Abstract
Objective
To compare the correlation of intracranial pressure (ICP) measurement and time to detection of ICP crises (defined as ICP ≥ 20 mm Hg for ≥ 5 min) between an intraparenchymal (IP) monitor and external ventricular drain (EVD) in children where continuous cerebrospinal fluid (CSF) diversion was used as a therapy for severe traumatic brain injury (TBI).
Setting
Academic, pediatric intensive care unit.
Design
Retrospective review of a prospectively-collected Pediatric Neurotrauma database.
Patients
Children with severe TBI (GCS ≤ 8) who underwent ICP monitoring with both IP and EVD techniques were studied. In Cohort 1 (n = 58), hourly ICP measurements were extracted from the medical record. In Cohort 2 (n = 4), ICP measurements were collected every minute by an automated data collection system.
Measurements and Main Results
The mean absolute difference in ICP (|ICP|) and intraclass correlation coefficients (ICC) were calculated. Timing to detection of ICP crises was analyzed. Data expressed as mean ± SEM. In cohort 1, 7,387 hours of data were analyzed and 399 hours (23,940 min) were analyzed in Cohort 2. In Cohort 1, |ICP| = 3.10 ± 0.04 mm Hg (ICC = 0.98, p < 0.001). |ICP| in Cohort 2 was 3.30 ± 0.05 mm Hg (ICC = 0.98, p < 0.001). In Cohort 2, a total of 75 ICP crises were observed. Fifty-five (73%) were detected first by the IP monitor, of which 35 were not identified by the EVD monitor. Time between IP and EVD detection of a crisis was 12.60 ± 2.34 min.
Conclusion
EVD and IP measurements of ICP were highly correlated, although intermittent EVD ICP measurements may fail to identify ICP events when continuously draining CSF. In institutions using continuous CSF diversion as a therapy, a two-monitor system may be valuable for accomplishing monitoring and therapeutic goals.
INTRODUCTION
Intracranial pressure (ICP) monitoring was among the first neurological monitoring systems employed in children, with its inception largely starting during Reye’s syndrome outbreaks in the 1970s. ICP monitoring is common for many pediatric neurocritical care disorders associated with brain edema, hemorrhage, or hydrocephalus. ICP monitoring is a mainstay in the management of children with severe traumatic brain injury (TBI). Current treatment guidelines strongly encourage ICP monitoring for children with GCS ≤ 8 and abnormal head CT findings at admission as the majority of the evidence suggests that treatment of intracranial hypertension is associated with improved outcome. As such, protocols for treatment of children with severe TBI incorporate ICP monitoring as part of a comprehensive plan to minimize secondary injuries, using either ICP and/or cerebral perfusion pressure (CPP) as the therapeutic target [1].
There are two approaches commonly used to monitor ICP, including placement of a device within the parenchyma (intraparenchymal - IP) or within the cerebral ventricular system (via an externalized ventricular drain - EVD). Various advantages have been outlined for both techniques with ease of placement and decreased local tissue damage cited by advocates for IP monitoring and therapeutic CSF diversion emphasized by advocates of EVD placement. A potential limitation of EVD monitoring is the inability of these systems to simultaneously drain CSF and monitor ICP. For the past several years in our institution, we have used concurrent placement of both IP and EVD systems to allow continuous ICP monitoring (via the IP catheter) and continuous drainage of CSF (via the EVD) as a preemptive therapy for intracranial hypertension. In addition, we intermittently confirm IP ICP readings with the EVD. Moreover, we have recently installed an automated data collection system for bedside monitors, allowing objective timing of pathophysiological events in patients and more precise comparisons of events identified by multiple monitoring systems.
There is a paucity of literature describing the relationship between EVD and IP ICP measurements in adults, with two relatively small studies completed in children [2, 3]. Furthermore, most of these studies included subjects without TBI. While several authors concluded that a strong correlation exists between EVD and IP ICP measurements [2, 4–7], others found striking differences in measurements between the two methods [8]. We hypothesized that a significant correlation would be observed between these two modalities in a cohort of our patients enrolled in a TBI registry for the last several years. Moreover, we hypothesized that ICP crises (ICP ≥ 20 mm Hg for ≥ 5 minutes) would first be identified by the continuously reading IP device as a proof of a concept that combined monitoring would effectively detect intracranial crises earlier while simultaneously allowing continuous CSF diversion. For this second hypothesis, a smaller subset of patients enrolled in our Pediatric Neurotrauma registry where automated data collection from the bedside monitor was available was studied.
