Abstract
S100A9 is a calcium binding protein with multiple ligands and post-translation modifications that is involved in inflammatory events and the initial development of the cancer cell through to the development of metastatic disease. This review has a threefold purpose: 1) describe S100A9 structural elements important for its biological activity, 2) describe S100A9 biology in the context of the immune system, and 3) illustrate the role of S100A9 in the development of malignancy via interactions with the immune system and other cellular processes.
S100A9 structure
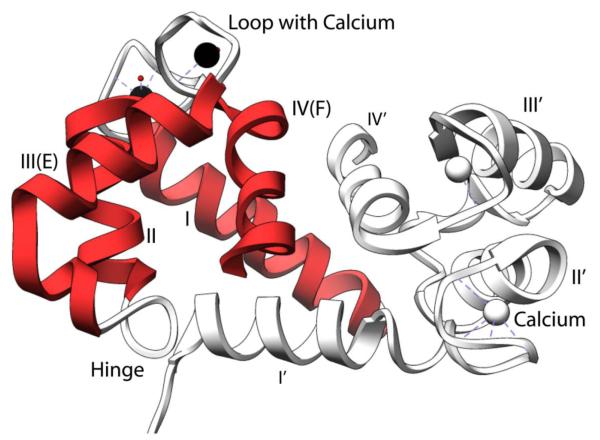
S100A9 is a calcium binding protein. Each S100A9 monomer contains a high affinity calcium binding site at the C-terminus and a low-affinity calcium binding site at the N-terminus. The canonical high affinity calcium binding site consists of the typical 12 amino acids of helix 3 (E), loop 2, and helix 4 (F) that has the shape of a human hand (EF-hand). The non-canonical low affinity calcium binding EF-hand is defined by 14 amino acids of helix 1 (E), loop 1, and helix 2 (F) (Figure 1). Helices 2 and 3 are connected by the hinge region. Upon binding to calcium there is a conformational change whereby helix 3 rotates, thus exposing a hydrophobic cleft that is postulated to serve as an anchoring point for macromolecular interactions (Figure 1) [1].
Figure 1.
Ribbon diagram of homodimeric calcium bound S100A9 from protein data bank file 1IRJ using the program Chimera.[113] Depicted in red is one subunit with the EF hand labeled. Secondary structure elements of homodimeric S100A9 include: helix one Q7-S23; helix two Q34-D44; hinge region L45-N55, calcium binding loop one: V24-N33, helix 3 E56-L66 (helix E of canonical EF Hand), calcium binding loop two D67-S75, and helix 4 F76-M94 (helix F of canonical EF hand). The C-terminal tail is disordered in the crystal structure and is therefore not shown in the figure. In this region, H92-E97 is the zinc binding motif. In the C-terminal disordered tail residues 103 to 105 represent the arachidonic acid binding region. There is also a truncated form of S100A9 of 12.7 kDa that is missing residues 1-4 found but has unclear biological function[114].
S100A9 may exist as a homodimer, heterodimer with an S100A8 partner (S100A8/A9)2, or as a heterotetramer with an S100A8 partner (S100A8/A9)4. The three dimensional structures of the calcium bound S100A9 homodimer, S00A8/A9 heterodimer, and heterotetramer of S100A8/A9 are known [2-4]. The natural state of the protein is dependent on the environment in which it resides, but from the above studies, and others, it appears that the S100A8/A9 heterodimer is found in most biological interactions; however, in many of these studies, the presence of the heterotetramer was not specifically evaluated. S100A8/A9 is highly protease resistant in a fashion similar to prion proteins [5]. In the heterodimer, the C-terminus of S100A9 and the N-terminus of S100A8 are aligned in an anti-parallel fashion similar to other homodimeric S100 proteins. The heterodimer is recognized by the E210 antibody [6, 7]. S100A8/A9 heterodimerization is not dependent on calcium but formation of heterotetramers is calcium dependent. Zinc also induces tetramer formation [8]. There is a truncated form of murine S100A9 (amino acids 1-102) that is the result of protease activity in vitro and exhibits reduced zinc binding, but this truncated peptide still retains the native disulfide bond formation between cysteine-79 and cysteine-90 [9]. Based on this data and structural data listed above, the zinc binding site is proposed to be located on the C-terminal region near a series of histidine residues but a Zn2+-S100A9 structure has not been determined to date. The structure of S100A9 has been conserved through evolution as evidenced by the fact that murine S100A9 heterodimerizes with human S100A8. This suggests biochemical functional equivalence of the human and the murine proteins despite a relatively low degree of sequence homology (59%) [10]. S100A9 appears to be specific in its dimerization partners as S100A12, another S100 protein involved in inflammation, does not dimerize with S100A9 [11].
S100A9 was first identified in the context of multiple inflammatory reactions which has led to confusing nomenclature in the literature (Table 1). In 1987, it was found in infiltrating macrophages of rheumatoid arthritis patients and named MRP-14 (myeloid related protein of molecular weight 14 kD) [12]. Other investigators have called it migration inhibitory factor related protein (MRP) of molecular weight 14 kD due to its ability to translocate to keratin intermediate filaments in response to calcium stimulation [13]. The abundance of p14 (synonym for S100A9) in neutrophils and monocytes was confirmed in 1991 by Edgeworth, et al. and this was followed by the first large scale purification of the protein for structure determination [14]. S100 proteins obtained their name due to the fact that they are soluble in 100% ammonium sulfate [15]. S100A9 is now considered to be a member of the S100 family of calcium binding proteins [16]. There are more than 20 members of the S100 family each with unique roles in signal transduction. Given the numerous contexts in which S100A9 was discovered, a guide to the nomenclature was published in 2006 (Table 1) [16].
Table 1.
Synonyms for S100A9 and S100A8.[16] Calprotectin = S100A8/A9
| Synonym | |
|---|---|
| S100A8 |
-Calgranulin A (CAGA)
-CGLA -P8 -Myeloid related protein of molecular weight 8 kDa -Migration inhibitory factor related protein of molecular weight 8 kDa (MRP8), -CFAG, -LiAg, -60B8AG |
| S100A9 |
-Calgranulin B (CAGB) -
-CGLB -p14 -Myeloid related protein of molecular weight 14 kDa -Migration inhibitory factor-related protein of molecular weight 14 kDa (MRP14) -CFAG -LiAg -60B8AG |
Calcium bound S100A9 binds to arachidonic acid, cytoskeletal elements (e.g. keratin intermediate filaments), Receptor for Advanced Glycation Endproducts (RAGE), Toll-Like Receptor 4 (TLR4), the major fatty acid transporter CD36, matrix metallo-proteinases (MMPs), fibronectin, and heparin sulfate glycosaminoglycans. The nature of S100A9 binding to these targets will be discussed later in this review.
S100A9 localization
The physical location of S100A9 varies according to the cell type and disease state. S100A9 is located in myeloid cells, cancer cells, and in tumor stroma. S100A9 is an abundant cytoplasmic protein in normal myeloid cells such as polymorphonuclear cells and monocytes. S100A8/A9 expressing macrophages are recruited to inflammatory sites in many cancers including pancreas adenocarcinoma, gastric adenocarcinoma, small cell lung carcinoma, pancreatic cystadenoma, lung adenocarcinoma, breast adenocarconoma, B-cell lymphoma, esophageal squamous cell carcinoma, and lung squamous cell carcinoma [17]. Supporting the concept of increasing levels of S100A9 with increasing inflammation is the finding of increased S100A9 protein levels both in gliobastoma multiforme treated with radiation [18] and in radiation-induced mammary carcinomas possibly due to increased infiltration of the tumor by inflammatory cells [19]. S100A9 is also up-regulated in other inflammatory diseases such as psoriasis. In this case, S100A9 is located both in the cytoplasm and plasma membrane in differentiated keratinocytes [20]. In the context of normal human gastrointestinal physiology, S100A9 is located in the cytoplasm and plasma membrane of pancreatic cell lines, whereas in the esophageal mucosa, S100A9 is located within the nuclei [21, 22]. S100A9 localization is important for its physiologic activities. S100A8 and S100A9 are minimally expressed in normal esophageal epithelium, but S100A9 is expressed across the spectrum of Barrett’s esophagus through adenocarcinoma [23]. In the normal esophagus, S100A9 is located in the basal aspect of the cells. With increasing dysplasia in Barrett’s esophagous S100A8/A9 is found diffusely within the cytoplasm rather than isolated to the basal aspect of the cells [24]. S100A9 protein expression is increased in poorly differentiated tumors including undifferentiated (anaplastic) thyroid carcinomas [25, 26] and invasive adenocarcinoma of the breast associated with poor tumor differentiation [27]. It has become clear that S100A9 localizes with its partner S100A8 in many biological processes but may act as a sole player in other cancers. One study in particular examined a variety of cell lines and found S100A9, S100A8, or S100A8/A9 located within multiple cancer cell types. This information is reviewed in Table 2 [28]. The common theme that has emerged is that localization of S100A9 correlates to centers of inflammation in cancer or other pathological processes.
Table 2.
