Abstract
The anterior thalamic nuclei form part of a network for episodic memory in humans. The importance of these nuclei for recognition and recency judgments remains, however, unclear. Rats with anterior thalamic nuclei lesions and their controls were tested on object recognition, along with two types of recency judgment. The spontaneous discrimination of a novel object or a novel odor from a familiar counterpart (recognition memory) was not affected by anterior thalamic lesions when tested after retention delays of 1 and 60 min. To measure recency memory, rats were shown two familiar objects, one of which had been explored more recently. In one condition, rats were presented with two lists (List A, List B) of objects separated by a delay, thereby creating two distinct blocks of stimuli. After an additional delay, rats were presented with pairs of objects, one from List A and one from List B (between-block recency). No lesion-induced deficit was apparent for recency discriminations between objects from different lists, despite using three different levels of task difficulty. In contrast, rats with anterior thalamic lesions were significantly impaired when presented with a continuous list of objects and then tested on their ability to distinguish between those items early and late in the same list (within-block recency). The contrasting effects on recognition and recency support the notion that interlinked hippocampal–anterior thalamic interconnections support aspects of both spatial and nonspatial learning, although the role of the anterior thalamic nuclei may be restricted to a subclass of recency judgments (within-block).
Keywords: anterior thalamic nuclei, memory, proactive interference, recency, recognition
The anterior thalamic nuclei are thought to comprise part of an “extended hippocampal system” (Aggleton & Brown, 1999) and therefore, together with the hippocampus, are critically involved in spatial memory (Aggleton, Hunt, Nagle, & Neave, 1996; Aggleton, Neave, Nagle, & Hunt, 1995; Henry, Petrides, St-Laurent, & Sziklas, 2004; Mitchell & Dalrymple-Alford, 2005; Sutherland & Rodriguez, 1989; Sziklas & Petrides, 1999, 2007; Warburton, Morgan, Baird, Muir, & Aggleton, 1999). It is far less clear, however, whether this correspondence extends to nonspatial learning (Wolff, Gibb, & Dalrymple-Alford, 2006). Although hippocampal lesions in rats often spare recognition memory (Aggleton, Desimone, & Mishkin, 1986; Barker, Bird, Alexander, & Warburton, 2007; Forwood, Winters, & Bussey, 2005; Mumby, 2001; Winters, Forwood, Cowell, Saksida, & Bussey, 2004; but see Broadbent, Gaskin, Squire, & Clark, 2010; Broadbent, Squire, & Clark, 2004; Clark, West, Zola, & Squire, 2001; Clark, Zola, & Squire, 2000; Gaskin et al., 2010), there is more consistent evidence of a hippocampal deficit for recency judgments (Albasser, Lin, Iordanova, Amin, & Aggleton, 2012; Barker & Warburton, 2011; Fortin, Agster, & Eichenbaum, 2002; Kesner, Gilbert, & Barua, 2002; Kesner, Hunsaker, & Ziegler, 2010). The present study, therefore, examined whether this profile of effects in rats with hippocampal damage (spared recognition, impaired recency) is shared by anterior thalamic nucleus lesions.
Previous studies have found that anterior thalamic lesions in rats spare the ability to discriminate novel from familiar objects (Aggleton et al., 1995; Mitchell & Dalrymple-Alford, 2005; Moran & Dalrymple-Alford, 2003; Warburton & Aggleton, 1998). A potential problem, however, is that these studies rely on a protocol (Ennaceur & Delacour, 1988) that involves spontaneous behavior and few trials. The resulting variance can make the task insensitive to mild deficits. The first goal was, therefore, to reexamine recognition memory but to include protocols that might prove more sensitive. The bow-tie maze (Albasser, Poirier, & Aggleton, 2010) combines aspects of delayed nonmatching to sample (Aggleton, 1985; Mishkin & Delacour, 1975) with spontaneous recognition (Ennaceur & Delacour, 1988) and therefore can provide multiple recognition trials within a session. Consequently, the task should reduce performance variance as well as increase proactive interference between objects.
The second goal was to examine recency memory, the discrimination of items by their order. Previous research appears, however, inconsistent. Rats with anterior thalamic lesions could successfully discriminate two objects separated by a 60 min interval (Mitchell & Dalrymple-Alford, 2005), when the recency test occurred 2 hr after the first sample object (i.e., Sample A → 60 min delay → Sample B → 60 min delay → Test [A vs. B]). In contrast, anterior thalamic lesions disrupted the ability to select an odor that was presented earlier in a list of odors in order to find food (Wolff et al., 2006). To investigate recency memory, this study compared when the test objects to be discriminated are separated by a clear interval (between-block recency, e.g., Mitchell & Dalrymple-Alford, 2005) with when the set of test objects form part of a continuous series (within-block recency, e.g., Wolff et al., 2006). Recognition and recency memory were tested both with objects and with odor cues to extend our knowledge of these forms of learning and therefore assess whether performance is differentially influenced by stimulus type. Finally, performance on a standard spontaneous object recognition task in an open arena was assessed (Ennaceur & Delacour, 1988), along with spatial alternation task in a T maze. The latter task is highly sensitive to anterior thalamic damage (e.g., Aggleton et al., 1995).
Materials and Methods
Subjects
The subjects were 25 male hooded rats that weighed 270–320 g at the beginning of the experiment (Harlan, Bicester, UK). The animals were housed in pairs under a 12-hr light–dark cycle. The animals were given free access to water but maintained at 85% of their free-feeding weight for the duration of the experiments. Fifteen rats received bilateral lesions of the anterior thalamic nuclei (ATNx), and 10 control rats received sham (Sham) surgeries. All animals were habituated to handling before the start of the first experiment. All experiments were performed in accordance with the U.K. Animals (Scientific Procedures) Act (1986) and associated guidelines.
Surgery
Surgery was performed under pentobarbitone sodium anesthesia (60 mg/kg ip, Sigma-Aldrich, Dorset, UK). Once anesthetized, the animal was placed in the head holder of the stereotaxic apparatus (Kopf Instruments, CA) with the incisor bar adjusted to +5.0 relative to the horizontal plane. Following an incision, the scalp was retracted to expose the skull. A craniotomy was made, and the dura was cut, exposing the cortex above the target location. Lesions to the anterior thalamic nuclei were made by injecting 0.12 M N-methyl-d-aspartic acid (NMDA; Sigma Chemical, UK) dissolved in sterile phosphate buffer (ph 7.4) over two separate sites within one hemisphere with the use of a 1-μl Hamilton syringe (Hamilton, Switzerland) that was attached to a stereotaxic frame. The lateral and medial sites were infused with 0.22 μl or 0.24 μl of NMDA over a period of 5 min, respectively. The syringe was left in situ for an addition 4 min before being retracted. The lesion coordinates relative to bregma were anteroposterior −0.6; mediolateral ±0.9 and ±1.8 from the midline; dorsoventral −7.0 and −6.3 from bregma for the medial site and the lateral site, respectively. For the sham surgeries, the syringe was lowered to +0.2 above the target site for a few seconds and then removed. No NMDA was injected in the control rats. After removal of the Hamilton syringe, the incision was cleaned and sutured. A topical antibiotic powder (Aureomycin, Fort Dodge, Animal Health, Southampton, UK) was applied. The rats also received glucose-saline (5 ml sc) for fluid replacement and then placed in a recovery chamber until they regained consciousness (i.e., movement and righting reflex). Rats were given the analgesic Metacam (0.06 ml sc; 5 mg/ml meloxicam; Boehringer Ingelheim Vetmedica, Germany). A respiratory stimulant millophylline (0.1 ml sc, Arnolds Veterinary Products, Shropshire, UK), an antimicrobial Baytril in their water (2.5%; Bayer, Animal Health Division, Ireland) and low dose of diazepam (0.07 ml sc, 5 mg/ml; CP Pharmaceuticals, UK) was administered to facilitate postoperative recovery as needed. All animals were monitored carefully until they had fully recovered.
Histology
Following behavioral testing, the animals were administered with an intraperitoneal injection of a lethal overdose of Euthatal (200 mg/ml sodium pentobarbital, Marial Animal Health, Harlow, Essex, UK) and perfused intracardially with 0.1 M phosphate buffer saline (PBS) followed by 4% paraformaldehyde in 0.1 M PBS (PFA). The brains were extracted from the skull and placed on a stirrer to postfix in PFA for 4 hr, after which the brains were placed in 25% sucrose overnight. The brains were frozen on a microtome (Leica, UK) and sectioned at 40 μm in the coronal plane. One-in-five sections were mounted and stained with cresyl violet, a Nissl stain. The remaining sections were divided into four (one-in-five sections) series and were frozen (approximately −20 °C) in cryoprotectant for later immunohistochemistry.
Immunohistochemistry for Neuronal Nuclei (NeuN)
One of the one-in-five series of frozen sections from every ATNx and one Sham animal was subsequently used for NeuN immunohistochemistry (approximately 12 sections through the anterior thalamic nuclei). Because NeuN only stains neural cell bodies (Mullen, Buck, & Smith, 1992), visualization of the lesion extent can often be improved. The sections were washed for 10 min in PBS four times. The sections were then rinsed in PBS containing 0.2% Triton X-100 (PBST) for 10 min two times. The sections were then washed in 0.3% hydrogen peroxide in PBST for 10 min to block endogenous peroxidase activity and rinsed four times for 10 min in PBST. Afterward, the sections were incubated at 4 °C for 48 hr in PBST with mouse antineuronal nuclei monoclonal antibody (NeuN; 1:5000, MAB377, lot number: 0703055636, Chemicon). The sections were then rinsed for 10 min in PBST four times. Following the four washes, the sections were incubated in biotinylated horse antimouse secondary antibody (diluted 1:200 in PBST; Vector Laboratories) and normal horse serum for 2 hr. The sections were washed again in PBST, and incubated for 1 hr in avidin-biotinylated horseradish peroxidase complex in PBST (Elite Kit, Vector Laboratories). Next, sections were rinsed for 10 min in 0.05 M Tris buffer (pH 7.4) two times. The reaction was visualized using diaminobenzidine (DAB Substrate Kit, Vector Laboratories) and stopped by washing in cold PBS. Finally, the sections were mounted on gelatin-coated slides, dehydrated through a graded series of alcohols, and coverslipped.
Volumetric Analysis of Lesions
The sizes of the anterior thalamic were estimated in all 15 rats. The extent of the thalamic lesion, based on both the cresyl violet and NeuN stained sections, was drawn by hand onto six equally spaced, standard coronal sections (Paxinos & Watson, 2005; from bregma −1.08 mm to −2.28 mm). The same procedure was repeated for 20 standard coronal sections through the length of the hippocampus (Paxinos & Watson, 2005). These drawings were scanned, and the area of damage was quantified using the program analySISD̂ (Soft-Imaging Systems, Olympus). The percentage of damage in the anterior thalamic nuclei and hippocampus was quantified by taking the area of damage within the region of interest and dividing it by the total area of that region summed across each drawing. (For these analyses, the hippocampus consisted of the dentate gyrus and the CA fields but not the subiculum.) The hippocampus was examined as the injection tracts passed through the fornix, and there was often some restricted tissue loss in the septal hippocampus. The volumetric analysis was conducted on each hemisphere separately as well as together. Those animals with anterior thalamic damage that involved less than 50% of the total structure were excluded from the behavioral analysis.
Behavioral Testing
The rats were first tested on a T maze alternation task. Next, the rats were trained and tested on a series of experiments in the bow-tie maze that assessed both recency and novelty judgments, using both objects and odors. Prior to testing object recognition in an open arena, the rats were retested on T maze alternation (see Table 1).
