Abstract
Capillary zone electrophoresis-multiple/single reaction monitoring (CZE-MRM/SRM), which employed an electrokinetically driven sheath-flow electrospray interface, was used for the rapid and highly sensitive detection of protein analytes in complex tryptic digests. MRM channels were developed against a commercial exponential mixture of bovine proteins. Five proteins spanning four orders of magnitude concentration range were confidently detected from only 2.5 ng of the digest mixture; the mass detection limits (S/N=3) of two detected proteins, alpha-casein and glutamate dehydrogenasewere about 600 zmole and 30 amole, respectively. This technique was then applied to a RAW 264.7 cell lysate digest. Three proteins were confidently and reproducibly detected from 100 pg of this digest. The sample amount corresponds to the approximate protein content from a single cell, which suggests that CZE-MRM may be a useful analytical tool in chemical cytometry. In addition to providing highly sensitive detection of proteins in complex mixtures, this system is highly rapid; migration time of the protein digests was less than 10 min.
Keywords: Capillary zone electrophoresis, electrokinetic sheath flow electrospray, multiple reaction monitoring, single reaction monitoring, protein digests, RAW 264.7 cell line, picogram tryptic digests analysis
Introduction
Capillary zone electrophoresis (CZE) provides fast and efficient separations of biological molecules [1–3]. CZE-electrospray ionization-mass spectrometry (CZE-ESI-MS) is a useful technique for protein analysis [4]. It has several advantages including shorter analysis time and much lower sample and solvent consumption compared with widely used reversed phase liquid chromatography (RPLC)-ESI-MS.
The performance of CZE-MS is largely determined by the electrospray interface, and the interfaces can be divided into two basic designs, sheath-flow and sheathless interfaces [4]. Recently, a sheathless CE-MS interface with an etched porous silica capillary as the electrospray emitter was used by several research groups for CE-MS based bottom-up proteomics analysis [5–8]. Results indicated that CE-MS was more efficient than RPLC-MS for protein identification when the loaded sample amount was mass limited [8], and peptide intensity from CE-MS was higher than that from RPLC-MS [7], which suggest that the CE-MS is more sensitive than RPLC-MS for peptide analysis.
Our group developed an electrokinetically driven sheath-flow CE-MS interface [9], employing an extremely low sheath flow-rate, circumventing the high-dilution factors typically observed in sheath-flow interfaces. This interface produces low amole peptide detection limits [10,11] when coupled to a modern FT-based mass spectrometer (LTQ-Velos Orbitrap). In addition, the electrokinetic sheath-flow interface based CZE system yielded more peptide identifications than RPLC-ESI-MS/MS system when mass limited BSA digests (60 amole to 3.5 fmol) were loaded for analysis [11]. Further refinement enabled the analysis of subnanogram amounts of tryptic digests of a cellular homogenate with the CZE-ESI-MS/MS system, and six proteins were confidently identified from triplicate runs, consuming 700 pg of digest per analysis [11]. This amount of protein for analysis is roughly equal to the protein content of ten typical eukaryotic cells [12], which indicates that an order of magnitude improved sensitivity of the CE-MS system alone should allow the analysis of the protein content of a single cell. Single cell analysis is particularly interesting when studying heterogeneous mixtures of cells. Information on that heterogeneity is inevitably lost in conventional analysis, where the mixture is homogenized. Instead, study of cellular heterogeneity can provide insight on differentiation and development, on stochastic gene expression, and on the clonal nature of cancer [12]. Of course, analysis of the proteome of a single cell is extremely challenging due to the minute amount of protein within a single cell.
Multiple/selected reaction monitoring (MRM/SRM) is an attractive way to further improve the sensitivity of the CE-MS system [13]. Aebersold’s group employed the MRM/SRM based approach to detect and quantify proteins with concentrations less than 50 copies/cell from total S. cerevisiae digests [14]. MRM/SRM experiments usually employ one-dimensional liquid chromatography (LC) coupled to a triple-quadrupole or hybrid linear ion-trap triple quadrupole (QqQ) mass spectrometer. Briefly, the parent ion of a targeted peptide is isolated in the first quadrupole (Q1) and fragmented in the second quadrupole (Q2). One or several fragment ions derived from the targeted peptide are isolated by the third quadrupole (Q3) for detection [15, 16]. Parent and fragment ion pairs are called transitions. MRM/SRM has been widely used for highly sensitive detection and precise quantification of target proteins and their modifications in complex biological samples [17–21]. Although MRM/SRM is usually used for target quantification, it also can be used for highly sensitive and specific detection of target peptides in biological samples [22, 23].
Recently, our group coupled CZE to a triple-quadrupole-based mass spectrometer for the accurate and precise-quantification of Leu-enkephalin in an excess of bovine serum albumin tryptic digest using MRM [24]. The limit of detection (3σ) was 60 pM, corresponding to 335 zmol of peptides, which was a 10–20-fold improvement in mass sensitivity over the mass detection limit obtained by nano HPLC-MRM and LC-MS/MS. The peptide detection limit of the CZE-MRM system was also improved about one order of magnitude compared with that obtained by CZE-MS/MS system with an LTQ-Orbitrap Velos as the detector [10,11]. In addition, the CZE-MRM system produced reproducible peak height, migration time, and robust electrospray performance. More recently, Wang et al. directly coupled transient capillary isotachophoresis (CITP)/CZE with a QqQ mass spectrometer for MRM based peptide quantitation [25]. A four-order of magnitude linear dynamic range was observed and the limit of quantitation of the system was well below 50 pM, demonstrating the high sensitivity of the approach.