Examples of cell lines determined by micro-array to express S100A8 or S100A9 [28].
| Cell Line | Tissue of Origin | S100A8 | S100A9 |
|---|---|---|---|
| Hs683 | Glioma | + | + |
| T47D | Breast | + | + |
| SNU484 | Gastric | + | + |
| HeLa | Cervical | + | + |
| SK-Hep-1 | Hepatocellular | − | + |
| MCF7 | Breast | − | + |
| LNCaP | Prostate | + | − |
S100A9 signal transduction is important for inflammatory signal cascades and the oxidative potential of the NADPH complex
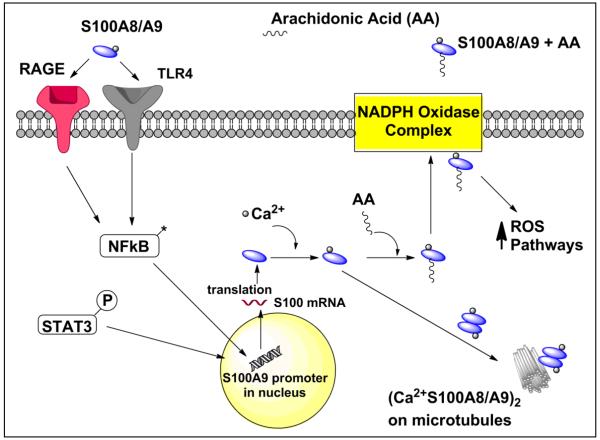
S100A9 transports arachidonic acid between the cytosol and the NADPH oxidase complex at the plasma membrane in neutrophils as part of an inflammatory signal cascade. Arachidonic acid is a polyunsaturated omega-6 fatty acid that is involved as a second messenger in cellular signaling. S100A9 transfers arachidonic acid to gp91phox of the NADPH complex while S100A8 binds to p67phox and rac-2 of the NADPH oxidase complex leading to the oxidative burst important in inflammatory cells (Figure 2) [29]. Thus is it is possible that a S100A8/A9 heterodimer could have multiple effects on the NADPH complex. The C-terminus of S100A9 (residues 103-105) in either the homodimeric or heterodimeric state with S100A8 facilitates arachidonic acid transport [30, 31]. S100A9 that has been phosphorylated at threonine-113 of the S100A8/A9 complex enhances activation of NADPH oxidase whereas zinc blocks arachidonic acid binding to S100A8/A9 [32]. The NADPH oxidative burst is decreased in neutrophils from S100A9 knockout mice. Two mutations, (H103A,H104A,H105A,K106A) S100A9 mutant and truncation of S100A9 to residues 1-100 eliminated the ability of S100A9 to activate the NADPH oxidase complex presumably due to lack of arachidonic acid migration to the plasma membrane [29]. Studies in HaCaT keratinocytes that over-express S100A9 demonstrate increased NADPH oxidase and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) activity whereas HeLa cells bearing the (H103A,H104A,H105A,K106A) S100A9 mutation are unable to bind arachidonic acid and fail to promote PMA (phorbol myristate acetate)-induced NADPH oxidase activity [33]. In general, S100A9 appears to contribute to the control of the oxidative potential of the NADPH complex.
Figure 2.
Intracellular S100A9 activity: S100A8/A9 binds to cell receptors initiating signal transduction through NFκB pathways promoting increased S100A9 transcription. Ca2+-S100A9 complexes with microtubules increasing cell motility. In addition, Ca2+-S100A9 increases the oxidative potential of cells by migrating to the NADPH complex via PKC dependent mechanisms and activating reactive oxygen species pathways. S100A9 is then released into the extra-cellular space.
Post-translation modifications of S100A9 also control its activities in inflammatory pathways
Both S100A8 and S100A9 have the capability of being nitrosylated (R-NO) which in general results in decreased inflammatory activity. However, S100A9 is nitrosylated in a calcium dependent manner. Translocation of cytosolic NADPH oxidase components such as p47phox and p67phox to the plasma membrane, and subsequent superoxide generation are inhibited by nitrosylated residues on the S100A8 subunit of the S100A8/A9 complex [29, 34]. Once outside the plasma membrane, the calcium-independent formation of S100A8 nitrosylation products function as a nitric oxide shuttle [34]. S-glutathionylation of S100A9 reduces its capacity to heterodimerize with S100A8 and bind fibronectin, but the S100A9 arachidonic acid binding capacity remains the same [35]. S-glutathionylation of S100A9 may therefore serve as a mechanism to limit the inflammatory response in the extracelullar matrix. Oxidation of methionine 63 and 83 abolishes S100A9’s chemo-repulsive (fugetactic) effect on peripheral neutrophils [36]. Therefore, S100A8/A9 nitrosylation, glutathionylation, and oxidation serve as post-translational mechanisms controlling both the magnitude and extent of the immune response.
S100A9 signaling is important in mediating inflammatory cascades in the vicinity of the plasma membrane via interaction with RAGE and TLR4
Current evidence supports myeloid secretion of S100A8/A9 which in turn binds to carboxylated glycans on RAGE or RAGE itself in vitro and in many cell types. The S100A8/A9-RAGE complex can activate signaling pathways including mitogen-activated protein kinase (MAPK) and NF-κB in colon tumor cells and NFκB in tissues from a murine model of skin carcinogenesis induced by DMBA/TPA [37-39]. The S100A9 subunit may be responsible for the association of S100A8/A9 to RAGE as in vitro binding data supports a thermodynamically favorable homo-dimeric S100A9-RAGE complex in the presence of zinc [40]. Controversy exists whether it is RAGE or TLR4-MD2 actions that are responsible for downstream S100A9 signaling in inflammatory processes. In actuality, these molecules appear to be important in different biological processes. For example, in a S100A9 null murine system, it was determined that TLR4-MD2 and not RAGE was responsible for S100A8/A9 mediated effects. In this model, S100A9 is upstream of TNF-α induction and the S100A8 subunit of the S100A8/A9 complex induced NF-κB via TLR4-MD2. When direct binding of S100A9 to RAGE and TLR4-MD2 is measured in vitro, the S100A9 homodimer has a greater affinity for RAGE or TLR4-MD2 than its heterodimeric counterpart, S100A8/A9 [40]. Therefore, it appears that S100A9 may be important for the structural interaction of the heterodimer with RAGE or TLR4-MD2, and S100A8 is important for regulation of the heterodimer’s ability to complex with RAGE or TLR4-MD2. In another example, extra-cellular S100A8/A9 enhances LPS signaling at the plasma membrane leading to increased LPS-induced mortality in mice. Interestingly, this is accomplished by S100A8 and not S100A9 of the S100A8/A9 heterodimer binding directly to the TLR4-MD2 complex [41]. S100A8 and S100A9 are likely both important for mediating inflammatory cascades through their interactions with RAGE and TLR4, but the structural and mechanistic reasons why each subunit is important for specific inflammatory reactions requires further investigation.
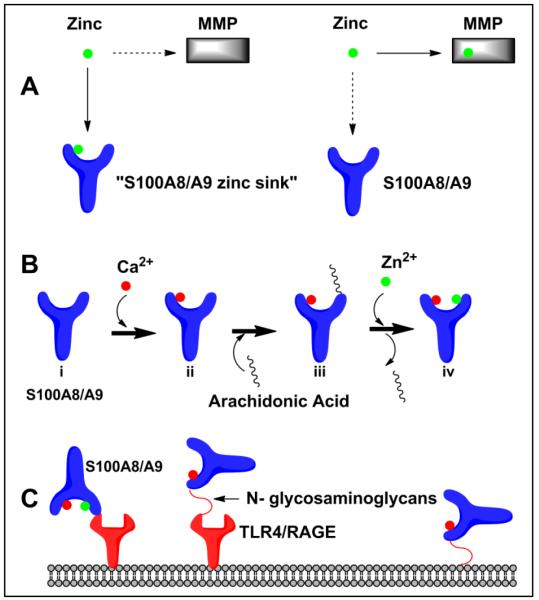
S100A9 activity within the intracellular cytoskeleton and extra-cellular matrix is dependent on calcium and zinc
Calcium dependent tetramer formation of S100A8/A9 is essential for the formation of microtubules and has been measured in vitro [42]. In the polymerization of microtubules, S100A8 interacts with tubulin and S100A9 serves as the regulatory subunit [42]. In epithelial cells, calcium serves as a secondary messenger and binds to S100A8 and S100A9 and induces their translocation to keratin intermediate filaments thought to mediate cellular migration [13]. S100A9 localizes to the cytoplasm in monocyte cell culture systems and an increase in calcium levels promotes translocation of S100A9 to the membrane probably by protein kinase C (PKC) dependent mechanisms [43]. It is unclear whether S100A9 directly transports arachidonic acid out of the cell, but S00A8/A9 is likely secreted from neutrophil-like HL-60 cells via a calcium dependent protein kinase C pathway. Once in the extracellular space, calcium bound S100A9 is found complexed to arachidonic acid (Figure 3) [44]. In the extra-cellular space, S100A8/A9 not only binds to arachidonic acid in a calcium dependent manner, but has interactions with the major fatty acid transporter of endothelial cells (CD36) to promote fatty acid uptake and heparin sulfate proteoglycans via the S100A9 subunit thereby promoting myeloid cell migration [45, 46]. MMP2 and MMP9 need zinc to cleave S100A9, thereby limiting its activity in inflammatory pathways (Figure 3) [47]. S100A8/A9 is itself able to block the metalloproteinase (MMP) degradation of the extra-cellular matrix by sequestration of zinc, thus forming a negative feedback loop [48]. The zinc binding motif on S100A9 (H92-E97) is responsible for inhibiting the spread and phagocytic activity of adherent peritoneal cells presumable by this mechanism [49]. By its interaction with cytoskeletal components and extracellular matrix factors, S100A8/A9 can promote inflammation and also create the molecular environment responsible for termination of biological activity in the presence of divalent ions such as calcium and zinc.
Figure 3.
