Abstract
The past several years have seen the accumulation of evidence demonstrating that tissue injury induced by diverse toxicants is due not only to their direct effects on target tissues but also indirectly to the actions of resident and infiltrating macrophages. These cells release an array of mediators with cytotoxic, pro- and anti-inflammatory, angiogenic, fibrogenic, and mitogenic activity, which function to fight infections, limit tissue injury, and promote wound healing. However, following exposure to toxicants, macrophages can become hyperresponsive, resulting in uncontrolled or dysregulated release of mediators that exacerbate acute tissue injury and/or promote the development of chronic diseases such as fibrosis and cancer. Evidence suggests that the diverse activity of macrophages is mediated by distinct subpopulations that develop in response to signals within their microenvironment. Understanding the precise roles of these different macrophage populations in the pathogenic response to toxicants is key to designing effective treatments for minimizing tissue damage and chronic disease and for facilitating wound repair.
Keywords: liver, lung, inflammation, ROS, RNS, fibrosis, cytokines
INTRODUCTION
The concept that macrophages accumulating in tissues in response to injury or infection can contribute to disease pathogenesis was first proposed in the late nineteenth century by Eli Metchnikoff. Considered one of the “fathers” of modern immunology, he described the inflammatory response as a “salutary reaction against some injurious influence” and postulated that “ferments” released by cells at the site of inflammation might be capable of damaging host tissues (1). The past hundred years have seen the accumulation of evidence supporting this concept in diverse target organs including the lung, liver, skin, kidney, and brain. Thus in each of these tissues, a characteristic response to toxicants involves increased numbers of “activated” macrophages at sites of injury along with enhanced production of cytotoxic and proinflammatory mediators. Additionally, in many experimental models, agents that block macrophages abrogate tissue injury. More recent data have suggested that macrophages also play an essential role in suppressing inflammation and initiating wound repair, and that aberrations in these activities can lead to an exaggerated response to toxicants and/or the development of fibrosis or cancer. Hence it appears that macrophages can function as agents of defense or agents of destruction, either protecting the host from toxins and pathogens or promoting tissue injury and chronic disease. Their specific response depends on the toxicant, the exposure levels, and the nature of the inflammatory mediators they encounter in the tissue microenvironment. In this review, the diverse functioning of macrophages and their contribution to liver and lung toxicity is described.
MACROPHAGES
Macrophages are mononuclear phagocytes derived from monocytic precursors in the blood and bone marrow. Once localized in tissues, macrophages acquire specialized functions depending on the requirements of the tissue. Thus in the liver, resident macrophages (also known as Kupffer cells) develop a high phagocytic capacity aimed at removing endotoxin and other foreign materials from the portal circulation, whereas in the lung, alveolar macrophages acquire the capacity to release large quantities of highly reactive cytotoxic oxidants to destroy inhaled pathogens and xenobiotics. Macrophages are essential cellular effectors of the innate immune response, ridding the body of worn-out cells and debris—as well as viruses, bacteria, apoptotic cells, and some tumor cells—and mounting an inflammatory response following injury or infection (2). Macrophages are also one of the most active secretory cell types in the body, releasing a multitude of mediators that regulate all aspects of host defense, inflammation, and homeostasis (Table 1). In addition, they are considered professional antigen-presenting cells, one of the major cell types involved in initiating specific immune responses of T lymphocytes.
Table 1.