Our earlier work evaluated CZE-MRM with a relatively simple sample. Here, we have extended MRM-CZE to the rapid, highly sensitive, and specific detection of target proteins from picogram amounts of a complex proteome sample, the RAW 264.7 cell lysate digest. The RAW 264.7 cell line is mouse leukaemic monocyte macrophage cell line, and it is useful for metabolic, inflammation, and apoptosis studies. Empirical transitions were determined for these MRM experiments by first analyzing the digests with UPLC-ESI-MS/MS (Orbitrap Velos) with higher energy collisional dissociation (HCD); HCD provides a good model for the QqQ-collisionally induced dissociation (CID) fragmentation spectra employed in MRM experiments [26]. This approach facilitates more robust detection of MRM transitions and demonstrates compatibility with existing processes in converting bottom-up derived protein lists to verified MRM transitions. We first evaluated the transition generation method and the CZE-MRM system by analyzing a five-order of magnitude exponential digest mixture of six proteins with the CZE-MRM system. A detection limit of alpha casein in the high zmole range was obtained in the presence of four-order-of-magnitude excess peptide background. We subsequently applied the CZE-MRM system for analysis of 100 pg of RAW 264.7 cell lysate digest. Thirty-six transitions targeting nine proteins were monitored by CZE-MRM with less than 10 min per analysis. Three proteins were reliably and reproducibly detected from the 100-pg sample.
Experimental section
Chemicals and reagents
Bovine pancreas TPCK-treated trypsin, urea, ammonium bicarbonate (NH4HCO3), dithiothreitol (DTT), and iodoacetamide (IAA) were purchased from Sigma–Aldrich (St. Louis, MO, USA). Six bovine tryptic digest exponential molar mix was purchased from Bruker-Michrom Inc. (Auburn, CA, USA). Acetonitrile (ACN) and formic acid (FA) were purchased from Fisher Scientific (Pittsburgh, PA, USA). Methanol was purchased from Honeywell Burdick & Jackson (Wicklow, IE, USA). Water was deionized by a Nano Pure system from Thermo scientific (Marietta, OH, USA). Fused silica capillaries (50 μm i.d. × 150 μm o.d.) were purchased from Polymicro Technologies (Phoenix, AZ, USA). ZipTip C18 (ZTC18S096) was purchased from Millipore (Bedford, MA, USA).
Dulbecco’s Modified Eagle’s Medium (DMEM) with L-glutamine and fetal bovine serum (FBS) were purchased from ATCC (Manassas, VA, USA). Mammalian Cell-PE LB™ Buffer for cell lysis was purchased from G-Biosciences (St. Louis, MO, USA). Complete, mini protease inhibitor cocktail (provided in EASYpacks) was purchased from Roche (Indianapolis, IN, USA).
Sample preparation
The six bovine tryptic digest exponential molar mixture was dissolved in 0.1% (v/v) FA, and desalted with ZipTip C18. The desalted digest was lyophilized, then redissolved in 2% ACN, 0.1% FA with concentration as 0.2 mg/mL, followed by UPLC-ESI-MS/MS (Orbitrap Velos) analysis. For CZE-MRM analysis, the lyophilized digests were redissolved in 5 mM NH4HCO3 at concentrations of 0.35 mg/mL and 0.014 mg/mL.
The procedure for RAW 264.7 cell lysate preparation was the same as used earlier [27, 28]. RAW 264.7 cells were cultured in a T25 flask at 37 °C and 5% CO2 in DMEM with L-glutamine and 10% FBS. After washing with cold PBS buffer twice, 1 mL mammalian cell-PE LB™ buffer (pH 7.5) supplemented with complete protease inhibitor was added to the flask, and shaken gently for 10 min on ice. The cell lysate was transferred to a 1.5 mL Eppendorf tube and incubated on ice for an additional 15 min. Subsequently, the cell lysate was centrifuged at 18,000 g for 15 min, and the supernatant was collected for measurement of protein concentration with the BCA method. Next, 300 μL of cell lysate (corresponding to ~54 μg protein) was denatured at 90 °C for 20 min, followed by reduction with DTT (3.3 mM) at 65 °C for 1 h and alkylation with IAA (8.3 mM) at room temperature for 30 min in the dark. Then, 1.2 mL of cold acetone was added to the protein solution and incubated at −20 °C for 12 h, followed by centrifugation at 18,000 g for 15 min. The protein pellet was washed with cold acetone again, and dried at room temperature. The protein pellet was re-dissolved in 100 μL 1 M urea and 100 mM NH4HCO3 buffer (pH 8.0), with protein concentration of ~0.5 mg/mL, followed by in-solution trypsin digestion at 37 °C for 12 h with a trypsin/protein ratio (w/w) of 1/30. After acidification, the digest was desalted with ZipTip C18 and lyophilized. The digest was redissolved in 2% ACN, 0.1% FA with concentration of 0.5 mg/mL for UPLC-ESI-MS/MS (Orbitrap Velos) analysis. For CZE-MRM analysis, the digest was redissolved in 5 mM NH4HCO3 with concentrations of 0.1 mg/mL and 0.014 mg/mL.