Extra-cellular S100A9 activity A) S100A8/A9 sequesters zinc inhibiting the extracellular matrix degradation capacity of MMPs
B) S100A8/A9 or S100A9 serves to transport arachidonic acid (AA). Calcium binding to Apo-S100A9 (i) forms Ca2+-S100A9 (ii). The Ca2+-S100A9 (ii) protein binds to arachidonic acid forming the Ca2+-S100A9 –AA complex (iii). Zinc binding to Ca2+-S100A9 (ii) forms the Zn2+Ca2+-S100A9 complex (iv) and can no longer bind arachidonic acid.
C) S100A9 or S100A8/A9 binds to cell surface receptors initiating signal transduction cascades.
S100A9 expression mediates the inflammatory and migratory potential of myeloid cells
It is the goal of this section to outline general mechanisms of S100A9 induced inflammation and the roles of the S100A8 and S100A9 sub-units in influencing the migratory potential of myeloid cells that will be important in the discussion of S100A9 in different cancers. In general, levels of S100A9 are increased at sites of inflammation [50-53]. As described in previous sections, S100A9 activates NF-κB signaling pathways, although the precise mechanism of this initiation is still unclear. The ability of S100A9 to influence the inflammatory cascade and migration in multiple types of myeloid cells is dependent on divalent ions such as calcium and zinc. S100A9 is recruited to sites of inflammation by its interaction with glycans. In a rheumatoid arthritis model, S100A8 and S100A9 induced the expression and secretion of pro-inflammatory cytokines by monocytes such as IL-6, CXCL8, IL-1β, and TNF-α [54]. Mice treated with an anti-glycan antibody in a LPS-based colitis model exhibited reduced S100A8/A9 expression in colon tissues and had reduced levels of TNF-α, nitric oxide, IL-23 mRNA, and mucosal addressin cell adhesion molecule 1 (MAdCAM-1) [55]. S100A9 null mice and S100A9 null granulocytes in vitro also demonstrate reduced recruitment of granulocytes. There was also a reduction in granulocyte infiltration when S100A9 phosphorylation was blocked by p38 MAPK inhibition in human monocytes. Mechanistically, it appears that the S100A8 subunit interacts with tubulin and the S100A9 subunit is the regulatory element that promotes movement such as kinesis of cytoskeletal elements [56]. The conclusion that S100A9 is important for myeloid migration is supported by the evidence that S100A9 null neutrophils demonstrated defective chemoattractant induced calcium signaling as mediated by phospholipase C pathways [57]. S100A9 phosphorylation at Threonine-113 by protein kinase C modulates S100A9 translocation to the cell membrane of human neutrophils [58, 59]. This phosphorylation event appears to control cytoskeletal rearrangements important for myeloid cell migration. Another way in which S100A9 promotes cell movement is in monocytes where it localizes to the type III intermediate filament vimentin in a calcium dependent manner [60]. S100A9 is therefore involved in both the recruitment, and containment of inflammatory cells during the inflammatory response.
S100A9 regulates the maturation of myeloid cells
In the inflammatory response, monocytes differentiate into mature macrophages, initially expressing both S100A8 and S100A9. Later, macrophages at inflammatory sites will lose S100A8 expression [61]. Even though S100A9 is not important until later in the development of different cell types, knock-out of its binding partner S100A8 in mice causes early resorption of the embryo [62]. Up-regulation of S100A9 correlates with differentiation of the promyelocyte to a myelocyte/granulocyte and correlates with the expression of CD11b in neutrophilic cells already expressing CD15. S100A9 is up-regulated prior to the expression of CD15 in monocytes. In both monocytes and macrophages, S100A9 production correlates with the expression of CD11b on the cell surface [63]. Kruppel-related zinc finger protein and the transcriptional intermediary factor 1 beta (TIF1 ) appear to be involved in a myeloid protein regulatory element (MRE) binding complex regulating S100A9 gene expression and promotion of differentiation [63]. However, differentiation of HL-60 cells in response to ATRA was reduced by 40% when treated with S100A9-siRNA so elements other than S100A9 may be important for maturation. S100A9 levels also correlate with the trans-differentiation of neutrophilic granulocytes to macrophages when stimulated with CSF-1 [64]. The role of S100A9 in the maturation of myeloid cells is not completely known, but it is interesting to note that early myeloid cells called myeloid derived suppressor cells (MDSC) may be induced by S100A9 and are able to suppress the immune response to cancer cells.
S100A9 recruits myeloid derived suppressor cells (MDSCs) promoting cancer growth via inflammatory pathways
The relationship between S100A9 and MDSC has been carefully studied [53, 65-67]. Murine MDSCs express both CD11b and Gr1. Human MDSCs are a heterogeneous population of early myeloid cells that exhibit a multitude of cell surface markers including: CD11b, HLADRlow/−, CD33, CD15, CD14, and IL4Rα [68]. MDSCs are also characterized by their ability to inhibit T cell function and the immune reaction to cancer cells by the release of reactive oxygen species (e.g. nitric oxide), cytokines, and arginase. Lymphoma tumors in S100A9 null mice grow less rapidly than in wild type mice and these results are dependent on reduced recruitment of MDSCs. Over-expression of S100A9 increases MDSC recruitment and inhibits differentiation of dendritic cells [69]. As a second example, S100A9 knockout mice are better able to reject EL4 lymphomas compared to wild type mice [70]. This appears to be due to lack of MDSC recruitment to tumor sites. On the biochemical level, it may be possible that S100A8/A9 on MDSCs binds to carboxylated glycans on endothelial surfaces or RAGE on the tumor cell to promote migration of MDSCs. S100A8/A9 is also expressed by tumor cells and may provide a mechanism for recruiting additional MDSC into the tumor microenvironment by binding to RAGE on MDSCs and promoting NFκB inflammatory pathway signal transduction [38, 71]. S100A9 appears to be important in the recruitment of MDSC to tumor sites and inhibition of the immune responses to cancers.
S100A9 is differentially expressed in various cancers
S100A9 is up-regulated in numerous cancer types including breast cancer (invasive ductal carcinoma), colitis-associated colon cancer, hepatocellular carcinoma, gastric cancer, pulmonary adenocarcinoma, colorectal cancer, breast apocrine carcinomas, non-small cell lung cancer, and squamous cervical cancer [27, 28, 38, 72-80]. S100A9 levels are also increased in the stroma of nasopharyngeal carcinoma, and in transitional cell carcinomas of the bladder [81, 82]. Given the number of cancers that appear to over-express S100A9, the biochemistry and molecular biology of this protein will be reviewed in the context of cancer biology.
In non-small cell lung adenocarcinoma, S100A9 is associated with a poor prognosis. Patients with early stage lung cancer who had over-expression of S100A9 within the cancer cells exhibited a significantly worse overall five year survival [83]. In a second small study, forty patients were divided into two groups consisting of poor survival (median 7.7 months) and extended survival (median 92.7 months). S100A8/A9 levels were increased in the poor survival group in the non-macrophage component of the stroma, but in the extended survival group S100A8/A9 was increased in the non-macrophage component of tumor cell islets [79]. This apparent controversy can be explained by the fact that M1 macrophages were increased in the extended survival group at the tumor site. S100A8/A9 may also function in M1 macrophages in conjunction with reactive oxygen species to suppress cancer. In a murine Lewis lung carcinoma model, it has been shown that S100A9 promotes both the development of metastatic disease and myeloid cell recruitment [84]. In this model, expression of S100A9 was decreased in the presence of an antibody to TGF-β. Addition of exogenous TGF-β to mice increased S100A9 levels in the presence of VEGF-A and TNF-α [84]. Therefore, S100A9 is likely linked to the TGF-β pathway that promotes metastasis. S100A8/A9 may function within M1 macrophages to suppress cancer, but may function with TGF-β, VEGF-A and TNF-α to promote cancer. Depending on the molecular environment S100A9 can promote or inhibit tumor growth in lung cancer.
In pancreatic adenocarcinoma, S100A9 co-localizes with CD14 on monocytes and macrophages in the stroma. In pancreatic cancer S100A9 may interact with the TGF-β signaling pathway to influence cell growth and migration. Over half of pancreatic cancers are proposed to have a mutated component of the Smad4-mediated TGF-β signaling pathway [85]. With the lack of Smad4, S100A9 may be preferentially expressed compared to S100A8 in pancreatic adenocarcinoma [86]. Over 50% of pancreatic cancers lose expression of Smad4 late in the disease course and knockdown of Smad4 in pancreatic cancer cells abolishes TGF-β-mediated cell cycle arrest. The abolition of cell cycle arrest permits cancer cell growth, but the TGF-β protein is still present. Smad4 knockdown does not inhibit the TGF-β induced epithelial mesenchymal transition that is responsible for the evolution of pancreatic cancer cell type that has the ability to migrate and metastasize [87]. As TGF-β promotes the development of metastatic disease in lung cancer, it is very likely that S100A9 may promote these activities in pancreatic adenocarcinoma through interactions with the TGF-β pathway, although the specifics have not yet been elucidated. One possibility is that Smad4 mutations may accentuate the S100A9-TGF-β pathway in pancreatic adenocarcinoma. Therefore, in lung, pancreas and other cancers, S100A9 likely contributes to tumor cell migration through the activity of TGF-β, and S100A9 also leads to the recruitment of myeloid cells such as inhibitory MDSCs.
In hepatocellular carcinoma, NFκB binds to the S100A9 promoter and activates transcription. S100A9 proceeds to activate reactive oxygen species dependent signaling pathways protecting hepatocellular carcinoma cells from apoptotic cell death [88]. In this system S100A9 appears to be co-expressed with S100A8. Nemeth et al. were able to validate increased S100A8 and S100A9 levels in hepatocellular carcinoma in murine and human tissue [88].
