Abstract
Recent studies describe a heterogeneous population of cells of the myeloid lineage, termed myeloid derived suppressor cells (MDSC), which are observed with increased prevalence in the peripheral blood and tumor microenvironment of cancer patients, including pancreatic cancer. Accumulation of MDSC in the peripheral circulation has been related to extent of disease, and correlates with stage. MDSC have primarily been implicated in promoting tumor growth by suppressing antitumor immunity. There is also compelling evidence MDSC are also involved in angiogenesis and metastatic spread.
Two main subsets of MDSC have been identified in cancer patients: a monocytic subset, characterized by expression of CD14, and a granulocytic subset characterized by expression of CD15. Both subsets of MDSC actively suppress host immunity through a variety of mechanisms including production of reactive oxygen species and arginase. Just as in humans, accumulation of monocytic and granulocytic MDSC has been noted in the bone marrow, spleen, peripheral circulation, and tumors of tumor bearing mice.
Successful targeting of MDSC in mice is associated with improved immune responses, delayed tumor growth, improved survival, and increased efficacy of vaccine therapy. By further elucidating mechanisms of MDSC recruitment and maintenance in the tumor environment, strategies could be developed to reverse immune tolerance to tumor. We discuss here what is currently known about MDSC as well as some potential strategies targeting MDSC in the context of our work on pancreatic cancer and recent literature. Due to the number of new reports on MDSC, the most pertinent ones have been selected.
Keywords: myeloid-derived suppressor cells, pancreatic cancer, immune suppression, bone marrow, metastasis
INTRODUCTION
Pancreatic adenocarcinoma: prognosis and clinical management
Pancreatic adenocarcinoma, referred to further in this review as pancreatic cancer, is a highly aggressive malignancy and the fourth leading cause of cancer deaths in the United States with over 43,000 estimated new cases in 2010 [1]. The prognosis of these patients is dismal with overall survival less than 2% and a median survival of 4 to 6 months. The significance of this malignancy is further illustrated by comparing the estimated 36,800 deaths from pancreatic cancer in 2010 to the estimated 32,050 and 40,230 deaths from prostate and breast cancer, respectively [1]. The poor prognosis of pancreatic cancer is related to a combination of late detection and ineffective treatment regimens. Surgical resection is the only known curative treatment for pancreatic cancer, however, only 15-20% of patients present with operable disease [2]. Among these patients median survival is 15-20 months with approximately 20% five-year survival rate [3, 4].
The current Federal Drug Administration-approved therapy for unresectable pancreatic cancer is gemcitabine monotherapy based on a Phase IIl clinical trial from 1997 demonstrating a median survival of 5.5 months and 18% 1-year survival [5]. Although gemcitabine-based chemotherapeutic regimens are considered to be first line treatments, pancreatic cancer is notoriously chemo-resistant and effectiveness of chemotherapeutics remains disappointing. Multiple combinations of chemotherapeutic drugs as well as combinations of chemotherapy and biological agents have been explored [6] in the interest of improving on this limitedly effective therapy in both non-surgical and surgical patients. Recently, the combination of erlotinib plus gemcitabine was shown to improve 1-year overall survival and median survival among patients with advanced pancreatic cancer but the overall benefit was modest at the cost of increased toxicity [7].
Investigators at the Virginia Mason Clinic in Seattle recently reported the results of their Phase II trial using an interferon-alpha (IFNα) based chemoradiation regimen in patients after resection in which the 2-year overall survival reached 64% [8]. We concluded a similar Phase II trial utilizing adjuvant IFN-α, gemcitabine, 5-FU, and radiation and demonstrated a similar 2-year overall survival of 56% [9]. This compares favorably to the outcomes of a variety of randomized clinical trials using radiation and chemotherapy following surgery [10-12]. More recently, novel neo-adjuvant gemcitabine-based chemoradiation protocols have been introduced with encouraging results [13, 14]. Given the above results using adjuvant immunomodulatory strategies and neo-adjuvant chemoradiation, combining the two is a logical next step. It is important to note that while gemcitabine blocks DNA synthesis resulting in inhibition of tumor replication and lysis of tumor cells [15], data from animal studies shows evidence that gemcitabine selectively depletes myeloid derived suppressor cells (MDSC) and improves T cell immune responses, raising the possibility of an immunologic component to gemcitabine monotherapy [16, 17].
THE IMMUNE RESPONSE TO PANCREATIC CANCER
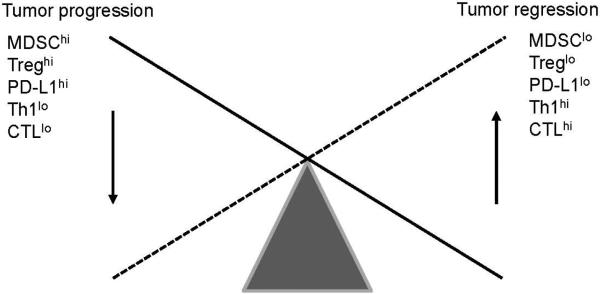
Human tumors of non-hematopoietic origin are generally characterized by infiltrating leukocytes. The presence of an immune cell infiltrate has been interpreted as the host's response to the developing tumor. Indeed, in several types of malignancies the extent of immune infiltrate correlates with good prognosis [18, 19]. A detailed analysis of the tumor infiltrate in colorectal carcinomas implicated cell type, density, and location as prognostic indicators [20, 21]. However, equally convincing and numerous are reports describing a worse prognosis with increasing intensity of infiltrate [20, 22]. Key in the discrepancy of these findings may be the composition of the immune infiltrate; a recent study suggests that the presence of a lymphocytic infiltrate, in particular the presence of memory Th1 and cytotoxic T cells (CTL) are strong prognostic factors for long term survival (Figure 1) [19]. The presence of immune suppressor cells such as T regulatory cells (Treg) and myeloid-derived suppressor cells (MDSC) on the other hand may worsen prognosis.
Figure 1.
Schematic representation of the balance between tumor growth and immune correlates.
Progress in basic and translational immunology has confirmed the importance of the immune system in cancer prevention and prognosis, and has renewed interest in vaccine therapy for cancer [19, 20, 23]. A number of proteins overexpressed by most pancreatic cancers have been explored as targets for immune-based therapies such as mucin-1, carcinoembryonic antigen, K-RAS, and mesothelin [24]. While it has been observed that patients with advanced pancreatic cancer exhibit depressed immuno-responsiveness [25, 26] and therefore may not be appropriate candidates for immunotherapy, several clinical studies [25, 27, 28] have demonstrated this patient population can effectively mount immune responses to vaccines. There is strong evidence for the presence of circulating tumor-specific immune effector cells in patients with pancreatic cancer [29-31] that can be expanded by active immunization [24, 27, 32]. Studies from our group have focussed on mesothelin as a immunologic target in pancreatic cancer. Mesothelin is a ubiquitous antigen that is nearly universally expressed by pancreatic adenocarcinoma which is the most common histologic type of pancreas cancers composing about 85% of all tumors [33, 34]. In vitro stimulation with purified mesothelin protein induced mesothelin-specific reactivity in both CD4 and CD8 T cells from patients with diverse MHC backgrounds [35], further confirming that patients with pancreatic cancer contain tumor-specific T cells that can be expanded and potentially exploited therapeutically.
In spite of these encouraging findings, significant clinical responses are rare [24, 25, 27], illustrating one of the major limitations immune-based therapies face: the induction of specific antitumor immune effector cells in peripheral blood does not necessarily translate into durable antitumor immune responses. It is interesting to note that two components of the immune suppressor network are independent predictors of prognosis in pancreatic cancer. Both the prevalence of Treg [36, 37] and expression levels of Programmed Death-1 ligand (PD-L1), a key immune regulatory molecule have prognostic value [38]. Treg suppress immune effector cells through a variety of mechanisms (discussed below), whereas PD-L1 induces cell death in PD-1-expressing cells such as activated T cells [39]. The finding that both Treg and PD-L1 are prognostic factors further argues that pancreatic cancer induces an immune response that, when harnessed appropriately, might have an impact on clinical responses.
Immune suppression in pancreatic cancer
Over the past several years, a number of reviews have described mechanisms of immune evasion [40, 41]. Although specific information on immune escape mechanisms in pancreatic cancers is limited, recent findings suggest that tumor-induced mechanisms of immune suppression are the main reason for the weak clinical responses to immune-based therapies [42-44]. Immune suppression is mediated by both soluble factors such as TGFβ, prostaglandins, and IL-10 [44], and suppressor cells such as MDSC and Treg. In addition to directly inhibiting antitumor immunity, soluble tumor-derived factors promote the generation of immune suppressor cells which can alter the immune balance and thus the type of predominant immune response. For example, reduced levels of IL-2 and increased levels of IL-10 promote differentiation of CD4+ Th2 cells over Th1 cells thereby limiting cellular antitumor immunity [26]. Cytokines in the tumor environment have also been shown to induce expression of the tryptophan degrading enzyme indoleamine 2, 3-dioxygenase (IDO) in macrophages and dendritic cells. As tryptophan is necessary for effective T cell activation, depletion of tryptophan by IDO results in T cell suppression [45]. Similarly, tumor-derived factors interfere with normal bone marrow myelopoiesis, promoting the formation of MDSC and inhibiting normal differentiation of antigen-presenting dendritic cells. Treg and, more recently, MDSC have been associated with immune suppression and tumor promotion (Figure 1) [46, 47].
MYELOID-DERIVED SUPPRESSOR CELLS (MDSC) IN TUMOR-BEARING HOSTS
Bone marrow-derived immune suppressor cells have been described in tumor-bearing mice since the late 1970s [48, 49]. These cells are of myeloid origin and were initially characterized as suppressor macrophages [48-51]. It was not until the 1990s that investigators focused more intensely on these cells in cancer patients [52-54]. Although initially termed “myeloid suppressor cells” or “immature myeloid cells” they have more accurately been renamed “myeloid-derived suppressor cells”, or MDSC to better reflect the origin and function of these undifferentiated myeloid cells [55]. MDSC comprise heterogeneous populations of immature cells arising from the myeloid lineage that share common functional and phenotypical characteristics. They are observed with increased prevalence in the peripheral blood and in the tumor microenvironment of patients with solid tumors [56], including pancreatic cancer [52, 57-60]. MDSC are also increased in many pathologic conditions such as infections, inflammatory diseases, sepsis, and traumatic shock, and serve to down regulate T cell responses. While MDSC were initially pursued because of their suppressive effect on antitumor immunity, investigators have learned that they may have a direct impact on cancer biology independent of the immune system such as by promoting tumor growth and formation of metastases.
MDSC origin and phenotype
MDSC are bone marrow-derived cells of myeloid origin that are present in blood, lymph nodes, spleen, tumors, and immune-activated tissues. The production of these cells results from an altered hematopoietic differentiation pathway leading to the expansion and accumulation of a heterogenous population of immature myeloid cells. Hematopoiesis is typically represented as a hierarchical process (Figure 2). Normal developmental hematopoietic pathways have largely been defined based on expression of surface and intracellular markers. The hematopoietic stem cell is the most important, which in addition to self-renewal can initiate hematopoiesis which gives rise to all blood cells [61]. The hematopoietic stem cell can differentiate into hematopoietic progenitor cells that give rise to multipotential progenitor cells (Figure 2). In turn these cells give rise to myeloid and lymphoid progenitor cells.
Figure 2.
Hierarchical development of mature peripheral blood cells and MDSC through differentiation of hematopoietic stem cells (HSC) in mice. Differentiation of HSC is considered normal until the Granulocyte/Monocyte Precursor (GMP) stage. GMPs fail to differentiate into mature PMN and monocytes in a tumor-bearing host. Instead, GMPs give rise to MDSC that bear markers of both PMN and monocytes, but do not express markers of fully-matured cells. Please note that this diagram is meant primarily to illustrate the origin and main phenotypic markers of MDSC rather than depict in great detail the various differentiation pathways.
The myeloid progenitor cells further differentiate into immature myeloid cells. It is at this stage that the cells can leave the bone marrow and enter the peripheral circulation. Normally, the myeloid cells will migrate to various peripheral organs where they undergo differentiation into mature myeloid cells such as macrophages, dendritic cells, or granulocytes. However, under conditions such as infections, sepsis/trauma [62, 63], or in a tumor environment [64], the differentiation of immature myeloid cells into normal mature myeloid cells is blocked, and at the same time the immature myeloid cells are activated and become MDSC (Figure 2).
Additionally, altered differentiation of immature myeloid cells has been noted, for example in a tumor environment. Instead of giving rise to mature macrophages, some immature myeloid cells differentiate into tumor-associated macrophages (TAM) that are functionally and phenotypically distinct from normal mature macrophages [65-67]. Based on expression of differentiation markers, TAM, tumor-infiltrating dendritic cells [68] and mast cells differ from MDSC [65, 66, 69-71], but caution should be taken with classification of myeloid cells into various cell types, as the distinction between “immature” and “alternatively differentiated” may not always be clear.