Table 1. List of The Experiments (Exp.) That the ATNx and Sham Groups Performed in the Order in Which They Were Conducted (Left Column).
Apparatus
The T maze was located in the center of a room (300 cm × 300 cm × 240 cm) and was elevated 100 cm above the ground with the use of metal supports. The floor of the maze was made of wood and painted white. Each arm was 70 cm long and 10 cm wide with walls made from clear Perspex (16.5 cm high). Sunken food wells, 3 cm in diameter and 0.75 cm deep, were located at the end of each arm. From the maze, the rats had full view of the distal extramaze cues (e.g., table, posters).
Pretraining
Pretraining began at least 2 weeks after surgery. During both pretraining and testing, the rats were brought into the room inside opaque aluminum carrying boxes. The rats were habituated to the apparatus for 4 days. On the first day, the T maze was blocked at the junction point with the use of a metal barrier. This created two straight alleys: (a) a start arm alley and (b) the choice arms alley (i.e., the top of the “T”). Separate habituation to these alleys ensured that the rats were not rewarded for specific arm turns during pretraining. Each rat was placed for 5 min in each alley with sucrose pellets (45 mg per pellet; Noyes Purified Rodent Diet, Lancaster, NH) scattered along the floor. On the second day, the rats were again placed into the two alleys, but the sucrose pellets were now only located within the food wells. On days 3 and 4, the food wells were only baited and rebaited with a single sucrose pellet.
Testing
The rats received six trials per day for 6 days. Each trial consisted of a sample phase and a choice phase. During the sample phase, the rat was given access to one of the two arms at the top of the T by blocking the entrance to the other arm with a metal barrier at the junction of the maze. On reaching the end of the sample arm, the rat was allowed to consume a single sucrose pellet. The rat was then picked up and confined behind a metal barrier in the start arm for approximately 15 s while the barrier at the choice point was removed. Following this 15 s delay, all barriers were removed so that the rat had free choice between the two arms of the T maze (choice phase). The rat was rewarded with a single sucrose pellet for choosing the arm that was not previously visited during the sample phase (i.e., the rat alternated arms between the sample and choice runs). The rat was deemed to have made a choice when it placed a hind foot down an arm. Following a correct choice, the rat was allowed to eat the reward before being returned to the metal carrying case. When the rat made an incorrect choice that arm was blocked behind the rat and it was allowed to run down the arm and reach the empty food well before being picked up and returned to the carrying box. The rats were run in squads of 3–4, each rat receiving one trial at a time. Consequently, the intertrial interval (ITI) was approximately 5 min.
Following extensive training in the bow-tie maze (see Table 1), the rats were retested in the T maze. The interval was approximately 5 months, and the rats were again tested in the same squads of 3–4 for six trials per day over 4 consecutive days.
Object Recognition and Object Recency in the Bow-Tie Maze: Experiments 2–4
All of these experiments used the spontaneous preference for new (object recognition) or less recent (recency) objects as measures for these respective forms of memory.
Apparatus
The rats were tested in a maze with the shape of a bow-tie (120 cm long, 50 cm wide, and 50 cm high) made of aluminum (Figure 1A). Each end of the maze consisted of a triangular area, and these areas were joined together at their apexes by a corridor (12 cm wide). In the center of the corridor, an opaque sliding door could be lowered or raised by the experimenter to allow passage from one end of the maze to the other. At the far wall of each of the triangles there were two food wells (3.5 cm in diameter and 2 cm deep), separated by a short, opaque, wall extending 15 cm from the middle of the end wall. The two food wells were 25 cm apart. Objects were placed above these two food wells during the experiment.
Figure 1. (A) The shape and dimensions (cm) of the bow-tie maze. Adapted from “New Behavioral Protocols to Extend Our Knowledge of Rodent Object Recognition Memory,” by Albasser, Poirier, & Aggleton, 2010European Journal of Neuroscience, 31.
Objects (Experiments 2–4)
Experiments 2–4 used 75 triplicate sets of identical objects that differed in size, shape, color, and texture, but without any obvious odor to the experimenter (see Figure 1B). The objects had to be large enough to cover one food well and light enough for the rats to displace. The presentation of the objects was counterbalanced, so that half of the rats experienced the list of objects presented in one order (e.g., A–K), whereas the other half experienced the list in the reverse order (e.g., K–A). The relative left and right positioning of the items was also counterbalanced across rats.
Although some of the same objects were reused across Experiments 2–4, no object was reused within 1 month. Furthermore, because the recency memory tests followed the recognition memory tests (see Table 1) the only object repeats were for the recency experiments.
Habituation and Pretraining
Animals were initially habituated for 7 days to the bow-tie maze, so that by the end of pretraining all rats would run from one end of the maze to the other and displace objects covering the food wells to obtain a reward (sucrose pellet). On Day 1, pairs of rats were placed in the maze for 20 min and allowed to explore and consume sucrose pellets scattered across the floor and in the food wells. On Day 2, rats were trained individually for 10 min to run back and forth for a reward located only in the food wells. From Day 3, the rats were introduced to the sliding door that restricted their movement from one compartment to the other. On Day 4, four identical wood blocks were introduced and gradually covered the food wells, so that by the end of the 10 min session, the rats would have to push the blocks to obtain the food reward. From Day 5, three other pairs of objects were introduced that varied in size, shape, color, and weight. These three objects were only used during pretraining and not during the experiment proper.
Behavioral Procedures
Object Recognition Memory (Experiment 2)
Object recognition was examined after two retention periods (1 and 60 min). The short retention condition used the standard recognition procedure, where consecutive trials are continuous and separated by 1 min (Albasser, Chapman, et al., 2010). The objects from the short retention period were then used as the familiar objects for longer retention period, tested 60 min later. Consequently, the short and long retention conditions formed two consecutive test sessions administered on the same day (see Table 2A).
Table 2. Presentation Order of Items in The Different Bow-Tie Maze protocols.
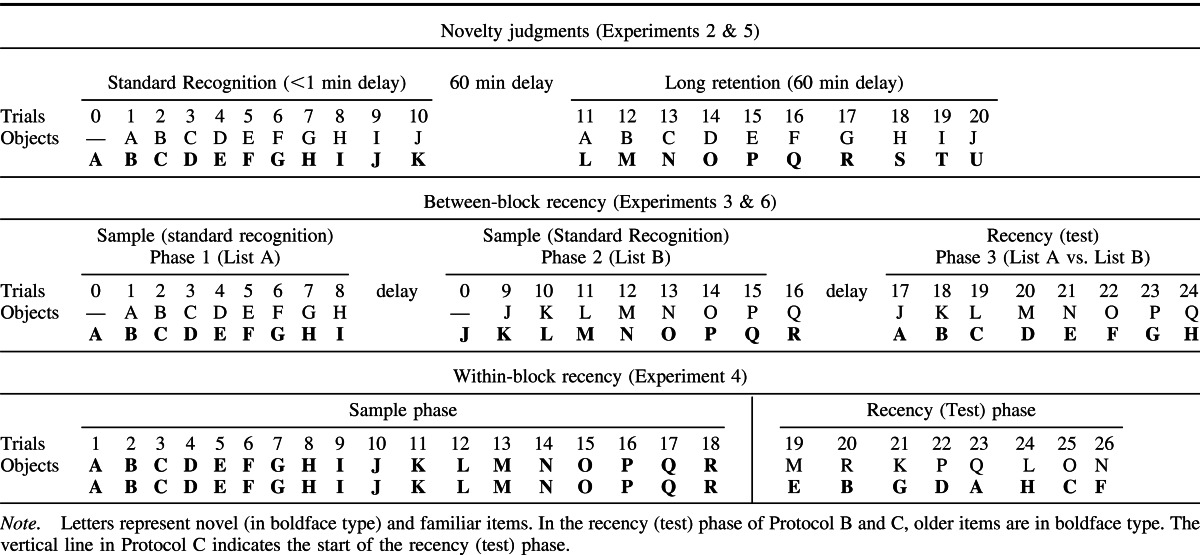
1 min retention
For the 1 min condition, the rats received 10 recognition trials within a single session (Table 2A). The session began with the rat being placed in one end of the maze that contained an object (Object A1) covering one food well and a wood block covering the other well. The rat was allowed to retrieve the food rewards and explore both objects for 1 min. The sliding door was then raised allowing access to the second compartment. Once the rat ran to the opposite side of the maze the sliding door was lowered, the rat could now explore a novel item (Object B1) and a familiar item (Object A2, a duplicate of Object A1 from Trial 0). Both the novel and familiar objects covered wells that contained a reward, so every object on every trial was rewarded. After a minute, the sliding door was raised again, and the rat ran back to the first compartment of the maze (Trial 2) where Object C (C1; novel) and a duplicate of Object B (B2; familiar) were presented. After 1 min, the sliding door was raised again (Trial 3), and the rat ran back into the second compartment to explore a copy of Object C (C2; familiar) and new Object D (D1; novel). This process was repeated with new objects until 10 trials had been completed. All objects covered only one food pellet. This arrangement motivated the rats to approach the objects, but did not affect the validity of the behavioral test of recognition, which relied on differential levels of exploration between the two baited objects. Animals were video-recorded during all sessions.
60 min retention
Following completion of the 1 min retention procedure, the rat was removed from the bow-tie maze and returned to its home cage in the colony room. After a 60 min delay from the first trial of the short retention procedure, the rat was returned to the bow-tie maze and received a further 10 trials (Table 2A). The session began by placing the rat into one compartment of the bow-tie maze with two objects covering the food wells: one object was a third copy of familiar Object A (A3; seen 60 min earlier) and the other was novel Object L (Trial 1). After a minute, the sliding door was raised and the rat ran to the second compartment where a copy of familiar Object B (B3; seen 60 min earlier) and novel Object M were presented (Trial 2). Trial 3 consisted of familiar Object C versus novel Object N and so on. All of the familiar items had triplicate copies.
Object Recency Memory: Between-Block Recency (Experiment 3)
Each of the four test sessions comprising Experiment 3, which was separated by at least 14 days from the previous test session, contained three phases (see Figure 2 and Table 2B). In sample Phase 1, rats received eight trials (plus Trial 0 in which the first object was sampled, see Table 2B) of standard object recognition, where the procedure was identical to the 1 min delay condition described above (see Experiment 2 procedure). Sample Phase 2, which followed after a variable interval, consisted of eight more trials of standard object recognition (1 min retention), using new sets of objects. In this way, objects were presented to the rats in two distinct, temporal blocks (see Figure 2). The third phase assessed recency judgments. Each of the eight trials in Phase 3 (see Table 2B) involved an object from sample Phase 1 paired with an object (more recent) from sample Phase 2 (Table 2B). The recency phase began by placing the rat back inside the bow-tie maze. Copies of the first object from each of the two standard recognition object lists (i.e., Object A3 and Object J3; see Table 2B) were presented. The rat had 1 min to explore the items and retrieve the sucrose pellets under both objects. The sliding door was then raised, and the rat ran to the second compartment where copies of the second object from each of the standard recognition phases were presented (i.e., Object B3 and Object K3) and so on for eight trials.
Figure 2. Illustration of the three different difficulty levels used in the between-block object recency task (Experiment 3). The different shaded boxes indicate the three different phases: white = sample (standard recognition) Phase 1, light gray = sample (standard recognition) Phase 2, and dark gray = recency (test) phase. The two standard recognition phases were 9 min in duration and the recency (test) phase was 8 min in duration. The time between the boxes and the length of the lines indicate the duration of the delays. SP1 = sample Phase 1; SP2 = sample Phase 2.