S100A9 is up-regulated in colon adenocarcinomas. Murine model exists where colon adenocarcinomas can be induced by the pro-inflammatory agent 1,2-dimethylhydrazine (DMH) or the genotoxic agent azoxymethane. Both S100A8 and S100A9 exhibit increased expression in the resultant tumor cells [89, 90]. In a series of colorectal cancers obtained from fresh surgical specimens, S100A9 levels were increased and a protein signature was identified that inversely correlated with levels of S100A9. This signature included liver fatty acid binding protein, actin-binding protein/smooth muscle protein 22-alpha and cyclooxygenase 2 [91]. Also, S100A9 containing macrophages and polymorphoneutrophils (PMNs) accumulate along the invasive margins of human colorectal carcinomas [92]. These data suggest that S100A9 is involved in the invasive phenotype and development of colorectal tumors.
S100A9 may have both tumor supportive and tumor suppressive roles in breast cancer. In invasive ductal carcinoma of the breast, immunopositivity for S100A9 correlated with mitotic activity, the MIB-1 proliferation index (monoclonal antibody to recombinant Ki-67), HER2 over-expression, poor tumor differentiation, vessel invasion, nodal metastasis and poor pathological stage [93]. S100A9 is associated with high grade, negative ER and PR status, high Ki67 and p53 expression, and ERBB2 and EGFR expression. S100A9 expression is also closely correlated to a 10 protein basal signature CK5/6, CD10, EGFR, CAV1, CD44, ETS1, MET, Moesin, GATA3, and CK19. The presence of S100A9 in node negative breast cancer patients has prognostic value [94]. However, other studies reveal a more complex relationship. For example, S100A8/A9 gene over-expression in the presence of calcium conferred an invasive/migratory phenotype to the breast cancer line MCF10A presumably via the H-Ras signaling pathway [95]. An increase in S100A8/A9 protein levels may also result in S100A9 zinc mediated sequestration, inability of MMP9 to cleave S100A9, and activation of RAGE signal transduction pathways all of which are known to be pro-tumorigenic. In contrast, S100A9 induced growth repression in infiltrating ductal carcinoma of the breast in MCF-7 cells. Smith et al. have shown that oncostatin (OM) inhibits the growth of infiltrating ductal carcinoma of the breast through the binding of oncostatin-activated STAT3 to the S100A9 promoter in MCF-7 breast cancer cells [96]. Activation of the oncostatin receptor can also activate the MEK/ERK pathway. In this system, blocking the ERK pathway inhibited S100A8 expression more efficiently and blocking the P38 MAPK pathway inhibited S100A9 expression more efficiently [95]. The dichotomy of S100A9 function needs to be reconciled with the fact that S00A8/A9 over-expression has been associated with adverse pathological features in infiltrating ductal carcinoma of the breast. Other evidence suggests that S100A9 appears to promote growth at low concentrations and inhibit growth at high concentrations. In a murine model, exogenous administration of S100A8/A9 was found to inhibit MM46 mammary carcinoma cells with a minimum inhibitory concentration between 50-100 g/mL. When zinc was added to this same system, it abrogated the ability of S100A8/A9 to inhibit MM46 apoptosis by at least 80% but was unaffected even by millimolar concentrations of either exogenous calcium or magnesium [97, 98]. Just like another S100 protein, S100B, p53 binds to the promoter of S100A9 [99, 100]. Also, increasing levels of S100A9 promotes apoptosis via p53-dependent and p53-independent pathways [99]. These activities of S100A9 are not only dependent on the native protein, but the relative local concentration gradients, its ligands and presumably its post-translational modifications. Thus, S100A9 appears to be inhibitory to breast cancers at higher concentrations and may be promoting tumor growth at lower concentrations, but additional research is needed to distinguish between dosage effects and model differences.
Esophageal adenocarcinoma is another cancer in which controversy exists over how S100A9 expression is involved in the pathogenesis of the disease. On the one hand, S100A9 is down-regulated in Barrett’s esophagus progression via increased expression of microRNA-196a [101] and is reduced in esophageal adenocarcinoma cancer [22] [102, 103]. There are reports which show increased expression of S100A9 in esophageal cancers while other groups document reduced expression of S100A9. Notably, RAGE expression correlates with S100A9 over-expression in esophageal preneoplasia in a rat model, supporting a role for S100A9-mediated inflammatory pathways in esophageal cancer [104]. Clearly additional work needs to be done to elucidate the role of S100A9 in esophageal adenocarcinoma.
Clinically, prostate tumors with less differentiation tend to express more S100A9. For example, tissue specimens were obtained from 75 patients and higher Gleason score prostate cancer patients tended to have higher levels of S100A9 and RAGE along with expression of S100A8 in prostatic tissue [105]. In prostate cancer cell lines, S100A8/A9 co-localizes with RAGE and likely activates inflammatory signaling pathways such as NF-κB [106]. However, circulating S100A8/A9 in blood is not associated with prostate cancer risk [107]. Other studies are underway to validate the relationship of S100A9 levels to prognosticate outcomes and response to therapy.
Pharmaceutical targeting of S100A9
Multiple therapeutic strategies for blocking S100A9 and/or its activity are currently under development in either inflammatory diseases or in the setting of cancer. Recently, a series of compounds have been shown to limit the inflammatory activities of S100A9. Quinoline-3-carboxamides bind to the S100A9 homodimer and limit the zinc and calcium dependent interactions of S100A9 with RAGE, TLR4-MD2 and arachidonic acid. [40]. More recently, these compounds were tested in patients with metastatic prostate cancer in a phase II trial for patients post-radiation and were found to be useful based on their anti-angiogenic properties. The progression free survival increased from an average of 3.3 to 7.6 months [108, 109]. Second, in PARP-1 knock-out mice and in mice treated with a Poly(ADP-ribose) polymerase (PARP) inhibitor S100A9 levels were reduced by approximately 66% [110]. These manipulations led to decreased 7,12-dimethylbenz(a)anthracene plus 12-O-tetradecanoylphorbol-13-acetate-induced skin carcinogenesis in a mouse model. S100A9 inhibitors may therefore be able to slow specific tumors.
Current S100A9 research may result in future pharmaceutical targets
In the cardiovascular system, the S100A8/A9 heterodimer enhanced secretion of the pro-inflammatory factors IL-6, ICAM-1, VCAM-1 and MCP1 in HUVEC cells in a dose dependent manner. The effects of S100A8/A9 were reduced by inhibition of ERK1/2 and p38 in the MAP kinase pathways. It has been postulated that blocking S100A8/A9 may represent a modality to treat atherosclerosis via the down-regulation of inflammatory pathways [111]. McCormick et al. found that arteries lacking atherosclerosis lacked expression of S100A8 or S100A9. S100A9 may associate with lipid structures and may promote dystrophic calcification by altering the ability of phospholipid to bind calcium [112]. These strategies all have the common theme of S100A9 involvement in aberrant regulation of the immune system and will likely also have therapeutic implications in cancer.
Summary
S100A9 is a calcium binding protein important in the pathogenesis of different cancer types. S100A9 structure was reviewed (Figure 1) including the nature of EF hands, the binding of divalent cations, and the structural basis for ligand binding. S100A9 is expressed in normal cell types such as myeloid cells, but its levels are generally increased in inflammation and cancer. The S100A9 protein is important for mediating inflammatory processes in myeloid cells and other cell types involved in inflammation (Figure 2). S100A9 activity is dependent on the concentration gradients of both calcium and zinc in the extra-cellular space (Figure 3). The review concluded with a discussion on development of targeted therapeutics. S100A9 levels may prove useful for prognosticating patient outcomes. Lastly, targeting S100A9 will likely prove useful in a number of inflammatory diseases and cancer states.
Acknowledgements
This review was supported by T32CA090223 (to J. Markowitz).
Molecular graphics images were produced using the UCSF Chimera package from the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco (supported by NIH P41 RR001081).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Joseph Markowitz, OSU Comprehensive Cancer Center The Ohio State University 320 West 10th Avenue Columbus, OH 43210 joseph.markowitz@osumc.edu.
William E. Carson, III, OSU Comprehensive Cancer Center The Ohio State University N924 Doan Hall 410 W. 10th Ave. Columbus, OH 43210-1228 Phone (614) 293-6306 or 292-5819 FAX: (614) 293-3465 william.carson@osumc.edu.