The diverse heterogeneity of MDSC has made characterization difficult [72]. In humans, MDSC express CD11b (common myeloid marker, alpha M-integrin, Mac-1) and myelomonocytic/macrophage markers such as CD14 and CD33 (common myeloid marker), or granulocyte/neutrophil markers including CD15 (neutrophil marker, Lewis X antigen), often without exhibiting markers of terminal differentiation (Lin-Drlo/-) [73-75]. Recent studies on MDSC in cancer patients have shown that at least two subsets of human MDSC exist: CD11b+CD33+ cells that are CD15+ [57, 59, 60] or CD66b+ [58, 76], and CD15-CD14+DRlo cells [77-80]. The prevalence of each subset appears disease-related, as the CD15+ cells were detected in patients with renal cell carcinoma [57], non-small cell lung cancer [60] and pancreatic adenocarcinoma [59], whereas monocytic MDSC (CD14+) were detected in patients with glioblastoma [81], ovarian carcinoma [82], hepatocellular carcinoma [77], malignant melanoma [78, 79, 83], bladder, and prostate cancer [84, 85].
Our preliminary data show that the tumor microenvironment in pancreatic adenocarcinoma contains both monocytic MDSC (CD11b+CD14+) and granulocytic MDSC (CD11b+CD15+) (Figure 3). There is also a subset that expresses both CD14 and CD15, confirming the immature phenotype of MDSC. In contrast, no MDSC infiltrate was detected in normal pancreas tissue (not shown). In the periphery, the CD11b+ subset co-expressing CD15 is significantly greater in patients compared to age-matched controls. Patients with advanced pancreatic cancer also have a significantly expanded CD11b+ subset that co-expresses CD14 instead of CD15, as determined by flow cytometry (unpublished observations).
Figure 3.
Both monocytic and granulocytic MDSC infiltrate human pancreatic adenocarcinoma. A: Immunohistochemistry demonstrates a high prevalence of CD15+CD11b+ MDSC in tissue samples resected from patients with pancreatic adenocarcinoma. Red (CD11b) and green (CD15) fluorescence is shown with co-localization of CD15+CD11b+ MDSC indicated by yellow. B: Co-localization of CD11b (blue) and CD14 (red) indicated by pink/purple, and CD11b and CD15 (green) indicated by aqua. Additionally, co-localization of CD14 and CD15 is noted in some cells (pink plus aqua).
The monocytic and granulocytic human MDSC subsets resemble those identified in murine tumors [86, 87]. In mice, MDSC are characterized by co-expression of CD11b and Gr-1 [64, 72, 75, 88, 89], a granulocyte marker also known as Ly6G (Figure 2). Some MDSC express high levels of Gr-1 and display a granulocytic morphology whereas other MDSC expressing low levels of Gr-1 resemble monocytes [65], and highly express Ly6C [87]. It has been observed that due to hypoxia monocytic MDSC rapidly differentiate into TAM in the tumor environment[90], expressing F4/80 (mouse) [71, 86, 91] or CD68 (human), hence the phenotypic and functional overlap between tumor-derived MDSC and TAM [67, 70, 92]. Other MDSC markers that have been reported are CD115 (M-CSF or GSF-1 receptor) [93] and CD124, the IL-4 receptor α (IL-4Rα) [65, 71, 86]. It should be noted, however, that unlike CD11b and Gr-1, CD115 and CD124 may not be involved with immunosuppressive MDSC in all tumor models [46].
MDSC actively suppress host immunity
MDSC from tumor-bearing hosts directly suppress antigen-specific T cell-mediated immunity [94]. This became evident when investigators explored why patients with advanced cancers responded so poorly to various vaccination protocols: poor responses were associated with an increased prevalence of immature myeloid cells and a decrease in dendritic cells. Increased numbers of MDSC in the tumor and peripheral blood were initially noted in patients with head and neck cancer [52, 95, 96], and their increased prevalence was confirmed in patients with other cancers [46, 97, 98]. Initial studies focused on CD34+ myeloid cells that are precursors of granulocytes and/or antigen presenting macrophages and dendritic cells. While the CD34+ cells could be induced to differentiate into functionally active dendritic cells in vitro, freshly isolated CD34+ cells from cancer patients inhibited IL-2 production by anti-CD3 antibody-stimulated intratumoral T cells. Likewise, in vitro depletion of CD15+ cells [57, 99] or CD66b+ cells [58] restored IFNγ secretion, T cell proliferation and TCR expression in anti-CD3-activated peripheral blood lymphocytes from patients with advanced renal cell carcinoma. Suppression of T cell function is not restricted to non-specific T cell activation, as shown by Kusmartsev et al. using CD33+DR- MDSC from metastatic renal cancer patients that inhibited antigen-specific T cell IFN-γ secretion and cytolytic activity [100]. In patients with metastatic pancreatic or colon cancer, dramatic accumulation of CD15+ low-density granulocytes actively suppressed TCR expression and cytokine production [59].
There is ample evidence in experimental tumor models that MDSC perturb antitumor immunity [91]. Mouse MDSC suppress both antigen-specific and non-specific T cell responses, but suppression is limited to the site where MDSC are exposed to stimuli [101]. This may depend on the compartment from which they are derived: tumor-derived MDSC suppress both antigen-specific and non-specific T cell responses whereas spleen-derived MDSC suppressed non-specific T responses only [46]. While this may be a reflection of how the tumor microenvironment influences MDSC, it is also possible that the subset composition of MDSC differs in various compartments. For example, recognizing that Gr-1+ cells comprise both Ly6G (Gr-1high) and Ly6C (Gr-1low) cells [87], it is possible that each subset employs its own unique migratory and functional characteristics. Further evidence for MDSC-mediated T cell suppression comes from studies in which MDSC were specifically targeted: reduction of MDSC prevalence restores T and NK cell activity, and promotes tumor immune surveillance and the host response to cancer vaccines [46, 97]. Lastly, the importance of MDSC in controlling antitumor immunity is also highlighted in aging mice that are at increased risk of developing tumors. Aging is associated with an increased prevalence of MDSC which may contribute to the increased incidence of tumor [102].
The regulation of innate immunity by MDSC is more controversial. Several studies demonstrated MDSC-mediated inhibition of NK antitumor cytotoxicity and IFNγ release [103], but another study showed MDSC activated NK cells through expression of Rae-1, a ligand for the activation receptor NKG2D on NK cells [104]. Further characterization of MDSC subsets may clarify this discrepancy.
MDSC also interact with NKT cells. While NKT cells comprise only a small subset of immune cells, they have profound effects on other immune subsets [105]. The type II NKT cells that are characterized by a variable T cell receptor facilitate MDSC recruitment and tumor growth through secretion of IL-13 [106] which can bind to the IL-4Rα on MDSC and activate MDSC [65, 105]. In contrast, type I NKT cells or invariant NKT cells produce IFNγ and thereby promote macrophage-mediated antitumor activity [105].
In addition to the direct suppression of immune effectors, MDSC can skew the immune repertoire from anti-tumor to pro-tumor, for example by promoting CD4 Th2 rather than Th1 cells [107], or by promoting the recruitment and expansion of suppressor Treg [93, 108].
MDSC promote Treg
MDSC have been implicated in the recruitment and maintenance of Treg [93, 98, 109, 110]. It has been shown that immature dendritic cells preferentially stimulate Treg [111]. Essential for the stimulation of Treg is the tumor-induced ability of the dendritic cells to produce TGFβ [112]. Treg, in turn, have been identified as master regulators of immune homeostasis by maintaining peripheral tolerance to self-antigens through suppression of self-reactive T cells [113-116]. As most tumor antigens are self-antigens, Treg also suppress antitumor immunity [117-119]. Elevated levels of Treg have been observed in many histologically-different types of cancer [117, 120] which has been shown in several of these to correlate with decreased survival [37, 121, 122]. The interplay between MDSC and Treg creates a powerful barrier against immune attack.
MDSC immune suppressor mechanisms
MDSC use a variety of mechanisms to control antitumor immunity mediated by secreted molecules and/or expression of particular cell surface molecules (Table 1). Most of these mechanisms have been identified in experimental models and await validation in cancer patients. One such mechanism is the release of reactive oxygen species (ROS) [59, 96, 123] and peroxynitrite that catalyze nitration of the TCR and thereby prevent interaction of the TCR with peptide-MHC complexes [124, 125]. A second, well-documented mechanism is the depletion of arginine from the environment [57, 58, 126]. L-arginine is an amino acid essential for T cell replication and production of the TCR signaling molecule, zeta. MDSC express high levels of arginase 1 and inducible nitric oxide synthase (iNOS), two enzymes that degrade arginine and the TCRζ chain, and thereby block T cell activation and proliferation [60, 87]. Arginase 1 converts arginine into ornithine and urea, whereas iNOS converts arginine into nitric oxide (NO) which can suppress T cell activation and induce apoptosis [127-129]. The finding that both arginase 1 and iNOS are activated in MDSC is further evidence that MDSC do not fit the strict separation into classically or alternatively activated macrophages [130]. T cell function can be restored by using specific arginase 1 or iNOS inhibitors. Similarly, MDSC can deprive the environment of cysteine, another amino acid that is essential for T cell activation [97, 131, 132]. MDSC are dependent on cysteine as well, and import cystine for conversion into cysteine. T cells lack the ability to import cystine, and are dependent on cysteine which is normally produced by mature dendritic cells and macrophages during antigen presentation [131]. Additional mechanisms of suppression include the recently described down-regulation of the lymph node homing receptor, CD62L on CD4 and CD8 T cells [132, 133]. Down regulation of CD62L results in lack of T cell activation, as T cells do not migrate to lymph nodes where they would otherwise be activated.
Table 1.
MDSC suppressor mechanisms
| Mechanism | Suppressor cell phenotype | Model | Refs. |
|---|---|---|---|
| ROS release | CD11b, GR-1 CD11b, Ly6G+Ly6Clo CD15 CD11b,GR-1 |
7 different tumor models (mice) 10 different tumor models (mice) Advanced cancer (human) CT26 colon, C-3 sarcoma (mice) |
[96] [87] [59] [123] |
| Peroxynitrite release | CD11b, GR-1 | EL4 (mice) | [124] |
| Arginase 1 release | CD11b, GR-1 CD11b, CD66b CD11b,GR-1,IL-4Rα CD11b,CD15 |
3LL (mice), NSCLC (human) RCC (human) 5 different tumor models (mice) RCC (human) |
[126] [58] [65] [57] |
| iNOS release | CD11b, Ly6G-Ly6Chi CD11b,GR-1,IL-4Rα CD11b,Ly6Chi |
10 different models (mice) 5 different tumor models (mice) EAE (mice) |
[87] [65] [127] |
| TGFβ secretion | GR-1, CD115 | MCA-26 colon carcinoma (mice) | [93] |
| IL-10 secretion | CD11b, GR-1 GR-1, CD115 |
4T1 breast carcinoma (mice) MCA-26 colon carcinoma (mice) |
[107] [93] |
| Cysteine depletion | CD11b, GR-1 | 4T1 breast carcinoma (mice) | [131] |
| CD62L down-regulation | CD11b, GR-1 | 4T1 breast carcinoma (mice) | [133] |
| B7-H4, PD-L1 (B7-H1) expression | CD68 CD1a,HLA-DR |
HCC (human) Ovarian cancer (human) |
[136] [134, 135] |
NSCLC, non-small cell lung carcinoma; RCC, renal cell carcinoma; EAE is Experimental Autoimmune Encephalomyelitis; HCC, hepatocellular carcinoma
Other suppressor mechanisms are related to the secretion of cytokines by activated MDSC. For example, MDSC can be activated by TAM to increase production of IL-10 which in turn down-regulates IL-12 secretion by macrophages [107]. Another immunosuppressive cytokine produced by MDSC is TGFβ which can block CTL activity. TGFβ has also been implicated in activation and expansion of Treg, although TGFβ-independent induction of Treg by MDSC has been described recently. In the latter case, expression of CTLA-4 (CD152) was required on MDSC. Other T cell inhibitory molecules may be expressed on MDSC such as B7-H4 [134] and PD-L1 (B7-H1) [135, 136] that when crosslinked to their respective receptors on activated T cells induce down-regulation.
MDSC promote tumor angiogenesis and metastasis
In addition to immune suppression, MDSC may directly promote tumor development by inducing angiogenesis [137, 138] and formation of distant metastasis [139-142] Through secretion of factors that induce bone marrow mobilization such as VEGF and G-CSF, tumors activate myeloid precursors in the bone marrow [143]. The mobilization is associated with changes in the mechanisms that regulate homing of myeloid precursors in the bone marrow, and the precursors enter the peripheral circulation and migrate to the site of primary tumor as well as to future sites of metastases (Figure 4). At the site of primary tumor, the MDSC are activated and in addition to immune suppressive factors and chemokines, expression of matrix metalloproteinases (MMPs) [138] and of VEGFR1 [140, 144] is induced. For example, in the murine Lewis Lung cancer model and a spontaneous breast cancer model, Gr-1+CD11b+ MDSC directly participated in tumor angiogenesis by liberating VEGF from tumor stroma via MMP9 [145]. In addition, a subpopulation of Gr-1+CD11b+CD31+ MDSC was found to directly participate in tumor neoangiogenesis [138]. In the tumor environment some MDSC differentiate into endothelial cells that further support the generation of new vasculature [138]. It has therefore been proposed that MDSC represent the angiogenic switch that allows tumors to expand beyond their natural boundaries.
