Abstract
Background
Behavioral strategies are recommended for menopausal symptoms, but little evidence exists regarding efficacy.
Purpose
Describe design and methodology of a randomized controlled 3 by 2 factorial trial of yoga, exercise and omega-3 fatty acids.
Methods
Women from three geographic areas with a weekly average of ≥14 hot flashes/night sweats, who met exclusion/inclusion criteria, were randomized to 12 weeks of: 1) yoga classes and daily home practice; 2) supervised, facility-based aerobic exercise training; or 3) usual activity. Women in each arm were further randomized to either omega-3 supplement or placebo. Standardized training, on-going monitoring, and site visits were adopted to ensure consistency across sites and fidelity to the intervention. Participant adherence to the intervention protocol was monitored continuously, and retention was actively encouraged by staff. Information on adverse events was systematically collected.
Results
Of 7,377 women who responded to mass mailings, 355 (4.8%) were randomized; mean age was 54.7 (sd=3.7), 26.2% were African American, 81.7% were post-menopausal, and mean baseline frequency of daily hot flashes/night sweats was 7.6 (sd=3.8). Adherence of ≥ 80% was 59% for yoga, 77% for exercise training, and 80% for study pills. Final week 12 data were collected from 95.2%
Conclusions
Conducting a multi-site, multi-behavioral randomized trial for menopausal symptoms is challenging but feasible. Benefits included cost-effective study design, centralized recruitment, and methodologic standardization.
Keywords: vasomotor symptoms, randomized controlled trial, yoga, exercise, omega-3 fatty acids, factorial design
Over 38 million U.S. women will transition from pre- to post-menopause during the next decade, and 30–80% of these women will experience hot flashes/night sweats, collectively referred to as vasomotor symptoms (VMS) (1). About 10% consider their symptoms severe (1), 44–82% report that VMS interfere with sleep, mood, and daily functioning (2), and about 60% seek medical advice and treatment for relief of menopausal symptoms (3). For many years, hormone therapy (HT, either estrogen plus progesterone, or estrogen alone for women without a uterus) was the standard of care and was generally effective for reducing the frequency and severity of hot flashes/night sweats. However, findings from the Women’s Health Initiative (WHI) published in 2002 (4) showed an increase in the risk of breast cancer, thromboembolism, myocardial infarction, stroke, gall bladder disease, and dementia in women randomized to combined HT (estrogen and progesterone). Subsequently, HT use declined dramatically (5, 6), and the on-going search for safe and effective alternative treatments took on added urgency.
To encourage that search, the National Institutes of Health awarded funding in 2008 to the MsFLASH (Menopause Strategies: Finding Lasting Answers for Symptoms and Health) Research Network to conduct a series of randomized controlled trials of innovative treatments for VMS. The MsFLASH Network is a collaborative project that includes a data coordinating center (DCC), located at the Fred Hutchison Cancer Research Center in Seattle, five clinical sites, located at the Group Health Research Institute in Seattle, Kaiser Permanente Northern California in Oakland, Indiana University in Indianapolis, University of Pennsylvania Medical School in Philadelphia, and the Massachusetts General Hospital/Harvard University in Boston, and NIH program officers from the National Institute on Aging (NIA), the Eunice Kennedy Shriver National Institute of Child Health and Development (NICHD), the National Center for Complementary and Alternative Medicine (NCCAM), and the Office of Research on Women’s Health (ORWH). The MsFLASH investigators have already published positive results from the first trial, which tested the efficacy of escitalopram vs. placebo (7). A second trial, known as Interventions for Menopausal Symptoms: the 3 by 2 Factorial Design Examining Yoga, Exercise and Omega-3 Supplementation, has recently been completed and primary results of that trial are expected shortly in a series of three published manuscripts. This trial was designed specifically to test the efficacy of a low risk nutritional supplement and two behavioral interventions, which many patients prefer as initial treatment options. The purpose of the current report is to describe the rationale for the 3 by 2 study design, to discuss issues relevant to intervention-specific methodology and implementation, and to present data on recruitment, eligibility, and baseline characteristics. We highlight issues faced in the implementation of this complex and challenging multi-behavioral, multi-site protocol.
Methods
Overview of Study Design
This study was a 12-week, randomized controlled, 3 by 2 factorial trial that evaluated the following interventions: yoga, aerobic exercise, and omega-3 fish oil supplementation. With this design, 30% of the women were randomized to yoga, 30% to exercise, and 40% to usual care. Within each of these groups, women were further randomized in a 1:1 ratio to active omega-3 supplementation capsules or matching placebo capsules three times a day. The primary aims of the trial were to compare changes in self-reported frequency, and bother of vasomotor symptoms in each specific intervention arm to those in the control group.
The sample size of each cell provided 90% statistical power to test hypotheses related to the efficacy of each intervention vs. placebo/usual activity on the primary outcomes of VMS frequency and bother. For each intervention, a Type I error rate of 2.5% was assumed to control for testing of two primary outcomes. The study did not provide sufficient power to detect small or moderate differences in efficacy between yoga and exercise, or to accurately estimate potential additive effects of the behavioral interventions and omega-3, both of which would have required a much larger sample. Nevertheless, those hypotheses can be explored in secondary analyses in a more rigorous fashion through the 3 by 2 factorial design than would be permitted by separate trials of each intervention.