UPLC-ESI-MS/MS analysis
A nanoACQUITY Ultra Performance LC® (UPLC®) system (Waters, Milford, MA, USA) was used for separation of the protein digests. Buffer A (0.1% FA in water) and buffer B (0.1% FA in ACN) were used as mobile phases for gradient separation. Protein digests were automatically loaded onto a commercial C18 reversed phase column (Waters, 100 μm×100 mm, 1.7 μm particle, BEH130 C18, with a column temperature of 40 °C); 2% buffer B for 5 min at a flow rate of 1.2 μL/min. The peptides were separated by a three-step gradient, 2 min from 2 % B to 10% B, 23 min to 30% B, 5 min to 85% B, and maintained for 10 min. The column was equilibrated for 14 min with 2% buffer B prior to the next sample analysis. The eluted peptides from the C18 column were analyzed by an LTQ-Orbitrap Velos instrument (Thermo Fisher Scientific). The electrospray voltage was 1.8 kV, and the ion transfer tube temperature was 300 °C. For each sample, 2 μL was loaded for analysis.
A programmed data dependent acquisition (TOP10) method was applied. Full MS scans were acquired in the Orbitrap mass analyzer over m/z 395–1800 range with resolution 30,000 (m/z 400). The target value was 1.00E+06. The ten most intense peaks with charge state ≥ 2 were fragmented in the HCD collision cell with normalized collision energy of 40%, and tandem mass spectra were acquired in the Orbitrap mass analyzer with resolution 7,500. The target value was 5.00E+04. The ion selection threshold was 5,000 counts, and the maximum allowed ion accumulation times were 500 ms for full scans and 250 ms for HCD. Dynamic exclusion was enabled, and peaks selected for fragmentation more than once within 15 s were excluded from selection for 15 s.
Transitions generation and CZE-MRM analysis
The raw files were first transferred into Mascot generic format (mgf) files with RAW2MSM from the Mann laboratory using the default settings [29]. Database searching was performed with the Paragon search engine within Protein Pilot 4.0 (ABSciex) [23, 30]. Iodoacetamide was selected as the cys. alkylation, and trypsin was selected as the digestion enzyme. Instrument parameters were set to Orbi (MS) and Orbi (MS/MS). A Uniprot database including common contaminant proteins was used for database searching. Mus musculus was selected as species for RAW 264.7 cell lysate data. Database searching for the reversed database was also performed in order to evaluate the false discovery rate (FDR) [31, 32]. Peptides with confidence higher than 95% were recognized as positive hits, and the corresponding global FDR on peptide level was less than 1%.
MRM Pilot 2.1 (ABSciex) and hand-selection were used to generate the MRM transitions from the database searching results. One to four unique peptides with highest intensity from each protein were chosen, and 2–4 fragment ions with highest intensity and also a higher m/z than the corresponding parent ion were chosen for each peptide. The collision energy for each peptide was based on the mass and charge of the peptide. In total, 46 transitions were generated for the six bovine tryptic digest exponential molar mixture and 36 transitions were generated for the 100-pg RAW 264.7 cell lysate digest. The information about m/z ratio of parent ions (Q1) and fragment ions (Q3) of transitions, dwell time, and collision energy are listed in S-Table 1 in supporting material I and Table 1. Dwell time was determined based on the expected peak width, number of transitions, and to ensure adequate point-spacing for detection.
Table 1.
MRM transitions for 100-pg RAW 264.7 cell lysate digest analysis.