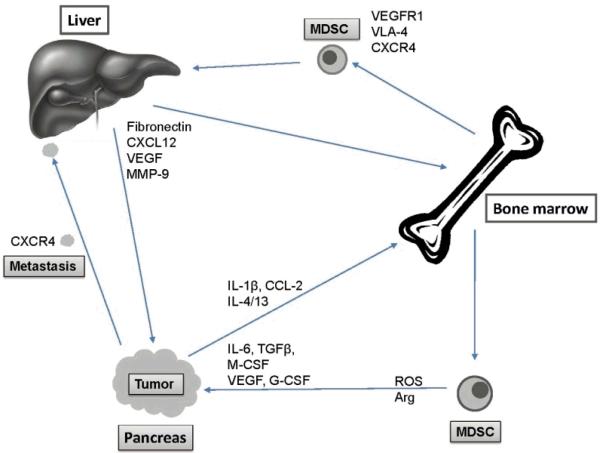
Figure 4.
Schematic overview of the role of MDSC in promoting tumor development. Developing tumors secrete factors such as VEGF, chemokines and inflammatory cytokines that induce myelopoiesis in the bone marrow. These factors also cause an imbalance in homing mechanisms of myeloid precursors which in turn leads to release of myeloid precursors into the circulation. Chemokine receptor expression on these myeloid cells directs them to various locations in the host where the corresponding ligand is expressed. For example, MDSC recruitment to primary tumor promotes angiogenesis and immune suppression, whereas MDSC recruitment to distant organs, e.g. liver, promotes the formation of metastasis. Mechanisms for MDSC recruitment, e.g. CXCR4 are also exploited by metastatic tumor cells.
Of note is the observation that recruitment of MDSC to primary tumor may also protect tumors from therapy. In animal tumor models, compensatory events have been observed between macrophages and neutrophils [146]; MDSC may increase the tumor's refractoriness to anti-VEGF antibody therapy, and MDSC infiltrate tumors after chemo – and radiation therapy and up-regulate pro-angiogenic factors such as VEGF [147]
Selected bone-marrow-derived MDSC also migrate to distant organs where they prepare what is known as the “pre-metastatic niche” (Figure 4) [140-142]. The pre-metastatic niche is characterized by clusters of myeloid cells that express VEGFR-1 and VLA-4 (integrin α4β1). They also express matrix metalloprotease-9 (MMP9). Tumor-derived growth factors induce fibronectin (the ligand for VLA-4) in resident fibroblasts and lysyl oxidase that modifies the extracellular matrix. This allows the infiltrating myeloid cells to interact with local cell types in the residing tissue, and provide a specialized area producing chemokines, growth factors, MMPs and adhesion molecules. Together the formation of these clusters prepares the site for arrival of tumor cells so that they can home to the site and develop into metastases [140, 141]. MDSC in pre-metastatic niches are also highly suppressive of T cell activation and function [142]. Elegant studies by Kaplan et al [140] demonstrated that the primary tumor determines where metastases are formed, primarily by regulating the induction of fibronectin expression in distant sites. For example, mice inoculated intradermally with Lewis Lung carcinoma developed metastasis restricted to the lung and liver whereas mice with implanted B16 melanoma developed widespread metastasis. However, mice treated with melanoma-derived conditioned medium before inoculation with Lewis Lung carcinoma developed widespread metastases resembling the B16 melanoma. The redirection of Lewis Lung metastases to non-conventional sites for this tumor was associated with high levels of VEGF and placental growth factor. Further analysis of the pre-metastatic lung tissue revealed that expression of the inflammatory proteins S100A8 and S100A9 was dramatically up-regulated by VEGF-A, TGFβ, and TNFα released by the primary tumor [148, 149]. The up-regulation of the S100 molecules was unique to the lung, the site of later metastasis, and not found in other organs. S100A8 and A9 are potent chemoattractants to CD11b+ myeloid cells and induce myeloid cell activation [150-152]. S100A9 also inhibits differentiation of MDSC into dendritic cells and macrophages. However, S100-A8 and A9 also mediated chemoattraction of tumor cells acting through Toll-like receptor 4 on tumor cells [148, 153].
At other sites of metastases chemokines different from S100-A8 and A9 might play an important role in recruitment and activation of MDSC and tumor cells (Figure 4). For example, production of keratinocyte-derived chemoattractant by abdominal tumors was essential for the recruitment and expansion of a distinct population of metastasis-enabling MDSC in the liver [142]. Likewise, the chemokine/ chemokine receptor pair CXCL12/CXCR4 is not only essential for migration of myeloid progenitor cells, but has also been implicated in migration of tumor cells, such as pancreatic, lung, breast, and prostate cancers [154-157]. In addition to regulating cell trafficking, signaling through CXCR4 also increases expression of cell surface integrin molecules and induces secretion of MMPs and angiogenic factors such as VEGF [155, 157].
MDSC recruitment, activation, and expansion in tumor-bearing hosts
There is ample evidence to suggest that recruitment of MDSC from the bone marrow to tumor and their activation is mediated mostly by chemokines [157, 158] and cytokines that are typical of inflammation [64, 97, 159-162]. Interestingly, investigators recently identified tumor-induced complement 5a as an activator of MDSC [163]. Those factors that control expansion of MDSC, i.e. myelopoiesis, are mostly tumor-derived whereas factors involved in activation of MDSC are mostly produced by stromal cells and activated T cells. Tumor-derived GM-CSF[89], G-CSF, IL-3, and VEGF have all been associated with accumulation and expansion of MDSC [143, 144, 164]. Likewise, IL-1β, IL-6, M-CSF, SCF, VEGF, prostaglandins, and COX-2 are all tumor-derived factors that can expand MDSC; inhibit their differentiation[165], and increase their resistance to apoptosis[166]. The importance of these cytokines on MDSC is supported by in vitro studies in which various combinations of these cytokines induced functionally active MDSC from normal peripheral blood mononuclear cells[167] Tumors capable of secreting these factors are more invasive and progress more rapidly, as was demonstrated in a mouse model using IL-1β-secreting and non-IL-1β-secreting tumors [168, 169]. Key in the signaling pathways triggered by these factors is the transcription factor STAT-3 that when activated promotes expansion and survival of myeloid progenitor cells [152, 170]. STAT-3 also induces accumulation of MDSC through expression of the chemoattractants S100-A8 and –A9 in MDSC, the receptors for which are also expressed on MDSC [151, 152]. Thus, the pro-inflammatory action of S100-A8/A9 involves autocrine and paracrine mechanisms [171]. Another important signaling pathway in MDSC activation is MyD88, a central adapter protein involved in signaling through most TLRs. Mice deficient in MyD88 do not develop increased levels of MDSC in response to sepsis [62].
On the other hand, T cell- and stroma-derived factors such as IFNγ [172], IL-4, IL-10, IL-13, and TGFβ induce activation of MDSC [173], meaning that they activate functional activity of MDSC as described earlier. Signaling of these molecules is mediated through STAT1, STAT6, and nuclear factor –κB. For example, IFNγ signals through STAT1 in MDSC which results in up-regulation of arginase 1 and iNOS [65]. It is interesting to note that IFNγ responsible for activating MDSC was found to be produced by activated tumor-infiltrating T cells with antitumor activity. As such, an antitumor immune response induces its own down-regulation through induction of MDSC. IFNγ can also induce expression of IDO in tumor-infiltrating dendritic cells and macrophages, and thereby induce T cell suppression [45]. IL-4 and IL-13 induce activation of STAT6 through binding to IL-4Rα in a subset of MDSC [65] which leads to expression of arginase 1 and production of TGFβ by MDSC.
The activation of MDSC also promotes crosstalk between MDSC and macrophages, and vice versa. Activation of CD14+ MDSC by pro-inflammatory cytokines such as IL1β leads to up-regulation of IL-10 production in MDSC that, in turn, down-regulates production of IL-12 in macrophages [107, 159].
TARGETING MDSC
The accumulation of MDSC is closely related to the extent of disease, and successful treatment of the inciting cancer with surgery or chemotherapy results in the regression of MDSC number [47].
In an interesting recent paper using an elegant model, Zhang et al [174] have shown that tumor growth is arrested by a single transfer of effector T cells that selectively target CD11b+Gr1+ MDSC that cross present antigens captured from surrounding cancer cells. Elimination of these tumor-associated MDSC resulted in tumor regression and sustained inhibition of tumor growth. The depletion of MDSC in murine models has been associated with an improved host immune response resulting in delayed tumor growth, improved survival, and increased efficacy of vaccine therapy. Elimination of MDSC has been shown to restore CTL and NK function, and decrease tumor angiogenesis [138, 145, 174].
A large number of different strategies have been employed to deplete and/or inhibit MDSC [175, 176]. Additionally, regimens have been tested that modulate MDSC suppressor function [177] or induce differentiation of MDSC into mature macrophages, granulocytes, and dendritic cells. An overview of the various MDSC targeting strategies is presented in Table 2; we will discuss a few of the strategies tested in humans in more detail.
Table 2.
Clinical studies specifically targeting MDSC
| Target | Reagent | Type of cancer | Refs |
|---|---|---|---|
| Induction of MDSC differentiation | ATRA | Renal cell carcinoma, | [88, 100, 178] |
| Induction of MDSC differentiation | Vitamin D3 | Head and neck cancer | [179] |
| Induction of MDSC differentiation | GM-CSF | Malignant melanoma | [180] |
| Blockade of MDSC expansion/ activation | SCF | Colon carcinoma | [181]b |
| Anti-VEGF Ab +/- IL-2 | Renal cell carcinoma | [58] | |
| VEGF-trap | Advanced tumors | [182] | |
| Sunitinib | Renal cell carcinoma | [99] | |
| Blockade of MDSC function | CDDO-Mea | Pancreatic cancer | [183] |
| Blockade of MDSC function | COX-2 inhibitorsb | Intestinal tumors Pancreatic cancer Lung cancer |
[184] [185] [186] |
| Elimination of MDSC | Gemcitabine | Lung cancer and Mesothelioma Colon cancer Breast cancer Pancreatic cancerc |
[16]b [187]b [17]a |
| Recruitment of MDSC | Aminobisphosphonates | Breast cancer, Pancreatic cancerc | [145]b |
CDDO-Me is the triterpenoid C-28 methyl ester of 2-cyano-3, 12-dioxooleana-1, 9-dien-28-oic acid
Mouse model
Linehan et al., ongoing clinical study
One strategy attempts to promote differentiation of MDSC into macrophages, dendritic cells or granulocytes using all-trans retinoic acid (ATRA) [88]. ATRA is a metabolite of vitamin A and is clinically used for induction therapy of patients with acute promyelocytic leukemia [188, 189]. ATRA has been found to induce differentiation of normal immature myeloid cells into monocytes, macrophages, and dendritic cells. Using MDSC (CD33+DR-) from patients with metastatic renal cell carcinoma in vitro, ATRA was found to restore cytolytic T cell activity, and together with GM-CSF induced differentiation of MDSC into antigen-presenting cell precursors [100]. Tested in patients with renal cell carcinoma, ATRA induced a substantial decrease in MDSC in the periphery and improved antigen-specific T cell responses [178]. Likewise, vitamin D3 can reduce the number of MDSC by inducing differentiation, and has shown promise in patients with head and neck cancer [179]. Lastly, GM-CSF-producing tumor vaccines have been used in an attempt to induce differentiation of MDSC in the tumor environment. However, there appears to be a threshold concentration in the circulation above which GM-CSF impairs antitumor activity through induction of MDSC [162, 190].
Alternative strategies focus on blocking the expansion of MDSC. For example, blockade of stem-cell factor function decreased MDSC expansion and tumor angiogenesis [181]. Targeting of VEGF using a specific antibody, bevacizumab (Avastin®), reduced the prevalence of CD11b+VEGFR1+ cells in the periphery [100]. However, a fusion protein known as VEGF-trap that binds all forms of VEGF and placental growth factor administered to patients with refractory solid tumors did not have any effect on MDSC prevalence although it did increase the proportion of mature dendritic cells [182]. Likewise, anti-VEGF antibody, bevacizumab treatment of patients with advanced metastatic renal cell carcinoma did not alter the prevalence of MDSC in the peripheral blood [58]. Another strategy aims to block signaling through VEGFR. The tyrosine kinase inhibitor, sunitinib for example blocks signaling through VEGFR, platelet-derived growth factor receptor, stem cell factor receptor (c-kit) and CSF-1R [191]. Sunitinib is being used in patients with clear cell renal cell carcinoma in which genetically-induced overproduction of VEGF has been associated with the pathogenesis of the disease. Treatment of patients with metastatic RCC significantly reduced the prevalence of CD33+DR- and CD15+CD14- MDSC subsets [99]. The reduction in MDSC was associated with a reduction in Treg and an increase in effector T cell populations in peripheral blood of patients. It should be noted that VEGF signaling pathways have been targeted also because of their role in promoting angiogenesis. While the therapies had a demonstrable inhibitory effect on tumor angiogenesis, the effect was transitory, most likely due to resistance to therapy [147].