METHODS
Study Population
Children (age < 18 yrs) who were admitted to the Pediatric Intensive Care Unit after suffering from severe TBI (post-resuscitation GCS ≤ 8) were eligible for enrollment into our Pediatric Neurotrauma registry. The registry was approved by the Institutional Review Board of the University of Pittsburgh and informed consent was obtained from parents/guardians for enrollment in this study.
A clinical protocol for treatment of children with severe TBI has been used at our institution for several years. Briefly, all children with severe TBI received comprehensive care to rapidly stabilize and assess for injuries, mitigate secondary insults and promote optimal neurological outcome in accordance with published guidelines [1]. This protocol includes early endotracheal intubation, administration of ventilatory support for promotion of oxygen saturation and rapid correction/normalization of hemodynamic parameters. All patients were maintained in a neutral position with their head midline and the head of the bed elevated to 30 degrees to improve cerebral blood return to the thorax. Once the children are stabilized, neurological assessment and imaging studies to determine the extent of neurological injury were performed. Placement of ICP monitoring devices occurred as soon as was feasible and both IP and EVD monitors were placed within the same frontal lobe. In general, the IP catheter was directed toward uninjured brain in the hemisphere opposite the side of injury, while the EVD was placed within the ventricle using standard neurosurgical techniques. EVD monitors were zeroed based at the tragus of the ear and continuously drained at 3 cm above the midbrain and IP monitors were zeroed at the level of the EVD, and used and maintained in accordance with the manufacturer’s instructions (Codman). Other invasive monitoring devices (arterial catheters, central venous catheters) were placed as part of our standard practice, and some children (n = 41) also underwent brain tissue oxygen monitoring using LICOX (Integra Neurosciences, NJ). Additionally, a subset of children were concurrently enrolled in randomized, controlled trials evaluating the safety (phase II) and efficacy (phase III) of early hypothermia as a neuroprotectant.
The clinical protocol was developed to standardize practice for periods of intracranial pressure crises (generally defined as ICP ≥ 20 mmHg for ≥ 5 minutes). Step-wise escalations of care were implemented via a protocol [1], and included sedation (with fentanyl), neuromuscular blockade (with vecuronium), mild hyperventilation (PaCO2 ~ 35 mm Hg), hyperosmolar therapies (mannitol or 3% NaCl) and barbiturate administration. As mentioned above, continuous CSF diversion was employed in all children.
Data Collection
All data were collected as part of the Pediatric Neurotrauma Data registry and included demographic, physiological and outcome data for all children. Two cohorts of patients were identified based on the ICP data collection process available at the time of injury. For the first cohort (Cohort 1: 1999 – 2009), data extraction occurred by direct examination of the medical record by research coordinators. Specifically, bedside nursing staff recorded physiological data (including ICP measurements from monitors read simultaneously) either during routine care (hourly) or during periods of instability (more frequently, if necessary). Research personnel extracted this information into the clinical database for statistical analysis. For the second cohort (Cohort 2: 2009 – present), an automated, electronic data collection system (Bedmaster EX, Excel Medical Systems, FL) was used to retrieve physiological parameters every 1 min for the duration of the monitoring period. With this system, simultaneous readings from devices were collected and available for analysis. Additionally, waveforms from the various monitors were also collected at each time point and the reliability of the data was assessed.
For the purpose of this study, an ICP crisis was defined as a measurement of ICP ≥ 20 mm Hg for ≥ 5 minutes in one or both monitors. Dual identification of a crisis was defined as simultaneous IP and EVD ICP measurements of ≥ 20 mm Hg for ≥ 5 minutes at any point during the time span of a particular episode. Resolution of a crisis was defined as the reduction of ICP to < 20 mm Hg for ≥ 10 minutes as measured by the identifying monitor(s).