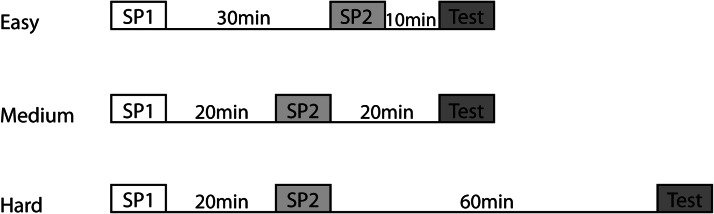
In order to vary the difficulty of the recency task, rats received four test sessions, categorized as easy, medium (two sessions), and hard. The rationale was that objects close together in time would be more difficult to distinguish and that performance would decline with extended retention delays before recency testing (see Figure 2). The initial recognition phases (sample Phases 1 and 2) were separated by either 30 min (easy) or 20 min (for both medium and hard). The final delay before the start of the recency phase (from sample Phase 2 to recency Phase 3) was varied from 10 min (easy) to 20 min (medium) and to 60 min (hard; see Figure 2). During each delay the rat was returned to its home cage but kept inside the test room. The medium difficulty condition was tested twice to help ensure that the performance of the control rats was above chance.
Within-Block Recency (Experiment 4)
The rats received two test sessions, with no object repeats across sessions. Each session involved an 18-trial sample phase and an eight-trial recency (test) phase (Table 2C), all within a continuous block of trials (see Table 2C). The first trial of the sample phase began by placing the rat inside one end of the bow-tie maze. The rat was given 1 min to push aside and explore two identical objects (A1, A2) that each covered a food well (Table 2C). The sliding door was then opened, allowing the rat to run across to the second compartment where two copies of novel Object B (B1, B2) were present (Trial 2). The rat had 1 min to explore these items and obtain the sucrose pellets. Then the sliding door was opened and the rat ran back to the first compartment where two copies of novel Object C (C1, C2) were presented (Trial 3). Following the final trial of this sample phase (Trial 18), the sliding door was raised allowing the rat to change compartments. The recency phase began immediately, so the rat was not removed from the apparatus nor was the rat handled between two phases.
For within-block recency testing, the rat could explore for 1 min two objects of different recency on each trial. As before, every object covered a food reward. Thus, Trial 1 of the recency (test) phase might consist of copies of Object E (E3) and Object M (M3). After a minute, the rat was allowed to run to the other side of the maze to find copies of Object B (B3) and Object R (R3; Trial 2; see Table 2C for remaining trials). The number of interleaving items between the two objects was set at 3, 7, 11, or 15. Every item was experienced in the same compartment end of the maze for both the sample and recency test phases. Trials with different numbers of interleaving items were intermixed.
Analysis of Behavior
Exploration of an object was defined as directing the nose at a distance of <1 cm to the item and/or touching it with the nose or the paws (including pushing). Sitting on or turning around the item was not included. It was also observed that the rats spent time chewing, carrying the items in their mouths, and freezing near or above the items (at a distance of <1 cm). These behaviors were also excluded. The videos were scored blind to lesion group assignment.
Two discrimination indices (D1 and D2) were calculated (Ennaceur & Delacour, 1988). The recognition index D1 was calculated by subtracting the time spent exploring the familiar item from the time spent exploring the novel item (i.e., time novel–time familiar) and was summed across trials (cumulative D1). The D2 index takes the differential exploration time for the pair of objects (i.e., the D1 score) and then divides it by the total time spent exploring both the novel and the familiar item. The D2 score yields a ratio between −1 and + 1, where a positive score indicates a preference for the novel item. The D2 score was updated after every trial by using the summed (updated D2) data. (Note that the final updated D2 score is not equivalent to the mean of each D2 score for every trial).
The D1 and D2 indices were also calculated for the between-block and within-block recency tasks (Experiments 3 and 4). However, because both of the items were familiar, the time spent exploring the recent item was subtracted from the time spent exploring the older item (i.e., time older–time recent). For the D2 ratio, a positive score indicates a preference for the older item.
Odor Recognition and Recency (Experiments 5 and 6)
Apparatus
The experiments used the bow-tie maze, as described above. The odors were presented in visually identical blue plastic cubes (Figure 1C). There were a total of 36 triplicate sets of cubes (5 cm × 5 cm × 5 cm), each containing a different aroma, for example, rose, red apple, popcorn (Vortex Cubes, Dale Air, UK). Every cube was pierced with six holes on the top to dispense the aromas. The odor cubes were repeated across some of the experiments, but no odor was experienced within the same month.
These experiments were conducted in the dark as previous research suggests that rats may use odor cues to guide spatial behaviors in darkness, but less so in the light (Lavenex & Schenk, 1995). Furthermore, previous studies of normal rats in the bow-tie maze indicated that the dark might aid odor recognition (Albasser et al., 2011). Consequently, all sources of illumination were switched off or blocked, resulting in a light intensity of 0.11 lux in the center of the maze. The darkness was such that the experimenters could not see their hands in front of their eyes and therefore wore night vision goggles (Productive Firm Dipol Ltd., Vitebsk, Belarus). The sessions were recorded with two infrared cameras (Maplin Electronics, UK) fixed directly above the maze, as there is no evidence that rats can see at these wavelengths (Burn, 2008).
Odor recognition
In Experiment 5, we used 21 triplicate sets of cubes, each with a different aroma. These cubes were randomly selected from the pool of 36 odor cubes. In Experiment 5, we matched the procedures used for object recognition in the light (Experiment 2) so that recognition was tested after 1 min (10 trials) and 60 min (10 trials) in two consecutive test sessions administered on the same day (see Table 2A). The presentation of the odors was counterbalanced. Half of the rats experienced the list of odors in one order (A–K), whereas the other half experienced the list in reverse order (K–A). The relative left and right positioning of the items was also counterbalanced across rats.
Odor recency (between-block)
The procedure for Experiment 6 matched that for between-block recency (Experiment 3), except that only one set of time intervals was used. For odor recency there was a 30 min delay between the two sample (standard recognition) phases and a 10 min delay between the second sample phase and the recency phase (i.e., the easy condition; see Figure 2). The rats received two test days using these particular delays. The rats were placed in an adjacent well-lit, quiet, test room during the delay periods. This room did not contain any odor cubes. (Rats were also tested on an odor within-block recency task, but the control rats could not perform above chance, and therefore this study is not reported).
Object Recognition in an Open Arena (Experiment 7)
Apparatus
The apparatus consisted of a large, square arena with wooden walls and floor, measuring 100 cm wide × 100 cm long × 46 cm high. The arena was located on the floor in the center of a room (300 cm × 300 cm × 240 cm), which was the same as used in Experiment 1. The walls of the arena were painted gray, and the floor was covered in sawdust. A checkered curtain divider (169 cm high and 242 cm wide) was placed outside much of the arena, thereby obscuring distal room cues.
Objects
Six identical copies of four objects were used so that the objects presented at the sample and test phases were duplicates and, hence, could not be odor marked. These objects were sufficiently heavy to prevent the rats from pushing them. The four objects were (a) a large tin can, (b) a clear plastic water bottle, (c) a rectangular baking tray, and (d) a large glass Nutella jar (Nutella, Ferrero, Watford, Hertfordshire, UK). All the labels were removed, and the objects were thoroughly cleaned prior to their use. Presentation of the objects was counterbalanced so that half of the rats experienced Set A as the sample objects and Set B as the novel objects, and the other half experienced Set B as the sample and Set A as novel.
Pretraining
The rats were habituated to the open field arena for 2 days. On Day 1, the rats were placed inside the arena in groups of two or three for 10 min. On Day 2, the rats were placed inside the arena one at a time for 5 min. Two objects (a blue beer can and a large ceramic figurine of Snoopy, 15 cm high from Peanuts, a comic strip by Charles M. Schulz) were placed 10 cm away from the middle of the left and right walls, respectively. These two objects were only used during the two days of habituation and were not present during testing.
Testing
The rats received two trials on consecutive days. Each trial consisted of a sample and choice phase. During the 5 min sample phase, the rat was placed in the center of the maze and allowed to explore four identical copies of the sample item (A1, A2, A3, A4) located in each corner, approximately 10 cm from the walls. The rat was then placed in a metal carrier box for 15 min before being returned to the open arena for the choice phase. During the choice phase, two new copies of the same sample objects (A5, A6) occupied two adjacent corners, and two identical novel objects (B1, B2) were placed in the remaining two corners. The location of the pairs of choice (test) objects was counterbalanced between rats as well as between the first and second test days. Assessment of the behavioral measures matched that described for Experiment 2.
Locomotor Activity (Experiment 8)
Apparatus and Room
On one wall of a novel room (272 cm × 135 cm × 240 cm), a 3 × 6 activity test cage rack was located. The cage rack contained 18 activity test cages (Paul Fray, Cambridge, UK). The cages measured 56 cm × 39 cm × 19 cm and contained two photobeams positioned 18 cm from the short walls 20 cm apart.
Procedure
The rats were tested in the activity boxes approximately 12 months postsurgery. Each rat was taken into the room and placed individually inside an activity test cage. The room was illuminated and the locomotor activity of the rat was recorded for 20 min. The activity period was divided into 20 one-min bins. The number of beam breaks (a single beam being repeatedly broken) as well as beam crossovers (both the front and back beams broken sequentially) for each bin was recorded.
Statistical Analyses
Recognition and Recency Memory (Experiments 2–7)
As D2 scores can better compensate for differences in the total amount of exploration of the items, the D1 scores are only reported when they differ qualitatively from the D2 scores. For recognition memory, one-sample t tests (one-tailed) were conducted using the cumulative data (D1 and D2) from the final test trials to assess whether the animals showed a preference for the novel (recognition) or the less recent (recency) object. For Experiments 2 and 5, the discrimination scores (D1 or D2) for the two groups (Sham and ATNx) were compared in a one between-subjects (Groups: Sham and ATNx) by one within-subject (Delays: <1 min and 60 min) analysis of variance (ANOVA). Simple effects for each condition were analyzed using the pooled error term when significant interactions were found, as recommended by Winer (1971). When the data violated the assumptions of parametric tests (e.g., homogeneity of variance, normality), nonparametric tests were used for statistical analysis (e.g., Mann–Whitney instead of a t test). When the data violated sphericity (repeated measures designs), the Greenhouse-Geisser correction was applied, as recommended by Fields (2005). Any exceptions to the above are signified.
For the between-block recency task using objects (Experiment 3), a one-way between-subjects factor (Groups: Sham and ATNx) × one within-subjects factor (Difficulty: easy, medium, hard) was used to analyze the discrimination scores (D1 and D2) of the recency phase data. A three-way mixed-model ANOVA was used to analyze the six sample phases that also taxed recognition (one between-subjects factor: Group [ATNx, Sham] and two within-subjects factors: Difficulty [easy, medium, hard] and Recognition performance [Phase 1, Phase 2]). Because the medium difficulty condition was run twice, the mean D1 and D2 scores of both test sessions was used for these statistical analyzes. Again, one-sample t tests (one-tailed) were used to determine whether the ATNx and Sham groups preferred the older object, that is, if their scores were above chance. Because the between-block recency task using odors (Experiment 6) only examined one delay condition (easy), a between-sample t test (two-tailed) compared the recency choices of the Sham and ATNx groups.