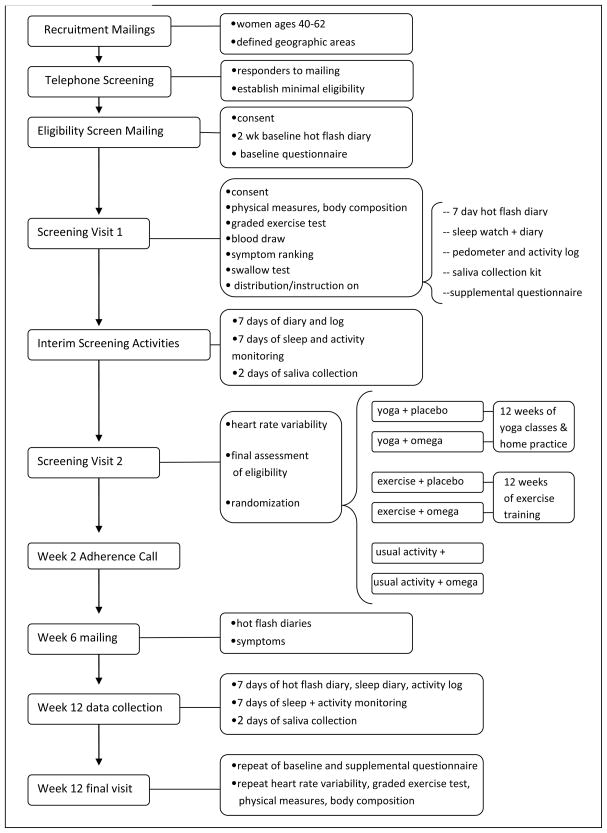
Figure 1 summarizes the basic design of the study. Briefly, following telephone screening of responders to mass mailings, women completed two weeks of a daily hot flash diary and a baseline questionnaire. They then attended a clinic examination that included a blood draw, blood pressure and body size measurements, and a graded exercise treadmill test. A week later, after completing an additional one-week hot flash diary, questionnaires that included items on outcome expectancy, one week of monitoring for sleep (actigraphy and diary) and usual activity (pedometry), and collection of saliva samples for cortisol measurement four times a day on two consecutive days, women returned to the clinic for randomization and a measure of heart rate variability. An interim 7-day hot flash diary was completed during week 6, and a final diary in week 12, the last week of the intervention period. At the end of Week 12, women returned to the clinic where baseline measurements were repeated.
Figure 1.
Study design of the MsFLASH 3 by 2 factorial intervention trial of yoga, exercise and omega 3 fatty acids
Rationale for Selected Interventions
Testing the efficacy of behavioral and complementary and alternative medicine (CAM) treatments was a priority for the MsFLASH Network because these treatments are widely available, acceptable, low risk, and provide many additional health benefits. The rationale for each intervention was based on existing evidence and potential biological mechanisms of efficacy. In the case of exercise, conflicting results from both observational studies and intervention trials suggested the need for a rigorous, adequately powered randomized trial (8). The evidence for the efficacy of yoga as a treatment for VMS was based on the results of several prior studies that also indicated that yoga practice contributed to positive changes in total menopausal symptoms, psychological symptoms, sleep, and quality of life (9–12). Hypothesized mechanisms for the efficacy of yoga included positive changes in stress reactivity and the balance of sympathetic to parasympathetic output (13). Physiological adaptations to exercise training, including decreased resting heart (14) and improved heart rate variability (15,16) suggested similar biological mechanisms might apply to that intervention as well. The evidence for omega 3 supplementation was a recent placebo-controlled study that demonstrated significantly more efficacy than placebo in the treatment of hot flashes in women who were experiencing psychological distress at baseline (17,18), as well as on studies showing benefits for cardiovascular health (19,20).
Rationale for Measured Outcomes and Other Variables
Given the high prevalence of VMS during the menopausal transition, the primary outcomes of interest were hot flash frequency and bother. Secondary outcomes included measures of insomnia and quality of sleep, anxiety, and depression, which are also common complaints of midlife women. Other outcomes of interest included sexual function, pain and health- and menopause-related quality of life.
A graded exercise test was conducted both to ensure safety of exercise training in those randomized to the exercise arm, and to provide a measure of change in aerobic fitness, an important indicator of the effectiveness of the training. Body composition was measured in order to evaluate whether changes in body fat mediated any observed effect of the exercise intervention. Similarly, heart rate variability and cortisol, assayed from saliva specimens, were assessed to allow for exploration of potential physiological mechanisms that might account for efficacy of the yoga intervention, as well as the exercise intervention. Pedometer recordings (model #NL-1000, New Lifestyles, Inc., Lee’s Summit, MO) for 7 days at baseline and week 12 were obtained in order to account for changes in regular physical activity behavior over the course of the intervention. Finally, the swallow test was conducted to ensure that participants would be able to swallow the study pills,
Sample Selection and Recruitment
The MsFLASH Research Network placed a priority on maintaining consistent inclusion/exclusion criteria across all of the Network trials in order to ensure comparability. The common inclusion criteria were 40–62 years of age, post-menopausal or in the late menopausal transition, defined as having skipped 2 or more menstrual cycles with an amenorrhea interval ≥60 days in the past 12 months, in good general health as established by blood pressure, heart rate, and medical history, having frequent VMS (at least 14 per week for 3 weeks, with a frequency in week 3 at least 50% of that in the first two weeks), rating the VMS as at least moderately bothersome or severe (at least 4 days or nights per week for 3 weeks), and providing a signed informed consent. Common exclusion criteria included use of hormone therapy or hormonal contraception, use of any other prescription, over the counter or herbal therapy taken specifically for treatment of vasomotor symptoms, any severe or unstable medical or psychiatric illness, and inability or unwillingness to complete study procedures or interventions. A number of other exclusion criteria specific to one or more of the intervention arms were defined for the 3 by 2 trial and are summarized in Table 1.
Table 1.