| Q1 (m/z) | Q3 (m/z) | dwell time (ms) | protein description_peptide sequence.peptide charge | collision energy (eV) |
|---|---|---|---|---|
| 661.68 | 884.94 | 30 | Peroxiredo_TIAQDYGVLKADEGISFR.3 | 37.084 |
| 661.68 | 785.39 | 30 | Peroxiredo_TIAQDYGVLKADEGISFR.3 | 37.084 |
| 613.35 | 770.42 | 30 | Peroxiredo_QITINDLPVGR.2 | 31.987 |
| 613.35 | 984.55 | 30 | Peroxiredo_QITINDLPVGR.2 | 31.987 |
| 400.24 | 441.27 | 30 | Actin_AVFPSIVGRPR.3 | 24.012 |
| 400.24 | 881.53 | 30 | Actin_AVFPSIVGRPR.3 | 24.012 |
| 488.73 | 630.28 | 30 | Actin_AGFAGDDAPR.2 | 26.504 |
| 488.73 | 701.32 | 30 | Actin_AGFAGDDAPR.2 | 26.504 |
| 728.38 | 773.45 | 30 | Profilin-1_SSFFVN[Dea]GLTLGGQK.2 | 37.049 |
| 728.38 | 888.48 | 30 | Profilin-1_SSFFVN[Dea]GLTLGGQK.2 | 37.049 |
| 437.78 | 561.34 | 30 | Profilin-1_TLVLLMGK.2 | 24.262 |
| 437.78 | 660.41 | 30 | Profilin-1_TLVLLMGK.2 | 24.262 |
| 473.75 | 549.27 | 30 | Pyruvate kinase_VNLAMDVGK.2 | 25.845 |
| 473.75 | 620.31 | 30 | Pyruvate kinase_VNLAMDVGK.2 | 25.845 |
| 420.77 | 559.36 | 30 | Pyruvate kinase_APIIAVTR.2 | 23.514 |
| 420.77 | 672.44 | 30 | Pyruvate kinase_APIIAVTR.2 | 23.514 |
| 589.32 | 624.31 | 30 | Alpha-enol_MILPVGASSFR.2 | 30.93 |
| 589.32 | 820.43 | 30 | Alpha-enol_MILPVGASSFR.2 | 30.93 |
| 450.28 | 614.39 | 30 | Alpha-enol_TIAPALVSK.2 | 24.812 |
| 450.28 | 685.42 | 30 | Alpha-enol_TIAPALVSK.2 | 24.812 |
| 568.31 | 761.42 | 30 | Plastin_QFVTATDVVR.2 | 30.006 |
| 568.31 | 860.48 | 30 | Plastin_QFVTATDVVR.2 | 30.006 |
| 751.88 | 845.4 | 30 | Plastin_MINLSVPDTIDER.2 | 38.083 |
| 751.88 | 944.47 | 30 | Plastin_MINLSVPDTIDER.2 | 38.083 |
| 513.31 | 685.4 | 30 | Elongation factor_IGGIGTVPVGR.2 | 27.586 |
| 513.31 | 912.53 | 30 | Elongation factor_IGGIGTVPVGR.2 | 27.586 |
| 468.91 | 652.32 | 30 | Elongation factor_YYVTIIDAPGHR.3 | 27.446 |
| 468.91 | 765.4 | 30 | Elongation factor_YYVTIIDAPGHR.3 | 27.446 |
| 595.33 | 660.39 | 30 | 14-3-3 protein_DSTLIMQLLR.2 | 31.195 |
| 595.33 | 773.47 | 30 | 14-3-3 protein_DSTLIMQLLR.2 | 31.195 |
| 774.86 | 876.41 | 30 | 14-3-3 protein_SVTEQGAELSNEER.2 | 39.094 |
| 774.86 | 1004.46 | 30 | 14-3-3 protein_SVTEQGAELSNEER.2 | 39.094 |
| 523.79 | 660.37 | 30 | Macrophage migration_LLC[CAM]GLLSDR.2 | 28.047 |
| 523.79 | 820.4 | 30 | Macrophage migration_LLC[CAM]GLLSDR.2 | 28.047 |
| 644.35 | 700.37 | 30 | Macrophage migration_PMFIVNTNVPR.2 | 33.351 |
| 644.35 | 799.44 | 30 | Macrophage migration_PMFIVNTNVPR.2 | 33.351 |
The CZE-MRM interface and mass spectrometer used in this work were the same as in our earlier work [24]. High voltage was supplied by two Spellman CZE 1000R power supplies. The separation capillary was coupled to a QTrap 5500 hybrid linear-ion trap triple-quadrupole instrument (AB Sciex) with an electrokinetically driven sheath-flow electrospray interface [9–11]. The emitter was pulled in a Sutter pipette puller to a ~10 μm ID. Voltage programming was controlled by LabView software. Protein digests were dissolved in 5 mM NH4HCO3 (pH 8.0), and injected onto the separation capillary (50 μm i.d. × 150 μm o.d., 35 cm) at 5 kV for 3 s. For CZE separation, 10 kV was applied at the injection end of the 35 cm separation capillary and 3 kV was applied at the electrospray interface. The separation buffer was 5 mM NH4HCO3 (pH 8.0), and the sheath liquid was 50% (v/v) methanol and 0.1% (v/v) FA. Each sample was analyzed in at least duplicate runs.
MRM data were acquired in manual acquisition mode on the QTrap 5500 mass spectrometer with the Nanospray III DCI interface heater installed and set to 160 °C. The values of declustering potential (DP) and collision exit potential (CXP) were set at 100 V and 35 V, respectively. Resolution for all transitions was performed at Q1, Q3 unit resolution (0.7 amu fwhm). All other parameters were the default settings, and neither period nor MRM scheduling was used.
Data analysis
Skyline 1.3 (MacCoss Lab, University of Washington) was used to analyze the MRM data [33]. Savitzky-Golay smoothing was applied for all transitions. Transitions with signal noise ratio (S/N) higher than 3 (based on a noise of 10 counts s−1) within 3 min. time range were considered to be confident detection. For each peptide, at least two transitions were detected, and co-migration of all the detected transitions was required. For each protein, at least one unique peptide was confidently detected. In addition, each protein was detected in at least duplicate runs. The information of the detected transitions was exported from the Skyline software and is listed in supporting material II.
Results and discussion
Overview
In this work, we sought to determine the applicability of the MRM based approach as a sensitive protein-detector and as an extension of the sensitivity gains afforded by CZE. We generated the transitions for MRM analysis based on an UPLC-ESI-MS/MS (Orbitrap Velos) analysis of the protein digests with HCD for peptide fragmentation. HCD was chosen for fragmentation because it provides greater similarity to QqQ-CID fragmentation than ion trap-CID [26].