Statistical Analysis
All data are presented as mean ± SEM unless otherwise indicated. The absolute difference between all simultaneous EVD and IP ICP measurements were determined, from which a mean absolute difference between measurements (|ICP|) was calculated. Intraclass correlation coefficients (ICC) were calculated using commercially available software and were used to compare the agreement between IP and EVD monitoring methods in both cohorts of children. ICC was also calculated during periods of intracranial crises in both cohorts to determine if any significant variability in techniques existed within these important clinical events. Additionally, in Cohort 2, the time difference between detection of ICP crises (defined as ICP ≥ 20 mm Hg for ≥ 5 min for this study) between the devices was calculated. Statistical significance was defined as p < 0.05.
RESULTS
Demographic information is provided in Table 1 for both cohorts of patients. There were 46 boys and 12 girls in Cohort 1 (n = 58) with a mean age of 110.4 ± 7.9 months, while Cohort 2 (n = 4) was composed of 3 boys and 1 girl with a mean age of 115.3 ± 32.3 months. There were 3 deaths in enrolled subjects (mortality = 4.8 %). There was no association between insertions of either device and clinically significant hemorrhage, nor were any devices removed for infectious reasons.
Table 1.
Demographic data for subjects in Cohort 1 and Cohort 2.
| Cohort 1 (n = 58) | Cohort 2 (n = 4) | |
|---|---|---|
| Age, months | 110.4 ± 7.9 | 115.3 ± 32.3 |
| Gender | 46 male (79%) | 3 male (75%) |
| 12 female (21%) | 1 female (25%) | |
| Mechanism of injury | 18 car vs. pedestrian (31%) | 3 falls (75%) |
| 16 falls (28%) | 1 recreational vehicle | |
| 13 MVC (23%) | crash (25%) | |
| 5 recreational injuries (8%) | ||
| 3 other (5%) | ||
| 2 inflicted brain injuries (3%) | ||
| 1 GSW to head (2%) | ||