Exclusion criteria specific to the MsFLASH 3 by 2 factorial intervention trial
| Criterion | Intervention Arm | Rationale |
|---|---|---|
| Body mass index >37 | Exercise | Rapid progression of exercise prescription not appropriate for heavier women |
| Severe uncorrected hearing or vision problems. | Yoga | Need to hear and see yoga instructor |
| Current, regular use (≥3 times/week) of anti-coagulants | Omega-3 | Potential omega-3 supplement interaction |
| Physical limitations, such as severely limited mobility, severe, recent or chronic back problems, and other musculoskeletal problems that limit the ability to walk on a treadmill or ride a stationary bicycle. | Exercise, yoga | Safety considerations and ensure ability to participate in yoga or exercise interventions, as designed |
| Presence of any absolute contraindications to exercise testing and training, as defined by the American College of Sports Medicinea | Exercise | Safety considerations |
| Practiced or attended yoga, tai chi, qi gong, or meditation more than one time per week on average in prior 3 months | Yoga | Maximize potential effect of yoga intervention |
| Participate in aerobic exercise more than 30 minutes a day on 3 or more days per week in prior 3 months | Exercise | Maximize potential effect of exercise intervention |
| Inability to achieve 85% of heart rate reserve (HRR) on graded exercise treadmill test. | Exercise | Provide standard measure of fitness and ensure ability to comply with exercise prescription |
| Allergy or sensitivity to fish | Omega-3 | Contents of omega-3 supplement |
| Severe allergy to soy | Omega-3 | Contents of omega-3 supplement |
| Currently eating 4 or more servings of fish per week. | Omega-3 | Maximize potential effect of omega-3 |
| Currently taking an omega-3 fish oil supplement and unwilling to stop | Omega-3 | Maximize potential effect of omega-3 |
absolute contraindications include resting ECG evidence of significant ischemia, unstable angina, and uncntrolled cardiac dysrhythmias; for complete list of absolute and relative contraindications, see Reference #24
Informed consent was obtained at each stage in the screening and enrollment process: verbal consent for the telephone screen, implied consent based on completion and return of the first hot flash diary and baseline questionnaire, and signed and witnessed consent at the first in-person screening visit. Similarly, women were informed of their eligibility status at each stage: over the telephone for the initial screening, by letter or phone call before an in-person visit was scheduled, and in person for final eligibility.
A centralized recruitment effort was undertaken with one site (NCKP) handling mass mailings to age-eligible women in defined geographic areas, utilizing both health plan membership records and commercial mailing lists, and another site (GHRI) handling initial telephone screening. This provided an accurate denominator for calculating response and eligibility rates and a systematic accounting of reasons for ineligibility.
Design and Delivery of Interventions
The yoga intervention consisted of the class series, “Yoga for Mid-Life Women”, which focused on relaxing and restorative poses, sequenced according to the principles of Viniyoga (21). The series was developed by a leading yoga instructor from the Seattle area (RR) and based on a review of the literature coupled with feedback from two of the investigators from the Seattle clinical site (CBLF and KJS). The intervention was further reviewed by a yoga expert from NCCAM. The practice emphasized special “cooling” breathing exercises, three groups of postures believed to be useful for relieving VMS (9,10,22,23), and Yoga Nidra, a type of visualization, meditative yoga practice. All yoga classes were taught by trained and experienced yoga instructors, whose expertise for teaching this form of yoga was further evaluated by the lead yoga instructor.
Participants assigned to the Yoga intervention were asked to attend one weekly, 90 minute class for 12 weeks, joining an on-going class as they were recruited and randomized. All classes started with a breathing exercise, followed by 6–9 postures, such as mountain pose, standing forward bend, lateral bends and twists, cobra lifts, supported inversion, and corpse pose and then concluded with a deep relaxation featuring Yoga Nidra. Three different sequences of poses were selected, which were then rotated sequentially so that each woman could attend 4 classes of each sequence. In addition, participants were asked to practice at home for 20 minutes on all non-class days. Two types of home practice were recommended each week: a simplified class sequence including an introductory breathing exercise and postures (with handouts and a DVD to guide the practice) and a guided Yoga Nidra (provided on a CD). Women were asked to alternate these two practices every other day. Yoga equipment (bolsters, straps, blankets) was provided to ensure safe home practice.
The exercise intervention was individual, facility-based aerobic exercise training on a treadmill, stationary bicycle, or elliptical trainer performed three times a week for 12 weeks. Although designing the intervention as facility-based, rather than home-based, was less convenient for participants who were required to go to the facility at a specific time arranged with their trainers, it allowed for careful monitoring of actual exercise dose, an important feature of an efficacy trial. All women had the same progressive energy expenditure goal, relative to body weight, specifically, 4 kcals/kg in week 1, 8 kcals/kg in week 2, 12 kcals/kg in week 3, and 16 kcals/kg in weeks 4–12. For most women, this translated to an energy expenditure of about 1,000–1,500 kcal/week by week 4. The duration of each training session, typically, 40–60 minutes, was determined for each woman based on her weekly total energy expenditure goal and the workload required to achieve her prescribed target heart rate. Women were encouraged to train at 50–60% heart rate reserve (HRR) for the first month and then to increase intensity to 60–70% HRR, which translated to a target heart rate ranging from about 125 to 145, depending on age and resting heart rate. To ensure that they were exercising at their prescribed heart rates, heart rate monitors were worn during all training sessions. The exercise prescription was based on the need to allow sedentary women time to adapt to a training program (24) while still achieving the minimum level of physical activity recommended for health benefits (25,26).
Exercise trainers, who were educated in exercise science and certified as personal trainers by the American College of Sports Medicine or other similar organization, supervised each training session. Responsibilities included: 1) scheduling training sessions and maintenance of attendance logs; 2) monitoring and documenting exercise heart rates every 5–10 minutes; 3) reporting and responding to adverse events related to exercise; 4) adjusting the exercise prescriptions as necessary to accommodate changes in body weight or fitness level; and 5) providing support, encouragement, and advice about training issues or concerns.
The usual activity group was asked to follow their usual activity routine during the 12-week intervention period and not to start new yoga practice or exercise. At the completion of the 12-week intervention period, they were offered their choice of attendance at a yoga workshop or a one-month gym membership.
The omega-3 study supplement contained omega-3 fatty acids from fish oils (also described as ω-3, n-3, or polyunsaturated fatty acids or PUFAs, manufactured as EPA by Nordic Naturals, Watsonville, CA). Each gel capsule had a total omega-3 dosage of 615 mg, which included the two major omega-3 components of ethyl eicosapentaenoic acid (EPA; 425 mg) and docosahexaenoic acid (DHA; 100 mg). Vitamin E (15 IU), an antioxidant, was added to each gel capsule to prevent oxidation of the fish oil and preserve its freshness. Participants assigned to placebo took an identical gel capsule containing olive oil. Both gel capsules (placebo and omega-3) contained natural lemon oil and rosemary extract to enhance taste and freshness and to ensure that they had a similar taste. At randomization, 3 bottles of supplements were dispensed (all omega-3 or all placebo), and participants were instructed to take three capsules per day, approximately 1.8 g/day of omega-3, for 12 weeks.