References
- [1].Chazin WJ. Relating form and function of EF-hand calcium binding proteins. Acc Chem Res. 2011;44:171–179. doi: 10.1021/ar100110d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Itou H, Fujita I, Ishikawa K, Yao M, Watanabe N, Suzuki M, Nishihira J, Tanaka I. Expression, purification, crystallization and preliminary X-ray diffraction analysis of the human calcium-binding protein MRP14 (S100A9) Acta Crystallogr D Biol Crystallogr. 2001;57:1174–1176. doi: 10.1107/s090744490100957x. [DOI] [PubMed] [Google Scholar]
- [3].Itou H, Yao M, Fujita I, Watanabe N, Suzuki M, Nishihira J, Tanaka I. The crystal structure of human MRP14 (S100A9), a Ca(2+)-dependent regulator protein in inflammatory process. J Mol Biol. 2002;316:265–276. doi: 10.1006/jmbi.2001.5340. [DOI] [PubMed] [Google Scholar]
- [4].Korndorfer IP, Brueckner F, Skerra A. The crystal structure of the human (S100A8/S100A9)2 heterotetramer, calprotectin, illustrates how conformational changes of interacting alpha-helices can determine specific association of two EF-hand proteins. J Mol Biol. 2007;370:887–898. doi: 10.1016/j.jmb.2007.04.065. [DOI] [PubMed] [Google Scholar]
- [5].Nacken W, Kerkhoff C. The hetero-oligomeric complex of the S100A8/S100A9 protein is extremely protease resistant. FEBS Lett. 2007;581:5127–5130. doi: 10.1016/j.febslet.2007.09.060. [DOI] [PubMed] [Google Scholar]
- [6].Leukert N, Sorg C, Roth J. Molecular basis of the complex formation between the two calcium-binding proteins S100A8 (MRP8) and S100A9 (MRP14) Biol Chem. 2005;386:429–434. doi: 10.1515/BC.2005.051. [DOI] [PubMed] [Google Scholar]
- [7].Hessian PA, Fisher L. The heterodimeric complex of MRP-8 (S100A8) and MRP-14 (S100A9). Antibody recognition, epitope definition and the implications for structure. Eur J Biochem. 2001;268:353–363. doi: 10.1046/j.1432-1033.2001.01894.x. [DOI] [PubMed] [Google Scholar]
- [8].Vogl T, Leukert N, Barczyk K, Strupat K, Roth J. Biophysical characterization of S100A8 and S100A9 in the absence and presence of bivalent cations. Biochim Biophys Acta. 2006;1763:1298–1306. doi: 10.1016/j.bbamcr.2006.08.028. [DOI] [PubMed] [Google Scholar]
- [9].Raftery MJ, Collinson L, Geczy CL. Overexpression, oxidative refolding, and zinc binding of recombinant forms of the murine S100 protein MRP14 (S100A9) Protein Expr Purif. 1999;15:228–235. doi: 10.1006/prep.1998.1015. [DOI] [PubMed] [Google Scholar]
- [10].Nacken W, Sopalla C, Propper C, Sorg C, Kerkhoff C. Biochemical characterization of the murine S100A9 (MRP14) protein suggests that it is functionally equivalent to its human counterpart despite its low degree of sequence homology. Eur J Biochem. 2000;267:560–565. doi: 10.1046/j.1432-1327.2000.01040.x. [DOI] [PubMed] [Google Scholar]
- [11].Vogl T, Propper C, Hartmann M, Strey A, Strupat K, van den Bos C, Sorg C, Roth J. S100A12 is expressed exclusively by granulocytes and acts independently from MRP8 and MRP14. J Biol Chem. 1999;274:25291–25296. doi: 10.1074/jbc.274.36.25291. [DOI] [PubMed] [Google Scholar]
- [12].Odink K, Cerletti N, Bruggen J, Clerc RG, Tarcsay L, Zwadlo G, Gerhards G, Schlegel R, Sorg C. Two calcium-binding proteins in infiltrate macrophages of rheumatoid arthritis. Nature. 1987;330:80–82. doi: 10.1038/330080a0. [DOI] [PubMed] [Google Scholar]
- [13].Goebeler M, Roth J, van den Bos C, Ader G, Sorg C. Increase of calcium levels in epithelial cells induces translocation of calcium-binding proteins migration inhibitory factor-related protein 8 (MRP8) and MRP14 to keratin intermediate filaments. Biochem J. 1995;309(Pt 2):419–424. doi: 10.1042/bj3090419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Edgeworth J, Gorman M, Bennett R, Freemont P, Hogg N. Identification of p8,14 as a highly abundant heterodimeric calcium binding protein complex of myeloid cells. J Biol Chem. 1991;266:7706–7713. [PubMed] [Google Scholar]
- [15].Moore BW. A soluble protein characteristic of the nervous system. Biochem Biophys Res Commun. 1965;19:739–744. doi: 10.1016/0006-291x(65)90320-7. [DOI] [PubMed] [Google Scholar]
- [16].Marenholz I, Lovering RC, Heizmann CW. An update of the S100 nomenclature. Biochim Biophys Acta. 2006;1763:1282–1283. doi: 10.1016/j.bbamcr.2006.07.013. [DOI] [PubMed] [Google Scholar]
- [17].Kurata A, Terado Y, Schulz A, Fujioka Y, Franke FE. Inflammatory cells in the formation of tumor-related sarcoid reactions. Hum Pathol. 2005;36:546–554. doi: 10.1016/j.humpath.2005.02.017. [DOI] [PubMed] [Google Scholar]
- [18].Deininger MH, Pater S, Strik H, Meyermann R. Macrophage/microglial cell subpopulations in glioblastoma multiforme relapses are differentially altered by radiochemotherapy. J Neurooncol. 2001;55:141–147. doi: 10.1023/a:1013805915224. [DOI] [PubMed] [Google Scholar]
- [19].Imaoka T, Yamashita S, Nishimura M, Kakinuma S, Ushijima T, Shimada Y. Gene expression profiling distinguishes between spontaneous and radiation-induced rat mammary carcinomas. J Radiat Res (Tokyo) 2008;49:349–360. doi: 10.1269/jrr.07126. [DOI] [PubMed] [Google Scholar]
- [20].Broome AM, Ryan D, Eckert RL. S100 protein subcellular localization during epidermal differentiation and psoriasis. J Histochem Cytochem. 2003;51:675–685. doi: 10.1177/002215540305100513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Fanjul M, Renaud W, Merten M, Guy-Crotte O, Hollande E, Figarella C. Presence of MRP8 and MRP14 in pancreatic cell lines: differential expression and localization in CFPAC-1 cells. Am J Physiol. 1995;268:C1241–1251. doi: 10.1152/ajpcell.1995.268.5.C1241. [DOI] [PubMed] [Google Scholar]
- [22].Wang J, Cai Y, Xu H, Zhao J, Xu X, Han YL, Xu ZX, Chen BS, Hu H, Wu M, Wang MR. Expression of MRP14 gene is frequently down-regulated in Chinese human esophageal cancer. Cell Res. 2004;14:46–53. doi: 10.1038/sj.cr.7290201. [DOI] [PubMed] [Google Scholar]
- [23].Sabo E, Meitner PA, Tavares R, Corless CL, Lauwers GY, Moss SF, Resnick MB. Expression analysis of Barrett’s esophagus-associated high-grade dysplasia in laser capture microdissected archival tissue. Clin Cancer Res. 2008;14:6440–6448. doi: 10.1158/1078-0432.CCR-08-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bax DA, Siersema PD, Haringsma J, Kuipers EJ, Vos AJ, Van Dekken H, Van Vliet AH, Kusters JG. High-grade dysplasia in Barrett’s esophagus is associated with increased expression of calgranulin A and B. Scand J Gastroenterol. 2007;42:902–910. doi: 10.1080/00365520601138189. [DOI] [PubMed] [Google Scholar]
- [25].Ito Y, Arai K, Ryushi, Nozawa, Yoshida H, Tomoda C, Uruno T, Miya A, Kobayashi K, Matsuzuka F, Kuma K, Kakudo K, Miyauchi A. S100A9 expression is significantly linked to dedifferentiation of thyroid carcinoma. Pathol Res Pract. 2005;201:551–556. doi: 10.1016/j.prp.2005.08.003. [DOI] [PubMed] [Google Scholar]
- [26].Ito Y, Miyauchi A, Arai K, Nozawa R, Miya A, Kobayashi K, Nakamura Y, Kakudo K. Usefulness of S100A9 for diagnosis of intrathyroid epithelial thymoma (ITET)/carcinoma showing thymus-like differentiation (CASTLE) Pathology. 2006;38:541–544. doi: 10.1080/00313020601024086. [DOI] [PubMed] [Google Scholar]
- [27].Arai K, Teratani T, Kuruto-Niwa R, Yamada T, Nozawa R. S100A9 expression in invasive ductal carcinoma of the breast: S100A9 expression in adenocarcinoma is closely associated with poor tumour differentiation. Eur J Cancer. 2004;40:1179–1187. doi: 10.1016/j.ejca.2004.01.022. [DOI] [PubMed] [Google Scholar]
- [28].Cross SS, Hamdy FC, Deloulme JC, Rehman I. Expression of S100 proteins in normal human tissues and common cancers using tissue microarrays: S100A6, S100A8, S100A9 and S100A11 are all overexpressed in common cancers. Histopathology. 2005;46:256–269. doi: 10.1111/j.1365-2559.2005.02097.x. [DOI] [PubMed] [Google Scholar]
- [29].Kerkhoff C, Nacken W, Benedyk M, Dagher MC, Sopalla C, Doussiere J. The arachidonic acid-binding protein S100A8/A9 promotes NADPH oxidase activation by interaction with p67phox and Rac-2. Faseb J. 2005;19:467–469. doi: 10.1096/fj.04-2377fje. [DOI] [PubMed] [Google Scholar]