In total, 7387 hours of monitoring were available for analysis in Cohort 1 (125.2 hours ± 4.6 per subject), and 399 hours (99.9 hours ± 25.9 per subject) for Cohort 2. For both cohorts, an extremely close agreement was observed between the two monitoring devices. In Cohort 1, a highly significant correlation (ICC = 0.98, p < 0.001) between IP and EVD ICP measurements was observed, with the mean absolute difference between IP and EVD measurements of 3.10 ± 0.04 mm Hg. A similarly significant correlation was observed in Cohort 2 (ICC = 0.98, p < 0.001), with a comparable mean absolute difference between measurements of 3.30 ± 0.05 mm Hg.
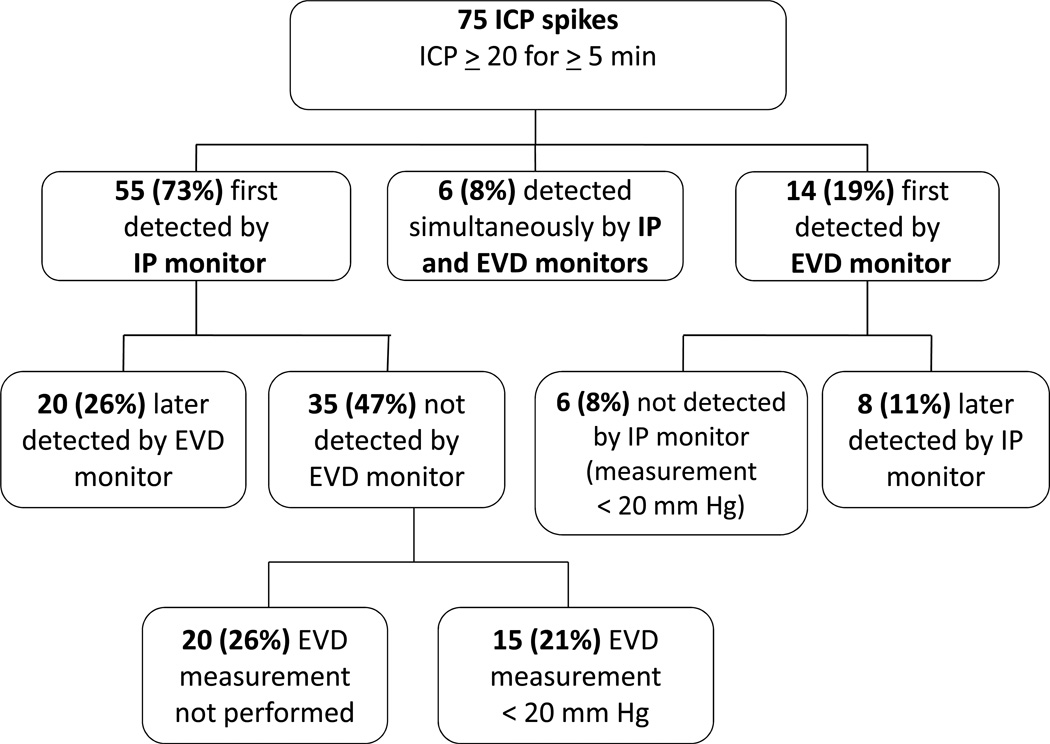
In Cohort 2, 75 ICP crises were identified by either monitoring system (see Figure 2). Fifty-five (73%) were detected first by the IP monitor, of which 35 (47%) were never identified by the EVD monitor [EVD not transduced (26%); EVD measurement remained <20 mm Hg (21%)] (see Figures 1a and 1b). Fourteen (19%) were detected first by the EVD monitor, of which 6 (8%) were never identified by the IP monitor. In these 6 instances, the EVD and IP monitor were located within the same cerebral hemisphere. On average, the IP monitor detected an ICP crisis 12.60 ± 2.34 minutes (range 1–43 minutes) before the EVD monitor.
Figure 2.
Description of ICP (intracranial pressure) crisis detection in Cohort 2 by monitoring method. External ventricular drain (EVD) monitors and intraparenchymal (IP) monitors were used to measure ICP in all subjects.
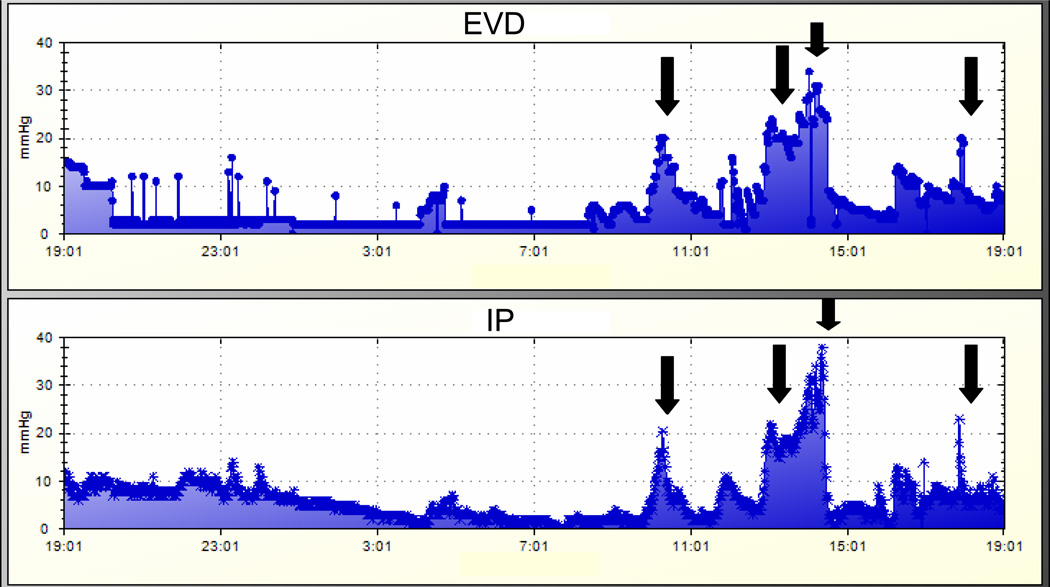
Figure 1.
a. Representative graphs of intracranial pressure (ICP) measurements in a patient in Cohort 2. Data were obtained by the automated collection system (Bedmaster Ex, Excel Medical). The top graph represents measurements made by the external ventricular drain (EVD) monitor; the bottom graph represents measurements made by the intraparenchymal (IP) monitor. In this particular patient, both monitors demonstrate consistent and timely detection of ICP crises (solid black arrows).