Targeting effector mechanisms of MDSC has also shown promise such as by administration of cyclooxygenase 2 (COX-2) inhibitors in patients with renal cell carcinoma. COX-2 is required for production of prostaglandin E2 which is essential for the up-regulation of arginase 1 in MDSC. PGE-E2 also inhibits effector T cell activity; stimulates Treg, and activates IDO, the enzyme that converts tryptophan, an essential amino acid for survival of effector T cells. In an animal model of spontaneous pancreatic cancer, COX-2 inhibitors significantly improved the antitumor immune response in combination with low-dose gemcitabine and vaccine [185]. Inhibition of phosphodiesterase-5 using sildenafil (Viagra) leads to an IL-4Rα-mediated decrease in arginase 1 and NOS-2 expression in MDSC, and restored T cell proliferation in vitro [192] and in vivo [193]. In a recent study, bardoxolone methyl (CDDO-Me) was tested in patients with pancreatic cancer together with gemcitabine in an attempt to block the production of reactive oxygen species from MDSC. CDDO-Me completely blocked MDSC-mediated suppression of T cell proliferation in vitro and significantly improved patients’ T cell responses to tetanus toxoid and phytohemagglutinin but did not alter the prevalence of MDSC [183].
Finally, certain chemotherapeutic drugs such as gemcitabine may be capable of directly eliminating MDSC. Gemcitabine has been shown in mouse tumor models to preferentially deplete MDSC through an unknown mechanism while leaving other immune cells intact [16, 17, 187]. Other pharmaceutical agents have been observed to target MDSC as well. Zoledronic acid, a potent aminobisphosphonate that inhibits farnesyl-pyrophosphate-transferase, has been shown to inhibit the tumor mediated increase in hematopoiesis which generates MDSC [145]. Although the mechanism of action in tumor bearing animals appears to involve modification of hematopoiesis, hematological toxicities of the drug on normal bone marrow are essentially not observed. Using pharmacological targeting of MDSC with zoledronic acid, improved anti-tumor effects have been observed including restoration of NK and CD8+ T cell activity [65] and decreased tumor angiogenesis [181]. The action of zoledronic acid on MDSC is of particular interest in pancreatic cancer as it appears that gemcitabine, the first-line agent and mainstay of treatment of this disease, also preferentially acts on MDSC. Thus, the combination of these two drugs is a potential method for overcoming the potent tumor-induced immunosuppression of pancreatic cancer.
The immunologic mechanism and anti-neoplastic effects of zoledronic acid
Aminobisphosphonates were originally developed as osteoclast inhibitors used to treat malignant and nonmalignant bone disease due to their high affinity for the bone matrix. Recent studies, however, have demonstrated impressive immunomodulatory, antitumor, and antimetastatic effects in patients with prostate and breast cancer [194]. Zoledronic acid is a third generation aminobisphosphonate and acts by inhibiting farnesyl-pyrophosphate synthase and prevention of downstream prenylation of various signaling proteins affecting multiple survival and trafficking pathways [195].
Zoledronic acid has been demonstrated to have direct antiproliferative, antimetastatic, and proapoptotic effects on human pancreatic cancer cell lines in vitro [196]. In addition, zoledronate has been shown to have positive immunomodulatory effects denoted by the up-regulation of the number and activity of gamma-delta-T cells [197], suppression of proangiogenic factors including VEGF and MMP-9, and the blockade of tumor-induced MDSC expansion [198-201].
The bone marrow is believed to serve as a reservoir of functional Treg [202], MDSC [145], and bone marrow derived cells which facilitate tumor growth and metastasis [140]. The CXCR4/CXCL12 chemotaxis pathway plays a critical role in maintaining homeostasis in the bone marrow among the various progenitor cells [155]. Disruption of this axis results in the efflux of myeloid precursors and immature myeloid cells which then can migrate to the primary tumor and sites of future metastasis and become MDSC (Figure 4) [140, 202, 203]. Zoledronic acid has been demonstrated to abrogate the effect of alterations in the CXCR4/CXCL12 axis, and thereby prevent pre-MDSC from entering the bloodstream.
Recently, aminobisphosphonates have been shown to inhibit the accumulation of MDSC in the tumor environment in a breast cancer animal model by preventing tumor-induced myeloid hematopoiesis [145]. Just as in previous investigations, reduction of MDSC in tumors was associated with decreased levels of VEGF and MMP9, conferring a relative inability of tumors to induce neoangiogenesis [145]. Our group has observed that treatment with zoledronic acid significantly inhibits the accumulation of MDSC in mice inoculated subcutaneously with pancreatic cancer cells (Figure 5, Porembka et al., submitted). The decrease in MDSC at the site of tumor correlated with decreased tumor growth and improved survival in the murine model of pancreatic cancer. In addition, a decrease in the number of Treg cells and mast cells was observed while the relative number of immune effectors cells increased (Porembka et al., submitted). Because of these findings, we hypothesize that the effect of zoledronic acid on MDSC may inhibit the immunosuppressive tumor microenvironment and potentially improve the efficacy of standard gemcitabine-based chemotherapy regimens.
Figure 5.
Zoledronate treated mice have smaller pancreatic tumors compared with untreated tumor-bearing mice. C57BL/6 mice (n=5) were challenged with 1.0 × 105 viable Pan02 cells on day 0 (injected subcutaneously in the left thigh). Treatment with either placebo or zoledronate was initiated on day 10, when subcutaneous pancreatic tumors were present by palpation. Zoledronate at a dose of 0.1 mg/kg was diluted in saline and administered daily subcutaneously for 5 days per week. Control mice (n=5) received 0.2 mL of saline daily subcutaneously. Shown is the average tumor volume per group; error bars indicate the standard error of the mean (p<0.01).
Targeting MDSC and Treg in human pancreatic cancer
Important considerations in the design of clinical studies in pancreatic cancer are the late detection of the disease, and the high recurrence rate after surgical resection. More than 80% of patients present with inoperable disease. Of the remaining patients that are candidates for surgery, approximately 50% will develop recurrent disease within one year from the time of surgery. This suggests that at the time of surgery micrometastatic disease may be already present in a large number of patients. As discussed above, bone marrow-derived MDSC are instrumental in the formation of metastases and in development of primary tumors (Figure 4). As such, targeting of MDSC in pancreatic cancer patients should arguably be initiated at the time of detection. This consideration is also supported by recent data from mouse models in which pancreatic adenocarcinoma spontaneously develops [42, 109, 204, 205], and is exacerbated by introducing additional genetic alterations [205, 206]. One of these models [204, 206] is based on activation of the Ras oncogene in the pancreas, and resembles pancreatic cancer development in humans by both genetic and histologic criteria. In the second model, transforming growth factor α expressed in the pancreas in p53 deficient mice also leads to spontaneous formation of pancreatic adenocarcinoma [205]. Interestingly, the development of pancreatic neoplasms, starting with dysplasia, is already associated with an increased prevalence of MDSC, Treg, and TAM with no or little evidence for an antitumor immune response in both models [109, 205]. These findings further support the notion that pancreatic cancers induce a strong immunosuppressive environment early on in the disease.
We have initiated a clinical trial in which patients with resectable pancreatic adenocarcinoma will be treated with peri-operative zoledronate plus standard adjuvant (gemcitabine) therapy. The two main objectives are to evaluate the safety and feasibility of perioperative neoadjuvant zoledronic acid, and to evaluate whether treatment with peri-operative zoledronic acid prolongs overall survival or disease free survival. When combined with current adjuvant chemotherapy, the use of zoledronic acid in the neoadjuvant and adjuvant setting might result in a more robust antitumor immune response leading to increased overall survival through the prevention of local disease and distant metastasis.
SUMMARY
There is compelling evidence that cancers induce a highly immunosuppressive environment mainly through MDSC that suppress antitumor immunity and promote tumor growth and formation of metastases. While the scientific interest in MDSC has dramatically increased over the past several years, their characterization, in particular the phenotypic characterization is still incomplete. In part this may be because the tumor-induced blockade of lineage differentiation may not be uniform and/or may change over time. Additionally, evidence has emerged that at least two main subsets of MDSC exist consisting of inflammatory monocytes and granulocytes/neutrophils.
In spite of these gaps in our knowledge, several MDSC targeting strategies have been developed with encouraging results in animal tumor models. Most promising is that MDSC can be targeted at multiple levels, ranging from recruitment and activation to differentiation or elimination, suggesting combination strategies are possible and perhaps most effective. As new data become available that more clearly define the biology of MDSC and its subset composition, improved strategies can be designed.
Clinical translation of MDSC targeting strategies is still in its infancy but will be critical to move the field forward. In this context, we have opened a clinical trial using perioperative zoledronic acid in pancreatic adenocarcinoma that we hope will contribute insights into MDSC biology and focus attention on the bone marrow as a key target to prevent tumor progression.
Acknowledgments
Supported in part by NIH T32 CA09621 (JBM, MRP, MCBT)
Abbreviations used
- MDSC
myeloid-derived suppressor cell
- Treg
T regulatory cell
- CTL
cytotoxic T lymphocyte
- Th
T helper
- IFN
interferon
- MHC
major histocompatibility complex
- PD-L1
programmed cell death ligand-1
- TGFβ
transforming growth factor beta
- IL
interleukin
- IDO
indoleamine 2,3-dioxygenase
- TAM
tumor-associated macrophage
- NK
natural killer
- iNOS
inducible nitric oxide synthase
- CTLA-4
cytoxic T lymphocyte-associated antigen-4
- VEGF
vascular endothelial growth factor
- M-CSF
macrophage-colony-stimulating factor, aka CSF-1
- GM-CSF
granulocyte/macrophage-colony-stimulating factor, aka CSF-2
- G-CSF
granulocyte colony-stimulating factor, aka CSF-3
- MMP
matrix metalloproteinase
- VLA
very late antigen
- TNF
tumor necrosis factor
- SCF
stem cell factor, aka Kit ligand (KITLG)
- COX
cyclooxygenase
- STAT
signal transducer and activator of transcription
- TLR
toll-like receptor
- ATRA
all-trans retinoic acid
- PGE2
prostaglandin E2
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010. CA Cancer J. Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Cress RD, Yin D, Clarke L, Bold R, Holly EA. Survival among patients with adenocarcinoma of the pancreas: a population-based study (United States). Cancer Causes Control. 2006;17:403–409. doi: 10.1007/s10552-005-0539-4. [DOI] [PubMed] [Google Scholar]
- 3.Garcea G, Dennison AR, Pattenden CJ, Neal CP, Sutton CD, Berry DP. Survival following curative resection for pancreatic ductal adenocarcinoma. A systematic review of the literature. J.O.P. 2008;9:99–132. [PubMed] [Google Scholar]
- 4.Winter JM, Cameron JL, Campbell KA, Arnold MA, Chang DC, Coleman J, Hodgin MB, Sauter PK, Hruban RH, Riall TS, Schulick RD, Choti MA, Lillemoe KD, Yeo CJ. 1423 pancreaticoduodenectomies for pancreatic cancer: A single-institution experience. J. Gastrointest. Surg. 2006;10:1199–1210. doi: 10.1016/j.gassur.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 5.Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J. Clin. Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 6.Mackenzie RP, McCollum AD. Novel agents for the treatment of adenocarcinoma of the pancreas. Expert Rev. Anticancer Ther. 2009;9:1473–1485. doi: 10.1586/era.09.109. [DOI] [PubMed] [Google Scholar]