Mediators released by classically (M1) and alternatively (M2) activated macrophages implicated in liver and lung injury and repair
| M1 mediators | Toxicant
|
|
|---|---|---|
| Liver | Lung | |
|
| ||
| Reactive oxygen species | Endotoxin (17, 18) | Ozone (59–61, 63, 64, 101) |
| Acetaminophen (6, 13, 104) | Radiation (65) | |
| Phenobarbital (15) | Bleomycin (66) | |
| Carbon tetrachloride (97, 98) | Silica (84) | |
| 1,2-Dichlorobenzene (100) | ||
|
| ||
| Reactive nitrogen species | Endotoxin (47, 184) | Ozone (59, 61–64, 101) |
| Acetaminophen (12, 25, 44) | Radiation (65, 112) | |
| Carbon tetrachloride (40) | Bleomycin (66, 111, 113) | |
| Ethanol (114) | Silica (115) | |
|
| ||
| Proteases | Endotoxin (118) | Bleomycin (93) |
| Acetaminophen (37, 44) | Endotoxin (78) | |
| Carbon tetrachloride (134) | Sulfur mustard (69, 121) | |
| Thioacetamide (120) | Particulate matter (117) | |
|
| ||
| Bioactive lipids | Endotoxin (47, 136, 140) | Ozone (59, 64, 131, 138, 145) |
| Acetaminophen (127, 128) | Silica (124, 125) | |
| Carbon tetrachloride (97, 126, 130, 134, 148) | Sulfur mustard (69) | |
|
| ||
| Proinflammatory cytokines (TNFα, IL-1β, chemokines) | Endotoxin (47) | Ozone (60, 63, 64, 68) |
| Acetaminophen (3, 6, 37, 38, 44, 127) | Radiation (162) | |
| Carbon tetrachloride (14, 40, 43, 126, 158) | Silica (85) | |
| Galactosamine (153) | Particulate matter (113, 176) | |
| Cadmium (154) | Endotoxin (78) | |
| Asbestos (155, 156) | ||
|
| ||
|
M2 mediators
| ||
| IL-10 | Endotoxin (47) | Ozone (63, 64) |
| Acetaminophen (37, 38, 44, 49, 50) | Silica (91) | |
| Carbon tetrachloride (51) | Endotoxin (178) | |
|
| ||
| IL-4 | Acetaminophen (38) | Radiation (87) |
| Concanavalin-A (42) | Silica (89) | |
| Endotoxin (178) | ||
| Hyperoxia (177) | ||
|
| ||
| IL-13 | Endotoxin (45) | Endotoxin (178) |
| Acetaminophen (46) | Hyperoxia (177) | |
| Diesel exhaust (176) | ||
|
| ||
| TGFβ | Endotoxin (185) | Bleomycin (179) |
| Acetaminophen (38) | Radiation (180) | |
| Carbon tetrachloride (43, 126, 182) | Asbestos (186) | |
| Thioacetamide (181) | ||
Abbreviations: IL, interleukin; TGFβ, transforming growth factor β; TNFα, tumor necrosis factor α.
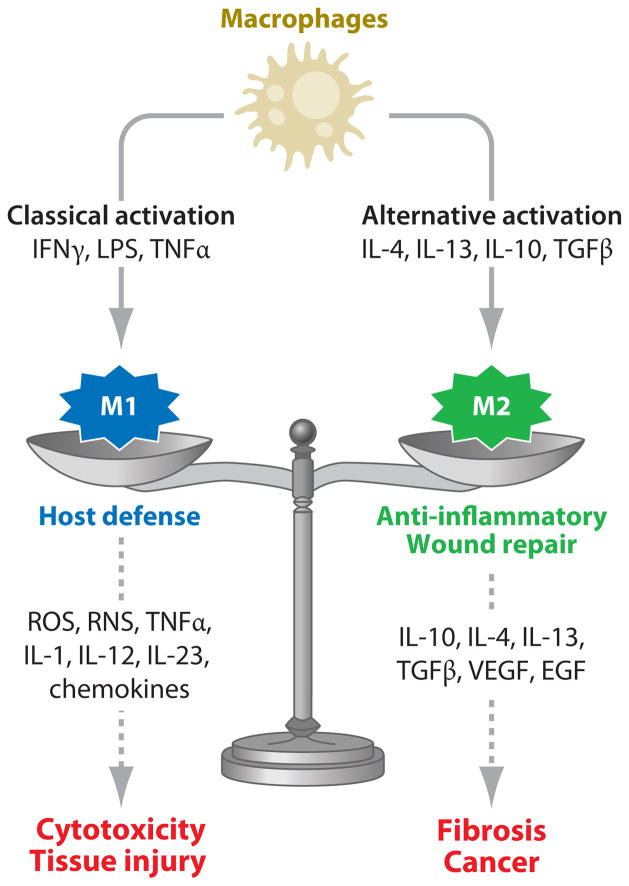
Evidence suggests that the diverse biological activity of macrophages is mediated by phenotypically distinct subpopulations of cells that develop in response to inflammatory mediators they encounter in their microenvironment. Two major populations have been characterized: classically activated M1 macrophages and alternatively activated M2 macrophages (see Figure 1). M1 macrophages are activated by type I cytokines [e.g., interferon-γ (IFNγ) and tumor necrosis factor α (TNFα)], or after recognition of pathogen-associated molecular patterns or PAMPs [e.g., lipopolysaccharide (LPS), lipoproteins, dsRNA, and lipoteichoic acid] and endogenous “danger” signals [e.g., heat shock proteins and high-mobility group protein 1 (HMGB1)]. These cells exhibit potent microbicidal and tumoricidal activity and release interleukin (IL)-12 and IL-23, promoting strong proinflammatory Th1 immune responses. In addition, they exert antiproliferative and cytotoxic activities, which result from the release of reactive oxygen species (ROS) and reactive nitrogen species (RNS) and proinflammatory cytokines (e.g., TNFα, IL-1, IL-6). The M1 population is thought to contribute to macrophage-mediated tissue injury (3–7). The activity of M1 macrophages is balanced by M2 macrophages, which are primarily involved in downregulating inflammation and initiating wound repair. This is accomplished through the release of anti-inflammatory cytokines such as IL-4, IL-10, and