We first evaluated the transition generation method and the CZE-MRM system by analyzing a five-order of magnitude exponential digest mixture of six proteins with the CZE-MRM system. We then applied the CZE-MRM system for analysis of 100 pg of RAW 264.7 cell lysate digest.
Evaluation of the transition generation method and the CZE-MRM system for protein digests analysis
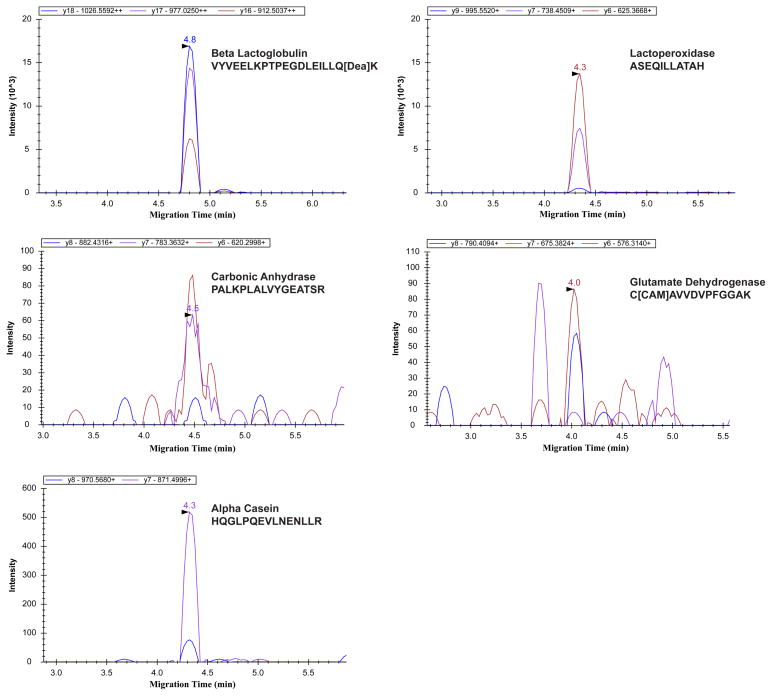
To evaluate the transition generation method and also the CZE-MRM system for protein digests analysis, a six-protein tryptic digest exponential molar mixture with five orders of magnitude concentration dynamic range (see S-Table 2 in supporting material I) was used as a model sample for analysis by CZE-MRM. In total, 46 transitions from the six proteins were monitored by CZE-MRM, S-Table 1 in supporting material I. When the sample loading amount was 2.5 ng, five proteins with four orders of magnitude concentration difference were confidently detected by the CZE-MRM system, Figure 1. The injection amounts for alpha-casein and glutamate dehydrogenase were ~10 amole and ~90 amole, respectively, and the signal of the highest transition for the two proteins was about 500 and 90 counts s−1, respectively. Therefore, the corresponding mass detection limits (S/N = 3) were ~600 zmole and ~30 amole, respectively, according to the transition with the highest intensity (calculated with noise as 10 counts s−1). It is worth mentioning that the detection limits of the two proteins were obtained in the presence of ion suppression produced by the other 4 proteins, whose total concentration is at least 1,000 times higher than alpha-casein and glutamate dehydrogenase. The total ion current chromatogram of the six-protein-digest mixture from UPLC-ESI-MS/MS (Orbitrap Velos) is shown in S-Figure 1 in supporting material I, which suggests the complexity of the sample. Besides the ion suppression of the huge background from other four proteins, there might be one benefit of the existence of huge background for low abundant protein detection, which is the obviously reduced potential adsorption of low abundant protein digests on the capillary inner wall.
Figure 1.
MRM transitions results of detected five proteins from 2.5 ng of the six bovine tryptic digest exponential molar mixture analyzed by the CZE-MRM system.
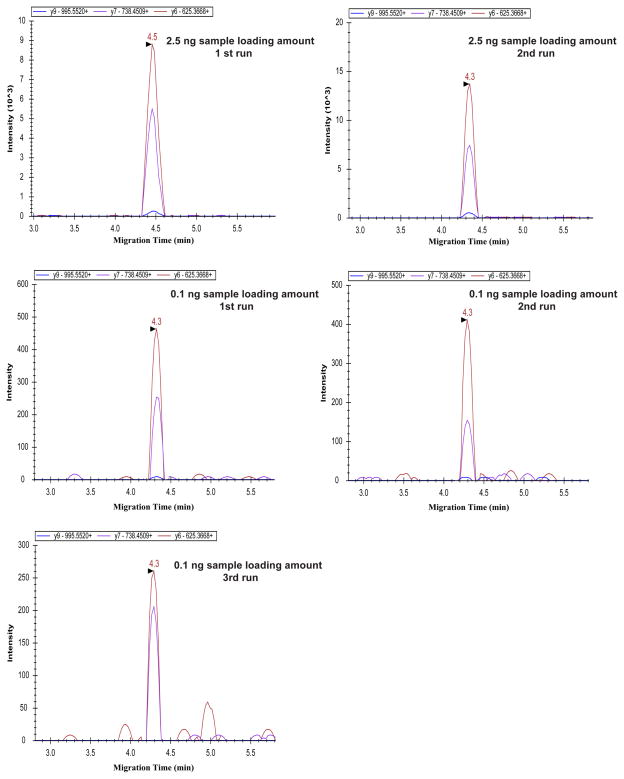
We also analyzed a 100 pg aliquot of the six bovine tryptic digest exponential molar mixture sample. The two highest abundant proteins (lactoperoxidase and beta lactoglobulin) in the protein mixture were reliably detected, Figure 2 and S-Figure 2 in supporting material I. The detected transitions of the two proteins from a 2.5 ng protein digests produced reasonably reproducible intensity between runs. For lactoperoxidase (Figure 2), the two detected transitions from the 100 pg sample yielded consistent intensity in triplicate runs, and the relative standard deviations (RSDs) were about 27% and 23%, respectively. For lactoperoxidase (Figure 2), the ratios of intensity between the two detected transitions from both 2.5 ng and 100 pg samples were also consistent for all the five runs, and the RSD was about 27%. We also determined the intensity ratios of the two detected transitions between 2.5 ng and 100 pg samples for lactoperoxidase, and the ratios of average intensity from duplicate or triplicate runs were about 30, which accurately reflects the 25-fold difference of sample loading amounts.