- 7.Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, Campos D, Lim R, Ding K, Clark G, Voskoglou-Nomikos T, Ptasynski M, Parulekar W. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J. Clin. Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 8.Picozzi VJ, Kozarek RA, Traverso LW. Interferon-based adjuvant chemoradiation therapy after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am. J. Surg. 2003;185:476–480. doi: 10.1016/s0002-9610(03)00051-5. [DOI] [PubMed] [Google Scholar]
- 9.Linehan DC, Tan MC, Strasberg SM, Drebin JA, Hawkins WG, Picus J, Myerson RJ, Malyapa RS, Hull M, Trinkaus K, Tan BR., Jr. Adjuvant interferon-based chemoradiation followed by gemcitabine for resected pancreatic adenocarcinoma: a single-institution phase II study. Ann. Surg. 2008;248:145–151. doi: 10.1097/SLA.0b013e318181e4e9. [DOI] [PubMed] [Google Scholar]
- 10.Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, Beger H, Fernandez-Cruz L, Dervenis C, Lacaine F, Falconi M, Pederzoli P, Pap A, Spooner D, Kerr DJ, Buchler MW. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N. Engl. J. Med. 2004;350:1200–1210. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 11.Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, Schramm H, Fahlke J, Zuelke C, Burkart C, Gutberlet K, Kettner E, Schmalenberg H, Weigang-Koehler K, Bechstein WO, Niedergethmann M, Schmidt-Wolf I, Roll L, Doerken B, Riess H. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. J.A.M.A. 2007;297:267–277. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 12.Regine WF, Winter KA, Abrams RA, Safran H, Hoffman JP, Konski A, Benson AB, Macdonald JS, Kudrimoti MR, Fromm ML, Haddock MG, Schaefer P, Willett CG, Rich TA. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. J.A.M.A. 2008;299:1019–1026. doi: 10.1001/jama.299.9.1019. [DOI] [PubMed] [Google Scholar]
- 13.Evans DB, Varadhachary GR, Crane CH, Sun CC, Lee JE, Pisters PW, Vauthey JN, Wang H, Cleary KR, Staerkel GA, Charnsangavej C, Lano EA, Ho L, Lenzi R, Abbruzzese JL, Wolff RA. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J. Clin. Oncol. 2008;26:3496–3502. doi: 10.1200/JCO.2007.15.8634. [DOI] [PubMed] [Google Scholar]
- 14.Varadhachary GR, Wolff RA, Crane CH, Sun CC, Lee JE, Pisters PW, Vauthey JN, Abdalla E, Wang H, Staerkel GA, Lee JH, Ross WA, Tamm EP, Bhosale PR, Krishnan S, Das P, Ho L, Xiong H, Abbruzzese JL, Evans DB. Preoperative gemcitabine and cisplatin followed by gemcitabine-based chemoradiation for resectable adenocarcinoma of the pancreatic head. J .Clin. Oncol. 2008;26:3487–3495. doi: 10.1200/JCO.2007.15.8642. [DOI] [PubMed] [Google Scholar]
- 15.Veltkamp SA, Pluim D, van Eijndhoven MA, Bolijn MJ, Ong FH, Govindarajan R, Unadkat JD, Beijnen JH, Schellens JH. New insights into the pharmacology and cytotoxicity of gemcitabine and 2′,2′-difluorodeoxyuridine. Mol. Cancer Ther. 2008;7:2415–2425. doi: 10.1158/1535-7163.MCT-08-0137. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin. Cancer Res. 2005;11:6713–6721. doi: 10.1158/1078-0432.CCR-05-0883. [DOI] [PubMed] [Google Scholar]
- 17.Le HK, Graham L, Cha E, Morales JK, Manjili MH, Bear HD. Gemcitabine directly inhibits myeloid derived suppressor cells in BALB/c mice bearing 4T1 mammary carcinoma and augments expansion of T cells from tumor-bearing mice. Int. Immunopharmacol. 2009;9:900–909. doi: 10.1016/j.intimp.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 18.Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904–5912. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pages F, Galon J, Dieu-Nosjean MC, Tartour E, Sautes-Fridman C, Fridman WH. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene. 2009;29:1093–1002. doi: 10.1038/onc.2009.416. [DOI] [PubMed] [Google Scholar]
- 20.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 21.Galon J, Fridman WH, Pages F. The adaptive immunologic microenvironment in colorectal cancer: a novel perspective. Cancer Res. 2007;67:1883–1886. doi: 10.1158/0008-5472.CAN-06-4806. [DOI] [PubMed] [Google Scholar]
- 22.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 24.Laheru D, Jaffee EM. Immunotherapy for pancreatic cancer - science driving clinical progress. Nat. Rev. Cancer. 2005;5:459–467. doi: 10.1038/nrc1630. [DOI] [PubMed] [Google Scholar]
- 25.Jaffee EM, Hruban RH, Biedrzycki B, Laheru D, Schepers K, Sauter PR, Goemann M, Coleman J, Grochow L, Donehower RC, Lillemoe KD, O'Reilly S, Abrams RA, Pardoll DM, Cameron JL, Yeo CJ. Novel allogeneic granulocyte-macrophage colony-stimulating factor-secreting tumor vaccine for pancreatic cancer: a phase I trial of safety and immune activation. J. Clin. Oncol. 2001;19:145–156. doi: 10.1200/JCO.2001.19.1.145. [DOI] [PubMed] [Google Scholar]
- 26.Poch B, Lotspeich E, Ramadani M, Gansauge S, Beger HG, Gansauge F. Systemic immune dysfunction in pancreatic cancer patients. Langenbecks Arch. Surg. 2007;392:353–358. doi: 10.1007/s00423-006-0140-7. [DOI] [PubMed] [Google Scholar]
- 27.Laheru D, Lutz E, Burke J, Biedrzycki B, Solt S, Onners B, Tartakovsky I, Nemunaitis J, Le D, Sugar E, Hege K, Jaffee E. Allogeneic granulocyte macrophage colony-stimulating factor-secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: a pilot study of safety, feasibility, and immune activation. Clin. Cancer Res. 2008;14:1455–1463. doi: 10.1158/1078-0432.CCR-07-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tseng JF, Willett CG, Fernandez-del Castillo C, Ryan DP, Clark JW, Zhu AX, Rattner DW, Winkelmann JL, Warshaw AL. Patients undergoing treatment for pancreatic adenocarcinoma can mount an effective immune response to vaccinations. Pancreatology. 2005;5:67–74. doi: 10.1159/000084492. [DOI] [PubMed] [Google Scholar]
- 29.Schmitz-Winnenthal FH, Escobedo LV, Beckhove P, Schirrmacher V, Bucur M, Ziouta Y, Volk C, Schmied B, Koch M, Antolovic D, Weitz J, Buchler MW, Z'Graggen K. Specific immune recognition of pancreatic carcinoma by patient-derived CD4 and CD8 T cells and its improvement by interferon-gamma. Int. J. Oncol. 2006;28:1419–1428. doi: 10.3892/ijo.28.6.1419. [DOI] [PubMed] [Google Scholar]
- 30.Schmitz-Winnenthal FH, Volk C, Z'Graggen K, Galindo L, Nummer D, Ziouta Y, Bucur M, Weitz J, Schirrmacher V, Buchler MW, Beckhove P. High frequencies of functional tumor-reactive T cells in bone marrow and blood of pancreatic cancer patients. Cancer Res. 2005;65:10079–10087. doi: 10.1158/0008-5472.CAN-05-1098. [DOI] [PubMed] [Google Scholar]
- 31.Kubuschok B, Neumann F, Breit R, Sester M, Schormann C, Wagner C, Sester U, Hartmann F, Wagner M, Remberger K, Schilling M, Pfreundschuh M. Naturally occurring T-cell response against mutated p21 ras oncoprotein in pancreatic cancer. Clin. Cancer Res. 2006;12:1365–1372. doi: 10.1158/1078-0432.CCR-05-1672. [DOI] [PubMed] [Google Scholar]
- 32.Thomas AM, Santarsiero LM, Lutz ER, Armstrong TD, Chen YC, Huang LQ, Laheru DA, Goggins M, Hruban RH, Jaffee EM. Mesothelin-specific CD8(+) T cell responses provide evidence of in vivo cross-priming by antigen-presenting cells in vaccinated pancreatic cancer patients. J. Exp. Med. 2004;200:297–306. doi: 10.1084/jem.20031435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Argani P, Iacobuzio-Donahue C, Ryu B, Rosty C, Goggins M, Wilentz RE, Murugesan SR, Leach SD, Jaffee E, Yeo CJ, Cameron JL, Kern SE, Hruban RH. Mesothelin is overexpressed in the vast majority of ductal adenocarcinomas of the pancreas: identification of a new pancreatic cancer marker by serial analysis of gene expression (SAGE). Clin. Cancer Res. 2001;7:3862–3868. [PubMed] [Google Scholar]
- 34.Iacobuzio-Donahue CA, Ashfaq R, Maitra A, Adsay NV, Shen-Ong GL, Berg K, Hollingsworth MA, Cameron JL, Yeo CJ, Kern SE, Goggins M, Hruban RH. Highly expressed genes in pancreatic ductal adenocarcinomas: a comprehensive characterization and comparison of the transcription profiles obtained from three major technologies. Cancer Res. 2003;63:8614–8622. [PubMed] [Google Scholar]
- 35.Johnston FM, Tan MC, Tan BR, Jr., Porembka MR, Brunt EM, Linehan DC, Simon PO, Jr., Plambeck-Suess S, Eberlein TJ, Hellstrom KE, Hellstrom I, Hawkins WG, Goedegebuure P. Circulating mesothelin protein and cellular antimesothelin immunity in patients with pancreatic cancer. Clin. Cancer Res. 2009;15:6511–6518. doi: 10.1158/1078-0432.CCR-09-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ikemoto T, Yamaguchi T, Morine Y, Imura S, Soejima Y, Fujii M, Maekawa Y, Yasutomo K, Shimada M. Clinical roles of increased populations of Foxp3+CD4+ T cells in peripheral blood from advanced pancreatic cancer patients. Pancreas. 2006;33:386–390. doi: 10.1097/01.mpa.0000240275.68279.13. [DOI] [PubMed] [Google Scholar]
- 37.Hiraoka N, Onozato K, Kosuge T, Hirohashi S. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin. Cancer Res. 2006;12:5423–5434. doi: 10.1158/1078-0432.CCR-06-0369. [DOI] [PubMed] [Google Scholar]
- 38.Nomi T, Sho M, Akahori T, Hamada K, Kubo A, Kanehiro H, Nakamura S, Enomoto K, Yagita H, Azuma M, Nakajima Y. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin. Cancer Res. 2007;13:2151–2157. doi: 10.1158/1078-0432.CCR-06-2746. [DOI] [PubMed] [Google Scholar]
- 39.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smyth MJ, Dunn GP, Schreiber RD. Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv. Immunol. 2006;90:1–50. doi: 10.1016/S0065-2776(06)90001-7. [DOI] [PubMed] [Google Scholar]
- 41.Mellor AL, Munn DH. Creating immune privilege: active local suppression that benefits friends, but protects foes. Nat. Rev. Immunol. 2008;8:74–80. doi: 10.1038/nri2233. [DOI] [PubMed] [Google Scholar]
- 42.Clark CE, Beatty GL, Vonderheide RH. Immunosurveillance of pancreatic adenocarcinoma: insights from genetically engineered mouse models of cancer. Cancer Lett. 2009;279:1–7. doi: 10.1016/j.canlet.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 43.Ungefroren H, Voss M, Bernstorff WV, Schmid A, Kremer B, Kalthoff H. Immunological escape mechanisms in pancreatic carcinoma. Ann. N. Y. Acad. Sci. 1999;880:243–251. doi: 10.1111/j.1749-6632.1999.tb09529.x. [DOI] [PubMed] [Google Scholar]
- 44.von Bernstorff W, Voss M, Freichel S, Schmid A, Vogel I, Johnk C, Henne-Bruns D, Kremer B, Kalthoff H. Systemic and local immunosuppression in pancreatic cancer patients. Clin. Cancer Res. 2001;7:925s–932s. [PubMed] [Google Scholar]
- 45.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat. Rev. Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 46.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol. Immunother. 2009;58:49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Young MR, Newby M, Wepsic HT. Hematopoiesis and suppressor bone marrow cells in mice bearing large metastatic Lewis lung carcinoma tumors. Cancer Res. 1987;47:100–105. [PubMed] [Google Scholar]
- 49.Allison AC. Mechanisms by which activated macrophages inhibit lymphocyte responses. Immunol. Rev. 1978;40:3–27. doi: 10.1111/j.1600-065x.1978.tb00399.x. [DOI] [PubMed] [Google Scholar]
- 50.Ye QW, Mokyr MB, Pyle JM, Dray S. Suppression of antitumor immunity by macrophages in spleens of mice bearing a large MOPC-315 tumor. Cancer Immunol. Immunother. 1984;16:162–169. doi: 10.1007/BF00205423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hardy CL, Balducci L. Early hematopoietic events during tumor growth in mice. J. Natl. Cancer Inst. 1986;76:535–540. [PubMed] [Google Scholar]
- 52.Garrity T, Pandit R, Wright MA, Benefield J, Keni S, Young MR. Increased presence of CD34+ cells in the peripheral blood of head and neck cancer patients and their differentiation into dendritic cells. Int. J. Cancer. 1997;73:663–669. doi: 10.1002/(sici)1097-0215(19971127)73:5<663::aid-ijc9>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 53.Gabrilovich DI, Chen HL, Girgis KR, Cunningham HT, Meny GM, Nadaf S, Kavanaugh D, Carbone DP. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat. Med. 1996;2:1096–1103. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- 54.Marx J. Cancer immunology. Cancer's bulwark against immune attack: MDS cells. Science. 2008;319:154–156. doi: 10.1126/science.319.5860.154. [DOI] [PubMed] [Google Scholar]
- 55.Gabrilovich DI, Bronte V, Chen SH, Colombo MP, Ochoa A, Ostrand-Rosenberg S, Schreiber H. The terminology issue for myeloid-derived suppressor cells. Cancer Res. 2007;67:425. doi: 10.1158/0008-5472.CAN-06-3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Greten TF, Manns MP, Korangy F. Myeloid derived suppressor cells in human diseases. Int. Immunopharmacol. 2011 doi: 10.1016/j.intimp.2011.01.003. Epub date 2011/01/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zea AH, Rodriguez PC, Atkins MB, Hernandez C, Signoretti S, Zabaleta J, McDermott D, Quiceno D, Youmans A, O'Neill A, Mier J, Ochoa AC. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res. 2005;65:3044–3048. doi: 10.1158/0008-5472.CAN-04-4505. [DOI] [PubMed] [Google Scholar]