IL-13. M2 macrophages also contribute to the resolution of inflammation by phagocytizing apoptotic neutrophils and synthesizing mediators important in tissue remodeling and angiogenesis, including transforming growth factor β (TGFβ), vascular endothelial growth factor (VEGF), and epidermal growth factor (EGF). M2 macrophages support Th2-associated effector functions and play a key role in regulating T cell functioning. Because of their diverse functioning, alternatively activated M2 macrophages have been further subdivided into subpopulations termed M2a (activated by IL-4 and IL-13), M2b (activated by immune complexes in combination with IL-1β or LPS), and M2c (activated by IL-10, TGFβ, or glucocorticoids). It should be noted, however, that classification of macrophages into two polarized states (M1 versus M2) oversimplifies the complex functional activity of these cells. Macrophage activation is in fact a dynamic process; the same cells may initially take part in proinflammatory and cytotoxic reactions and later participate in the resolution of inflammation and wound healing (5, 8, 9). This illustrates the plasticity of macrophages and their ability to modulate their responses as a consequence of a changing microenvironment (5, 8, 10). To exemplify this more clearly, Mosser & Edwards (9) proposed an alternative grouping of macrophages on the basis of three homeostatic functions of the cells: host-defense macrophages, wound-healing macrophages, and regulatory macrophages that “blend” together into various states of activation. Regardless of how they are classified, dysregulation in the functioning of macrophages can have detrimental effects. Consequently, hyperresponsive, classically activated macrophages can cause tissue damage, whereas overactive macrophages involved in wound healing can promote fibrosis and exacerbate cytotoxic and allergic responses. Similarly, uncontrolled activation of regulatory macrophages can contribute to the progression of hyperplasia and, by releasing IL-10, can predispose the host to infection. Specific examples of the consequences of these aberrant macrophage activities in the pathogenesis of hepatotoxicity and pulmonary toxicity are illustrated below.
Figure 1.
Model for the role of classically and alternatively activated macrophages in tissue injury and repair. Abbreviations: EGF, epidermal growth factor; IFNγ, interferon-γ; IL, interleukin; LPS, lipopolysaccharide; RNS, reactive nitrogen species; ROS, reactive oxygen species; TGFβ, transforming growth factor β; TNFα, tumor necrosis factor α; VEGF, vascular endothelial growth factor.
MACROPHAGES AND INFLAMMATORY MEDIATORS IN HEPATOTOXICITY
The liver contains the largest population (80–90%) of macrophages in the body. Located within the hepatic sinusoids, resident liver macrophages (Kupffer cells) are well positioned to rid the body of foreign substances that they encounter in the portal circulation, primarily through the process of phagocytosis. Kupffer cells possess several types of receptors that facilitate this activity: Fc, C3, and CRIg receptors; scavenger receptors; pattern-recognition receptors such as Toll-like receptor (TLR)-4; and CD14 (reviewed in References 7 and 11). Kupffer cells are among the most active secretory cells in the body. They release a myriad of different products with inflammatory, growth-promoting, and regulatory activity (Table 1). They also express major histocompatibility complex (MHC) class II antigens, CD40 and CD80, thereby acting as antigen-presenting cells for the initiation of specific T lymphocyte responses, and they are responsible for the induction of immunological tolerance in the liver.