Randomization and Blinding
Randomization was blocked on intervention arm and further stratified by study site. The participants and all clinical staff were blinded to supplement versus placebo assignment, and all clinical staff involved with data collection were also blinded to participants’ assignments to yoga, exercise, or usual activity.
Standardization of Intervention Delivery, Fidelity, and Quality Control
Several measures were adopted to ensure standardization of the study protocol and delivery of the interventions across the participating sites. The first involved centralized training for all staff having contact with participants. Two additional trainings were conducted, one in Oakland for the staff involved in exercise testing and the exercise intervention, and one by the developers of the yoga intervention who traveled to each site for a two day training of the yoga instructors. Detailed manuals of operations were developed for the delivery of each of the interventions that included: 1) the use of relevant study forms and equipment (e.g. heart rate monitors used for the exercise training); 2) the importance of standardization of all classes and approaches to exercise training; 3) safety; and 4) measures to encourage participant adherence. Mock yoga classes and exercise training sessions were also conducted. Yoga instructors were given training CDs, DVDs and handbooks that illustrated each of the poses and the different sequences of poses, as well as the scripts for the Yoga Nidra meditation. The exercise trainers were given detailed written instructions regarding the exercise prescription, the progression of the exercise dose, and the content of each training session. The importance of strict adherence to the intervention protocol was emphasized repeatedly during trainings.
Secondly, fidelity to the intervention was monitored throughout the trial. For the yoga intervention, a Yoga Fidelity Monitoring Form was developed that included the number of class participants, the starting and ending times of the class, whether the class sequence was followed as designed (i.e. order and repetitions of poses) and the time allotted to the introduction, yoga postures and Yoga Nidra practice. The form also asked whether or not the instructor embellished or gave inappropriate attributes to specific postures (e.g. this pose will help you cool down or improve your mood). The form was completed at each yoga class by an un-blinded staff member (not the yoga instructor) and was reviewed regularly by one investigator with yoga expertise at the Seattle site (KJS). In addition, the yoga instructors communicated weekly via email with the Seattle investigators to describe how classes were proceeding and whether they had any questions or concerns.
Fidelity to the exercise training sessions was monitored by the exercise supervisor at each site who observed each exercise trainer at least once a week leading a training session. A quality control check sheet was used to document that the trainer was following each step of the protocol as designed, and those check sheets were reviewed on a regular basis by the exercise supervisor (ALC) and PI at the Oakland site (BS). The trainers were also required to complete a training log for each session, on which they recorded the participant’s exercise heart rate from the heart rate monitor every 5–10 minutes, the mode, duration, and absolute intensity (i.e. workload) of exercise, a rating of perceived exertion, and the overall energy expenditure for the session (from the heart rate monitor). Data from the training logs were entered into the central study database, and summary reports were generated and reviewed regularly in order to monitor the extent to which the prescribed exercise dose was achieved. Finally, exercise trainers and supervisors from all sites, along with the investigators with expertise in exercise training at the Oakland site (BS and ALD), participated in regularly scheduled conference calls during which specific problems and questions were discussed and resolved.
For both exercise and yoga, a list of “Frequently Asked Questions” was compiled and distributed monthly that summarized the input from the yoga instructors and exercise trainers and was used to ensure a standardized approach to any issues that arose that had not been specified in the protocol. In addition, site visits were conducted that included observation, review of relevant charts and forms, and meetings with intervention staff and investigators. One investigator with yoga expertise from the Seattle site conducted the yoga site visits (CBLF), while the investigators and staff with exercise expertise from the Oakland site conducted the exercise site visits (BS, ALD, TEP, ALC).
Safety and Adverse Events
Although the risk of serious adverse events was judged to be very low for all three of the intervention arms, potential adverse events associated with yoga, exercise, and omega-3 fish oils were defined a priori, and classified by how frequently they were thought to occur. For example, muscle soreness or aches from yoga or exercise and fish oil aftertaste from omega-3 were considered common, while heart attack from exercise and increased bleeding time from omega 3 were considered very rare.
Measures adopted to minimize the occurrence of these and other adverse events associated with yoga and exercise included: 1) slow, steady progression and adherence to principles of exercise training, including warming up and cooling down; 2) monitoring of heart rate and signs and symptoms (e.g. dizziness, faintness, chest pain) by exercise trainers during the training sessions; 3) carefully selected and sequenced yoga poses; 4) use of instructional material (e.g. handouts, DVDs, CDs, and yoga equipment) for proper and safe yoga home practice; and 5) documented modification of exercise modality or yoga poses, based on health concerns of the participants.
To assist with safety monitoring of adverse events that might occur with yoga and exercise, participants were given an information sheet after randomization that described possible side effects and strategies for managing symptoms, and yoga instructors and exercise trainers regularly asked about potential adverse events at classes and training sessions. Similarly, all participants received an information sheet regarding safety and pill administration, and a 2-week follow-up phone call gave participants the opportunity to discuss their experience with and tolerance of the study pills. In addition, all participants were asked to call the study clinic promptly to report any side effects that were distressing to them, any hospitalization, surgery, or emergency room visit, any increased tendency to bleed or bruise easily, or a pregnancy. Finally, at the 12-week study visit, participants reported adverse events and completed a symptom checklist of specific expected side effects for the interventions.
Adherence and Retention
Prior to enrollment, all potential participants were asked whether they believed they could attend regularly scheduled yoga classes or training sessions. A negative response resulted in study exclusion. Because the likelihood of adherence to the intervention had to be established prior to randomization, women had to be generally available for both the yoga classes and the exercise training, even though they would be participating in only one (or neither) of these study activities.
Once a participant was enrolled, study staff maintained personal contact with the participant at regularly scheduled in-person visits and follow-up calls to encourage high levels of retention (i.e. participation in follow-up visits, accepting phone calls, and completing data collection) and adherence (i.e. taking the study pills; doing the exercise training at the assigned dose; attending yoga classes and practicing at home).
