Abstract
HCV has surpassed HIV as a cause of death in the United States and is particularly prevalent among injection drug users. I examined the availability of on-site HCV testing in a nationally representative sample of opioid treatment programs. Nearly 68% of these programs had the staff required for HCV testing, but only 34% offered on-site testing. Availability of on-site testing increased only slightly with the proportion of injection drug users among clients. The limited HCV testing services in opioid treatment programs is a key challenge to reducing HCV in the US population.
HCV recently surpassed HIV as a cause of death in the United States.1,2 Approximately 3.2 million people nationwide are living with chronic hepatitis, but most are unaware of their status because of limited opportunities for testing.3–6 Persons who inject drugs are particularly at risk for HCV infection as a result of sharing and reusing of needles.4,7 The estimated prevalence of antibodies to HCV (anti-HCV) among injection drug users ranges from 35% to 65%.8 The Centers for Disease Control and Prevention (CDC) thus recommends routine HCV testing for all current or former injection drug users.1,9 Offering HCV testing services in drug abuse treatment programs could help increase HCV case finding and reduce transmission.10,11 It could also help foster the adoption of preventive behaviors: knowledge of one’s anti-HCV status may indeed lead to safer injection practices (or other protective behaviors).12,13
I examined the availability of on-site HCV testing services in opioid treatment programs (i.e., physical facilities with resources dedicated specifically to treating opiate dependence with methadone, buprenorphine, or both).14,15 Opioid treatment programs treat both persons who inject drugs and people who have opiate addiction but do not inject drugs. The current recommended HCV testing protocol requires the collection of venous blood, performed by qualified staff (i.e., phlebotomists).16 However, the availability of (1) human resources required to offer HCV testing services and (2) on-site HCV testing services at opioid treatment programs nationwide is not known. I examined relations among the availability of on-site HCV testing services, human resources for HCV testing, and the proportion of injection drug users among opioid treatment program clients.
METHODS
I analyzed data from the 2011 National Drug Abuse Treatment System Survey (NDATSS).14,17,18 In total, a nationally representative sample of 200 opioid treatment programs completed the survey. Twenty-two opioid treatment programs refused to participate, and 90 initially screened were unable to complete interviews. A response rate of 87% was calculated with the Council of American Survey Research Organization method.19 I found no significant differences between responders and nonresponders.
I used 3 data elements from the 2011 NDATSS: (1) the proportion of injection drug users among clients of an opioid treatment program, (2) the presence of staff who perform blood collection, and (3) the availability of HCV testing services on site. I calculated the proportion of opioid treatment programs with human resources capacity and on-site HCV testing. I categorized opioid treatment programs by the prevalence of injection drug users among their clients (0%–24%, 25%–49%, 50%–74%, or 75%–100%). I used logistic regression with controls for opioid treatment program size (i.e., total number of clients in past year) to examine the association between human resources capacity and on-site HCV testing and the proportion of injection drug users among clients. I report predicted probabilities from these regressions. I used the simple Wald test to determine whether the proportion of opioid treatment programs with human resources capacity or on-site HCV testing differed between levels of the injection drug users variable. Among opioid treatment programs that did not offer on-site HCV testing, I examined the proportion of facilities that referred clients to off-site HCV testing services.
RESULTS
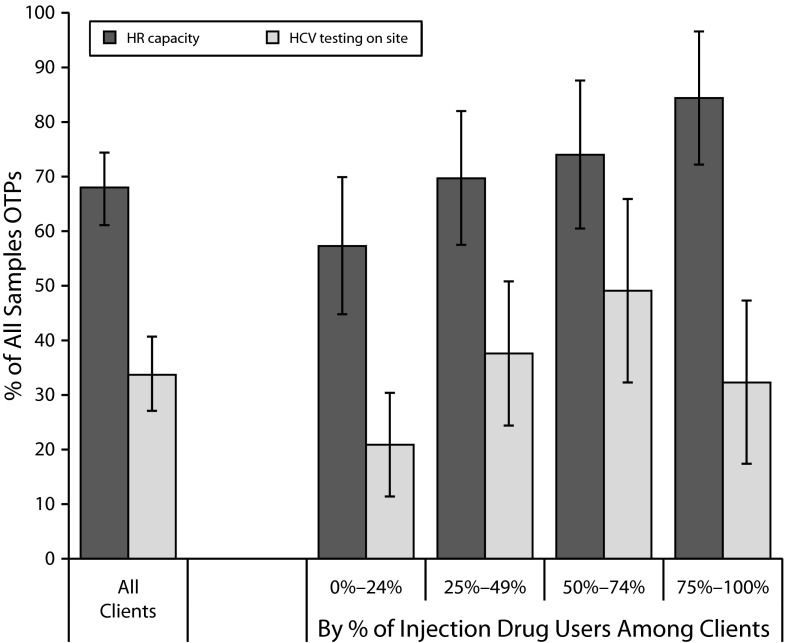
The results showed that 68.0% (95% confidence interval [CI] = 61.1, 74.4) of opioid treatment programs nationwide had staff capacity for HCV testing, but only 33.7% (95% CI = 27.1, 40.7) actually offered on-site HCV testing (Figure 1). The availability of HCV testing on site increased with the proportion of injection drug users among clients; however, only 32.3% (95% CI = 17.4, 47.3) of the opioid treatment programs with the most injection drug users among their clients (≥ 75%) offered on-site HCV testing. Human resources capacity increased significantly in proportion to higher prevalence of injection drug users among the clients of opioid treatment programs. However, more than half (58.5%) of the opioid treatment programs with staff capacity to provide on-site HCV testing did not offer such services. Among opioid treatment programs that did not offer on-site HCV testing, 84.1% referred their clients to off-site testing facilities. This proportion did not vary with the proportion of injection drug users among the clients of opioid treatment programs.