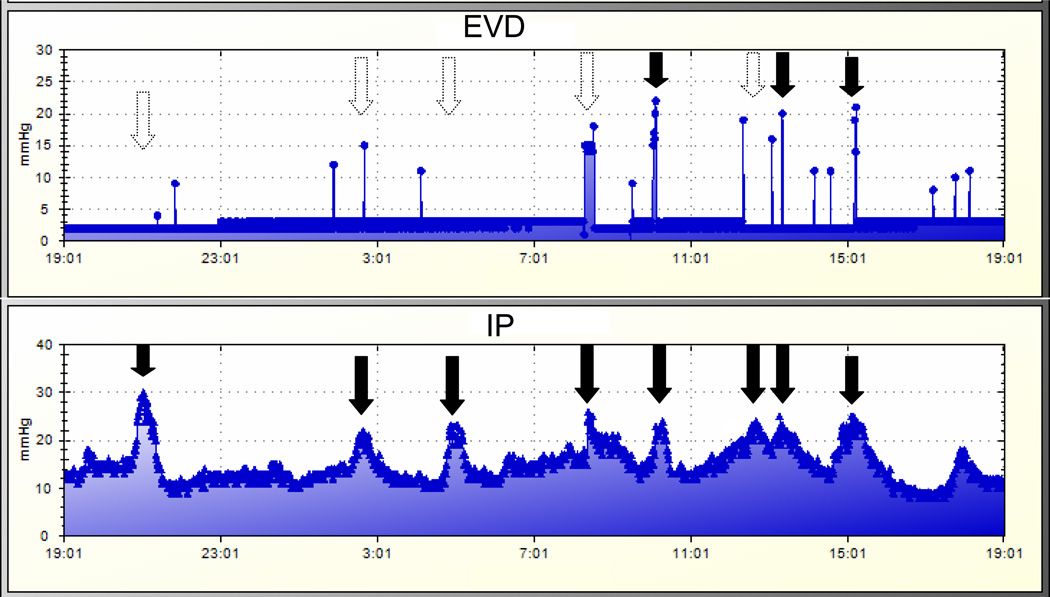
b. Representative graphs of intracranial pressure (ICP) measurements in a patient in Cohort 2. Data were obtained by the automated collection system (Bedmaster Ex, Excel Medical). The top graph represents measurements made by the external ventricular (EVD) monitor; the bottom graph represents measurements made by the intraparenchymal (IP) monitor. ICP crises are identified by solid black arrows. In contrast to Figure 1a, the EVD monitor fails to detect several significant ICP crises identified by the IP monitor (dashed line arrows).
DISCUSSION
Our data demonstrate a highly significant correlation between ICP measurements made by IP and EVD monitors reported both within the medical record and by an automated data collection system, arguing that either device can effectively monitor this important clinical parameter after TBI in children. Additionally, we believe that our data suggest that if continuous CSF diversion is planned, use of both monitors may be necessary since intermittent recordings of ICP via an EVD used for continuous drainage could result in a delay in detection of significant events.
ICP Monitoring after Pediatric TBI
A fundamental tenet of pediatric neurocritical care over the past decades has been to preserve neurological function and minimize second insults. As a part of this philosophy, ICP monitoring after TBI for children has been advocated as a means of directing therapy to both optimize cerebral perfusion and prevent herniation. In the most recent published guidelines [1], neither a “standard” nor a “guideline” could be adopted as only Class III evidence existed for the use of any type of ICP monitoring for children, although at least 500 children enrolled in 9 studies have demonstrated at least some association between ICP and outcome [9–17]. As a result, most centers adopted ICP monitoring years ago, while some have argued against the need for such an approach [18].
Since both EVD and IP catheters are in common use, it is important to determine the relationship between the readings from these different devices. As neither is a “gold-standard”, we chose to compare the reliability between the two catheters placed as part of the standard care of children on our neurocritical care service. Our findings are consistent with the relatively sparse literature. The correlation between EVD and IP measurements of ICP observed in individual children from Gambardella and colleagues were slightly lower (r = 0.73 – 0.89) than we observed (r = 0.98 in both data acquisition approaches). Moreover, the absolute differences between the readings was very similar to other published reports which found between 1 and 8 mm Hg absolute differences [2–7, 19]. We believe that our data support the conclusion that both devices reliably measure ICP in children.