Kupffer cells are active participants in inflammatory responses to liver damage. They rapidly accumulate at sites of injury in response to locally generated chemokines. Once localized at these sites, they phagocytize damaged cells and debris and release cytotoxic/proinflammatory mediators. Kupffer cells also elaborate chemokines, contributing to the recruitment of additional inflammatory macrophages into the tissue. Some of the earliest evidence that macrophages might contribute to toxicity was based on observations that there are increased numbers of these cells in the liver following exposure of animals to hepatotoxicants (reviewed in References 7 and 11). In addition, their specific location within the liver lobule varies with the toxicant and directly correlates with areas that subsequently exhibit damage. For example, after administration of acetaminophen, carbon tetrachloride, or thioacetamide, all of which induce centrilobular hepatic necrosis, macrophages are observed in these regions of the liver (11–14). In contrast, macrophages that localize in the liver following endotoxin, phenobarbital, Corynebacterium parvum, or galactosamine treatment of rodents are scattered in clusters throughout the liver lobule, which is consistent with the patterns of injury observed after exposure to these toxicants (11, 15–17). Further evidence for a role of macrophages in hepatotoxicity comes from findings that they are morphologically and functionally “activated” following hepatotoxicant exposure. Thus these cells, which consist of resident Kupffer cells and newly infiltrated macrophages, appear larger and more stellate than cells from control animals, are highly vacuolated, and display an increased cytoplasmic:nuclear ratio (13, 15, 17, 18). Additionally, they release increased quantities of cytotoxic, matrix-degrading, and proinflammatory mediators that have been implicated in hepatotoxicity (see below).
Direct evidence supporting a role for macrophages in hepatotoxicity is based on observations that tissue injury is correlated with their functional status. Hence, when liver macrophage cytotoxic/inflammatory activity is blocked with hydrocortisone or synthetic steroids, hepatotoxicity induced by acetaminophen and carbon tetrachloride is ameliorated (19, 20). Similarly, the accumulation of macrophages in the liver and subsequent toxicity of these xenobiotics is abrogated in rodents by pretreatment with macrophage inhibitors such as gadolinium chloride or dextran sulfate (21–25). Protection against early damage induced by acetaminophen has also been reported in animals depleted of macrophages by pretreatment with liposome-encapsulated dichloromethylene diphosphonate (clodronate) (26). Both gadolinium chloride and clodronate liposomes also prevent liver damage induced by allyl alcohol, ethanol, endotoxin, thioacetamide, cadmium chloride, and diethyldithiocarbamate (27–32). Additional support for macrophage involvement in the pathogenesis of liver injury comes from findings that activation of these cells can augment tissue damage induced by hepatotoxicants. In these studies, pretreatment of rodents with agents such as LPS or polyinosinic-polycytidylic acid, which induce a marked accumulation of activated macrophages in the liver, results in an exaggerated hepatotoxic response to agents such as acetaminophen and galactosamine (33–35).
Recent studies have focused on analysis of the phenotype of the macrophage population involved in promoting the hepatotoxic response. The fact that these cells release cytotoxic mediators such as ROS, RNS, proteolytic enzymes, and proinflammatory cytokines suggests that they are classically activated M1 macrophages. This is consistent with findings that TNFα, a prototypical M1 inducer, is rapidly upregulated in the liver following hepatotoxicant exposure (14, 36–40). Furthermore, specific depletion or inhibition of M1 macrophages correlates with protection against liver injury induced by diverse hepatotoxicants (21, 23, 25, 26, 30, 31, 41, 42).
Accumulating experimental data suggest that alternatively activated M2 macrophages also contribute to hepatotoxicity. As indicated above, these macrophages are important in downregulating the inflammatory response and initiating wound repair. Following hepatotoxicant exposure, expression of the M2 macrophage chemokine MCP-1 [monocyte chemotactic protein-1, also known as chemokine (C-C motif) ligand 2] and its receptor CCR2 [chemokine (C-C motif) receptor 2] is upregulated in the liver, as is expression of the M2-inducing cytokines IL-4, IL-10, and IL-13 (3, 6, 37, 43–46). This correlates with increased numbers of macrophages expressing M2 markers including arginase, STK/RON (stem cell–derived tyrosine kinase/recepteur d’origine nantais), Ym1, and/or Fizz1 at sites of injury (47, 48). Production of anti-inflammatory and mitogenic proteins, such as TGFβ and VEGF, is also increased (3, 36–38, 44–47, 49–52). These data support a protective function of M2 macrophages in acute liver injury. Additional support comes from findings that administration of the M2 inducer IL-10 or IL-13 protects mice from acetaminophen-induced hepatotoxicity and lethal endotoxemia, whereas transgenic mice lacking the gene for CCR2, IL-10, IL-13, or IL-4 plus IL-10 and wild-type mice treated with anti-IL-13 antibodies are hypersensitive to hepatotoxicants (6, 7, 45, 46, 50, 51, 53). The fact that the exaggerated hepatotoxicity is associated with increased production of cytotoxic/proinflammatory mediators—including ROS and RNS, IFNγ, and TNFα—suggests that prolonged M1 macrophage activity plays a key role in the pathogenic response.