For the within-block recency test (Experiment 4), the mean discrimination scores (D1 and D2) across trials with the same number of interleaving items were calculated across the two test sessions (each test session contained eight trials, two at each of the four different ITIs, 3, 7, 11, 15). These data were first divided into a low (3 + 7) and a high (11 + 15) number of interleaving items and then compared by using a two-way mixed factors ANOVA (Group [ATNx, Sham] × No. of interleaving items [low, high]). As a significant effect of number of interleaving items (low, high) was not found, these data were pooled across all numbers of interleaving items (mean scores), and one-sample t tests (one-tailed) examined whether the rats significantly preferred the older item.
Results
Histology
Figure 3 shows the extent of the lesions in those rats with the smallest and largest amount of tissue loss. Three animals (not depicted) were excluded from all analyses as their lesions involved less than 50% of the anterior thalamic nuclei. In the remaining 12 cases, cell loss was always centered in the anterior thalamic nuclei, making them the only consistent lesion site across all cases. The total cellular volume loss within the anterior thalamic nuclei was from 52%–94% (M = 76%, Mdn = 76%). The lesions were slightly asymmetrical with most rats (n = 8) having more damage to the left hemisphere. Any sparing typically occurred caudally in the most ventral portion of the anterior medial nucleus. However, two rats exhibited the opposite pattern; that is, rostral sparing with a more complete lesion at the caudal end of the anterior thalamic nuclei. These two animals had some sparing to the anterior dorsal nucleus. In 11 out of the 12 cases, there was partial damage to the rostral and dorsal portions of the laterodorsal nucleus, which in three cases was unilateral. In those rats with larger lesions, there was also some damage to the parataenial nucleus (n = 7; unilateral in two cases), the paraventricular nucleus of the thalamus (n = 3), the reticular nucleus (n = 6; unilateral in three cases), and rostral nucleus reuniens (n = 7).
Figure 3. The extent of the anterior thalamic lesion in the rat with the smallest (dark grey) and largest (light grey) anterior thalamic lesion. The coronal sections are taken and adapted with permission from The Rat Brain in Stereotaxic Coordinates (5th ed., pp. 42–52), by G. Paxinos & C. Watson, 2005.
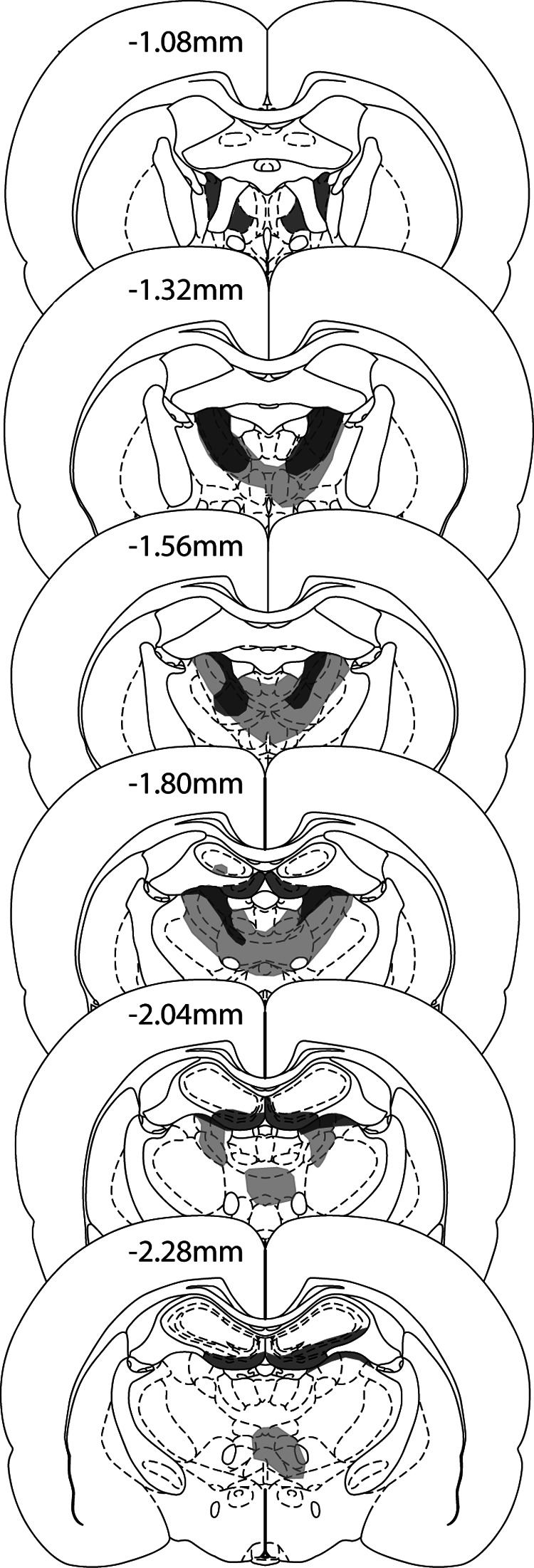
In all but one case, there was limited bilateral cell loss in the hippocampus; the exception had unilateral damage. Most of the cell loss was restricted to the most rostral part of the ventral (inferior) blade of the dentate gyrus (n = 12; in one case the damage was unilateral) and sometimes extended into the immediately adjacent CA3 (n = 9; although in three cases this damage was unilateral). A mean of 3.3% of the total hippocampus was damaged with a range of 0.2%–5.8%. In four cases there was some indication of fornix distortion in addition to the NMDA injection track lines. In one of these cases, the fornix damage appeared restricted and primarily placed unilaterally, whereas in the remaining three cases, the fornix appeared shrunken. Inspection of the behavioral data indicated that these four rats with additional fornix changes did not perform significantly differently to rest of the lesion group.
Behavioral Testing: T Maze Alternation (Experiment 1)
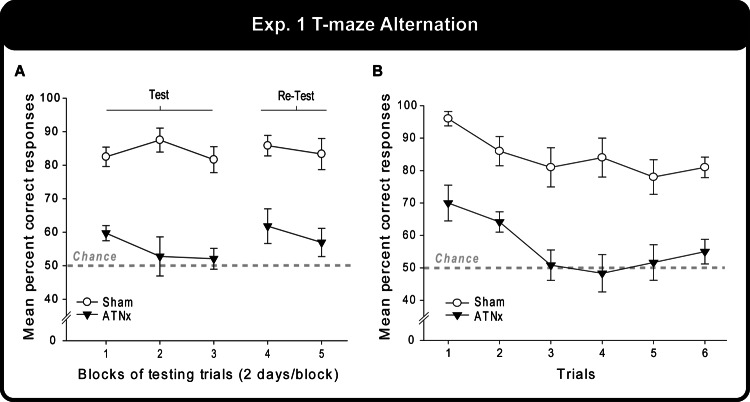
Figure 4A shows the very striking difference in performance between the Sham animals (over 80%) and the ATNx animals (close to chance). Using data combined over pairs of successive sessions, the ATNx group was significantly impaired compared with Sham animals: test, F(1, 20) = 61.5, p < .001; re-test, F(1, 20) = 20.1, p < .001. The size of the apparent deficit remained stable across the two test periods, and there were no differences in levels of performance across the two test periods (F < 1).
Figure 4. (A) The mean percentage of correct responses over blocks of testing trials on initial acquisition of a T maze alternation task and during re-test. (B) The same data recalculated for each individual trial, with the mean correct responses displayed. Data shown are group means, and the vertical bars are the standard error of the means (SEM). Fifty percent represents chance (i.e., the likelihood of choosing either arm in the T maze).
The correct responses of the Sham and ATNx animals were also tabulated for each of the six trials across all 10 test days (total correct out of 10; Figure 4B). The main effect of trial showed that the rats performed best on the earlier alternation problems, F(5, 100) = 5.36, p < .001. The Sham group performed at 91% correct on the first trial but dropped to 80% by the sixth, whereas the ATNx group chose the correct arm of the T maze 67% of the time on the first trial—which is significantly above chance, t(11) = 3.63, p = .004—but dropped to chance performance (53%) by the last trial.
Object Recognition in the Bow-Tie Maze (1 and 60 min retention; Experiment 2)
The cumulative D1 and the updated D2 scores are shown in Figure 5A-C. As can be seen in Figure 5B, the D2 score stabilizes across the session, and its variance diminishes as trial numbers increase. This reduction in variance is typical for this apparatus and aids group comparisons. The D1 score, meanwhile, continues to increase across trials.
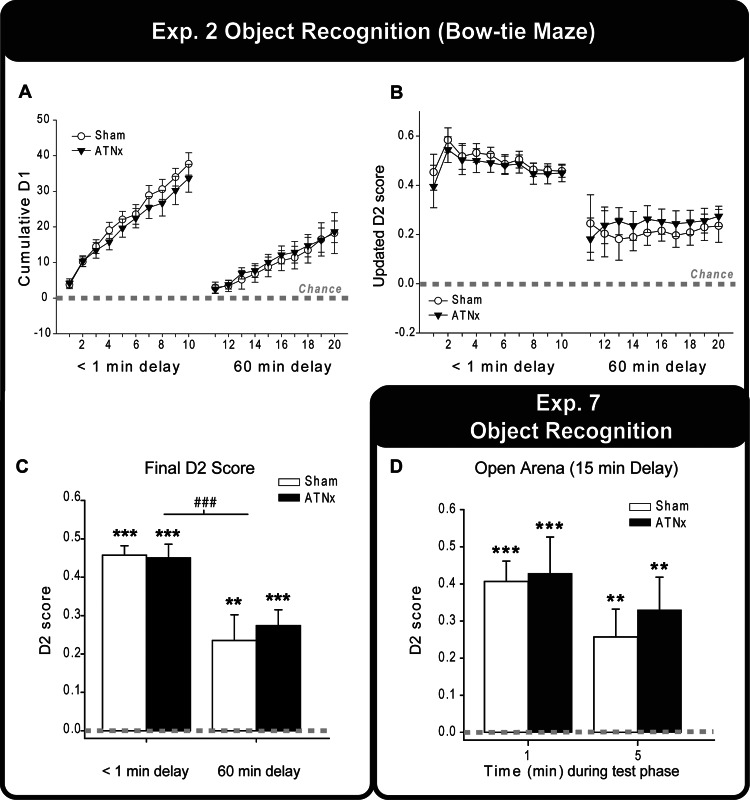
Figure 5. The cumulative D1 scores (A) and updated D2 scores (B) of the Sham and ATNx groups across trials during the 1 min and 60 min retention object recognition task. As the trials progress, the variance in the D2 scores decreases. (C) The final updated D2 scores of the <1 min and 60 min delay for the Sham and ATNx groups are depicted. (D) The updated D2 scores of the Sham and ATNx animals for the first or the entire 5 min test session of an object recognition task in an open arena (15 min delay; Experiment 7). Data shown are group means, and the vertical bars are the standard error of the means (SEM). Zero represents chance (i.e., exploring novel and familiar items equally). Significantly different from chance: **p < .01. ***p < .001. Significant effect of delay: ### p < .001.
The D2 scores (Figure 5B and 5C) indicated that the recognition performance of both groups was significantly above chance both when the delay period approached 1 min, that is, standard recognition test—Sham, t(9) = 18.5, p < .001; ATNx, t(11) = 12.6, p < .001—and when the retention period was 60 min—Sham, t(9) = 3.52, p = .003; ATNx, t(11) = 6.65, p < .001. The two-way mixed model ANOVA revealed that there was no difference between the Sham and ATNx groups (p > .1), nor was there an interaction with delay (p > .1). There was, however, a significant main effect of delay indicating a fall in recognition performance from the 1 min to the 60 min retention periods, F(1, 20) = 20.1, p < .001.