- 58.Rodriguez PC, Ernstoff MS, Hernandez C, Atkins M, Zabaleta J, Sierra R, Ochoa AC. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 2009;69:1553–1560. doi: 10.1158/0008-5472.CAN-08-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schmielau J, Finn OJ. Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of t-cell function in advanced cancer patients. Cancer Res. 2001;61:4756–4760. [PubMed] [Google Scholar]
- 60.Wang YM, Wang CL, Feng PH, Ko HW, Liu YH, Wu YC, Chu Y, Chung FT, Kuo CH, Lee KY, Lin SM, Lin HC, Wang CH, Yu CT, Kuo HP. Population alterations of L: -arginase- and inducible nitric oxide synthase-expressed CD11b(+)/CD14 (-)/CD15 (+)/CD33 (+) myeloid-derived suppressor cells and CD8 (+) T lymphocytes in patients with advanced-stage non-small cell lung cancer. J. Cancer Res. Clin. Oncol. 2010;136:35–45. doi: 10.1007/s00432-009-0634-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nemeth MJ, Bodine DM. Regulation of hematopoiesis and the hematopoietic stem cell niche by Wnt signaling pathways. Cell Res. 2007;17:746–758. doi: 10.1038/cr.2007.69. [DOI] [PubMed] [Google Scholar]
- 62.Delano MJ, Scumpia PO, Weinstein JS, Coco D, Nagaraj S, Kelly-Scumpia KM, O'Malley KA, Wynn JL, Antonenko S, Al-Quran SZ, Swan R, Chung CS, Atkinson MA, Ramphal R, Gabrilovich DI, Reeves WH, Ayala A, Phillips J, Laface D, Heyworth PG, Clare-Salzler M, Moldawer LL. MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. J. Exp. Med. 2007;204:1463–1474. doi: 10.1084/jem.20062602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Makarenkova VP, Bansal V, Matta BM, Perez LA, Ochoa JB. CD11b+/Gr-1+ myeloid suppressor cells cause T cell dysfunction after traumatic stress. J. Immunol. 2006;176:2085–2094. doi: 10.4049/jimmunol.176.4.2085. [DOI] [PubMed] [Google Scholar]
- 64.Serafini P, Borrello I, Bronte V. Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin. Cancer Biol. 2006;16:53–65. doi: 10.1016/j.semcancer.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 65.Gallina G, Dolcetti L, Serafini P, De Santo C, Marigo I, Colombo MP, Basso G, Brombacher F, Borrello I, Zanovello P, Bicciato S, Bronte V. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J. Clin. Invest. 2006;116:2777–2790. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 67.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 68.Norian LA, Rodriguez PC, O'Mara LA, Zabaleta J, Ochoa AC, Cella M, Allen PM. Tumor-infiltrating regulatory dendritic cells inhibit CD8+ T cell function via L-arginine metabolism. Cancer Res. 2009;69:3086–3094. doi: 10.1158/0008-5472.CAN-08-2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ma G, Pan PY, Eisenstein S, Divino CM, Lowell CA, Takai T, Chen SH. Paired immunoglobin-like receptor-B regulates the suppressive function and fate of myeloid-derived suppressor cells. Immunity. 2011;34:385–395. doi: 10.1016/j.immuni.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Biswas SK, Gangi L, Paul S, Schioppa T, Saccani A, Sironi M, Bottazzi B, Doni A, Vincenzo B, Pasqualini F, Vago L, Nebuloni M, Mantovani A, Sica A. A distinct and unique transcriptional program expressed by tumor-associated macrophages (defective NF-kappaB and enhanced IRF-3/STAT1 activation). Blood. 2006;107:2112–2122. doi: 10.1182/blood-2005-01-0428. [DOI] [PubMed] [Google Scholar]
- 71.Umemura N, Saio M, Suwa T, Kitoh Y, Bai J, Nonaka K, Ouyang GF, Okada M, Balazs M, Adany R, Shibata T, Takami T. Tumor-infiltrating myeloid-derived suppressor cells are pleiotropic-inflamed monocytes/macrophages that bear M1- and M2-type characteristics. J. Leukoc. Biol. 2008;83:1136–1144. doi: 10.1189/jlb.0907611. [DOI] [PubMed] [Google Scholar]
- 72.Youn JI, Gabrilovich DI. The biology of myeloid-derived suppressor cells: the blessing and the curse of morphological and functional heterogeneity. Eur. J. Immunol. 2010;40:2969–2975. doi: 10.1002/eji.201040895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vieweg J, Su Z, Dahm P, Kusmartsev S. Reversal of tumor-mediated immunosuppression. Clin. Cancer Res. 2007;13:727s–732s. doi: 10.1158/1078-0432.CCR-06-1924. [DOI] [PubMed] [Google Scholar]
- 74.Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, Carbone DP, Gabrilovich DI. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J. Immunol. 2001;166:678–689. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 75.Peranzoni E, Zilio S, Marigo I, Dolcetti L, Zanovello P, Mandruzzato S, Bronte V. Myeloid-derived suppressor cell heterogeneity and subset definition. Curr. Opin. Immunol. 2010;22:238–244. doi: 10.1016/j.coi.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 76.Brandau S, Trellakis S, Bruderek K, Schmaltz D, Steller G, Elian M, Suttmann H, Schenck M, Welling J, Zabel P, Lang S. Myeloid-derived suppressor cells in the peripheral blood of cancer patients contain a subset of immature neutrophils with impaired migratory properties. J. Leukoc. Biol. 2011;89:311–317. doi: 10.1189/jlb.0310162. [DOI] [PubMed] [Google Scholar]
- 77.Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Kruger C, Manns MP, Greten TF, Korangy F. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology. 2008;135:234–243. doi: 10.1053/j.gastro.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 78.Filipazzi P, Valenti R, Huber V, Pilla L, Canese P, Iero M, Castelli C, Mariani L, Parmiani G, Rivoltini L. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J. Clin. Oncol. 2007;25:2546–2553. doi: 10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]
- 79.Poschke I, Mougiakakos D, Hansson J, Masucci GV, Kiessling R. Immature immunosuppressive CD14+HLA-DR-/low cells in melanoma patients are Stat3hi and overexpress CD80, CD83, and DC-sign. Cancer Res. 2010;70:4335–4345. doi: 10.1158/0008-5472.CAN-09-3767. [DOI] [PubMed] [Google Scholar]
- 80.Brimnes MK, Vangsted AJ, Knudsen LM, Gimsing P, Gang AO, Johnsen HE, Svane IM. Increased level of both CD4+FOXP3+ regulatory T cells and CD14+HLA-DR/low myeloid-derived suppressor cells and decreased level of dendritic cells in patients with multiple myeloma. Scand. J. Immunol. 2010;72:540–547. doi: 10.1111/j.1365-3083.2010.02463.x. [DOI] [PubMed] [Google Scholar]
- 81.Gustafson MP, Lin Y, New KC, Bulur PA, O'Neill BP, Gastineau DA, Dietz AB. Systemic immune suppression in glioblastoma: the interplay between CD14+HLADRlo/neg monocytes, tumor factors, and dexamethasone. Neuro. Oncol. 2010;12:631–644. doi: 10.1093/neuonc/noq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Loercher AE, Nash MA, Kavanagh JJ, Platsoucas CD, Freedman RS. Identification of an IL-10-producing HLA-DR-negative monocyte subset in the malignant ascites of patients with ovarian carcinoma that inhibits cytokine protein expression and proliferation of autologous T cells. J. Immunol. 1999;163:6251–6260. [PubMed] [Google Scholar]
- 83.Ugurel S, Uhlig D, Pfohler C, Tilgen W, Schadendorf D, Reinhold U. Down-regulation of HLA class II and costimulatory CD86/B7-2 on circulating monocytes from melanoma patients. Cancer Immunol. Immunother. 2004;53:551–559. doi: 10.1007/s00262-003-0489-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Herr HW. Suppressor cells in immunodepressed bladder and prostate cancer patients. J. Urol. 1980;123:635–639. [PubMed] [Google Scholar]
- 85.Vuk-Pavlovic S, Bulur PA, Lin Y, Qin R, Szumlanski CL, Zhao X, Dietz AB. Immunosuppressive CD14+HLA-DRlow/- monocytes in prostate cancer. Prostate. 2010;70:443–455. doi: 10.1002/pros.21078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, De Baetselier P, Van Ginderachter JA. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 2008;111:4233–4244. doi: 10.1182/blood-2007-07-099226. [DOI] [PubMed] [Google Scholar]
- 87.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J. Immunol. 2008;181:5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gabrilovich DI, Velders MP, Sotomayor EM, Kast WM. Mechanism of immune dysfunction in cancer mediated by immature Gr-1+ myeloid cells. J. Immunol. 2001;166:5398–5406. doi: 10.4049/jimmunol.166.9.5398. [DOI] [PubMed] [Google Scholar]
- 89.Dolcetti L, Peranzoni E, Ugel S, Marigo I, Fernandez Gomez A, Mesa C, Geilich M, Winkels G, Traggiai E, Casati A, Grassi F, Bronte V. Hierarchy of immunosuppressive strength among myeloid-derived suppressor cell subsets is determined by GM-CSF. Eur. J. Immunol. 2010;40:22–35. doi: 10.1002/eji.200939903. [DOI] [PubMed] [Google Scholar]
- 90.Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P, Cho HI, Celis E, Quiceno DG, Padhya T, McCaffrey TV, McCaffrey JC, Gabrilovich DI. HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J. Exp. Med. 2010;207:2439–2453. doi: 10.1084/jem.20100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kusmartsev S, Nagaraj S, Gabrilovich DI. Tumor-associated CD8+ T cell tolerance induced by bone marrow-derived immature myeloid cells. J. Immunol. 2005;175:4583–4592. doi: 10.4049/jimmunol.175.7.4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Elgert KD, Alleva DG, Mullins DW. Tumor-induced immune dysfunction: the macrophage connection. J. Leukoc. Biol. 1998;64:275–290. doi: 10.1002/jlb.64.3.275. [DOI] [PubMed] [Google Scholar]
- 93.Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, Divino CM, Chen SH. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–1131. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 94.Solito S, Bronte V, Mandruzzato S. Antigen specificity of immune suppression by myeloid-derived suppressor cells. J. Leukoc. Biol. 2011 doi: 10.1189/jlb.0111021. Epub date 2011/04/14. [DOI] [PubMed] [Google Scholar]
- 95.Almand B, Resser JR, Lindman B, Nadaf S, Clark JI, Kwon ED, Carbone DP, Gabrilovich DI. Clinical significance of defective dendritic cell differentiation in cancer. Clin Cancer Res. 2000;6:1755–1766. [PubMed] [Google Scholar]
- 96.Corzo CA, Cotter MJ, Cheng P, Cheng F, Kusmartsev S, Sotomayor E, Padhya T, McCaffrey TV, McCaffrey JC, Gabrilovich DI. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J. Immunol. 2009;182:5693–5701. doi: 10.4049/jimmunol.0900092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J. Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Marigo I, Dolcetti L, Serafini P, Zanovello P, Bronte V. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol. Rev. 2008;222:162–179. doi: 10.1111/j.1600-065X.2008.00602.x. [DOI] [PubMed] [Google Scholar]
- 99.Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, Golshayan A, Rayman PA, Wood L, Garcia J, Dreicer R, Bukowski R, Finke JH. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin. Cancer Res. 2009;15:2148–2157. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- 100.Kusmartsev S, Su Z, Heiser A, Dannull J, Eruslanov E, Kubler H, Yancey D, Dahm P, Vieweg J. Reversal of myeloid cell-mediated immunosuppression in patients with metastatic renal cell carcinoma. Clin. Cancer Res. 2008;14:8270–8278. doi: 10.1158/1078-0432.CCR-08-0165. [DOI] [PubMed] [Google Scholar]
- 101.Haverkamp JM, Crist SA, Elzey BD, Cimen C, Ratliff TL. In vivo suppressive function of myeloid-derived suppressor cells is limited to the inflammatory site. Eur. J.Immunol. 2011;41:749–759. doi: 10.1002/eji.201041069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Grizzle WE, Xu X, Zhang S, Stockard CR, Liu C, Yu S, Wang J, Mountz JD, Zhang HG. Age-related increase of tumor susceptibility is associated with myeloid-derived suppressor cell mediated suppression of T cell cytotoxicity in recombinant inbred BXD12 mice. Mech. Ageing Dev. 2007;128:672–680. doi: 10.1016/j.mad.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 103.Li H, Han Y, Guo Q, Zhang M, Cao X. Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-beta 1. J. Immunol. 2009;182:240–249. doi: 10.4049/jimmunol.182.1.240. [DOI] [PubMed] [Google Scholar]
- 104.Nausch N, Galani IE, Schlecker E, Cerwenka A. Mononuclear myeloid-derived “suppressor” cells express RAE-1 and activate natural killer cells. Blood. 2008;112:4080–4089. doi: 10.1182/blood-2008-03-143776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Terabe M, Berzofsky JA. The role of NKT cells in tumor immunity. Adv. Cancer Res. 2008;101:277–348. doi: 10.1016/S0065-230X(08)00408-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sinha P, Clements VK, Ostrand-Rosenberg S. Interleukin-13-regulated M2 macrophages in combination with myeloid suppressor cells block immune surveillance against metastasis. Cancer Res. 2005;65:11743–11751. doi: 10.1158/0008-5472.CAN-05-0045. [DOI] [PubMed] [Google Scholar]