Figure 2.
CZE-MRM results of Lactoperoxidase (ASEQILLATAH) for 2.5 ng and 100 pg loading amounts of the six bovine tryptic digest exponential molar mixture.
We further analyzed the data of beta lactoglobulin, S-Figure 2 and 3 in supporting material I. For the 2.5 ng loading amount data (S-Figure 2), a reasonably reproducible transition intensity was obtained between runs, and the intensity ratios between transitions were also consistent in duplicate runs. However, for the 100 pg loading amount data (S-Figure 2), the intensity of transitions was more than 100 times lower than that from the 2.5 ng samples, and the intensity ratios between transitions were also not consistent with that from 2.5 ng samples. In order to well understand the results from 100 pg samples, we checked the peaks of generated by the transitions without Savitzky-Golay smoothing, S-Figure 3 in supporting material I. The peak width of the original transition in S-Figure 3 is about 5 s or less. The cycle time of the CZE-MRM analysis is about 1.4 s, and fewer than 4 points were acquired for each transition. Therefore, the peak shape of the transitions was not reproducible, and the intensity of the transitions was out of the linear range. For the intensity ratios between transitions, because only 4 or fewer points were acquired for each transition and the transition intensity is very low, it is possible that the ratios were not consistent with that from 2.5 ng samples. Because the detected peptides were unique to the proteins and the detected 2–3 transitions for each peptide were also specific, the protein detection with the CZE-MRM from 2.5 ng and 100 pg samples was confident. In addition, the migration time of transitions between 2.5 ng and 100 pg samples was consistent, which further confirmed the transitions detection from the 100 pg samples. These results indicate that the method for transition generation works well and the CZE-MRM system is suitable for highly sensitive and specific protein detection in complex samples.
It is worth mentioning that the migration time of all the detected transitions was less than 10 min, and the separation window was about three minutes. The results indicate that the CZE-MRM system is valuable for high throughput target protein analysis.
Application for 100 pg RAW 264.7 cell lysate digest analysis
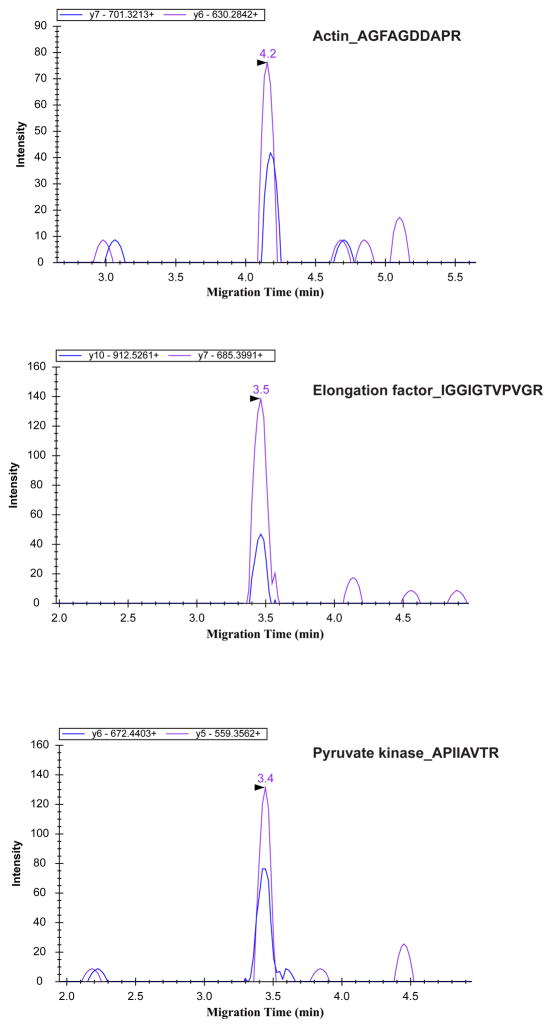
We applied the CZE-MRM system for the analysis of 100 pg of a RAW 264.7 cell lysate digest. This amount corresponds to the protein content of a single cell [12]. In order to develop the transitions, we first obtained 26 high abundant proteins in the RAW 264.7 cell lysate based on protein spectral count data using UPLC-ESI-MS/MS (LTQ-Orbitrap Velos). Then, 104 transitions were generated based on these empirical data, and monitored and detected by the CZE-MRM at a 700 pg sample load. For these data, a 10 ms dwell time was used. These 36 transitions corresponding to abundant targets from 9 proteins were ultimately monitored from 100 pg of RAW 264.7 cell lysate digest, Table 1.