The mean cumulative total exploration of the objects with a 1 min delay was 82.2 s for the Sham group and 72.9 s for the ATNx group. Likewise, the mean cumulative total exploration following a 60 min delay was 81.1 s for the Sham group and 70.6 s for the ATNx group. There was no significant effect of Group, Delay, or interaction in the amount of time the rats spent exploring both the familiar and the novel objects (i.e., total exploration; all p > .1).
Object Recency, Between-Block (List 1 vs. List 2) Testing (Easy, Hard, Difficult; Experiment 3)
Recency discrimination (test phase)
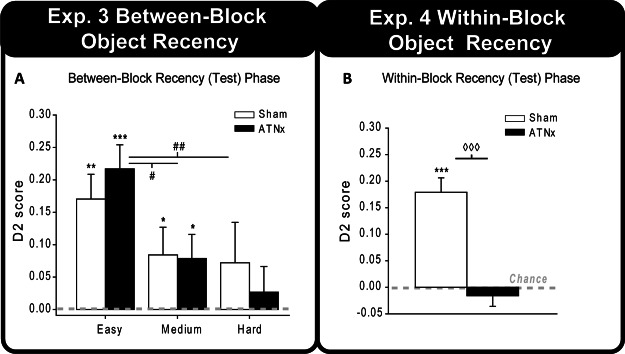
The final D2 scores for the recency (test) phase for the three different levels of difficult (easy, medium, and hard) are displayed in Figure 6A. For both groups the D2 index was significantly above chance in the easy recency task—Sham, t(9) = 4.47, p = .001; ATNx, t(11) = 5.85, p < .001—and the medium condition—Sham, t(9) = 1.97, p = .04; ATNx, t(11) = 2.13, p = .029. In contrast, when the difficulty was increased to hard, neither group showed a preference for the less recent objects, both p > .1. Analysis of the recency task with a two-way ANOVA helped to confirm the effect of task difficulty, F(2, 40) = 5.05, p = .011, though there was no difference between the Sham and ATNx groups, nor was there a Group × Task Difficulty interaction (p > .1, for both). Pairwise comparisons of difficulty levels collapsed across the two groups revealed that the D2 scores of the rats differed between the easy and hard conditions, t(21) = 3.56, p = .002, as well as the easy and medium conditions, t(21) = 2.75, p = .012, but not between the medium and hard conditions (p > .1; all comparisons corrected using Bonferroni).
Figure 6. (A) The updated D2 scores for the recency (test) phase of the between-block recency task with objects. (B) The mean D2 scores of the ATNx and Sham groups for the within-block recency test. Data shown are group means, and the vertical bars are the standard error of the means (SEM). Zero represents chance (i.e., exploring novel and familiar, or older and more recent, items equally). Significantly different from chance: * p < .05. ** p < .01. *** p < .001. Significant effect of task difficulty: # p < .05. ## p < .01. Significant group difference: p < .001.
Object recognition (Sample Phases 1 and 2)
Throughout the two sample phases, the rats were also tested on object recognition (1 min retention). One-sample t tests using the D2 Index revealed that both groups of rats had a consistent preference for the novel objects during the sample phases for all three recency difficulty levels (i.e., a total of six standard recognition phases, two for each of the three difficulty conditions for the Sham and the ATNx groups; all ps < .001). A three-way mixed model ANOVA assessed the D2 scores during the two standard recognition phases for the three different recency tasks (i.e., easy, medium, hard). There was a significant effect of group, but this indicated that overall the ATNx group performed better than Sham rats, F(1, 20) = 5.33, p = .032. No interactions were significant nor was there a significant effect of standard recognition phase (i.e., Phase 1 vs. Phase 2; all ps > .1). Comparable analyses with the D1 scores failed to yield a significant effect of group, standard recognition phases, or interactions (all ps > .1).
The total amounts of exploration did not differ between the Sham and ATNx groups during the sample stage (Phases 1 and 2) or the recency phase (p > .1, for both). However, there was a significant effect of task difficulty for the recency phase, as greater difficulty increased object exploration, F(2, 40) = 6.82, p = .003. It was also found, overall, that rats explored the objects in Phase 1 of the sample stage more than those in Phase 2, F(1, 20) = 9.79, p = .005.
Object Recency, Within-Block Testing (Experiment 4)
The recency (test) phase was analyzed by first grouping the number of interleaving objects into low (3 or 7 interleaving objects) and high (11 or 15 interleaving objects) categories. Using the D2 index, an ANOVA indicated that the ATNx rats were significantly impaired compared with Sham rats, F(1, 20) = 34.5, p < .001. However, there was no effect of the number of interleaving items (i.e., no effect of high and low numbers of interleaving items) or a Group × Interleaving Item interaction (p > .1, for both). Because of the lack of effect of the number of interleaving items, the data were collapsed across the high and low interleaving items categories (Figure 6B). One-sample t tests of the D2 scores indicated that the Sham group showed a clear preference for the older items, t(9) = 6.59, p < .001, whereas the ATNx group scores did not differ from chance (p > .1).
The mean total amount of time spent exploring the objects during the choice phase did not differ between the Sham and ATNx groups when the data were divided into low and high categories or collapsed across the number of interleaving items (for both, p > .1). Furthermore, the cumulative total exploration during the sample phase did not differ between the Sham and ATNx groups (p > .1). Because the within-block recency design does not allow for standard measures of object recognition to be analyzed (e.g., D2), an ANOVA examining the mean total exploration during the sample and choice phase was used to examine any subsidiary recognition measures (Albasser et al., 2011). The results confirmed that the rats showed some measures of recognition (or habituation) as they explored the objects significantly less during the test phase compared with the sample phase, F(1, 20) = 84.6, p < .001. The main effect of group and the interaction term were both nonsignificant (F < 1).
A final set of analyses looked at the relationship between the extent of pathology in the target area and performance on the recency task using all 15 animals that had received thalamic surgeries. A significant correlation was found between extent of anterior thalamic damage and the D2 recency scores (r = −.54, p = .038), such that poorer performance was associated with greater cell loss. The same analysis was conducted for the amount of unintended hippocampal damage. This correlation was not significant (r = .48, p = .072) and was in the opposite direction, that is, better performance with more hippocampal damage. The unexpected direction of this second correlation can be readily explained as there was a significant negative correlation between the extent of anterior thalamic damage and the extent of hippocampal damage across the 15 animals (r = −.76, p = .001), that is, those with the greatest thalamic damage tended to have the least hippocampal damage.
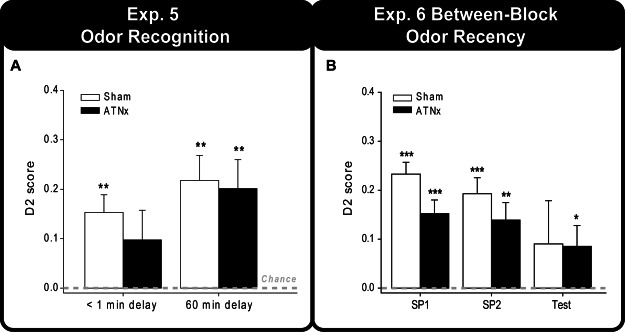
Odor Recognition (Experiment 5)
Figure 7A displays the final updated D2 score for both the standard recognition phase (less than 1 min delay) and the 60 min delay phase. One-sample t tests demonstrated that the Sham group was significantly above chance at delays of less than a minute and 60 min—1 min, t(9) = 4.29, p = .001; 60 min, t(9) = 4.30, p = .001—whereas the ATNx group showed a preference for the novel odors only at the 60 min retention—1 min, t(11) = 1.62, p = .067; 60 min, t(11) = 3.45, p = .0025. However, there is no significant effect of group, delay, or interaction (p > .1 for all). Inspection of Figure 7A suggests that the large variance in the performance of the ATNx group accounts for the failure to detect a significant preference for the novel odors in the standard recognition task.
Figure 7. (A) The updated D2 scores of the Sham and ATNx animals for the odor recognition task in the bow-tie maze. (B) The updated D2 scores for the two sample phases and the recency (test) phase of the between-block odor recency task. Data shown are group means, and the vertical bars are the standard error of the means (SEM). Zero represents chance (i.e., exploring novel and familiar items equally). SP1 = sample Phase 1; SP2 = sample Phase 2. Significantly different from chance: *p < .05. **p < .01. ***p < .001.
The mean cumulative total exploration of the odors with a 1 min delay was 65.8 s for the Sham group and 63.4 s for the ATNx group. The mean cumulative total exploration following a 60 min delay was 43.9 s for the Sham group and 45.3 s for the ATNx group. Although there were no group differences in total exploration of the odors (p > .1), both groups spent significantly more time exploring the odors in the standard recognition condition compared with the 60 min delay condition, F(1, 20) = 10.1, p = .005.
Odor Recency, Between-Block (List 1 vs. List 2) Test (Experiment 6)
The final D2 scores for the two sample phases (i.e., standard recognition) and the recency test phase are displayed in Figure 7B. For the recency discrimination scores (D2), there was no statistical difference between the Sham and ATNx groups (p > .1). Nevertheless, although the ATNx group performed above chance, ATNx, t(11) = 1.99, p = .036; the Sham group failed to show a significant preference for the older odor (Sham, p > .1). The same pattern was found for the D1 scores as the ATNx group performed above chance, ATNx, t(11) = 2.03, p = .034, but the Sham rats failed to do so (p > .1).
Once again, it was possible to look at performance on the recognition tests (1 min retention) embedded within the sample phases. One-sample t tests revealed that both groups preferred the novel odor during the two sample phases—Sham recognition Phase 1, t(9) = 9.65, p < .001; Sham recognition Phase 2, t(9) = 5.87, p < .001; ATNx recognition Phase 1, t(11) = 5.44, p < .001; ATNx recognition Phase 2, t(11) = 3.91, p = .001. An ANOVA that compared the D2 scores of the ATNx and Sham groups across the two standard recognition phases revealed a significant effect of group; although the ATNx rats preferred the novel odor, their level of discrimination was significantly lower than that of the Sham rats, F(1, 20) = 5.81, p = .026. However, unlike the D2 scores, an ANOVA examining the D1 scores of the two standard recognition phases did not yield any significant group differences (p > .1).
There was no difference in the total amount of exploration of the odor cubes between the Sham and ATNx groups during both the sample and recency choice phases (p > .1, for both). However, there was a significant order effect in the sample phase indicating that animals explored the odors in the second standard recognition phase less than the first, F(1, 20) = 4.93, p = .038.
Object Recognition in an Open Arena (Experiment 7)
The D2 recognition index scores for the first minute and the whole 5 min of the choice phase are shown in Figure 5D. The exploration data showed that both sets of rats could discriminate the novel objects when assessed after the first minute—one-sample t-tests Sham, t(9) = 7.39, p < .001; ATNx, t(11) = 4.38, p < .001—and after the whole choice session—Sham, t(9) = 3.41, p = .004; ATNx, t(11) = 3.73, p = .002. Furthermore, the D2 scores of the ATNx and Sham groups did not differ after either the first minute or the whole 5-min choice period (both p > .1).
Although the discrimination indices of the groups did not differ (D1 and D2), the Sham group spent significantly more time exploring both objects within the first minute of the choice phase, t(20) = 2.64, p = .016 (mean total exploration was 38.6 s and 31.4 s for the Sham and ATNx groups, respectively), but not for the whole 5 min of the choice phase (p > .1). There was no group difference for the total amount of exploration during the 5 min sample phase (p > .1).