- 107.Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand-Rosenberg S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J. Immunol. 2007;179:977–983. doi: 10.4049/jimmunol.179.2.977. [DOI] [PubMed] [Google Scholar]
- 108.Hoechst B, Gamrekelashvili J, Manns MP, Greten TF, Korangy F. Plasticity of human Th17 cells and iTregs is orchestrated by different subsets of myeloid cells. Blood. 2011 doi: 10.1182/blood-2010-11-317321. Epub date 2011/04/16. [DOI] [PubMed] [Google Scholar]
- 109.Clark CE, Hingorani SR, Mick R, Combs C, Tuveson DA, Vonderheide RH. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res. 2007;67:9518–9527. doi: 10.1158/0008-5472.CAN-07-0175. [DOI] [PubMed] [Google Scholar]
- 110.Lu LF, Lind EF, Gondek DC, Bennett KA, Gleeson MW, Pino-Lagos K, Scott ZA, Coyle AJ, Reed JL, Van Snick J, Strom TB, Zheng XX, Noelle RJ. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006;442:997–1002. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- 111.Steinbrink K, Mahnke K, Grabbe S, Enk AH, Jonuleit H. Myeloid dendritic cell: From sentinel of immunity to key player of peripheral tolerance? Hum. Immunol. 2009;70:289–293. doi: 10.1016/j.humimm.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 112.Ghiringhelli F, Puig PE, Roux S, Parcellier A, Schmitt E, Solary E, Kroemer G, Martin F, Chauffert B, Zitvogel L. Tumor cells convert immature myeloid dendritic cells into TGF-beta-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J. Exp. Med. 2005;202:919–929. doi: 10.1084/jem.20050463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 114.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 115.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 116.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat. Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 117.Linehan DC, Goedegebuure PS. CD25+ CD4+ regulatory T-cells in cancer. Immunol. Res. 2005;32:155–168. doi: 10.1385/IR:32:1-3:155. [DOI] [PubMed] [Google Scholar]
- 118.Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res. 1999;59:3128–3133. [PubMed] [Google Scholar]
- 119.Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J. Immunol. 1999;163:5211–5218. [PubMed] [Google Scholar]
- 120.Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, Drebin JA, Strasberg SM, Eberlein TJ, Goedegebuure PS, Linehan DC. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J. Immunol. 2002;169:2756–2761. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 121.Fu J, Xu D, Liu Z, Shi M, Zhao P, Fu B, Zhang Z, Yang H, Zhang H, Zhou C, Yao J, Jin L, Wang H, Yang Y, Fu YX, Wang FS. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132:2328–2339. doi: 10.1053/j.gastro.2007.03.102. [DOI] [PubMed] [Google Scholar]
- 122.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 123.Kusmartsev S, Gabrilovich DI. Inhibition of myeloid cell differentiation in cancer: the role of reactive oxygen species. J. Leukoc. Biol. 2003;74:186–196. doi: 10.1189/jlb.0103010. [DOI] [PubMed] [Google Scholar]
- 124.Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, Herber DL, Schneck J, Gabrilovich DI. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat. Med. 2007;13:828–835. doi: 10.1038/nm1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nagaraj S, Schrum AG, Cho HI, Celis E, Gabrilovich DI. Mechanism of T cell tolerance induced by myeloid-derived suppressor cells. J. Immunol. 2010;184:3106–3116. doi: 10.4049/jimmunol.0902661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rodriguez PC, Quiceno DG, Zabaleta J, Ortiz B, Zea AH, Piazuelo MB, Delgado A, Correa P, Brayer J, Sotomayor EM, Antonia S, Ochoa JB, Ochoa AC. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. 2004;64:5839–5849. doi: 10.1158/0008-5472.CAN-04-0465. [DOI] [PubMed] [Google Scholar]
- 127.Zhu B, Bando Y, Xiao S, Yang K, Anderson AC, Kuchroo VK, Khoury SJ. CD11b+Ly-6C(hi) suppressive monocytes in experimental autoimmune encephalomyelitis. J. Immunol. 2007;179:5228–5237. doi: 10.4049/jimmunol.179.8.5228. [DOI] [PubMed] [Google Scholar]
- 128.Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat. Rev. Immunol. 2005;5:641–654. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 129.Rodriguez PC, Ochoa AC. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: mechanisms and therapeutic perspectives. Immunol. Rev. 2008;222:180–191. doi: 10.1111/j.1600-065X.2008.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J .Leukoc. Biol. 2009;86:1065–1073. doi: 10.1189/jlb.0609385. [DOI] [PubMed] [Google Scholar]
- 131.Srivastava MK, Sinha P, Clements VK, Rodriguez P, Ostrand-Rosenberg S. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 2010;70:68–77. doi: 10.1158/0008-5472.CAN-09-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ostrand-Rosenberg S. Myeloid-derived suppressor cells: more mechanisms for inhibiting antitumor immunity. Cancer Immunol. Immunother. 2010;59:1593–1600. doi: 10.1007/s00262-010-0855-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hanson EM, Clements VK, Sinha P, Ilkovitch D, Ostrand-Rosenberg S. Myeloid-derived suppressor cells down-regulate L-selectin expression on CD4+ and CD8+ T cells. J. Immunol. 2009;183:937–944. doi: 10.4049/jimmunol.0804253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kryczek I, Zou L, Rodriguez P, Zhu G, Wei S, Mottram P, Brumlik M, Cheng P, Curiel T, Myers L, Lackner A, Alvarez X, Ochoa A, Chen L, Zou W. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J. Exp. Med. 2006;203:871–881. doi: 10.1084/jem.20050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, David O, Burow M, Gordon A, Dhurandhar N, Myers L, Berggren R, Hemminki A, Alvarez RD, Emilie D, Curiel DT, Chen L, Zou W. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat. Med. 2003;9:562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 136.Kuang DM, Zhao Q, Peng C, Xu J, Zhang JP, Wu C, Zheng L. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J. Exp. Med. 2009;206:1327–1337. doi: 10.1084/jem.20082173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat. Rev. Cancer. 2008;8:618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 138.Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, Matrisian LM, Carbone DP, Lin PC. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6:409–421. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 139.Yang L, Huang J, Ren X, Gorska AE, Chytil A, Aakre M, Carbone DP, Matrisian LM, Richmond A, Lin PC, Moses HL. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell. 2008;13:23–35. doi: 10.1016/j.ccr.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, Zhu Z, Hicklin D, Wu Y, Port JL, Altorki N, Port ER, Ruggero D, Shmelkov SV, Jensen KK, Rafii S, Lyden D. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Psaila B, Lyden D. The metastatic niche: adapting the foreign soil. Nat. Rev. Cancer. 2009;9:285–293. doi: 10.1038/nrc2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Connolly MK, Mallen-St Clair J, Bedrosian AS, Malhotra A, Vera V, Ibrahim J, Henning J, Pachter HL, Bar-Sagi D, Frey AB, Miller G. Distinct populations of metastases-enabling myeloid cells expand in the liver of mice harboring invasive and preinvasive intra-abdominal tumor. J. Leukoc. Biol. 2010;87:713–725. doi: 10.1189/jlb.0909607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Melani C, Chiodoni C, Forni G, Colombo MP. Myeloid cell expansion elicited by the progression of spontaneous mammary carcinomas in c-erbB-2 transgenic BALB/c mice suppresses immune reactivity. Blood. 2003;102:2138–2145. doi: 10.1182/blood-2003-01-0190. [DOI] [PubMed] [Google Scholar]
- 144.Kusmartsev S, Eruslanov E, Kubler H, Tseng T, Sakai Y, Su Z, Kaliberov S, Heiser A, Rosser C, Dahm P, Siemann D, Vieweg J. Oxidative stress regulates expression of VEGFR1 in myeloid cells: link to tumor-induced immune suppression in renal cell carcinoma. J. Immunol. 2008;181:346–353. doi: 10.4049/jimmunol.181.1.346. [DOI] [PubMed] [Google Scholar]
- 145.Melani C, Sangaletti S, Barazzetta FM, Werb Z, Colombo MP. Amino-biphosphonate-mediated MMP-9 inhibition breaks the tumor-bone marrow axis responsible for myeloid-derived suppressor cell expansion and macrophage infiltration in tumor stroma. Cancer Res. 2007;67:11438–11446. doi: 10.1158/0008-5472.CAN-07-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pahler JC, Tazzyman S, Erez N, Chen YY, Murdoch C, Nozawa H, Lewis CE, Hanahan D. Plasticity in tumor-promoting inflammation: impairment of macrophage recruitment evokes a compensatory neutrophil response. Neoplasia. 2008;10:329–340. doi: 10.1593/neo.07871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat. Rev. Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hiratsuka S, Watanabe A, Aburatani H, Maru Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat. Cell.Biol. 2006;8:1369–1375. doi: 10.1038/ncb1507. [DOI] [PubMed] [Google Scholar]
- 149.Rafii S, Lyden D. S100 chemokines mediate bookmarking of premetastatic niches. Nat. Cell Biol. 2006;8:1321–1323. doi: 10.1038/ncb1206-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Newton RA, Hogg N. The human S100 protein MRP-14 is a novel activator of the beta 2 integrin Mac-1 on neutrophils. J. Immunol. 1998;160:1427–1435. [PubMed] [Google Scholar]
- 151.Sinha P, Okoro C, Foell D, Freeze HH, Ostrand-Rosenberg S, Srikrishna G. Proinflammatory S100 proteins regulate the accumulation of myeloid-derived suppressor cells. J. Immunol. 2008;181:4666–4675. doi: 10.4049/jimmunol.181.7.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Cheng P, Corzo CA, Luetteke N, Yu B, Nagaraj S, Bui MM, Ortiz M, Nacken W, Sorg C, Vogl T, Roth J, Gabrilovich DI. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J. Exp. Med. 2008;205:2235–2249. doi: 10.1084/jem.20080132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hiratsuka S, Watanabe A, Sakurai Y, Akashi-Takamura S, Ishibashi S, Miyake K, Shibuya M, Akira S, Aburatani H, Maru Y. The S100A8-serum amyloid A3-TLR4 paracrine cascade establishes a pre-metastatic phase. Nat. Cell Biol. 2008;10:1349–1355. doi: 10.1038/ncb1794. [DOI] [PubMed] [Google Scholar]
- 154.Matsuo Y, Ochi N, Sawai H, Yasuda A, Takahashi H, Funahashi H, Takeyama H, Tong Z, Guha S. CXCL8/IL-8 and CXCL12/SDF-1alpha co-operatively promote invasiveness and angiogenesis in pancreatic cancer. Int. J. Cancer. 2009;124:853–861. doi: 10.1002/ijc.24040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kucia M, Reca R, Miekus K, Wanzeck J, Wojakowski W, Janowska-Wieczorek A, Ratajczak J, Ratajczak MZ. Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4 axis. Stem Cells. 2005;23:879–894. doi: 10.1634/stemcells.2004-0342. [DOI] [PubMed] [Google Scholar]
- 156.Marechal R, Demetter P, Nagy N, Berton A, Decaestecker C, Polus M, Closset J, Deviere J, Salmon I, Van Laethem JL. High expression of CXCR4 may predict poor survival in resected pancreatic adenocarcinoma. Br. J. Cancer. 2009;100:1444–1451. doi: 10.1038/sj.bjc.6605020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Burger JA, Kipps TJ. CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood. 2006;107:1761–1767. doi: 10.1182/blood-2005-08-3182. [DOI] [PubMed] [Google Scholar]
- 158.Huang B, Lei Z, Zhao J, Gong W, Liu J, Chen Z, Liu Y, Li D, Yuan Y, Zhang GM, Feng ZH. CCL2/CCR2 pathway mediates recruitment of myeloid suppressor cells to cancers. Cancer Lett. 2007;252:86–92. doi: 10.1016/j.canlet.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 159.Bunt SK, Clements VK, Hanson EM, Sinha P, Ostrand-Rosenberg S. Inflammation enhances myeloid-derived suppressor cell cross-talk by signaling through Toll-like receptor 4. J. Leukoc. Biol. 2009;85:996–1004. doi: 10.1189/jlb.0708446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bunt SK, Yang L, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Res. 2007;67:10019–10026. doi: 10.1158/0008-5472.CAN-07-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tu S, Bhagat G, Cui G, Takaishi S, Kurt-Jones EA, Rickman B, Betz KS, Penz-Oesterreicher M, Bjorkdahl O, Fox JG, Wang TC. Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell. 2008;14:408–419. doi: 10.1016/j.ccr.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bronte V, Chappell DB, Apolloni E, Cabrelle A, Wang M, Hwu P, Restifo NP. Unopposed production of granulocyte-macrophage colony-stimulating factor by tumors inhibits CD8+ T cell responses by dysregulating antigen-presenting cell maturation. J. Immunol. 1999;162:5728–5737. [PMC free article] [PubMed] [Google Scholar]