Utility of Simultaneous Monitoring Systems
In our institution, we adopted continuous CSF drainage as a standard strategy to limit the development of intracranial hypertension. This decision was made based on the clinical experiences of our faculty and data from several studies demonstrating the utility of this technique [20, 21]. Our group [22] has previously demonstrated that continuous CSF drainage resulted in drainage of a much larger volume of CSF, decreased concentrations of a number of biomarkers (including cytokines) and may have produced lower ICP when compared to intermittent drainage. In the current study, children in Cohort 2 drained 189 ml ± 19 of CSF/day, which is comparable to the 192 ml ± 15 in children with continuous CSF drainage reported by Shore and colleagues [13], indicating that the populations were comparable for this therapeutic maneuver. A disadvantage of this approach is that continuous measurement of ICP is not possible while CSF is being drained, potentially delaying the detection of ICP events warranting therapy. Because of this concern, we implemented a protocol using both monitors to continuously screen for intracranial hypertension while allowing for continuous CSF drainage.
Although advocated by others for use in adult TBI patients [8, 23], our approach of dual monitoring is relatively unique in children with TBI. A study describing the frequency of intracranial pressure monitoring in children reported the simultaneous use of EVD and IP monitoring systems [24] in 7 children, but ICP measurements between the two systems were not directly compared. Our approach afforded us a valuable opportunity to (i) detect correlations between the devices in a large number of children after TBI and (ii) compare the timing of identification of ICP ≥ 20 mm Hg between devices while CSF diversion was being practiced. In our small subset of children with automated data collection, we were surprised by the large number of ICP crises that met our a priori definition of requiring an intervention (n = 75 in 4 children). To our knowledge, this report is the first to describe the use of a continuous data collection system to study physiologic variables in the pediatric intensive care unit, and we believe that this system of automated data collection could greatly increase the sensitivity of detecting these clinical events over hourly measurements in future studies of TBI.
We also demonstrated that using our protocol, the IP monitor generally detected ICP crises before the EVD monitor by several minutes, likely related simply to intermittent recording by the bedside nurse, which could ultimately have led to delays in care with possible clinical consequences in the absence of the IP system. We consider this a significant advantage to our approach, as children received potential benefits from both monitoring systems. Other groups using continuous CSF drainage as the sole monitoring system may implement maneuvers (such as frequent mandatory nursing audits of ICP readings from the EVD) that might similarly detect ICP crises in a rapid fashion. However, any deviation from such a protocol would jeopardize the ability to detect ICP crises and intervene rapidly with therapies for intracranial hypertension.
In patients for whom placement of a second monitor is considered undesirable and intermittent CSF drainage is being used, it may be possible to garner some of the benefits provided by continuous drainage by adjusting the drainage threshold (from 20 mm Hg to 10 mm Hg, as an example). In theory, this may increase the total amount of CSF drained, thereby treating intracranial hypertension. Currently there are no data describing the use of such a treatment protocol, and studies of its efficacy and safety would be needed. Overall, we have found our dual monitoring approach (i) comprehensively assesses the brain for intracranial events, (ii) allows for valuable therapeutic maneuvers after TBI and (iii) should be considered by other centers in their protocol development.
Our study has several limitations. First, as a retrospective analysis, there is obvious concern for inaccuracies and biases in the medical record in Cohort 1. However, we believe that this concern is minimal since the relationship between IP and EVD measurements was remarkably consistent between the cohorts. Our findings of time to EVD confirmation of an IP-detected ICP crisis must be understood within the context of our protocol. Since our bedside nurses had both monitoring systems to screen for ICP crises, it is not possible based on our data to determine how long a delay would be observed or how many crises would be missed by using only an EVD-based detection system. Studies from other centers will be required to answer this important question. Our study included a relatively small number of children and should be replicated with a larger sample size to better define the scope of this discrepancy. Our patients with TBI possessed a wide range of intracranial pathology, including contusions, hemorrhages, diffuse axonal injury among other findings. A larger study would allow stratification of patients with divergent injuries and may determine an optimal monitoring system, based on this or other factors, including the cost-benefit ratio of this strategy, which we did not evaluate for this study. Finally, we recognize that in some cases it would not be expected for ICP readings to correlate when obtained in two separate locations in the same patient. The monitors detect two different pressures- the IP monitor measures the regional parenchymal pressure at its tip, while the EVD measurement represents a summary of all pressures at the ventricular-parenchymal interface. In addition, compartmentalization of ICP has been described in the injured brain, and is influenced by the nature, extent, and location of injury [25, 26]. In such cases, lack of agreement between devices may accurately reflect the heterogeneity of regional pressures, and may not be discrepant pressure measurements. In conclusion, ICP measurements by EVD and IP monitors were highly correlated and automated data collection demonstrated a large number of ICP events. For patients receiving continuous CSF drainage therapy, in which ICP was not continuously monitored by the EVD, an IP monitor was helpful for both maximally identifying the presence of an ICP crisis and accomplishing this more rapidly. Our data suggests that placement of a second IP monitor may be valuable for identifying episodes of intracranial hypertension that would not be recognized by intermittent EVD ICP measurements and should be considered by centers interested in continuous CSF diversion.