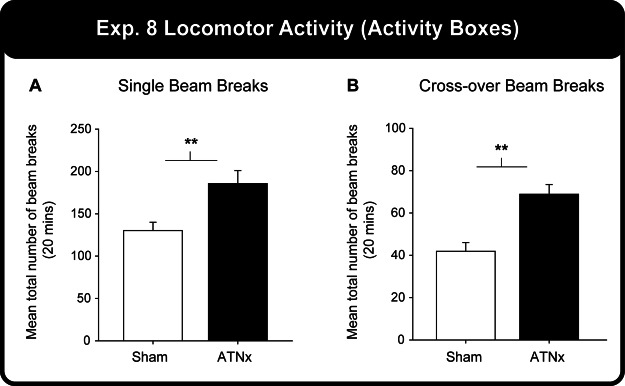
Locomotor Activity (Experiment 8)
Evidence that the ATNx group was hyperactive came from the total number of single and crossover beam breaks (see Figure 8). The ATNx group made significantly more single beam breaks (Mann–Whitney U = 16.0, p = .004) and crossover beam breaks (Mann–Whitney U = 10.0, p = .001) than the Sham group. (Nonparametric tests were used because of a violation of data normality).
Figure 8. (A) The total number of single and (B) crossover beam breaks for the Sham and ATNx groups. Significant group difference: **p < .01.
Discussion
Both recognition memory and recency memory were examined in rats with cytotoxic lesions of the anterior thalamic nuclei. Object recognition memory consistently appeared intact, despite using more than one procedure and testing different levels of performance. Likewise, odor recognition memory appeared largely unaffected. The same animals were also tested on their ability to discriminate two items based on their temporal separation (recency memory). Rats with anterior thalamic lesions could successfully discriminate those objects or odors belonging to the earlier of two lists of items presented with a distinct gap between list one and list two (between-block recency). In contrast, the anterior thalamic lesions impaired object recency discriminations when early and late objects were taken from within the same continuous list of objects (within-block recency). Within-block odor recognition is not, however, reported as normal rats could not solve the task.
Additional findings included severely impaired T maze alternation following anterior thalamic damage. This persistent deficit not only helps to confirm the effectiveness of the present surgeries (see also Aggleton, Amin, Jenkins, Pearce, & Robinson, 2011; Aggleton et al., 1995; Aggleton, Poirier, Aggleton, Vann, & Pearce, 2009) but also is of interest as the task taxes recency judgments. That is, the rat selects between two familiar locations or directions and avoids the most recent. Both the Sham and ATNx rats performed best on the first trial of the session (least proactive interference), but it was not possible to determine whether this effect was more or less pronounced in rats with anterior thalamic lesions as there were still marked differences in performance levels, that is, there were scaling effects. Consequently, the pattern can be explained by underlying deficits with spatial memory (Aggleton et al., 1995; Sutherland & Rodriguez, 1989; Sziklas & Petrides, 1999, 2007) without recourse to a specific recency deficit.
All previous studies of object recognition memory after anterior thalamic damage in rats have measured the spontaneous exploration of new and old items within a large arena. No significant lesion effects have been reported (Aggleton et al., 1995; Mitchell & Dalrymple-Alford, 2005; Moran & Dalrymple-Alford, 2003; Warburton & Aggleton, 1998). The behavioral measure is, however, spontaneous and consequently prone to much variance, a problem compounded when rats receive few trials. To remedy these problems, the bow-tie maze task can provide multiple trials per session (Table 2; see also Albasser, Chapman, et al., 2010). One benefit was the reduction in variance for the D2 index as the trial data accumulated (Figure 5B).
There was no evidence that anterior thalamic damage disrupts the discrimination of novel from familiar objects (in both the bow-tie maze and open arena) across different levels of task difficulty (Experiments 2, 7). The related finding from Experiment 5, that odor recognition appears intact, matches a previous study of odor recognition that used both match-to-sample and nonmatch-to-sample procedures (Wolff et al., 2006). In that study, rats received a sample list of six odors before being rewarded for selecting an odor when it was subsequently repeated. The rule was then reversed to nonmatching, that is, select the novel odor. In fact, because there was only a total pool of 18 odors, no odor was truly novel but rather had not been presented for days rather than minutes (Wolff et al., 2006). In addition, recognition data in this study came from the sample phases of the between-block recency tests (Experiments 3 and 6). To familiarize the stimuli for subsequent recency tests, all rats received two lists of objects (or odors) presented as for the standard recognition procedure (1 min delay). There was no evidence of a lesion-induced object recognition deficit on any of the six sets of sample–recognition phases (each eight trials). Although the ATNx rats significantly preferred the novel odor during the sample phases of the odor recency test, their level of preference appeared depressed compared with the Sham group (D2 only). This small deficit with the 1 min retention periods was not, however, found when the same stimuli were tested for recency judgments after a 60 min delay.
Throughout the study, the sampling levels for both object exploration and odor exploration remained unaffected by the anterior thalamic lesions. The only exception was during the first minute of the 5 min choice phase in Experiment 7 (open arena, object recognition). Despite these null effects, locomotor activity in a novel environment was increased in the ATNx rats (Experiment 8). This hyperactivity is consistent both with previous anterior thalamic lesion studies (Jenkins, Vann, Amin, & Aggleton, 2004; Poirier & Aggleton, 2009; Warburton et al., 1999) and with the effects of hippocampal damage (Davidson & Jarrard, 2004; Gray & McNaughton, 1983) and may partially reflect spatial impairments.
The present findings for rat recognition memory are potentially discrepant with studies of macaque monkeys (Macaca fascicularis). Lesions centered in the anterior thalamic nuclei of monkeys disrupt delayed nonmatching to sample for visual stimuli (Aggleton & Mishkin, 1983a, 1983b). Several points should, however, be made. First, the surgeries in monkeys were more extensive, including thalamic midline nuclei such as nucleus reuniens. Second, the task was food rewarded (not spontaneous). In attempting to explain these apparent cross-species differences, it should be noted that monkeys with lesions in the adjacent medial dorsal thalamic nucleus are also consistently impaired on visual recognition memory (Aggleton & Mishkin, 1983a, 1983b; Parker, Eacott, & Gaffan, 1997; Zola-Morgan & Squire, 1985). In contrast, medial dorsal thalamic lesions in rats spare object recognition performance, once the task rules have been acquired (Aggleton, Dumont, & Warburton, 2011; Cross, Aggleton, Brown, & Warburton, 2013; Hunt & Aggleton, 1998; Mitchell & Dalrymple-Alford, 2005; Mumby, Pinel, & Dastur, 1993). Thus, despite the various procedural differences across species, there is the likelihood that in rats, unlike monkeys, the medial and anterior thalamic nuclei are less critical for visual recognition. This potential species difference could reflect the more focused importance of the perirhinal cortex and area Te2 for rodent visual recognition memory, which contrasts to the arrangement in primates where wider networks of structures, including the prefrontal cortex, anterior thalamic, and medial thalamic nuclei, all appear vital (Aggleton, Dumont, et al., 2011; Bachevalier & Mishkin, 1986). An alternative possibility is that object recognition memory can be solved equally effectively by rats using either visual or nonvisual cues, for example, tactile–vibrissae or olfactory information (Albasser et al., 2011; Albasser, Olarte-Sanchez et al., 2013; Winters & Reid, 2010), and that these nonvisual pathways for recognition are largely unaffected by discrete thalamic damage in rats (so sparing performance). An unresolved issue is, therefore, whether anterior thalamic lesions in rats can impair object recognition when the task relies on vision alone.
The second goal was to examine recency memory, a form of memory more consistently associated with hippocampal function (Albasser et al., 2012; Barker & Warburton., 2011; Fortin et al., 2002; Kesner et al., 2002; Kesner et al., 2010; Warburton & Brown, 2010). No deficits were found when both object recency and odor recency were tested with a well-defined interval (between-block) separating the two lists of stimuli to be compared (Experiments 3 and 6). Furthermore, the between-block protocol made it possible to test the rats for their recognition of the same objects subsequently used for the recency judgments (see also Albasser et al., 2012). The null results matched findings from testing in an open arena, where rats with anterior thalamic lesions could successfully discriminate two objects separated by a 60 min interval, when tested 60 min after the second object (Mitchell & Dalrymple-Alford, 2005). The contrasting deficit for within-block recognition (Experiment 4) is also matched by the previous finding that anterior thalamic lesions disrupt the ability to select an odor presented earlier in a continuous list of odors (Wolff et al., 2006). The extended sequence of behavioral testing (see Table 1) does, however, raise the potential issue of transfer and interference effects. Comparisons across the various recognition memory tests (e.g., comparing performance on the sampling phases of the between-block recency tests, Experiment 3) did show differences in overall performance level but in no systematic order (the second of the three recognition tests proved the most difficult). Although the within-session recency memory task (Experiment 4) was the last to be tested in the bow-tie maze when using objects as test items, the immediately preceding recognition test was solved very effectively despite following several previous tests. Performance on the T maze remained stable across this same period, as demonstrated by the re-test scores (Figure 4A).
Several implications can be inferred from the recency experiments. The first is that the within-block object recency deficit is unlikely to reflect abnormal trace strengths associated with the various stimuli as any such changes would presumably have also affected recognition memory. In fact, the present dissociation between recognition memory (spared) and recency memory (impaired) has been found following lesions in a number of sites in the rat brain, including the hippocampus and prefrontal cortex (Barker et al., 2007; Barker & Warburton, 2011; Hannesson, Vacca, Howland, & Phillips, 2004; Warburton & Brown, 2010), sites reciprocally linked with the anterior thalamic nuclei (Shibata, 1993; Shibata & Naito, 2005; van Groen, Kadish, & Wyss, 1999; Wright, Erichsen, Vann, O’Mara, & Aggleton, 2010; Wyss, Swanson, & Cowan, 1979). Although localized hippocampal cell loss was found in the ANTx rats, it was found that the D2 scores on the within-block recency test correlated with the extent of anterior thalamic damage (more damage, poorer performance).
These lesion dissociations, along with electrophysiological findings, suggest that recency discriminations rely on mnemonic processes distinct from those for recognition memory (Brown & Aggleton, 2001; DeVito & Eichenbaum, 2011; Zhu, Brown, & Aggleton, 1995). A further implication is that there may be a qualitative difference between recency judgments made when there is a clear defining break (between-block) and when there is no such break (within-block) between the temporal orders of the objects to be discriminated (DeVito & Eichenbaum, 2011). Although the within-block condition is arguably the more difficult, the use of three different between-block conditions that tested performance across a range of task difficulties, makes an explanation based on task difficulty alone unlikely. At first sight, the present within-block recency deficit might seem inconsistent with the finding that rats with anterior thalamic lesions can learn the temporal order of pairs of auditory and visual stimuli in an operant chamber (Aggleton, Amin, et al., 2011). The two temporal order tasks are, however, very different. The present task was based on single sample exposures while the operant box task required 39 sessions, each with multiple trials, for the rats to acquire the rule, reinforce if A occurs before B but not if B occurs before A.
The underlying goal was to determine the extent to which anterior thalamic lesions mimic the impact of hippocampal lesions in rats. It is, therefore, significant that a dissociation between recognition memory and recency memory has not only been seen for rats with hippocampal lesions tested in the open arena (Barker et al., 2007; Barker & Warburton, 2011) but has also been demonstrated in the bow-tie maze (Albasser et al., 2012). As the deficits in the latter study were found using a between-block recency design, the effects of hippocampal lesions on the more difficult within-block recency was not examined. Other studies, however, show that hippocampal deficits are found in both within-block and between-block recency tasks (Albasser et al., 2012; Fortin et al., 2002; Kesner et al., 2002, 2010). It can, therefore, be seen that while a parallel between anterior thalamic and hippocampal lesion deficits exists, there is the caveat that the anterior thalamic lesion deficit may be more restricted to within-block recency.