- [30].Sopalla C, Leukert N, Sorg C, Kerkhoff C. Evidence for the involvement of the unique C-tail of S100A9 in the binding of arachidonic acid to the heterocomplex S100A8/A9. Biol Chem. 2002;383:1895–1905. doi: 10.1515/BC.2002.213. [DOI] [PubMed] [Google Scholar]
- [31].Kerkhoff C, Klempt M, Kaever V, Sorg C. The two calcium-binding proteins, S100A8 and S100A9, are involved in the metabolism of arachidonic acid in human neutrophils. J Biol Chem. 1999;274:32672–32679. doi: 10.1074/jbc.274.46.32672. [DOI] [PubMed] [Google Scholar]
- [32].Kerkhoff C, Vogl T, Nacken W, Sopalla C, Sorg C. Zinc binding reverses the calcium-induced arachidonic acid-binding capacity of the S100A8/A9 protein complex. FEBS Lett. 1999;460:134–138. doi: 10.1016/s0014-5793(99)01322-8. [DOI] [PubMed] [Google Scholar]
- [33].Benedyk M, Sopalla C, Nacken W, Bode G, Melkonyan H, Banfi B, Kerkhoff C. HaCaT keratinocytes overexpressing the S100 proteins S100A8 and S100A9 show increased NADPH oxidase and NF-kappaB activities. J Invest Dermatol. 2007;127:2001–2011. doi: 10.1038/sj.jid.5700820. [DOI] [PubMed] [Google Scholar]
- [34].Lim SY, Raftery M, Cai H, Hsu K, Yan WX, Hseih HL, Watts RN, Richardson D, Thomas S, Perry M, Geczy CL. S-nitrosylated S100A8: novel anti-inflammatory properties. J Immunol. 2008;181:5627–5636. doi: 10.4049/jimmunol.181.8.5627. [DOI] [PubMed] [Google Scholar]
- [35].Lim SY, Raftery MJ, Goyette J, Geczy CL. S-glutathionylation regulates inflammatory activities of S100A9. J Biol Chem. 2010;285:14377–14388. doi: 10.1074/jbc.M109.075242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sroussi HY, Berline J, Palefsky JM. Oxidation of methionine 63 and 83 regulates the effect of S100A9 on the migration of neutrophils in vitro. J Leukoc Biol. 2007;81:818–824. doi: 10.1189/jlb.0706433. [DOI] [PubMed] [Google Scholar]
- [37].Ichikawa M, Williams R, Wang L, Vogl T, Srikrishna G. S100A8/A9 activate key genes and pathways in colon tumor progression. Mol Cancer Res. 2011;9:133–148. doi: 10.1158/1541-7786.MCR-10-0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Turovskaya O, Foell D, Sinha P, Vogl T, Newlin R, Nayak J, Nguyen M, Olsson A, Nawroth PP, Bierhaus A, Varki N, Kronenberg M, Freeze HH, Srikrishna G. RAGE, carboxylated glycans and S100A8/A9 play essential roles in colitis-associated carcinogenesis. Carcinogenesis. 2008;29:2035–2043. doi: 10.1093/carcin/bgn188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Gebhardt C, Riehl A, Durchdewald M, Nemeth J, Furstenberger G, Muller-Decker K, Enk A, Arnold B, Bierhaus A, Nawroth PP, Hess J, Angel P. RAGE signaling sustains inflammation and promotes tumor development. J Exp Med. 2008;205:275–285. doi: 10.1084/jem.20070679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bjork P, Bjork A, Vogl T, Stenstrom M, Liberg D, Olsson A, Roth J, Ivars F, Leanderson T. Identification of human S100A9 as a novel target for treatment of autoimmune disease via binding to quinoline-3-carboxamides. PLoS Biol. 2009;7:e97. doi: 10.1371/journal.pbio.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Vogl T, Tenbrock K, Ludwig S, Leukert N, Ehrhardt C, van Zoelen MA, Nacken W, Foell D, van der Poll T, Sorg C, Roth J. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med. 2007;13:1042–1049. doi: 10.1038/nm1638. [DOI] [PubMed] [Google Scholar]
- [42].Leukert N, Vogl T, Strupat K, Reichelt R, Sorg C, Roth J. Calcium-dependent tetramer formation of S100A8 and S100A9 is essential for biological activity. J Mol Biol. 2006;359:961–972. doi: 10.1016/j.jmb.2006.04.009. [DOI] [PubMed] [Google Scholar]
- [43].Roth J, Burwinkel F, van den Bos C, Goebeler M, Vollmer E, Sorg C. MRP8 and MRP14, S-100-like proteins associated with myeloid differentiation, are translocated to plasma membrane and intermediate filaments in a calcium-dependent manner. Blood. 1993;82:1875–1883. [PubMed] [Google Scholar]
- [44].Kerkhoff C, Klempt M, Sorg C. Novel insights into structure and function of MRP8 (S100A8) and MRP14 (S100A9) Biochim Biophys Acta. 1998;1448:200–211. doi: 10.1016/s0167-4889(98)00144-x. [DOI] [PubMed] [Google Scholar]
- [45].Kerkhoff C, Sorg C, Tandon NN, Nacken W. Interaction of S100A8/S100A9-arachidonic acid complexes with the scavenger receptor CD36 may facilitate fatty acid uptake by endothelial cells. Biochemistry. 2001;40:241–248. doi: 10.1021/bi001791k. [DOI] [PubMed] [Google Scholar]
- [46].Robinson MJ, Tessier P, Poulsom R, Hogg N. The S100 family heterodimer, MRP-8/14, binds with high affinity to heparin and heparan sulfate glycosaminoglycans on endothelial cells. J Biol Chem. 2002;277:3658–3665. doi: 10.1074/jbc.M102950200. [DOI] [PubMed] [Google Scholar]
- [47].Greenlee KJ, Corry DB, Engler DA, Matsunami RK, Tessier P, Cook RG, Werb Z, Kheradmand F. Proteomic identification of in vivo substrates for matrix metalloproteinases 2 and 9 reveals a mechanism for resolution of inflammation. J Immunol. 2006;177:7312–7321. doi: 10.4049/jimmunol.177.10.7312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Isaksen B, Fagerhol MK. Calprotectin inhibits matrix metalloproteinases by sequestration of zinc. Mol Pathol. 2001;54:289–292. doi: 10.1136/mp.54.5.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Pagano RL, Sampaio SC, Juliano L, Juliano MA, Giorgi R. The C-terminus of murine S100A9 inhibits spreading and phagocytic activity of adherent peritoneal cells. Inflamm Res. 2005;54:204–210. doi: 10.1007/s00011-005-1344-y. [DOI] [PubMed] [Google Scholar]
- [50].Roth J, Vogl T, Sorg C, Sunderkotter C. Phagocyte-specific S100 proteins: a novel group of proinflammatory molecules. Trends Immunol. 2003;24:155–158. doi: 10.1016/s1471-4906(03)00062-0. [DOI] [PubMed] [Google Scholar]
- [51].Nacken W, Roth J, Sorg C, Kerkhoff C. S100A9/S100A8: Myeloid representatives of the S100 protein family as prominent players in innate immunity. Microsc Res Tech. 2003;60:569–580. doi: 10.1002/jemt.10299. [DOI] [PubMed] [Google Scholar]
- [52].Foell D, Wittkowski H, Vogl T, Roth J. S100 proteins expressed in phagocytes: a novel group of damage-associated molecular pattern molecules. J Leukoc Biol. 2007;81:28–37. doi: 10.1189/jlb.0306170. [DOI] [PubMed] [Google Scholar]
- [53].Ehrchen JM, Sunderkotter C, Foell D, Vogl T, Roth J. The endogenous Toll-like receptor 4 agonist S100A8/S100A9 (calprotectin) as innate amplifier of infection, autoimmunity, and cancer. J Leukoc Biol. 2009;86:557–566. doi: 10.1189/jlb.1008647. [DOI] [PubMed] [Google Scholar]
- [54].Sunahori K, Yamamura M, Yamana J, Takasugi K, Kawashima M, Yamamoto H, Chazin WJ, Nakatani Y, Yui S, Makino H. The S100A8/A9 heterodimer amplifies proinflammatory cytokine production by macrophages via activation of nuclear factor kappa B and p38 mitogen-activated protein kinase in rheumatoid arthritis. Arthritis Res Ther. 2006;8:R69. doi: 10.1186/ar1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Srikrishna G, Turovskaya O, Shaikh R, Newlin R, Foell D, Murch S, Kronenberg M, Freeze HH. Carboxylated glycans mediate colitis through activation of NF-kappa B. J Immunol. 2005;175:5412–5422. doi: 10.4049/jimmunol.175.8.5412. [DOI] [PubMed] [Google Scholar]
- [56].Vogl T, Ludwig S, Goebeler M, Strey A, Thorey IS, Reichelt R, Foell D, Gerke V, Manitz MP, Nacken W, Werner S, Sorg C, Roth J. MRP8 and MRP14 control microtubule reorganization during transendothelial migration of phagocytes. Blood. 2004;104:4260–4268. doi: 10.1182/blood-2004-02-0446. [DOI] [PubMed] [Google Scholar]
- [57].McNeill E, Conway SJ, Roderick HL, Bootman MD, Hogg N. Defective chemoattractant-induced calcium signalling in S100A9 null neutrophils. Cell Calcium. 2007;41:107–121. doi: 10.1016/j.ceca.2006.05.004. [DOI] [PubMed] [Google Scholar]
- [58].Guignard F, Mauel J, Markert M. Phosphorylation of myeloid-related proteins MRP-14 and MRP-8 during human neutrophil activation. Eur J Biochem. 1996;241:265–271. doi: 10.1111/j.1432-1033.1996.0265t.x. [DOI] [PubMed] [Google Scholar]
- [59].Hessian PA, Edgeworth J, Hogg N. MRP-8 and MRP-14, two abundant Ca(2+)-binding proteins of neutrophils and monocytes. J Leukoc Biol. 1993;53:197–204. [PubMed] [Google Scholar]