For the yoga arm, adherence was defined as attendance at weekly classes and consistency of home yoga practice. Class attendance was tracked on the Yoga Fidelity Monitoring Form described above; adherence to home practice was based on daily diaries that participants were asked to complete in which they recorded whether they had practiced yoga postures and/or Yoga Nidra.
Adherence to exercise was defined in terms of achievement of the prescribed exercise dose, including frequency (attendance at training sessions), intensity (time at target heart rate), and duration (total energy expenditure). To facilitate adherence, the intervention was designed as individual, supervised, facility-based training. However, the protocol allowed for some flexibility with the requirement that all of the exercise sessions be completed in the exercise facility with the trainer present. On a case-by-case basis, women who could not attend all of their scheduled exercise sessions were asked to exercise on their own. During the unsupervised sessions, women were asked to monitor their heart rates, either with a heart rate monitor or manually, and to record their heart rates and mode and duration of exercise on a form designed for that purpose, allowing capture of that data in the central study database.
Adherence to the omega-3 arm was determined by counting the unused pills contained in pill bottles returned at the final study visit
Results
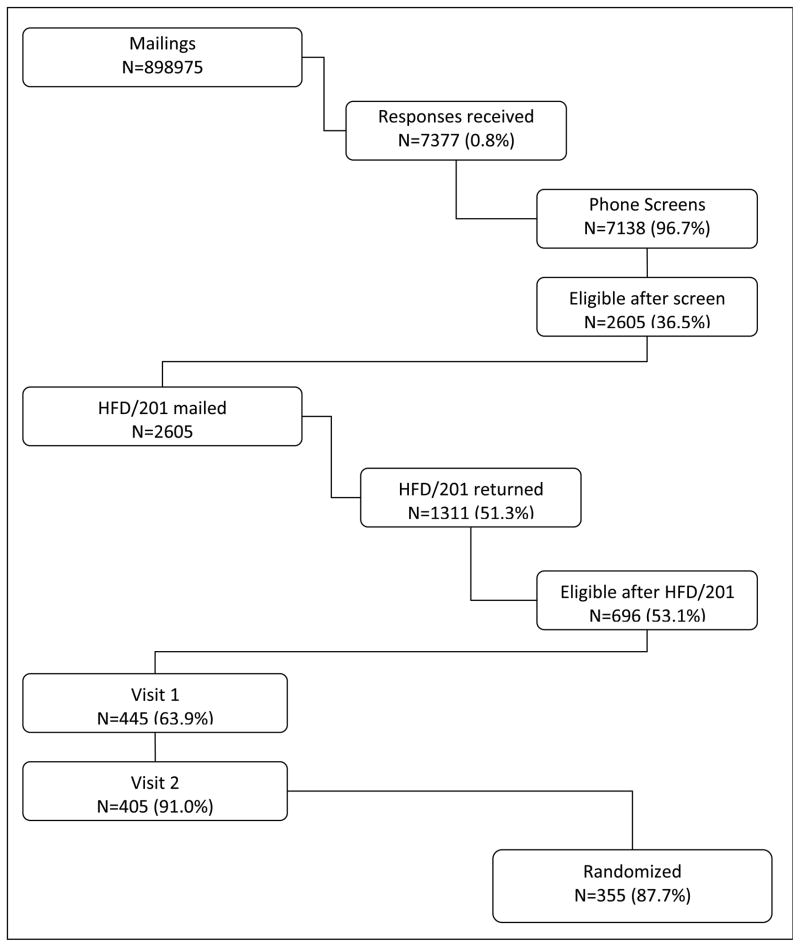
Recruitment was challenging. Figure 2 shows that only 355 out of the 7,377 (4.8%) of the women who responded to the mailing were randomized. An even smaller proportion of women (7,377 out of 898,975 or less than 1%) responded to the mailing.
Figure 2.
Eligibility rates for the MsFLASH 3 by 2 factorial intervention trial of yoga, exercise and omega 3 fatty acids
As shown in Table 2, rates of ineligibility were high. The most common reason for ineligibility was too few hot flashes/night sweats or too few VMS rated as bothersome (30.2%). In addition, a sizable proportion of women were ineligible due to medical conditions (18.7%). This included women who were using hormone preparations or other prescription or over the counter medications for hot flashes and those with any history of cardiovascular disease or uncontrolled metabolic disease. Some women were excluded because they already participated in regular aerobic exercise (11.2%), yoga or meditation (11.4%) or regularly took omega 3 supplements and were not willing to stop (9.0%). Few women were excluded because of too high a BMI (2.7%) or for musculoskeletal or mental health reasons (1.4% for each).
Table 2.
Reasons for exclusion
| Reason | Na (%) |
|---|---|
| Too few or not bothersome VMS | 1304 (30.2) |
| Prior intervention use | |
| Regular exercise | 484 (11.2) |
| Regular yoga practice, meditation | 490 (11.4) |
| Use of omega-3 | 389 (9.0) |
| Other exclusions/contraindications | |
| Medical/medication exclusionsb | 807 (18.7) |
| Musculoskeletal limitations | 60 (1.4) |
| Mental health exclusions | 59 (1.4) |
| Body Mass Index too high | 116 (2.7) |
| Refusalc | 2820 (39.5) |
239 women who responded to the mass mailing could not be screened, leaving 7,138 in the denominator; percentages for each reason for exclusion are based on 4,318 (those remaining after refusals)
medical/medication exclusions included the following: regular use of hormone preparations or other therapies (prescription, OTC or herbal) for hot flashes, regular use of anti-coagulants, uncontrolled hypertension, metabolic disease or seizure disorder, history of MI, angina, cardiovascular, cerebrovascular or pulmonary disease or events, liver or renal disease and chronic infectious disease
1133 refused during phone screen, 1494 refused after phone screen but prior to in-person visit, and 193 refused during clinic visits
The final study sample (N=355), mean age = 54.7 years (sd=3.7), had an average BMI of 27.0 (sd=4.4), 40.6% were overweight (BMI of 25–29), and 24.8% were obese (BMI of 30 or above) (Table 3). 26.2% were African American and 3% Hispanic, 9.0% were current smokers, and almost two thirds had a college degree (62.3%). Most of the women were post-menopausal (81.7%), and 61.9% rated their overall health as excellent or very good.
