion 484. [DOI] [PMC free article] [PubMed] [Google Scholar]