- [60].Burwinkel F, Roth J, Goebeler M, Bitter U, Wrocklage V, Vollmer E, Roessner A, Sorg C, Bocker W. Ultrastructural localization of the S-100-like proteins MRP8 and MRP14 in monocytes is calcium-dependent. Histochemistry. 1994;101:113–120. doi: 10.1007/BF00269357. [DOI] [PubMed] [Google Scholar]
- [61].Zwadlo G, Bruggen J, Gerhards G, Schlegel R, Sorg C. Two calcium-binding proteins associated with specific stages of myeloid cell differentiation are expressed by subsets of macrophages in inflammatory tissues. Clin Exp Immunol. 1988;72:510–515. [PMC free article] [PubMed] [Google Scholar]
- [62].Passey RJ, Williams E, Lichanska AM, Wells C, Hu S, Geczy CL, Little MH, Hume DA. A null mutation in the inflammation-associated S100 protein S100A8 causes early resorption of the mouse embryo. J Immunol. 1999;163:2209–2216. [PubMed] [Google Scholar]
- [63].Kerkhoff C, Hofmann HA, Vormoor J, Melkonyan H, Roth J, Sorg C, Klempt M. Binding of two nuclear complexes to a novel regulatory element within the human S100A9 promoter drives the S100A9 gene expression. J Biol Chem. 2002;277:41879–41887. doi: 10.1074/jbc.M207990200. [DOI] [PubMed] [Google Scholar]
- [64].Sasmono RT, Ehrnsperger A, Cronau SL, Ravasi T, Kandane R, Hickey MJ, Cook AD, Himes SR, Hamilton JA, Hume DA. Mouse neutrophilic granulocytes express mRNA encoding the macrophage colony-stimulating factor receptor (CSF-1R) as well as many other macrophage-specific transcripts and can transdifferentiate into macrophages in vitro in response to CSF-1. J Leukoc Biol. 2007;82:111–123. doi: 10.1189/jlb.1206713. [DOI] [PubMed] [Google Scholar]
- [65].Gebhardt C, Nemeth J, Angel P, Hess J. S100A8 and S100A9 in inflammation and cancer. Biochem Pharmacol. 2006;72:1622–1631. doi: 10.1016/j.bcp.2006.05.017. [DOI] [PubMed] [Google Scholar]
- [66].Srikrishna G. S100A8 and S100A9: new insights into their roles in malignancy. J Innate Immun. 2011;4:31–40. doi: 10.1159/000330095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Youn JI, Gabrilovich DI. The biology of myeloid-derived suppressor cells: the blessing and the curse of morphological and functional heterogeneity. Eur J Immunol. 2009;40:2969–2975. doi: 10.1002/eji.201040895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Filipazzi P, Huber V, Rivoltini L. Phenotype, function and clinical implications of myeloid-derived suppressor cells in cancer patients. Cancer Immunol Immunother. 2012;61:255–263. doi: 10.1007/s00262-011-1161-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Cheng P, Corzo CA, Luetteke N, Yu B, Nagaraj S, Bui MM, Ortiz M, Nacken W, Sorg C, Vogl T, Roth J, Gabrilovich DI. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med. 2008;205:2235–2249. doi: 10.1084/jem.20080132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Ko JS, Bukowski RM, Fincke JH. Myeloid-derived suppressor cells: a novel therapeutic target. Curr Oncol Rep. 2009;11:87–93. doi: 10.1007/s11912-009-0014-6. [DOI] [PubMed] [Google Scholar]
- [71].Sinha P, Okoro C, Foell D, Freeze HH, Ostrand-Rosenberg S, Srikrishna G. Proinflammatory S100 proteins regulate the accumulation of myeloid-derived suppressor cells. J Immunol. 2008;181:4666–4675. doi: 10.4049/jimmunol.181.7.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].El-Rifai W, Moskaluk CA, Abdrabbo MK, Harper J, Yoshida C, Riggins GJ, Frierson HF, Jr., Powell SM. Gastric cancers overexpress S100A calcium-binding proteins. Cancer Res. 2002;62:6823–6826. [PubMed] [Google Scholar]
- [73].Arai K, Yamada T, Nozawa R. Immunohistochemical investigation of migration inhibitory factor-related protein (MRP)-14 expression in hepatocellular carcinoma. Med Oncol. 2000;17:183–188. doi: 10.1007/BF02780526. [DOI] [PubMed] [Google Scholar]
- [74].Arai K, Teratani T, Nozawa R, Yamada T. Immunohistochemical investigation of S100A9 expression in pulmonary adenocarcinoma: S100A9 expression is associated with tumor differentiation. Oncol Rep. 2001;8:591–596. doi: 10.3892/or.8.3.591. [DOI] [PubMed] [Google Scholar]
- [75].Kim HJ, Kang HJ, Lee H, Lee ST, Yu MH, Kim H, Lee C. Identification of S100A8 and S100A9 as serological markers for colorectal cancer. J Proteome Res. 2009;8:1368–1379. doi: 10.1021/pr8007573. [DOI] [PubMed] [Google Scholar]
- [76].Celis JE, Gromova I, Gromov P, Moreira JM, Cabezon T, Friis E, Rank F. Molecular pathology of breast apocrine carcinomas: a protein expression signature specific for benign apocrine metaplasia. FEBS Lett. 2006;580:2935–2944. doi: 10.1016/j.febslet.2006.03.080. [DOI] [PubMed] [Google Scholar]
- [77].Chao A, Wang TH, Lee YS, Hsueh S, Chao AS, Chang TC, Kung WH, Huang SL, Chao FY, Wei ML, Lai CH. Molecular characterization of adenocarcinoma and squamous carcinoma of the uterine cervix using microarray analysis of gene expression. Int J Cancer. 2006;119:91–98. doi: 10.1002/ijc.21813. [DOI] [PubMed] [Google Scholar]
- [78].Zhu X, Lv J, Yu L, Zhu X, Wu J, Zou S, Jiang S. Proteomic identification of differentially-expressed proteins in squamous cervical cancer. Gynecol Oncol. 2009;112:248–256. doi: 10.1016/j.ygyno.2008.09.045. [DOI] [PubMed] [Google Scholar]
- [79].Ohri CM, Shikotra A, Green RH, Waller DA, Bradding P. The tissue microlocalisation and cellular expression of CD163, VEGF, HLA-DR, iNOS, and MRP 8/14 is correlated to clinical outcome in NSCLC. PLoS One. 2011;6:e21874. doi: 10.1371/journal.pone.0021874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Zhu H, Pei HP, Zeng S, Chen J, Shen LF, Zhong MZ, Yao RJ, Shen H. Profiling protein markers associated with the sensitivity to concurrent chemoradiotherapy in human cervical carcinoma. J Proteome Res. 2009;8:3969–3976. doi: 10.1021/pr900287a. [DOI] [PubMed] [Google Scholar]
- [81].Li MX, Xiao ZQ, Liu YF, Chen YH, Li C, Zhang PF, Li MY, Li F, Peng F, Duan CJ, Yi H, Yao HX, Chen ZC. Quantitative proteomic analysis of differential proteins in the stroma of nasopharyngeal carcinoma and normal nasopharyngeal epithelial tissue. J Cell Biochem. 2009;106:570–579. doi: 10.1002/jcb.22028. [DOI] [PubMed] [Google Scholar]
- [82].Yao R, Lopez-Beltran A, Maclennan GT, Montironi R, Eble JN, Cheng L. Expression of S100 protein family members in the pathogenesis of bladder tumors. Anticancer Res. 2007;27:3051–3058. [PubMed] [Google Scholar]
- [83].Kawai H, Minamiya Y, Takahashi N. Prognostic impact of S100A9 overexpression in non-small cell lung cancer. Tumour Biol. 2011;32:641–646. doi: 10.1007/s13277-011-0163-8. [DOI] [PubMed] [Google Scholar]
- [84].Hiratsuka S, Watanabe A, Aburatani H, Maru Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol. 2006;8:1369–1375. doi: 10.1038/ncb1507. [DOI] [PubMed] [Google Scholar]
- [85].Massague J, Blain SW, Lo RS. TGFbeta signaling in growth control, cancer, and heritable disorders. Cell. 2000;103:295–309. doi: 10.1016/s0092-8674(00)00121-5. [DOI] [PubMed] [Google Scholar]
- [86].Sheikh AA, Vimalachandran D, Thompson CC, Jenkins RE, Nedjadi T, Shekouh A, Campbell F, Dodson A, Prime W, Crnogorac-Jurcevic T, Lemoine NR, Costello E. The expression of S100A8 in pancreatic cancer-associated monocytes is associated with the Smad4 status of pancreatic cancer cells. Proteomics. 2007;7:1929–1940. doi: 10.1002/pmic.200700072. [DOI] [PubMed] [Google Scholar]
- [87].Levy L, Hill CS. Smad4 dependency defines two classes of transforming growth factor {beta} (TGF-{beta}) target genes and distinguishes TGF-{beta}-induced epithelial-mesenchymal transition from its antiproliferative and migratory responses. Mol Cell Biol. 2005;25:8108–8125. doi: 10.1128/MCB.25.18.8108-8125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Nemeth J, Stein I, Haag D, Riehl A, Longerich T, Horwitz E, Breuhahn K, Gebhardt C, Schirmacher P, Hahn M, Ben-Neriah Y, Pikarsky E, Angel P, Hess J. S100A8 and S100A9 are novel nuclear factor kappa B target genes during malignant progression of murine and human liver carcinogenesis. Hepatology. 2009;50:1251–1262. doi: 10.1002/hep.23099. [DOI] [PubMed] [Google Scholar]