Table 3.
Characteristics of the study sample (N=355)
| Baseline Characteristics | N | (%) |
|---|---|---|
| Age at screening, mean (SD) | 54.7 (3.7) | |
| < 50 | 19 | 5.4 |
| 50 – 54 | 162 | 45.6 |
| 55 – 59 | 130 | 36.6 |
| 60+ | 44 | 12.4 |
|
| ||
| Race | ||
| White | 228 | 64.2 |
| African American | 93 | 26.2 |
| Other | 34 | 9.6 |
|
| ||
| Vasomotor symptoms, daily frequency, mean (SD) | 7.6 (3.8) | |
|
| ||
| BMI (m/kg2), mean (SD) | 27.0 (4.4) | |
| <25 | 123 | 34.6 |
| 25 – 29 | 144 | 40.6 |
| ≥ 30 | 88 | 24.8 |
|
| ||
| Education | ||
| School/training after high school | 112 | 31.5 |
| College graduate | 221 | 62.3 |
|
| ||
| Employment status | ||
| Retired or no employment | 49 | 13.8 |
| Full-time | 215 | 60.6 |
| Part-time | 52 | 14.6 |
| Homemaker | 13 | 3.7 |
| Other | 25 | 7.0 |
|
| ||
| Marital Status | ||
| Never married | 34 | 9.6 |
| Divorced | 76 | 21.4 |
| Widowed | 7 | 2.0 |
| Married/living with partner | 236 | 66.5 |
|
| ||
| Smoking | ||
| Never | 232 | 65.4 |
| Past | 89 | 25.1 |
| Current | 32 | 9.0 |
|
| ||
| Menopause status | ||
| Postmenopausal | 290 | 81.7 |
| Late transition | 58 | 16.3 |
| Early transition | 7 | 2.0 |
|
| ||
| Hysterectomy | 64 | 18.0 |
|
| ||
| Self-reported health | ||
| Excellent | 58 | 16.3 |
| Very Good | 162 | 45.6 |
| Good | 119 | 33.5 |
| Fair | 15 | 4.2 |
|
| ||
| Clinical Center | ||
| Indianapolis | 118 | 33.2 |
| Oakland | 110 | 31.0 |
| Seattle | 127 | 35.8 |
Table 4 presents the number of participants randomized into each cell of the 3 by 2 factorial design, and demonstrates that the randomization system was successful at achieving the desired proportions in the treatment and comparison arms.
Table 4.
Number (%) of participants in each cell of the 3×2 Factorial Design
| Exercise | Yoga | Usual Activity | Total | |
|---|---|---|---|---|
| Omega-3 | 54 (15) | 52 (15) | 71 (20) | 177 (50) |
| Placebo | 52 (15) | 55 (15) | 71 (20) | 178(50) |
| Total | 106 (30) | 107 (30) | 142(40) | 355 |
Retention and adherence were generally high, although adherence to yoga was somewhat more modest than adherence to exercise. Completion of the week 12 hot flash diary, the primary measure of retention, was 95.2% across all study participants. As shown in Table 5, there was variability in adherence in all intervention arms, but adherence of 80%, defined in terms of completion of training sessions for exercise, class attendance and recording of home practice for yoga, and pill counts for omega 3, or more was achieved by 59% of those in yoga, 80% of those in omega 3 and 77% of those in exercise.
Table 5.
Adherencea by Intervention Arm
| <50% | 50–<80% | ≥80% | |
|---|---|---|---|
| Exercise (N=106) | 11 (10) | 13 (12) | 82 (77) |
| Omega 3 (N=355) | 50 (14) | 20 (6) | 285 (80) |
| Yoga (N=107) | 13 (12) | 31 (29) | 63 (59) |
Adherence defined as: a) % training sessions completed out of 3 expected per week for exercise; b) % pills taken for Omega 3; and c) % classes and practices completed out of 4 expected per week for yoga
Fidelity to the exercise and yoga interventions was generally consistent. Although most women in the exercise arm (77%) substituted at least one home-based training session for the supervised session, half of those women (51%, N=41) trained on their own for no more than 3 out of the 36 sessions. Only 2% performed home-based training more than half of the time. Trainers completed training logs for 100% of the sessions they supervised. Out of 290 total yoga classes, 231 (80%) were audited using the fidelity checklist described above, and of those, only two classes deviated from the protocol, one in which an alternate week’s series was taught, and the other in which, one pose was omitted. In addition, only five out of 216 monitored yoga nidras mistakenly used a substitute script. Eighty nine percent of the women in the yoga intervention turned in more than 50% of their yoga diaries, documenting their home practice.
Two reports of serious adverse events were reviewed by the MsFLASH Data and Safety Monitoring Board, and both were determined to be unrelated to the study interventions. One was a two night hospitalization for pneumonia, and the other was a recurrence of a colon cancer.
Discussion
As described above, the design, methods and implementation of this multi-site, multi-behavioral randomized trial of treatments of vasomotor symptoms posed significant challenges. The large pool of women required to achieve the desired, relatively modest, sample size suggests that this type of trial could not be conducted at a single geographic site. On the other hand, the need to conduct the trial at multiple sites required careful and on-going attention to standardization of the study protocol.
Although the low response rate to the study mailing might suggest a limited need for or interest in this type of trial, the more likely interpretation relates to questions of the prevalence of VMS at any specific point in time, the reticence to participate in clinical trials in general, and the time commitment and general inconvenience required of the specific study interventions and the need to be available at pre-determined times, days, and places. It could also be that the women most interested in participating in the 3 by 2 trial were those most likely to be practicing a healthy lifestyle that already included regular aerobic exercise or yoga practice.