REFERENCES
- 1.Adelson PD, Bratton SL, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Pediatr Crit Care Med. 2003;4(3) Suppl:S1–S75. doi: 10.1097/01.CCM.0000067635.95882.24. [DOI] [PubMed] [Google Scholar]
- 2.Ostrup RC, Luerssen TG, Marshall LF, Zornow MH. Continuous monitoring of intracranial pressure with a miniaturized fiberoptic device. J Neurosurg. 1987;67(2):206–209. doi: 10.3171/jns.1987.67.2.0206. [DOI] [PubMed] [Google Scholar]
- 3.Gambardella G, Zaccone C, Cardia E, Tomasello F. Intracranial pressure monitoring in children: comparison of external ventricular device with the fiberoptic system. Childs Nerv Syst. 1993;9(8):470–473. doi: 10.1007/BF00393552. [DOI] [PubMed] [Google Scholar]
- 4.Chambers KR, Kane PJ, Choksey MS, Mendelow AD. An evaluation of the camino ventricular bolt system in clinical practice. Neurosurgery. 1993;33(5):866–868. doi: 10.1227/00006123-199311000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Piek J, Bock WJ. Continuous monitoring of cerebral tissue pressure in neurosurgical practice--experiences with 100 patients. Intensive Care Med. 1990;16(3):184–188. doi: 10.1007/BF01724800. [DOI] [PubMed] [Google Scholar]
- 6.Shapiro S, Bowman R, Callahan J, Wolfla C. The fiberoptic intraparenchymal cerebral pressure monitor in 244 patients. Surg Neurol. 1996;45(3):278–282. doi: 10.1016/0090-3019(95)00359-2. [DOI] [PubMed] [Google Scholar]
- 7.Piek J, Kosub B, Kuch F, Bock WJ. A practical technique for continuous monitoring of cerebral tissue pressure in neurosurgical patients. Preliminary results. Acta Neurochir (Wien) 1987;87(3–4):144–149. doi: 10.1007/BF01476066. [DOI] [PubMed] [Google Scholar]
- 8.Schickner DJ, Young RF. Intracranial pressure monitoring: fiberoptic monitor compared with the ventricular catheter. Surg Neurol. 1992;37(4):251–254. doi: 10.1016/0090-3019(92)90147-f. [DOI] [PubMed] [Google Scholar]
- 9.Alberico AM, Ward JD, Choi SC, Marmarou A, Young HF. Outcome after severe head injury. Relationship to mass lesions, diffuse injury, and ICP course in pediatric and adult patients. J Neurosurg. 1987;67(5):648–656. doi: 10.3171/jns.1987.67.5.0648. [DOI] [PubMed] [Google Scholar]
- 10.Barzilay Z, Augarten A, Sagy M, Shahar E, Yahav Y, Boichis H. Variables affecting outcome from severe brain injury in children. Intensive Care Med. 1988;14(4):417–421. doi: 10.1007/BF00262899. [DOI] [PubMed] [Google Scholar]
- 11.Chambers IR, Treadwell L, Mendelow AD. Determination of threshold levels of cerebral perfusion pressure and intracranial pressure in severe head injury by using receiver-operating characteristic curves: an observational study in 291 patients. J Neurosurg. 2001;94(3):412–416. doi: 10.3171/jns.2001.94.3.0412. [DOI] [PubMed] [Google Scholar]
- 12.Downard C, Hulka F, Mullins RJ, Piatt J, Chesnut R, Quint P, Mann NC. Relationship of cerebral perfusion pressure and survival in pediatric brain-injured patients. J Trauma. 2000;49(4):654–658. doi: 10.1097/00005373-200010000-00012. discussion 658-659. [DOI] [PubMed] [Google Scholar]
- 13.Eder HG, Legat JA, Gruber W. Traumatic brain stem lesions in children. Childs Nerv Syst. 2000;16(1):21–24. doi: 10.1007/s003810050005. [DOI] [PubMed] [Google Scholar]