The results suggest that the anterior thalamic nuclei and hippocampus, potentially in association with the prefrontal cortex (Barker et al., 2007; Hannesson et al., 2004; Warburton & Brown, 2010), function together to support specific aspects of temporal discrimination learning. This particular role for the anterior thalamic nuclei may relate to theta, an oscillatory activity pattern that could theoretically help separate a temporal stream of events (Aggleton et al., 2010; Buzsáki, 2002, 2005; Hasselmo & Eichenbaum, 2005). Neurons are present within the anterior ventral nucleus that oscillate within the theta band and are in synchrony with hippocampal theta (Tsanov et al., 2011; Vertes, Albo, & Viana Di Prisco, 2001). Consequently, anterior thalamic lesions might disrupt theta’s role as a temporal organizer for adjacent stimuli through the loss of synchronous theta oscillations within the extended hippocampal system (Bland, Konopacki, Kirk, Oddie, & Dickson, 1995; Colom, Christie, & Bland, 1988; Kocsis & Vertes, 1994; Kocsis & Vertes, 1997; see also Vann & Aggleton, 2004). Such a function could help explain why distinguishing items within a continuous stream of information might be particularly sensitive to anterior thalamic damage yet spare between-block recognition, where additional information types are potentially available. In particular, the present findings indicate that each block of stimuli (e.g., objects) becomes a distinguishable chunk that can be used to make coarse-grained temporal distinctions. The implication is that this coarser, parsing ability is spared after anterior thalamic damage, while more fine-grained temporal distinctions that are enabled by an additional mechanism are impaired. A further implication is that both of these mechanisms are lost after hippocampal damage in rats. Last, there is evidence that damage to the adjacent medial dorsal thalamic nucleus impairs recency memory when tested in an open arena using a between-block procedure (Cross et al., 2013; Mitchell & Dalrymple-Alford, 2005), raising the possibility of complementary temporal mechanisms operating in adjacent thalamic nuclei that are both interconnected with the medial prefrontal cortex.
Acknowledgments
This work was supported by the Wellcome Trust [WT087855] and NSERC. We thank Seralynne Vann.
References
- Aggleton J. P. (1985). One-trial object recognition by rats. Quarterly Journal of Experimental Psychology, 37, 279–294. [Google Scholar]
- Aggleton J. P., Amin E., Jenkins T. A., Pearce J. M., & Robinson J. (2011). Lesions in the anterior thalamic nuclei of rats do not disrupt acquisition of stimulus sequence learning. Quarterly Journal of Experimental Psychology, 64, 65–73. doi:10.1080/17470218.2010.495407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggleton J. P., & Brown M. W. (1999). Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. Behavioral and Brain Sciences, 22, 425–444. doi:10.1017/S0140525X99002034 [PubMed] [Google Scholar]
- Aggleton J. P., Desimone R., & Mishkin M. (1986). The origin, course, and termination of the hippocampo-thalamic projections in the macaque. Journal of Comparative Neurology, 243, 409–421. doi:10.1002/cne.902430310 [DOI] [PubMed] [Google Scholar]
- Aggleton J. P., Dumont J. R., & Warburton E. C. (2011). Unraveling the contributions of the diencephalon to recognition memory: A review. Learning & Memory, 18, 384–400. doi:10.1101/lm.1884611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggleton J. P., Hunt P. R., Nagle S., & Neave N. (1996). The effects of selective lesions within the anterior thalamic nuclei on spatial memory in the rat. Behavioural Brain Research, 81, 189–198. doi:10.1016/S0166-4328(96)89080-2 [DOI] [PubMed] [Google Scholar]
- Aggleton J. P., & Mishkin M. (1983a). Memory impairments following restricted medial thalamic lesions in monkeys. Experimental Brain Research, 52, 199–209. doi:10.1007/BF00236628 [DOI] [PubMed] [Google Scholar]
- Aggleton J. P., & Mishkin M. (1983b). Visual recognition impairments following medial thalamic lesions in monkeys. Neuropsychologia, 21, 189–197. doi:10.1016/0028-3932(83)90037-4 [DOI] [PubMed] [Google Scholar]
- Aggleton J. P., Neave N., Nagle S., & Hunt P. R. (1995). A comparison of the effects of anterior thalamic, mamillary body and fornix lesions on reinforced spatial alternation. Behavioural Brain Research, 68, 91–101. doi:10.1016/0166-4328(94)00163-A [DOI] [PubMed] [Google Scholar]
- Aggleton J. P., O’Mara S. M., Vann S. D., Wright N. F., Tsanov M., & Erichsen J. T. (2010). Hippocampal-anterior thalamic pathways for memory: Uncovering a network of direct and indirect actions. European Journal of Neuroscience, 31, 2292–2307. doi:10.1111/j.1460-9568.2010.07251.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggleton J. P., Poirier G. L., Aggleton H. S., Vann S. D., & Pearce J. M. (2009). Lesions of the fornix and anterior thalamic nuclei dissociate different aspects of hippocampal-dependent spatial learning: Implications for the neural basis of scene learning. Behavioral Neuroscience, 123, 504–519. doi:10.1037/a0015404 [DOI] [PubMed] [Google Scholar]
- Albasser M. M., Amin E., Iordanova M. D., Brown M. W., Pearce J. M., & Aggleton J. P. (2011). Perirhinal cortex lesions uncover subsidiary systems in the rat for the detection of novel and familiar objects. European Journal of Neuroscience, 34, 331–342. doi:10.1111/j.1460-9568.2011.07755.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albasser M. M., Chapman R. J., Amin E., Iordanova M. D., Vann S. D., & Aggleton J. P. (2010). New behavioral protocols to extend our knowledge of rodent object recognition memory. Learning & Memory, 17, 407–419. doi:10.1101/lm.1879610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albasser M. M., Lin T.-C. E., Iordanova M. D., Amin E., & Aggleton J. P. (2012). Evidence that the rat hippocampus has contrasting roles in object recognition memory and object recency memory. Behavioral Neuroscience, 126, 659–669. doi:10.1037/a0029754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albasser M. M., Olarte-Sánchez C. M., Amin E., Horne M., Newton M. J., Warburton E. C., & Aggleton J. P. (2013). The neural basis of nonvisual object recognition memory in the rat. Behavioral Neuroscience, 127, 70–85. doi:10.1037/a0031216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albasser M. M., Poirier G. L., & Aggleton J. P. (2010). Qualitatively different modes of perirhinal-hippocampal engagement when rats explore novel vs. familiar objects as revealed by c-Fos imaging. European Journal of Neuroscience, 31, 134–147. doi:10.1111/j.1460-9568.2009.07042.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachevalier J., & Mishkin M. (1986). Visual recognition impairment follows ventromedial but not dorsolateral prefrontal lesions in monkeys. Behavioural Brain Research, 20, 249–261. doi:10.1016/0166-4328(86)90225-1 [DOI] [PubMed] [Google Scholar]
- Barker G. R., Bird F., Alexander V., & Warburton E. C. (2007). Recognition memory for objects, place, and temporal order: A disconnection analysis of the role of the medial prefrontal cortex and perirhinal cortex. The Journal of Neuroscience, 27, 2948–2957. doi:10.1523/JNEUROSCI.5289-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker G. R., & Warburton E. C. (2011). When is the hippocampus involved in recognition memory? The Journal of Neuroscience, 31, 10721–10731. doi:10.1523/JNEUROSCI.6413-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland B. H., Konopacki J., Kirk I. J., Oddie S. D., & Dickson C. T. (1995). Discharge patterns of hippocampal theta-related cells in the caudal diencephalon of the urethane-anesthetized rat. Journal of Neurophysiology, 74, 322–333. [DOI] [PubMed] [Google Scholar]
- Broadbent N. J., Gaskin S., Squire L. R., & Clark R. E. (2010). Object recognition memory and the rodent hippocampus. Learning & Memory, 17, 5–11. doi:10.1101/lm.1650110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent N. J., Squire L. R., & Clark R. E. (2004). Spatial memory, recognition memory, and the hippocampus. Proceedings of the National Academy of Sciences of the United States of America, 101, 14515–14520. doi:10.1073/pnas.0406344101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. W., & Aggleton J. P. (2001). Recognition memory: What are the roles of the perirhinal cortex and hippocampus? Nature Reviews Neuroscience, 2, 51–61. doi:10.1038/35049064 [DOI] [PubMed] [Google Scholar]
- Burn C. C. (2008). What is it like to be a rat? Rat sensory perception and its implications for experimental design and rat welfare. Applied Animal Behaviour Science, 112, 1–32. doi:10.1016/j.applanim.2008.02.007 [Google Scholar]
- Buzsáki G. (2002). Theta oscillations in the hippocampus. Neuron, 33, 325–340. doi:10.1016/S0896-6273(02)00586-X [DOI] [PubMed] [Google Scholar]
- Buzsáki G. (2005). Theta rhythm of navigation: Link between path integration and landmark nagivation, episodic and semantic memory. Hippocampus, 15, 827–840. doi:10.1002/hipo.20113 [DOI] [PubMed] [Google Scholar]
- Clark R. E., West A. N., Zola S. M., & Squire L. R. (2001). Rats with lesions of the hippocampus are impaired on the delayed nonmatching-to-sample task. Hippocampus, 11, 176–186. doi:10.1002/hipo.1035 [DOI] [PubMed] [Google Scholar]
- Clark R. E., Zola S. M., & Squire L. R. (2000). Impaired recognition memory in rats after damage to the hippocampus. The Journal of Neuroscience, 20, 8853–8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colom L. V., Christie B. R., & Bland B. H. (1988). Cingulate cell discharge patterns related to hippocampal EEG and their modulation by muscarinic and nicotinic agents. Brain Research, 460, 329–338. doi:10.1016/0006-8993(88)90377-0 [DOI] [PubMed] [Google Scholar]
- Cross L., Aggleton J. P., Brown M. W., & Warburton E. C. (2013). The medial dorsal thalamic nucleus and the medial prefrontal cortex of the rat function together to support associative but not item recognition. Learning & Memory, 20, 41–50. doi:10.1101/lm.028266.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson T. L., & Jarrard L. E. (2004). The hippocampus and inhibitory learning: A ‘“gray’” area? Neuroscience & Biobehavioral Reviews, 28, 261–271. doi:10.1016/j.neubiorev.2004.02.001 [DOI] [PubMed] [Google Scholar]
- DeVito L. M., & Eichenbaum H. (2011). Memory for the order of events in specific sequences: Contributions of the hippocampus and medial prefrontal cortex. The Journal of Neuroscience, 31, 3169–3175. doi:10.1523/JNEUROSCI.4202-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennaceur A., & Delacour J. (1988). A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behavioural Brain Research, 31, 47–59. doi:10.1016/0166-4328(88)90157-X [DOI] [PubMed] [Google Scholar]