On the other hand, many demographic, psychosocial, and environmental factors contribute to an individual’s behavior and to an individual’s readiness to change that behavior (27–29). For many mid-life women, who are frequently balancing work, family, and community involvement, all while experiencing the physiological and psychological changes that accompany the menopause transition, the idea of adopting a new health behavior may simply demand more effort than they can spare. The factorial design, while efficient in terms of resources and statistical power, could have compounded this situation by requiring participants to agree to randomization to either exercise or yoga or no change. Requiring that inclusion criteria for all three interventions be met limited participation further since women who might have been eligible for one or even two of the interventions were excluded entirely.
On the other hand, a notable strength of the 3 by 2 study design was that all women received an intervention (yoga, exercise, and/or study pill). Providing all enrolled women with the active omega-3 supplement or placebo addressed the question of treatment expectancy, which can obviate the need to control for attention (30,31). A criticism frequently directed at randomized trials of behavioral interventions is that the attention paid to the experimental group, if not equal to the attention given to the control group,, could account for any observed treatment effect, rather than the behavior itself (30,31). However, the MsFLASH 3 by 2 trial was purposely designed to control for expectancy, rather than attention. The rationale for this decision was that the attention needed to successfully adopt a behavior such as regular aerobic exercise or a yoga practice is, in many ways, integral to the behavior. Ensuring that all groups received a treatment with a reasonable expectation of efficacy, rather than providing equal attention, was considered the best way to control for the anticipated placebo response. Nevertheless, in this type of a multi-site behavioral trial, the attention inherent in the delivery of the intervention had to be consistent across sites, both in quantity and quality. As described earlier, this necessitated the adoption of several strategies designed to ensure standardization and achieve that consistency.
Despite some of the challenges the factorial design posed in terms of recruitment, it was a more cost efficient approach than conducting separate trials to test each intervention against usual care or placebo. It also allows for side-by-side comparisons of each behavioral intervention against the same usual care comparison group.
Notwithstanding the challenges and complexities of the MsFLASH 3 by 2 trial, the study was brought to a successful conclusion: the desired sample size was recruited, reasonable standardization of intervention delivery and fidelity to the intervention were achieved, retention and adherence were acceptable, and safety of the study participants was maintained. Given the rigor of the study design and methodology, the findings from this trial should have a high degree of validity, applicability, and relevance to the many women who seek behavioral approaches to the management of menopausal symptoms, as well as to the clinicians who provide them with advice and treatment.
Acknowledgments
This study was supported by a cooperative agreement issued by the National Institute of Aging (NIA), in collaboration with the Eunice Kennedy Shriver National Institute of Child Health and Development (NICHD), the National Center for Complementary and Alternative Medicine (NCCAM) and the Office of Research and Women’s Health (ORWH), and grants U01 AG032656, U01AG032659, U01AG032669, U01AG032682, U01AG032699, U01AG032700 from the NIA. At the Indiana University site, the project was funded in part with support from the Indiana Clinical and Translational Sciences Institute, funded in part by grant UL1 RR025761 from the National Institutes of Health, National Center for Research Resources, Clinical and Translational Sciences Award.
The authors wish to acknowledge Lynn Fleckenstein, MPH, from the Data Coordinating Center for her overall project coordination, Teresa Picchi, RN, MNS, the project manager at the Oakland site, for her overall leadership of the exercise testing, Adrienne Castillo, RD, MS, the intervention supervisor at the Oakland site, for her overall leadership of the exercise intervention, and Robin Rothenberg from Seattle for developing and overseeing the yoga practice.
The authors also wish to thank all of the study staff and the participants for their contribution to this trial.
ROLE OF SPONSORS:
NIH staff critically reviewed the study protocol and drafts of the manuscript prior to journal submission.
Footnotes
Clinical Trials.gov Identifier: NCT01178892
AUTHOR CONTRIBUTIONS:
All authors made substantial contributions to the study and this manuscript. None were compensated for the manuscript preparation.
DISCLOSURES:
Drs. Newton and Reed reported research support from Otsuka Pharmaceutical Co., Ltd.
Dr. Cohen reported research support from Astra-Zeneca Pharrmaceuticals, Bayer HealthCare Pharmaceuticals, Bristol-Myers Squibb, Cephalon, Inc., Forest Laboratories, Inc., GlaxoSmithKline, Ortho-McNeil Janssen; Pfizer Inc., and Sunovion Pharmaceuticals, Inc. and has served as an advisor/consultant to Eli Lilly & Company, Noven Pharmaceuticals, and PamLab LLC.
Dr. Freeman has receivec research support from Forest, Lilly, and GlaxoSmithKline, and has served on an Advisory Board for Bristol Myers Squibb, Otsuka, Lundbeck/Takeda, and as a consultant for PamLab. She has received a stipend for medical editing for DSM nutrionals.
Dr. Ensrud serves as a consultant on a Data Monitoring Committee for Merck, Sharp & Dohme.