- 14.Esparza J, J MP, Sarabia M, Yuste JA, Roger R, Lamas E. Outcome in children with severe head injuries. Childs Nerv Syst. 1985;1(2):109–114. doi: 10.1007/BF00706691. [DOI] [PubMed] [Google Scholar]
- 15.Kasoff SS, Lansen TA, Holder D, Filippo JS. Aggressive physiologic monitoring of pediatric head trauma patients with elevated intracranial pressure. Pediatr Neurosci. 1988;14(5):241–249. doi: 10.1159/000120397. [DOI] [PubMed] [Google Scholar]
- 16.Michaud LJ, Rivara FP, Grady MS, Reay DT. Predictors of survival and severity of disability after severe brain injury in children. Neurosurgery. 1992;31(2):254–264. doi: 10.1227/00006123-199208000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Shapiro K, Marmarou A. Clinical applications of the pressure-volume index in treatment of pediatric head injuries. J Neurosurg. 1982;56(6):819–825. doi: 10.3171/jns.1982.56.6.0819. [DOI] [PubMed] [Google Scholar]
- 18.Salim A, Hannon M, Brown C, Hadjizacharia P, Backhus L, Teixeira PG, Chan LS, Ford H. Intracranial pressure monitoring in severe isolated pediatric blunt head trauma. Am Surg. 2008;74(11):1088–1093. [PubMed] [Google Scholar]
- 19.Gambardella G, d'Avella D, Tomasello F. Monitoring of brain tissue pressure with a fiberoptic device. Neurosurgery. 1992;31(5):918–921. doi: 10.1227/00006123-199211000-00014. discussion 921-912. [DOI] [PubMed] [Google Scholar]
- 20.Caruselli G, Recchioni MA, Occhipinti C, Bernardini M, Caruselli M. The role of CSF ventricular drainage in controlling intracranial hypertension in patients with brain lesions. Comparison of three methods. Preliminary results. J Neurosurg Sci. 1992;36(4):219–225. [PubMed] [Google Scholar]
- 21.Kerr ME, Weber BB, Sereika SM, Wilberger J, Marion DW. Dose response to cerebrospinal fluid drainage on cerebral perfusion in traumatic brain-injured adults. Neurosurg Focus. 2001;11(4):E1. doi: 10.3171/foc.2001.11.4.2. [DOI] [PubMed] [Google Scholar]
- 22.Shore PM, Thomas NJ, Clark RS, Adelson PD, Wisniewski SR, Janesko KL, Bayir H, Jackson EK, Kochanek PM. Continuous versus intermittent cerebrospinal fluid drainage after severe traumatic brain injury in children: effect on biochemical markers. J Neurotrauma. 2004;21(9):1113–1122. doi: 10.1089/neu.2004.21.1113. [DOI] [PubMed] [Google Scholar]
- 23.Luerssen TGaW CE. Pathophysiology and management of elevated intracranial pressure in children. Park Ridge: AANS; 1997. [Google Scholar]
- 24.Keenan HT, Nocera M, Bratton SL. Frequency of intracranial pressure monitoring in infants and young toddlers with traumatic brain injury. Pediatr Crit Care Med. 2005;6(5):537–541. doi: 10.1097/01.PCC.0000164638.44600.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenwasser RH, Kleiner LI, Krzeminski JP, Buchheit WA. Intracranial pressure monitoring in the posterior fossa: a preliminary report. J Neurosurg. 1989;71(4):503–505. doi: 10.3171/jns.1989.71.4.0503. [DOI] [PubMed] [Google Scholar]
- 26.Qureshi AI, Suri MF, Ringer AJ, Guterman LR, Hopkins LN. Regional intraparenchymal pressure differences in experimental intracerebral hemorrhage: effect of hypertonic saline. Crit Care Med. 2002;30(2):435–441. doi: 10.1097/00003246-200202000-00028. [DOI] [PubMed] [Google Scholar]