- Field A. (2005). Discovering Statistics Using SPSS (2nd ed.). London: Sage. [Google Scholar]
- Fortin N. J., Agster K. L., & Eichenbaum H. (2002). Critical role for the hippocampus in memory for sequences of events. Nature Neuroscience, 5, 458–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forwood S. E., Winters B. D., & Bussey T. J. (2005). Hippocampal lesions that abolish spatial maze performance spare object recognition memory at delays of up to 48 hours. Hippocampus, 15, 347–355. doi:10.1002/hipo.20059 [DOI] [PubMed] [Google Scholar]
- Gaskin S., Tardif M., Cole E., Piterkin P., Kayello L., & Mumby D. G. (2010). Object familiarization and novel-object preference in rats. Behavioural Processes, 83, 61–71. [DOI] [PubMed] [Google Scholar]
- Gray J. A., & McNaughton N. (1983). Comparison between the behavioural effects of septal and hippocampal lesions: A review. Neuroscience & Biobehavioral Reviews, 7, 119–188. doi:10.1016/0149-7634(83)90014-3 [DOI] [PubMed] [Google Scholar]
- Hannesson D. K., Vacca G., Howland J. G., & Phillips A. G. (2004). Medial prefrontal cortex is involved in spatial temporal order memory but not spatial recognition memory in tests relying on spontaneous exploration in rats. Behavioural Brain Research, 153, 273–285. doi:10.1016/j.bbr.2003.12.004 [DOI] [PubMed] [Google Scholar]
- Hasselmo M. E., & Eichenbaum H. (2005). Hippocampal mechanisms for the context-dependent retrieval of episodes. Neural Networks, 18, 1172–1190. doi:10.1016/j.neunet.2005.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry J., Petrides M., St-Laurent M., & Sziklas V. (2004). Spatial conditional associative learning: Effects of thalamo-hippocampal disconnection in rats. NeuroReport, 15, 2427–2431. doi:10.1097/00001756-200410250-00025 [DOI] [PubMed] [Google Scholar]
- Hunt P. R., & Aggleton J. P. (1998). An examination of the spatial working memory deficit following neurotoxic medial dorsal thalamic lesions in rats. Behavioural Brain Research, 97, 129–141. doi:10.1016/S0166-4328(98)00033-3 [DOI] [PubMed] [Google Scholar]
- Jenkins T. A., Vann S. D., Amin E., & Aggleton J. P. (2004). Anterior thalamic lesions stop immediate early gene activation in selective laminae of the retrosplenial cortex: Evidence of covert pathology in rats? European Journal of Neuroscience, 19, 3291–3304. doi:10.1111/j.0953-816X.2004.03421.x [DOI] [PubMed] [Google Scholar]
- Kesner R. P., Gilbert P. E., & Barua L. A. (2002). The role of the hippocampus in memory for the temporal order of a sequence of odors. Behavioral Neuroscience, 116, 286–290. doi:10.1037/0735-7044.116.2.286 [DOI] [PubMed] [Google Scholar]
- Kesner R. P., Hunsaker M. R., & Ziegler W. (2010). The role of the dorsal CA1 and ventral CA1 in memory for the temporal order of a sequence of odors. Neurobiology of Learning and Memory, 93, 111–116. doi:10.1016/j.nlm.2009.08.010 [DOI] [PubMed] [Google Scholar]
- Kocsis B., & Vertes R. P. (1994). Characterization of neurons of the supramammillary nucleus and mammillary body that discharge rhythmically with the hippocampal theta rhythm in the rat. Journal of Neuroscience, 14, 7040–7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocsis B., & Vertes R. P. (1997). Phase relations of rhythmic neuronal firing in the supramammillary nucleus and mammillary body to the hippocampal theta in urethane anesthetized rats. Hippocampus, 7, 204–214. doi:10.1002/(SICI)1098-1063(1997)7:2<204::AID-HIPO7>3.0.CO;2-M [DOI] [PubMed] [Google Scholar]
- Lavenex P., & Schenk F. (1995). Influence of local environmental olfactory cues on place learning in rats. Physiology & Behavior, 58, 1059–1066. doi:10.1016/0031-9384(95)02002-0 [DOI] [PubMed] [Google Scholar]
- Mishkin M., & Delacour J. (1975). An analysis of short-term visual memory in the monkey. Journal of Experimental Psychology: Animal Behavior Processes, 1, 326–334. doi:10.1037/0097-7403.1.4.326 [DOI] [PubMed] [Google Scholar]
- Mitchell A. S., & Dalrymple-Alford J. C. (2005). Dissociable memory effects after medial thalamus lesions in the rat. European Journal of Neuroscience, 22, 973–985. doi:10.1111/j.1460-9568.2005.04199.x [DOI] [PubMed] [Google Scholar]
- Moran J. P., & Dalrymple-Alford J. C. (2003). Perirhinal cortex and anterior thalamic lesions: Comparative effects on learning and memory. Behavioral Neuroscience, 117, 1326–1341. doi:10.1037/0735-7044.117.6.1326 [DOI] [PubMed] [Google Scholar]
- Mullen R. J., Buck C. R., & Smith A. M. (1992). NeuN, a neuronal specific nuclear protein in vertebrates. Development (Cambridge, England), 116, 201–211. [DOI] [PubMed] [Google Scholar]
- Mumby D. G. (2001). Perspectives on object-recognition memory following hippocampal damage: Lessons from studies in rats. Behavioural Brain Research, 127, 159–181. doi:10.1016/S0166-4328(01)00367-9 [DOI] [PubMed] [Google Scholar]
- Mumby D. G., Pinel J. P. J., & Dastur F. N. (1993). Mediodorsal thalamic lesions and object recognition in rats. Psychobiology, 21, 27–36. [Google Scholar]
- Parker A., Eacott M. J., & Gaffan D. (1997). The recognition memory deficit caused by mediodorsal thalamic lesion in non-human primates: A comparison with rhinal cortex lesion. European Journal of Neuroscience, 9, 2423–2431. doi:10.1111/j.1460-9568.1997.tb01659.x [DOI] [PubMed] [Google Scholar]
- Paxinos G., & Watson C. (2005). The rat brain in stereotaxic coordinates (5th ed.). San Diego, CA: Academic Press. [Google Scholar]
- Poirier G. L., & Aggleton J. P. (2009). Post-surgical interval and lesion location within the limbic thalamus determine extent of retrosplenial cortex hypoactivity. Neuroscience, 160, 452–469. doi:10.1016/j.neuroscience.2009.02.021 [DOI] [PubMed] [Google Scholar]
- Shibata H. (1993). Direct projections from the anterior thalamic nuclei to the retrohippocampal region in the rat. Journal of Comparative Neurology, 337, 431–445. doi:10.1002/cne.903370307 [DOI] [PubMed] [Google Scholar]
- Shibata H., & Naito J. (2005). Organization of anterior cingulate and frontal cortical projections to the anterior and laterodorsal thalamic nuclei in the rat. Brain Research, 1059, 93–103. doi:10.1016/j.brainres.2005.08.025 [DOI] [PubMed] [Google Scholar]
- Sutherland R. J., & Rodriguez A. J. (1989). The role of the fornix/fimbria and some related subcortical structures in place learning and memory. Behavioural Brain Research, 32, 265–277. doi:10.1016/S0166-4328(89)80059-2 [DOI] [PubMed] [Google Scholar]
- Sziklas V., & Petrides M. (1999). The effects of lesions to the anterior thalamic nuclei on object-place associations in rats. European Journal of Neuroscience, 11, 559–566. doi:10.1046/j.1460-9568.1999.00448.x [DOI] [PubMed] [Google Scholar]
- Sziklas V., & Petrides M. (2007). Contribution of the anterior thalamic nuclei to conditional learning in rats. Hippocampus, 17, 456–461. doi:10.1002/hipo.20286 [DOI] [PubMed] [Google Scholar]
- Tsanov M., Chah E., Wright N., Vann S. D., Reilly R., Erichsen J. T., et al. O’Mara S. M. (2011). Oscillatory entrainment of thalamic neurons by theta rhythm in freely moving rats. Journal of Neurophysiology, 105, 4–17. doi:10.1152/jn.00771.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Groen T., Kadish I., & Wyss J. M. (1999). Efferent connections of the anteromedial nucleus of the thalamus of the rat. Brain Research Reviews, 30, 1–26. doi:10.1016/S0165-0173(99)00006-5 [DOI] [PubMed] [Google Scholar]
- Vann S. D., & Aggleton J. P. (2004). The mammillary bodies–two memory systems in one? Nature Reviews Neuroscience, 5, 35–44. doi:10.1038/nrn1299 [DOI] [PubMed] [Google Scholar]
- Vertes R. P., Albo Z., & Viana Di Prisco G. (2001). Theta-rhythmically firing neurons in the anterior thalamus: Implications for mnemonic functions of Papez’s circuit. Neuroscience, 104, 619–625. doi:10.1016/S0306-4522(01)00131-2 [DOI] [PubMed] [Google Scholar]
- Warburton E. C., & Aggleton J. P. (1998). Differential deficits in the Morris water maze following cytotoxic lesions of the anterior thalamus and fornix transaction. Behavioural Brain Research, 98, 27–38. doi:10.1016/S0166-4328(98)00047-3 [DOI] [PubMed] [Google Scholar]
- Warburton E. C., & Brown M. W. (2010). Findings from animals concerning when interactions between perirhinal cortex, hippocampus and medial prefrontal cortex are necessary for recognition memory. Neuropsychologia, 48, 2262–2272. doi:10.1016/j.neuropsychologia.2009.12.022 [DOI] [PubMed] [Google Scholar]
- Warburton E. C., Morgan A., Baird A. L., Muir J. L., & Aggleton J. P. (1999). Does pretraining spare the spatial deficit associated with anterior thalamic damage in rats? Behavioral Neuroscience, 113, 956–967. doi:10.1037/0735-7044.113.5.956 [DOI] [PubMed] [Google Scholar]
- Winer B. J. (1971). Statistical Principles in Experimental Design. New York, NY: McGraw-Hill. [Google Scholar]
- Winters B. D., Forwood S. E., Cowell R. A., Saksida L. M., & Bussey T. J. (2004). Double dissociation between the effects of peri-postrhinal cortex and hippocampal lesions on tests of object recognition and spatial memory: Heterogeneity of function within the temporal lobe. The Journal of Neuroscience, 24, 5901–5908. doi:10.1523/JNEUROSCI.1346-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters B. D., & Reid J. M. (2010). A distributed cortical representation underlies crossmodal object recognition in rats. The Journal of Neuroscience, 30, 6253–6261. doi:10.1523/JNEUROSCI.6073-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff M., Gibb S. J., & Dalrymple-Alford J. C. (2006). Beyond spatial memory: The anterior thalamus and memory for the temporal order of a sequence of odour cues. The Journal of Neuroscience, 26, 2907–2913. doi:10.1523/JNEUROSCI.5481-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright N. F., Erichsen J. T., Vann S. D., O’Mara S. M., & Aggleton J. P. (2010). Parallel but separate inputs from limbic cortices to the mammillary bodies and anterior thalamic nuclei in the rat. The Journal of Comparative Neurology, 518, 2334–2354. doi:10.1002/cne.22336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss J. M., Swanson L. W., & Cowan W. M. (1979). A study of subcortical afferents to the hippocampal formation in the rat. Neuroscience, 4, 463–476. doi:10.1016/0306-4522(79)90124-6 [DOI] [PubMed] [Google Scholar]
- Zhu X. O., Brown M. W., & Aggleton J. P. (1995). Neuronal signalling of information important to visual recognition memory in rat rhinal and neighbouring cortices. European Journal of Neuroscience, 7, 753–765. doi:10.1111/j.1460-9568.1995.tb00679.x [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S., & Squire L. R. (1985). Amnesia in monkeys after lesions of the mediodorsal nucleus of the thalamus. Annals of Neurology, 17, 558–564. doi:10.1002/ana.410170605 [DOI] [PubMed] [Google Scholar]