All other authors have no disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Williams RE, Kalilani L, DiBenedetti DB, et al. Frequency and severity of vasomotor symptoms among peri- and postmenopausal women in the United States. Climacteric. 2008;11:32–43. doi: 10.1080/13697130701744696. [DOI] [PubMed] [Google Scholar]
- 2.Williams RE, Levine KB, Kalilani L, Lewis J, Clark RV. Menopause-specific questionnaire assessment in US population-based study shows negative impact on health-related quality of life. Maturitas. 2009;62:153–159. doi: 10.1016/j.maturitas.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 3.Williams RE, Kalilani L, DiBenedetti DB, Zhou X, Fehnel SE, Clark RV. Healthcare seeking and treatment for menopausal symptoms in the United States. Maturitas. 2007;58:348–358. doi: 10.1016/j.maturitas.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 5.Haas JS, Kaplan CP, Gerstenberger EP, Kerlikowske K. Changes in the use of postmenopausal hormone therapy after the publication of clinical trial results. Ann Intern Med. 2004;140:184–188. doi: 10.7326/0003-4819-140-3-200402030-00009. [DOI] [PubMed] [Google Scholar]
- 6.Tsai SA, Stefanick ML, Stafford RS. Trends in menopausal hormone therapy use of US office-based physicians, 2000–2009. Menopause. 2011;18:385–392. doi: 10.1097/gme.0b013e3181f43404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freeman EW, Guthrie KA, Caan B, et al. Efficacy of escitalopram for hot flashes in healthy menopausal women: a randomized controlled trial. JAMA. 2011;305:267–274. doi: 10.1001/jama.2010.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sternfeld B, Dugan S. Physical activity and health during the menopausal transition. Obstet Gynecol Clin North Am. 2011;38:537–566. doi: 10.1016/j.ogc.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Booth-LaForce C, Thurston RC, Taylor MR. A pilot study of a Hatha yoga treatment for menopausal symptoms. Maturitas. 2007;57:286–295. doi: 10.1016/j.maturitas.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 10.Cohen BE, Kanaya AM, Macer JL, Shen H, Chang AA, Grady D. Feasibility and acceptability of restorative yoga for treatment of hot flushes: a pilot trial. Maturitas. 2007;56:198–204. doi: 10.1016/j.maturitas.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Elavsky S, McAuley E. Physical activity and mental health outcomes during menopause: a randomized controlled trial. Ann Behav Med. 2007;33:132–142. doi: 10.1007/BF02879894. [DOI] [PubMed] [Google Scholar]
- 12.Chattha R, Raghuram N, Venkatram P, Hongasandra NR. Treating the climacteric symptoms in Indian women with an integrated approach to yoga therapy: a randomized control study. Menopause. 2008;15:862–870. doi: 10.1097/gme.0b013e318167b902. [DOI] [PubMed] [Google Scholar]
- 13.Kronenberg F. Hot flashes: epidemiology and physiology. Ann NY Acad Sci. 1990;592:52–86. doi: 10.1111/j.1749-6632.1990.tb30316.x. [DOI] [PubMed] [Google Scholar]
- 14.Astrand PO, Rodahl K. Textbook of Work Physiology. 3. New York: McGraw-Hill Company; 1986. [Google Scholar]
- 15.Davy KP, Miniclier NL, Taylor JA, Stevenson ET, Seals DR. Elevated heart rate variability in physically active postmenopausal women: a cardioprotective effect? Am J Physiol. 1996;271:H455–H460. doi: 10.1152/ajpheart.1996.271.2.H455. [DOI] [PubMed] [Google Scholar]
- 16.Davy KP, Willis WL, Seals DR. Influence of exercise training on heart rate variability in post-menopausal women with elevated arterial blood pressure. Clin Physiol. 1997;17:31–40. doi: 10.1046/j.1365-2281.1997.01010.x. [DOI] [PubMed] [Google Scholar]
- 17.Lucas M, Asselin G, Merette C, et al. Effects of an enriched eicosapentaenoic acid (E-EPA) omega-3 fatty acid supplement on hot flashes among midlife women: A double-blind, placebo-controlled, randomized clinical trial [abstract]Lucas M, Asselin G, Merette C, et al. Menopause. 2012;14:1109. [Google Scholar]
- 18.Campagnoli C, Abba C, Ambroggio S, Peris C, Perona M, Sanseverino P. Polyunsaturated fatty acids (PUFAs) might reduce hot flushes: an indication from two controlled trials on soy isoflavones alone and with a PUFA supplement. Maturitas. 2005;51:127–134. doi: 10.1016/j.maturitas.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Simopoulos AP. Omega-3 fatty acids in health and disease and in growth and development. Am J Clin Nutr. 1991;54:438–463. doi: 10.1093/ajcn/54.3.438. [DOI] [PubMed] [Google Scholar]
- 20.Kris-Etherton PM, Harris WS, Appel LJ. Omega-3 fatty acids and cardiovascular disease: new recommendations from the American Heart Association. Arterioscler Thromb Vasc Biol. 2003;23:151–152. doi: 10.1161/01.atv.0000057393.97337.ae. [DOI] [PubMed] [Google Scholar]
- 21.Kraftsow G. Yoga for wellness: Healing with the timeless teaching of viniyoga. New York, New York: Peguin Putnam, Inc; 1999. [Google Scholar]
- 22.Francina S. Yoga and the Wisdom of Menopause: A Guide to Physical, Emotional, and Spiritual Health at Midlife and Beyond. Deerfield, Fl: Health Communications, Inc; 2003. [Google Scholar]
- 23.Lasater JH. Relax and Renew: Restful Yoga for Stressful Times. Berkeley, CA: Rodmell Press; 1995. [Google Scholar]
- 24.American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. 8. 2009. [DOI] [PubMed] [Google Scholar]
- 25.U.S. Department of Health and Human Services. Physical Activity Guidelines for America. Washington DC: U.S. Department of Health and Human Services; 2009. [Google Scholar]
- 26.Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee Report. Washington, DC: U.S. Department of Health and Human Services; 2008. [Google Scholar]
- 27.King AC, Stokols D, Talen E, Brassington GS, Killingsworth R. Theoretical approaches to the promotion of physical activity: forging a transdisciplinary paradigm. Am J Prev Med. 2002;23:15–25. doi: 10.1016/s0749-3797(02)00470-1. [DOI] [PubMed] [Google Scholar]
- 28.King AC, Blair SN, Bild DE, et al. Determinants of physical activity and interventions in adults. Med Sci Sports Exerc. 1992;24:S221–S236. [PubMed] [Google Scholar]
- 29.Prochaska JO, Velicer WF. The transtheoretical model of health behavior change. Am J Health Promot. 1997;12:38–48. doi: 10.4278/0890-1171-12.1.38. [DOI] [PubMed] [Google Scholar]
- 30.Bootzin RR. Placebo: Theory, Research, and Mechanisms. New York: Guilford Press; 1985. The Role of expectancy in behavior change. [Google Scholar]
- 31.Critelli JW, Neumann KF. The placebo. Conceptual analysis of a construct in transition. Am Psychol. 1984;39:32–39. doi: 10.1037//0003-066x.39.1.32. [DOI] [PubMed] [Google Scholar]