FIGURE 1—
Human resource (HR) capacity to test and availability of on-site HCV testing services in opioid treatment programs (OTPs): 2011 National Drug Abuse Treatment System Survey.
DISCUSSION
I found large gaps in the availability of on-site HCV testing services and the human resources capacity of opioid treatment programs to provide testing, especially to high-risk populations (e.g., injection drug users). Even in opioid treatment programs with staff capacity, more than half did not offer on-site testing, thus creating large missed opportunities for HCV case finding and early treatment. Several factors may account for these findings, including policy and organizational factors (i.e., affiliation, ownership) and client characteristics (e.g., race, sex). Opioid treatment programs that do not offer on-site HCV testing appear to have referral agreements in place for their clients to undergo HCV testing off site. However, findings from other studies suggest that uptake of off-site HCV testing is likely to be much lower.20,21
My findings had several limitations. First, the sample was limited to programs that treat opiate dependence with methadone or buprenorphine. Second, I did not measure the uptake of HCV testing among clients of opioid treatment programs where on-site HCV testing was offered. Third, opioid treatment programs must have the required human resources to provide on-site HCV testing, but this is far from the only requirement: other factors, including state-level requirements, certification to test, availability of funding, and changes in payment systems, may limit the capacity of opioid treatment programs to offer on-site HCV testing to their clients.
Notwithstanding these limitations, results from this study have important implications for strategies to curb the HCV epidemic in the United States. These results indicated that increasing human resources capacity (i.e., hiring or training phlebotomists) alone is not sufficient to increase the availability of HCV testing services in opioid treatment programs. However, promoting the use of rapid HCV tests, which do not necessarily require phlebotomists, could help rapidly increase the availability of HCV testing services in opioid treatment programs. State-level requirements for certification may influence the use and availability of rapid testing.22 In addition, on-site HCV testing could serve as an important complementary service to prevention initiatives.
Overall, HCV prevention and testing services must become an integral component of services delivered by opioid treatment programs,23 particularly those with injection drug users. Additionally, client preferences for mode of testing, which may influence uptake of testing, should be considered in any initiative to promote HCV testing.24,25 Policies and investments similar to those adopted for HIV testing and counseling (e.g., opt-out testing) may be required to avert increasing mortality linked to HCV.
Acknowledgments
This publication was supported by the National Center for Advancing Translational Sciences, National Institutes of Health (grant KL2 TR000081), formerly, the National Center for Research Resources (grant KL2 RR024157), and the National Institute of Drug Abuse (grant R01 DA030459 02S1).
The author thanks Thomas D’Aunno for his valuable feedback.
Note. The content is solely the responsibility of the author and does not represent the official views of National Institute on Drug Abuse or the National Institutes of Health.
Human Participant Protection
The institutional review board at the Columbia University Medical Center approved all aspects of this study.