Three proteins were confidently detected by the CZE-MRM system from 100 pg of the RAW 264.7 cell lysate digest, Figure 3. The transition intensities of the three detected proteins were reasonably reproducible in duplicate runs, and the intensity ratios between transitions for the detected proteins were also consistent with that from 700 pg cell lysate digest, S-Figure 4, 5 and 6 in supporting material I. For 100 pg cell lysate digest analysis, at least two specific transitions from one unique peptide were reliably and reproducibly detected for each protein. Of course, only the most highly expressed proteins could be detected due to the minute amount of sample employed. Because the dwell time of the transitions for 700 pg and 100 pg cell lysate digests analysis is different (10 ms vs. 30 ms), the intensity ratios of transitions between 700 pg and 100 pg samples differ from the ratio of sample loading amounts (700pg/100pg). In addition, the consistent migration time between runs and between the 700 pg and 100 pg sample amounts further confirms the detection. The results demonstrate that “bottom-up” based single cell protein analysis may be extended with the use of CZE-MRM as a more sensitive and selective protein detector.
Figure 3.
CZE-MRM results of 100-pg RAW 264.7 cell lysate digest.
One potential application of the CZE-MRM system for single cell protein analysis is to couple the system with single cell injection, online cell lysis, [34, 35] and online protein digestion with an immobilized trypsin microreactor [36, 37]. Compared with current techniques for single cell protein analysis, one potential advantage of the CZE-MRM based approach is the depth of protein profiling due to its high sensitivity. However, one challenge for this approach is the efficient online digestion of trace amounts of proteins from a single cell with immobilized trypsin. That digestion will require efficient protein denaturation with MS compatible reagents.
Conclusion
The CZE-MRM/SRM system with an electrokinetically driven sheath-flow electrospray interface was used for the rapid and highly sensitive detection of protein analytes in ca omplex tryptic digests. For the commercial exponential mixture of bovine proteins, five proteins spanning four orders of magnitude concentration were confidently detected from only 2.5 ng of the digest mixture. For 100 pg of a RAW 264.7 cell lysate digest, three proteins were reliably and reproducibly detected, and the sample amount corresponds to the approximate protein content from a single cell, which suggests that CZE-MRM may be a valuable detector in single cell protein analysis. In addition to providing highly sensitive detection of proteins in complex mixtures, this system is rapid; the migration time of the protein digests was less than 10 min.
Supplementary Material
Acknowledgments
We thank Dr. William Boggess in the Notre Dame Mass Spectrometry and Proteomics Facility for his help with this project. This project was supported by a grant from the National Institutes of Health (R01GM096767).
References
- 1.Krylov SN, Dovichi NJ. Anal Chem. 2000;72:111R–128R. doi: 10.1021/a1000014c. [DOI] [PubMed] [Google Scholar]
- 2.Geiger M, Hogerton AL, Bowser MT. Anal Chem. 2012;84:577–596. doi: 10.1021/ac203205a. [DOI] [PubMed] [Google Scholar]
- 3.Jorgenson JW, Lukacs KD. Science. 1983;222:266–272. doi: 10.1126/science.6623076. [DOI] [PubMed] [Google Scholar]
- 4.Maxwell EJ, Chen DD. Anal Chim Acta. 2008;627:25–33. doi: 10.1016/j.aca.2008.06.034. [DOI] [PubMed] [Google Scholar]
- 5.Moini M. Anal Chem. 2007;79:4241–4246. doi: 10.1021/ac0704560. [DOI] [PubMed] [Google Scholar]
- 6.Busnel JM, Schoenmaker B, Ramautar R, Carrasco-Pancorbo A, Ratnayake C, Feitelson JS, Chapman JD, Deelder AM, Mayboroda OA. Anal Chem. 2010;82:9476–9483. doi: 10.1021/ac102159d. [DOI] [PubMed] [Google Scholar]
- 7.Faserl K, Sarg B, Kremser L, Lindner H. Anal Chem. 2011;83:7297–7305. doi: 10.1021/ac2010372. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Fonslow BR, Wong CCL, Nakorchevsky A, Yates JR., 3rd Anal Chem. 2012;84:8505–8513. doi: 10.1021/ac301091m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wojcik R, Dada OO, Sadilek M, Dovichi NJ. Rapid Commun Mass Spectrom. 2010;24:2554–2560. doi: 10.1002/rcm.4672. [DOI] [PubMed] [Google Scholar]