- 163.Markiewski MM, DeAngelis RA, Benencia F, Ricklin-Lichtsteiner SK, Koutoulaki A, Gerard C, Coukos G, Lambris JD. Modulation of the antitumor immune response by complement. Nat. Immunol. 2008;9:1225–1235. doi: 10.1038/ni.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Serafini P, De Santo C, Marigo I, Cingarlini S, Dolcetti L, Gallina G, Zanovello P, Bronte V. Derangement of immune responses by myeloid suppressor cells. Cancer Immunol. Immunother. 2004;53:64–72. doi: 10.1007/s00262-003-0443-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Eruslanov E, Daurkin I, Ortiz J, Vieweg J, Kusmartsev S. Pivotal Advance: Tumor-mediated induction of myeloid-derived suppressor cells and M2-polarized macrophages by altering intracellular PGE catabolism in myeloid cells. J. Leukoc. Biol. 2010;88:839–848. doi: 10.1189/jlb.1209821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chornoguz O, Grmai L, Sinha P, Artemenko K, Zubarev R, Ostrand-Rosenberg S. Proteomic pathway analysis reveals inflammation increases myeloid-derived suppressor cell resistance to apoptosis. Mol. Cell. Proteomics. 2011;10:1–9. doi: 10.1074/mcp.M110.002980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lechner MG, Liebertz DJ, Epstein AL. Characterization of cytokine-induced myeloid-derived suppressor cells from normal human peripheral blood mononuclear cells. J. Immunol. 2010;185:2273–2284. doi: 10.4049/jimmunol.1000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bunt SK, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. J. Immunol. 2006;176:284–290. doi: 10.4049/jimmunol.176.1.284. [DOI] [PubMed] [Google Scholar]
- 169.Song X, Krelin Y, Dvorkin T, Bjorkdahl O, Segal S, Dinarello CA, Voronov E, Apte RN. CD11b+/Gr-1+ immature myeloid cells mediate suppression of T cells in mice bearing tumors of IL-1beta-secreting cells. J. Immunol. 2005;175:8200–8208. doi: 10.4049/jimmunol.175.12.8200. [DOI] [PubMed] [Google Scholar]
- 170.Nefedova Y, Huang M, Kusmartsev S, Bhattacharya R, Cheng P, Salup R, Jove R, Gabrilovich D. Hyperactivation of STAT3 is involved in abnormal differentiation of dendritic cells in cancer. J. Immunol. 2004;172:464–474. doi: 10.4049/jimmunol.172.1.464. [DOI] [PubMed] [Google Scholar]
- 171.Ehrchen JM, Sunderkotter C, Foell D, Vogl T, Roth J. The endogenous Toll-like receptor 4 agonist S100A8/S100A9 (calprotectin) as innate amplifier of infection, autoimmunity, and cancer. J. Leukoc. Biol. 2009;86:557–566. doi: 10.1189/jlb.1008647. [DOI] [PubMed] [Google Scholar]
- 172.Mazzoni A, Bronte V, Visintin A, Spitzer JH, Apolloni E, Serafini P, Zanovello P, Segal DM. Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J. Immunol. 2002;168:689–695. doi: 10.4049/jimmunol.168.2.689. [DOI] [PubMed] [Google Scholar]
- 173.Bronte V, Serafini P, De Santo C, Marigo I, Tosello V, Mazzoni A, Segal DM, Staib C, Lowel M, Sutter G, Colombo MP, Zanovello P. IL-4-induced arginase 1 suppresses alloreactive T cells in tumor-bearing mice. J. Immunol. 2003;170:270–278. doi: 10.4049/jimmunol.170.1.270. [DOI] [PubMed] [Google Scholar]
- 174.Zhang B, Zhang Y, Bowerman NA, Schietinger A, Fu YX, Kranz DM, Rowley DA, Schreiber H. Equilibrium between host and cancer caused by effector T cells killing tumor stroma. Cancer Res. 2008;68:1563–1571. doi: 10.1158/0008-5472.CAN-07-5324. [DOI] [PubMed] [Google Scholar]
- 175.Kao J, Ko EC, Eisenstein S, Sikora AG, Fu S, Chen SH. Targeting immune suppressing myeloid-derived suppressor cells in oncology. Crit. Rev. Oncol. Hematol. 2011;77:12–19. doi: 10.1016/j.critrevonc.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, Martin F, Apetoh L, Rebe C, Ghiringhelli F. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70:3052–3061. doi: 10.1158/0008-5472.CAN-09-3690. [DOI] [PubMed] [Google Scholar]
- 177.Steding CE, Wu ST, Zhang Y, Jeng MH, Elzey BD, Kao C. The role of interleukin-12 on modulating myeloid-derived suppressor cells, increasing overall survival and reducing metastasis. Immunology. 2011;133:221–238. doi: 10.1111/j.1365-2567.2011.03429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Mirza N, Fishman M, Fricke I, Dunn M, Neuger AM, Frost TJ, Lush RM, Antonia S, Gabrilovich DI. All-trans-retinoic acid improves differentiation of myeloid cells and immune response in cancer patients. Cancer Res. 2006;66:9299–9307. doi: 10.1158/0008-5472.CAN-06-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lathers DM, Clark JI, Achille NJ, Young MR. Phase 1B study to improve immune responses in head and neck cancer patients using escalating doses of 25-hydroxyvitamin D3. Cancer Immunol. Immunother. 2004;53:422–430. doi: 10.1007/s00262-003-0459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Daud AI, Mirza N, Lenox B, Andrews S, Urbas P, Gao GX, Lee JH, Sondak VK, Riker AI, Deconti RC, Gabrilovich D. Phenotypic and functional analysis of dendritic cells and clinical outcome in patients with high-risk melanoma treated with adjuvant granulocyte macrophage colony-stimulating factor. J. Clin. Oncol. 2008;26:3235–3241. doi: 10.1200/JCO.2007.13.9048. [DOI] [PubMed] [Google Scholar]
- 181.Pan PY, Wang GX, Yin B, Ozao J, Ku T, Divino CM, Chen SH. Reversion of immune tolerance in advanced malignancy: modulation of myeloid-derived suppressor cell development by blockade of stem-cell factor function. Blood. 2008;111:219–228. doi: 10.1182/blood-2007-04-086835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Fricke I, Mirza N, Dupont J, Lockhart C, Jackson A, Lee JH, Sosman JA, Gabrilovich DI. Vascular endothelial growth factor-trap overcomes defects in dendritic cell differentiation but does not improve antigen-specific immune responses. Clin. Cancer Res. 2007;13:4840–4848. doi: 10.1158/1078-0432.CCR-07-0409. [DOI] [PubMed] [Google Scholar]
- 183.Nagaraj S, Youn JI, Weber H, Iclozan C, Lu L, Cotter MJ, Meyer C, Becerra CR, Fishman M, Antonia S, Sporn MB, Liby KT, Rawal B, Lee JH, Gabrilovich DI. Anti-inflammatory triterpenoid blocks immune suppressive function of MDSCs and improves immune response in cancer. Clin. Cancer Res. 2010;16:1812–1823. doi: 10.1158/1078-0432.CCR-09-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Talmadge JE, Hood KC, Zobel LC, Shafer LR, Coles M, Toth B. Chemoprevention by cyclooxygenase-2 inhibition reduces immature myeloid suppressor cell expansion. Int. Immunopharmacol. 2007;7:140–151. doi: 10.1016/j.intimp.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 185.Mukherjee P, Basu GD, Tinder TL, Subramani DB, Bradley JM, Arefayene M, Skaar T, De Petris G. Progression of pancreatic adenocarcinoma is significantly impeded with a combination of vaccine and COX-2 inhibition. J. Immunol. 2009;182:216–224. [PMC free article] [PubMed] [Google Scholar]
- 186.Rodriguez PC, Hernandez CP, Quiceno D, Dubinett SM, Zabaleta J, Ochoa JB, Gilbert J, Ochoa AC. Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J. Exp. Med. 2005;202:931–939. doi: 10.1084/jem.20050715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ko HJ, Kim YJ, Kim YS, Chang WS, Ko SY, Chang SY, Sakaguchi S, Kang CY. A combination of chemoimmunotherapies can efficiently break self-tolerance and induce antitumor immunity in a tolerogenic murine tumor model. Cancer Res. 2007;67:7477–7486. doi: 10.1158/0008-5472.CAN-06-4639. [DOI] [PubMed] [Google Scholar]
- 188.Degos L, Dombret H, Chomienne C, Daniel MT, Miclea JM, Chastang C, Castaigne S, Fenaux P. All-trans-retinoic acid as a differentiating agent in the treatment of acute promyelocytic leukemia. Blood. 1995;85:2643–2653. [PubMed] [Google Scholar]
- 189.Miyauchi J. All-trans retinoic acid and hematopoietic growth factors regulating the growth and differentiation of blast progenitors in acute promyelocytic leukemia. Leuk. Lymphoma. 1999;33:267–280. doi: 10.3109/10428199909058426. [DOI] [PubMed] [Google Scholar]
- 190.Serafini P, Carbley R, Noonan KA, Tan G, Bronte V, Borrello I. High-dose granulocyte-macrophage colony-stimulating factor-producing vaccines impair the immune response through the recruitment of myeloid suppressor cells. Cancer Res. 2004;64:6337–6343. doi: 10.1158/0008-5472.CAN-04-0757. [DOI] [PubMed] [Google Scholar]
- 191.Roskoski R., Jr. Sunitinib: a VEGF and PDGF receptor protein kinase and angiogenesis inhibitor. Biochem. Biophys. Res. Commun. 2007;356:323–328. doi: 10.1016/j.bbrc.2007.02.156. [DOI] [PubMed] [Google Scholar]
- 192.Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, Dolcetti L, Bronte V, Borrello I. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J. Exp. Med. 2006;203:2691–2702. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Capuano G, Rigamonti N, Grioni M, Freschi M, Bellone M. Modulators of arginine metabolism support cancer immunosurveillance. B.M.C. Immunol. 2009;10:1–13. doi: 10.1186/1471-2172-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Fleisch H. Development of bisphosphonates. Breast Cancer Res. 2002;4:30–34. doi: 10.1186/bcr414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Santini D, Galluzzo S, Vincenzi B, Schiavon G, Fratto E, Pantano F, Tonini G. New developments of aminobisphosphonates: the double face of Janus. Ann. Oncol. 2007;18(Suppl 6):vi164–167. doi: 10.1093/annonc/mdm249. [DOI] [PubMed] [Google Scholar]
- 196.Tassone P, Tagliaferri P, Viscomi C, Palmieri C, Caraglia M, D'Alessandro A, Galea E, Goel A, Abbruzzese A, Boland CR, Venuta S. Zoledronic acid induces antiproliferative and apoptotic effects in human pancreatic cancer cells in vitro. Br. J. Cancer. 2003;88:1971–1978. doi: 10.1038/sj.bjc.6600986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Marten A, Lilienfeld-Toal M, Buchler MW, Schmidt J. Zoledronic acid has direct antiproliferative and antimetastatic effect on pancreatic carcinoma cells and acts as an antigen for delta2 gamma/delta T cells. J. Immunother. 2007;30:370–377. doi: 10.1097/CJI.0b013e31802bff16. [DOI] [PubMed] [Google Scholar]
- 198.Ferretti G, Fabi A, Carlini P, Papaldo P, Cordiali Fei P, Di Cosimo S, Salesi N, Giannarelli D, Alimonti A, Di Cocco B, D'Agosto G, Bordignon V, Trento E, Cognetti F. Zoledronic-acid-induced circulating level modifications of angiogenic factors, metalloproteinases and proinflammatory cytokines in metastatic breast cancer patients. Oncology. 2005;69:35–43. doi: 10.1159/000087286. [DOI] [PubMed] [Google Scholar]
- 199.Giraudo E, Inoue M, Hanahan D. An amino-bisphosphonate targets MMP-9-expressing macrophages and angiogenesis to impair cervical carcinogenesis. J. Clin. Invest. 2004;114:623–633. doi: 10.1172/JCI22087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Santini D, Vincenzi B, Dicuonzo G, Avvisati G, Massacesi C, Battistoni F, Gavasci M, Rocci L, Tirindelli MC, Altomare V, Tocchini M, Bonsignori M, Tonini G. Zoledronic acid induces significant and long-lasting modifications of circulating angiogenic factors in cancer patients. Clin. Cancer Res. 2003;9:2893–2897. [PubMed] [Google Scholar]
- 201.Santini D, Vincenzi B, Galluzzo S, Battistoni F, Rocci L, Venditti O, Schiavon G, Angeletti S, Uzzalli F, Caraglia M, Dicuonzo G, Tonini G. Repeated intermittent low-dose therapy with zoledronic acid induces an early, sustained, and long-lasting decrease of peripheral vascular endothelial growth factor levels in cancer patients. Clin. Cancer Res. 2007;13:4482–4486. doi: 10.1158/1078-0432.CCR-07-0551. [DOI] [PubMed] [Google Scholar]
- 202.Zou L, Barnett B, Safah H, Larussa VF, Evdemon-Hogan M, Mottram P, Wei S, David O, Curiel TJ, Zou W. Bone marrow is a reservoir for CD4+CD25+ regulatory T cells that traffic through CXCL12/CXCR4 signals. Cancer Res. 2004;64:8451–8455. doi: 10.1158/0008-5472.CAN-04-1987. [DOI] [PubMed] [Google Scholar]
- 203.Hirbe AC, Rubin J, Uluckan O, Morgan EA, Eagleton MC, Prior JL, Piwnica-Worms D, Weilbaecher KN. Disruption of CXCR4 enhances osteoclastogenesis and tumor growth in bone. Proc. Natl. Acad. Sci. U. S. A. 2007;104:14062–14067. doi: 10.1073/pnas.0705203104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, Ross S, Conrads TP, Veenstra TD, Hitt BA, Kawaguchi Y, Johann D, Liotta LA, Crawford HC, Putt ME, Jacks T, Wright CV, Hruban RH, Lowy AM, Tuveson DA. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 205.Zhao F, Obermann S, von Wasielewski R, Haile L, Manns MP, Korangy F, Greten TF. Increase in frequency of myeloid-derived suppressor cells in mice with spontaneous pancreatic carcinoma. Immunology. 2009;128:141–149. doi: 10.1111/j.1365-2567.2009.03105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, Rustgi AK, Chang S, Tuveson DA. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]