References
- 1.Ly KN, Xing J, Klevens RM, Jiles RB, Ward JW, Holmberg SD. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med. 2012;156(4):271–278 [DOI] [PubMed] [Google Scholar]
- 2.Wise M, Bialek S, Finelli L, Bell BP, Sorvillo F. Changing trends in hepatitis C-related mortality in the United States, 1995-2004. Hepatology. 2008;47(4):1128–1135 [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention Viral Hepatitis Surveillance - United States, 2009. September 22, 2011.Available at: http://www.cdc.gov/hepatitis/statistics/2009surveillance/index.htm. Accessed February 2012 [Google Scholar]
- 4.Armstrong GL, Wasley AM, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144(10):705–714 [DOI] [PubMed] [Google Scholar]
- 5.Dhopesh VP, Taylor KR, Burke WM. Survey of hepatitis B and C in addiction treatment unit. Am J Drug Alcohol Abuse. 2000;26(4):703–707 [DOI] [PubMed] [Google Scholar]
- 6.Cohen MH, Grey D, Cook JAet al. Awareness of hepatitis C infection among women with and at risk for HIV. J Gen Intern Med. 2007;22(12):1689–1694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garfein RS, Doherty MC, Monterroso ER, Thomas DL, Nelson KE, Vlahov D. Prevalence and incidence of hepatitis C virus infection among young adult injection drug users. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;18(suppl 1):S11–S19 [DOI] [PubMed] [Google Scholar]
- 8.Amon JJ, Garfein RS, Ahdieh-Grant Let al. Prevalence of hepatitis C virus infection among injection drug users in the United States, 1994-2004. Clin Infect Dis. 2008;46(12):1852–1858 [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recomm Rep. 1998;47(RR-19):1–39 Available at: http://www.aidsinfo.nih.gov/contentfiles/Adult_OI.pdf. Accessed August 2012 [PubMed] [Google Scholar]
- 10.Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality The TEDS Report: Injection Drug Abuse Admissions to Substance Abuse Treatment: 1992 and 2009. Rockville, MD: December 1, 2011. Available at: http://www.samhsa.gov/data/2k11/WEB_TEDS_012/WEB_TEDS_012.htm. Accessed August 2012 [Google Scholar]
- 11.Vassilev ZP, Strauss SM, Astone JM, Friedmann PD, Des Jarlais DC. Provision of on-site medical care to patients with hepatitis C in drug treatment units. J Health Care Poor Underserved. 2004;15(4):663–671 [DOI] [PubMed] [Google Scholar]
- 12.Burt RD, Thiede H, Hagan H. Serosorting for hepatitis C status in the sharing of injection equipment among Seattle area injection drug users. Drug Alcohol Depend. 2009;105(3):215–220 [DOI] [PubMed] [Google Scholar]
- 13.Hahn JA, Evans JL, Davidson PJ, Lum PJ, Page K. Hepatitis C virus risk behaviors within the partnerships of young injecting drug users. Addiction. 2010;105(7):1254–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D’Aunno T, Pollack HA. Changes in methadone treatment practices: results from a national panel study, 1988-2000. JAMA. 2002;288(7):850–856 [DOI] [PubMed] [Google Scholar]
- 15.Substance Abuse and Mental Health Services Administration, Office of Applied Studies The N-SSATS Report: Overview of Opioid Treatment Programs Within the United States: 2008. Rockville, MD: Substance Abuse and Mental Health Services Administration; January 28, 2010. Available at: http://www.oas.samhsa.gov/2k10/222/222USOTP2k10Web.pdf. Accessed August 2012 [Google Scholar]
- 16.Drobnik A, Judd C, Banach D, Egger J, Konty K, Rude E. Public health implications of rapid hepatitis C screening with an oral swab for community-based organizations serving high-risk populations. Am J Public Health. 2011;101(11):2151–2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D’Aunno T, Vaughn TE, McElroy P. An institutional analysis of HIV prevention efforts by the nation’s outpatient drug abuse treatment units. J Health Soc Behav. 1999;40(2):175–192 [PubMed] [Google Scholar]
- 18.Pollack HA, D’Aunno T. HIV testing and counseling in the nation’s outpatient substance abuse treatment system, 1995-2005. J Subst Abuse Treat. 2010;38(4):307–316 [DOI] [PubMed] [Google Scholar]
- 19.Frankel LR. The report of the CASRO Task Force on response rates. In: Wiseman F, ed. Improving Data Quality in a Sample Survey. Cambridge, MA: Marketing Science Institute; 1983;1–11 [Google Scholar]
- 20.Metsch LR, Feaster DJ, Gooden Let al. Implementing rapid HIV testing with or without risk-reduction counseling in drug treatment centers: results of a randomized trial. Am J Public Health. 2012;102(6):1160–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris KA, Jr, Arnsten JH, Litwin AH. Successful integration of hepatitis C evaluation and treatment services with methadone maintenance. J Addict Med. 2010;4(1): 20–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.US Food and Drug Administration FDA News Release: FDA approves rapid test for antibodies to hepatitis C virus. June 25, 2010. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm217318.htm. Accessed April 25, 2012 [Google Scholar]
- 23.Bini EJ, Kritz S, Brown LS, Jret al. Hepatitis B virus and hepatitis C virus services offered by substance abuse treatment programs in the United States. J Subst Abuse Treat. 2012;42(4):438–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marsh KA, Reynolds GL, Rogala BE, Fisher DG, Napper LE. Who chooses a rapid test for HIV in Los Angeles County, California? Eval Health Prof. 2010;33(2):177–196 [DOI] [PubMed] [Google Scholar]
- 25.Spielberg F, Branson BM, Goldbaum GMet al. Overcoming barriers to HIV testing: preferences for new strategies among clients of a needle exchange, a sexually transmitted disease clinic, and sex venues for men who have sex with men. J Acquir Immune Defic Syndr. 2003;32(3):318–327 [DOI] [PubMed] [Google Scholar]