- 10.Wojcik R, Li Y, MacCoss MJ, Dovichi NJ. Talanta. 2012;88:324–329. doi: 10.1016/j.talanta.2011.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun L, Zhu G, Li Y, Wojcik R, Yang P, Dovichi NJ. Proteomics. 2012;12:3013–3019. doi: 10.1002/pmic.201200100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen D, Dickerson JA, Whitmore CD, Turner EH, Palcic MM, Hindsgaul O, Dovichi NJ. Annu Rev Anal Chem. 2008;1:165–190. doi: 10.1146/annurev.anchem.1.031207.113104. [DOI] [PubMed] [Google Scholar]
- 13.Picotti P, Lam H, Campbell D, Deutsch E, Mirzaei H, Ranish J, Domon B, Aebersold R. Nat Methods. 2008;5:913–914. doi: 10.1038/nmeth1108-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Picotti P, Bodenmiller B, Mueller LN, Domon B, Aebersold R. Cell. 2009;138:795–806. doi: 10.1016/j.cell.2009.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuhn E, Wu J, Karl J, Liao H, Zolg W, Guild B. Proteomics. 2004;4:1175–1186. doi: 10.1002/pmic.200300670. [DOI] [PubMed] [Google Scholar]
- 16.Lange V, Picotti P, Domon B, Aebersold R. Mol Syst Biol. 2008;4:222. doi: 10.1038/msb.2008.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Picotti P, Aebersold R. Nat Methods. 2012;9:555–566. doi: 10.1038/nmeth.2015. [DOI] [PubMed] [Google Scholar]
- 18.Anderson L, Hunter CL. Mol Cell Proteomics. 2006;5:573–588. doi: 10.1074/mcp.M500331-MCP200. [DOI] [PubMed] [Google Scholar]
- 19.Kuzyk MA, Smith D, Yang J, Cross TJ, Jackson AM, Hardie DB, Anderson NL, Borchers CH. Mol Cell Proteomics. 2009;8:1860–1877. doi: 10.1074/mcp.M800540-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lund H, Løvsletten K, Paus E, Halvorsen TG, Reubsaet L. Anal Chem. 2012;84:7926–7932. doi: 10.1021/ac301418f. [DOI] [PubMed] [Google Scholar]
- 21.Wolf-Yadlin A, Hautaniemi S, Lauffenburger DA, White FM. Proc Natl Acad Sci USA. 2007;104:5860–5865. doi: 10.1073/pnas.0608638104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Unwin RD, Griffiths JR, Whetton AD. Nat Protoc. 2009;4:870–877. doi: 10.1038/nprot.2009.57. [DOI] [PubMed] [Google Scholar]
- 23.Llarrull LI, Toth M, Champion MM, Mobashery S. J Biol Chem. 2011;286:38148–38158. doi: 10.1074/jbc.M111.288985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y, Wojcik R, Dovichi NJ, Champion MM. Anal Chem. 2012;84:6116–6121. doi: 10.1021/ac300926h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang C, Lee CS, Smith RD, Tang K. Anal Chem. 2012;84:10395–10403. doi: 10.1021/ac302616m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Graaf EL, Altelaar AFM, Breukelen BV, Mohammed S, Heck AJR. J Proteome Res. 2011;10:4334–4341. doi: 10.1021/pr200156b. [DOI] [PubMed] [Google Scholar]
- 27.Sun L, Li Y, Yang P, Zhu G, Dovichi NJ. J Chromatogr A. 2012;1220:68–74. doi: 10.1016/j.chroma.2011.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun L, Zhu G, Dovichi NJ. Rapid Commun Mass Spectrom. 2013;27:157–162. doi: 10.1002/rcm.6437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olsen JV, de Godoy LMF, Li G, Macek B, Mortensen P, Pesch R, Makarov A, Lange O, Horning S, Mann M. Mol Cell Proteomics. 2005;4:2010–2021. doi: 10.1074/mcp.T500030-MCP200. [DOI] [PubMed] [Google Scholar]
- 30.DiGiuseppe Champion PA, Champion MM, Manzanillo P, Cox JS. Mol Microbiol. 2009;73:950–962. doi: 10.1111/j.1365-2958.2009.06821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keller A, Nesvizhskii AI, Kolker E, Aebersold R. Anal Chem. 2002;74:5383–5392. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- 32.Elias JE, Gygi SP. Nat Methods. 2007;4:207–214. doi: 10.1038/nmeth1019. [DOI] [PubMed] [Google Scholar]
- 33.MacLean B, Tomazela DM, Shulman N, Chambers M, Finney GL, Frewen B, Kern R, Tabb DL, Liebler DC, MacCoss MJ. Bioinformatics. 2010;26:966–968. doi: 10.1093/bioinformatics/btq054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu S, Le Z, Krylov S, Dovichi NJ. Anal Chem. 2003;75:3495–3501. doi: 10.1021/ac034153r. [DOI] [PubMed] [Google Scholar]
- 35.Krylov SN, Zhang Z, Chan NW, Arriaga E, Palcic MM, Dovichi NJ. Cytometry. 1999;37:14–20. doi: 10.1002/(sici)1097-0320(19990901)37:1<14::aid-cyto2>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 36.Li Y, Wojcik R, Dovichi NJ. J Chromatogr A. 2011;1218:2007–2011. doi: 10.1016/j.chroma.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun L, Zhu G, Li Y, Yang P, Dovichi NJ. Anal Chem. 2012;84:8715–8721. doi: 10.1021/ac3019608. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.