- [89].Chaurand P, DaGue BB, Pearsall RS, Threadgill DW, Caprioli RM. Profiling proteins from azoxymethane-induced colon tumors at the molecular level by matrix-assisted laser desorption/ionization mass spectrometry. Proteomics. 2001;1:1320–1326. doi: 10.1002/1615-9861(200110)1:10<1320::AID-PROT1320>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- [90].Femia AP, Luceri C, Toti S, Giannini A, Dolara P, Caderni G. Gene expression profile and genomic alterations in colonic tumours induced by 1,2-dimethylhydrazine (DMH) in rats. BMC Cancer. 2010;10:194. doi: 10.1186/1471-2407-10-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Stulik J, Koupilova K, Osterreicher J, Knizek J, Macela A, Bures J, Jandik P, Langr F, Dedic K, Jungblut PR. Protein abundance alterations in matched sets of macroscopically normal colon mucosa and colorectal carcinoma. Electrophoresis. 1999;20:3638–3646. doi: 10.1002/(SICI)1522-2683(19991201)20:18<3638::AID-ELPS3638>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- [92].Stulik J, Osterreicher J, Koupilova K, Knizek, Macela A, Bures J, Jandik P, Langr F, Dedic K, Jungblut PR. The analysis of S100A9 and S100A8 expression in matched sets of macroscopically normal colon mucosa and colorectal carcinoma: the S100A9 and S100A8 positive cells underlie and invade tumor mass. Electrophoresis. 1999;20:1047–1054. doi: 10.1002/(SICI)1522-2683(19990101)20:4/5<1047::AID-ELPS1047>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- [93].Arai K, Takano S, Teratani T, Ito Y, Yamada T, Nozawa R. S100A8 and S100A9 overexpression is associated with poor pathological parameters in invasive ductal carcinoma of the breast. Curr Cancer Drug Targets. 2008;8:243–252. doi: 10.2174/156800908784533445. [DOI] [PubMed] [Google Scholar]
- [94].Goncalves A, Charafe-Jauffret E, Bertucci F, Audebert S, Toiron Y, Esterni B, Monville F, Tarpin C, Jacquemier J, Houvenaeghel G, Chabannon C, Extra JM, Viens P, Borg JP, Birnbaum D. Protein profiling of human breast tumor cells identifies novel biomarkers associated with molecular subtypes. Mol Cell Proteomics. 2008;7:1420–1433. doi: 10.1074/mcp.M700487-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Moon A, Yong HY, Song JI, Cukovic D, Salagrama S, Kaplan D, Putt D, Kim H, Dombkowski A, Kim HR. Global gene expression profiling unveils S100A8/A9 as candidate markers in H-ras-mediated human breast epithelial cell invasion. Mol Cancer Res. 2008;6:1544–1553. doi: 10.1158/1541-7786.MCR-08-0189. [DOI] [PubMed] [Google Scholar]
- [96].Li C, Zhang F, Lin M, Liu J. Induction of S100A9 gene expression by cytokine oncostatin M in breast cancer cells through the STAT3 signaling cascade. Breast Cancer Res Treat. 2004;87:123–134. doi: 10.1023/B:BREA.0000041594.36418.f6. [DOI] [PubMed] [Google Scholar]
- [97].Yui S, Mikami M, Yamazaki M. Induction of apoptotic cell death in mouse lymphoma and human leukemia cell lines by a calcium-binding protein complex, calprotectin, derived from inflammatory peritoneal exudate cells. J Leukoc Biol. 1995;58:650–658. doi: 10.1002/jlb.58.6.650. [DOI] [PubMed] [Google Scholar]
- [98].Yui S, Mikami M, Yamazaki M. Purification and characterization of the cytotoxic factor in rat peritoneal exudate cells: its identification as the calcium binding protein complex, calprotectin. J Leukoc Biol. 1995;58:307–316. doi: 10.1002/jlb.58.3.307. [DOI] [PubMed] [Google Scholar]
- [99].Li C, Chen H, Ding F, Zhang Y, Luo A, Wang M, Liu Z. A novel p53 target gene, S100A9, induces p53-dependent cellular apoptosis and mediates the p53 apoptosis pathway. Biochem J. 2009;422:363–372. doi: 10.1042/BJ20090465. [DOI] [PubMed] [Google Scholar]
- [100].Lin J, Yang Q, Yan Z, Markowitz J, Wilder PT, Carrier F, Weber DJ. Inhibiting S100B restores p53 levels in primary malignant melanoma cancer cells. J Biol Chem. 2004;279:34071–34077. doi: 10.1074/jbc.M405419200. [DOI] [PubMed] [Google Scholar]
- [101].Maru DM, Singh RR, Hannah C, Albarracin CT, Li YX, Abraham R, Romans AM, Yao H, Luthra MG, Anandasabapathy S, Swisher SG, Hofstetter WL, Rashid A, Luthra R. MicroRNA-196a is a potential marker of progression during Barrett’s metaplasia-dysplasia-invasive adenocarcinoma sequence in esophagus. Am J Pathol. 2009;174:1940–1948. doi: 10.2353/ajpath.2009.080718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Luo A, Kong J, Hu G, Liew CC, Xiong M, Wang X, Ji J, Wang T, Zhi H, Wu M, Liu Z. Discovery of Ca2+-relevant and differentiation-associated genes downregulated in esophageal squamous cell carcinoma using cDNA microarray. Oncogene. 2004;23:1291–1299. doi: 10.1038/sj.onc.1207218. [DOI] [PubMed] [Google Scholar]
- [103].Ji J, Zhao L, Wang X, Zhou C, Ding F, Su L, Zhang C, Mao X, Wu M, Liu Z. Differential expression of S100 gene family in human esophageal squamous cell carcinoma. J Cancer Res Clin Oncol. 2004;130:480–486. doi: 10.1007/s00432-004-0555-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Taccioli C, Wan SG, Liu CG, Alder H, Volinia S, Farber JL, Croce CM, Fong LY. Zinc replenishment reverses overexpression of the proinflammatory mediator S100A8 and esophageal preneoplasia in the rat. Gastroenterology. 2009;136:953–966. doi: 10.1053/j.gastro.2008.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Hermani A, Hess J, De Servi B, Medunjanin S, Grobholz R, Trojan L, Angel P, Mayer D. Calcium-binding proteins S100A8 and S100A9 as novel diagnostic markers in human prostate cancer. Clin Cancer Res. 2005;11:5146–5152. doi: 10.1158/1078-0432.CCR-05-0352. [DOI] [PubMed] [Google Scholar]
- [106].Hermani A, De Servi B, Medunjanin S, Tessier PA, Mayer D. S100A8 and S100A9 activate MAP kinase and NF-kappaB signaling pathways and trigger translocation of RAGE in human prostate cancer cells. Exp Cell Res. 2006;312:184–197. doi: 10.1016/j.yexcr.2005.10.013. [DOI] [PubMed] [Google Scholar]
- [107].Ludwig S, Stephan C, Lein M, Loening SA, Jung K. S100A8, S100A9, and the S100A8/A9 complex in circulating blood are not associated with prostate cancer risk-A re-evaluation study. Prostate. 2007;67:1301–1307. doi: 10.1002/pros.20619. [DOI] [PubMed] [Google Scholar]
- [108].Pili R, Haggman M, Stadler WM, Gingrich JR, Assikis VJ, Bjork A, Nordle O, Forsberg G, Carducci MA, Armstrong AJ. Phase II Randomized, Double-Blind, Placebo-Controlled Study of Tasquinimod in Men With Minimally Symptomatic Metastatic Castrate-Resistant Prostate Cancer. J Clin Oncol. 2011;29:4022–4028. doi: 10.1200/JCO.2011.35.6295. [DOI] [PubMed] [Google Scholar]
- [109].Dalrymple SL, Becker RE, Zhou H, Deweese TL, Isaacs JT. Tasquinimod prevents the angiogenic rebound induced by fractionated radiation resulting in an enhanced therapeutic response of prostate cancer xenografts. Prostate. 2012;72(6):638–48. doi: 10.1002/pros.21467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Martin-Oliva D, Aguilar-Quesada R, O’Valle F, Munoz-Gamez JA, Martinez-Romero R, Del Moral R. Garcia, de Almodovar J.M. Ruiz, Villuendas R, Piris MA, Oliver FJ. Inhibition of poly(ADP-ribose) polymerase modulates tumor-related gene expression, including hypoxia-inducible factor-1 activation, during skin carcinogenesis. Cancer Res. 2006;66:5744–5756. doi: 10.1158/0008-5472.CAN-05-3050. [DOI] [PubMed] [Google Scholar]
- [111].Ehlermann P, Eggers K, Bierhaus A, Most P, Weichenhan D, Greten J, Nawroth PP, Katus HA, Remppis A. Increased proinflammatory endothelial response to S100A8/A9 after preactivation through advanced glycation end products. Cardiovasc Diabetol. 2006;5:6. doi: 10.1186/1475-2840-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].McCormick MM, Rahimi F, Bobryshev YV, Gaus K, Zreiqat H, Cai H, Lord RS, Geczy CL. S100A8 and S100A9 in human arterial wall. Implications for atherogenesis. J Biol Chem. 2005;280:41521–41529. doi: 10.1074/jbc.M509442200. [DOI] [PubMed] [Google Scholar]
- [113].Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera--a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- [114].Teigelkamp S, Bhardwaj RS, Roth J, Meinardus-Hager G, Karas M, Sorg C. Calcium-dependent complex assembly of the myeloic differentiation proteins MRP-8 and MRP-14. J Biol Chem. 1991;266:13462–13467. [PubMed] [Google Scholar]