It is well established that liver macrophages also contribute to the development of hepatic fibrosis, the end stage of chronic liver disease (54). These cells release TGFβ and other mediators that activate hepatic stellate cells, and they stimulate the production of extracellular matrix proteins. The observation that macrophage production of profibrotic cytokines is upregulated by IL-4 and IL-13 indicates that alternatively activated M2 macrophages are involved in this process. This is supported by studies demonstrating that M2 macrophage accumulation in the liver and the development of fibrosis is dependent on MCP-1/CCR2 (43, 55). Thus it appears that prolonged hyperactivity of M2 macrophages in the liver can also lead to pathologic responses.
One approach to a better understanding of the role of M1 and M2 macrophages in response to hepatotoxicants has been to use agents that specifically block their activity. Most common are gadolinium chloride, which suppresses M1 cells, and clodronate liposomes, which deplete tissues of M2 macrophages (reviewed in Reference 7). The fact that acetaminophen-induced hepatotoxicity is suppressed in animals pretreated with gadolinium chloride but exacerbated when they are administered clodronate liposomes demonstrates the dual role of macrophages in hepatotoxic responses (3, 21, 22, 49). Providing additional support for this idea are studies using conditional knockout mice. These studies showed that carbon tetrachloride–induced fibrosis is ameliorated when macrophages are depleted during the initial inflammatory injury, whereas loss of these cells during recovery leads to a failure of resolution and the development of fibrosis (56).
MACROPHAGES AND INFLAMMATORY MEDIATORS IN PULMONARY TOXICITY
The lung is continuously exposed to the external environment. As a consequence, it is particularly sensitive to the adverse effects of inhaled gases, acid aerosols, and particles. The specific pathologic response depends on the nature of the toxic agent and the dose and duration of exposure. The lung is also sensitive to systemically administered drugs such as bleomycin and nitrogen mustard, as well as radiation, which cause acute injury and pneumonitis followed by pulmonary fibrosis. Macrophages are located throughout the lung, most prominently in the alveoli and interstitium, where they function as the first line of defense against xenobiotics. Lung macrophages possess receptors for complement; receptors for the Fc portion of IgG, IgA, and IgE; TLRs; and other pattern-recognition receptors. In addition, they can phagocytize a variety of foreign substances, although this activity is not as well developed as in Kupffer cells (57). Like other tissue macrophages, alveolar and interstitial macrophages respond to injury by mounting an inflammatory response and releasing ROS and RNS, proteases, proinflammatory lipids, and cytokines aimed at protecting the host. However, as observed in the liver, excessive or uncontrolled release of these mediators by macrophages can promote lung damage. In this regard, macrophages have been implicated in lung injury induced by a number of pulmonary toxicants and have been shown to play a role in the pathogenesis of a variety of lung diseases, including chronic obstructive pulmonary disease (COPD), asthma, and acute respiratory distress syndrome (Table 1 and Reference 58).
Experimental evidence supporting a role for macrophages in pulmonary toxicity is similar to that described above for hepatotoxicity. Accordingly, increased numbers of macrophages are observed in the lung and/or airways following toxicant exposure (59–64). In addition, these cells are rapidly activated to release cytotoxic and proinflammatory mediators. For example, following exposure of animals to ozone, particulate matter, bleomycin, sulfur mustard, or radiation, alveolar macrophages generate increased quantities of ROS, RNS, IL-1, TNFα, proteases, and bioactive lipids, which have the capacity to induce or amplify tissue injury (Table 1 and References 59–61, 63–69). Additionally, as observed in the liver, pulmonary damage induced by a number of these toxicants is ameliorated or prevented by blocking macrophages or macrophage-derived inflammatory mediators. In this regard, the use of anti-inflammatory steroids can ameliorate lung damage induced by ozone, silica, bleomycin, and sulfur mustard (70–74). Protection against lung injury has also been described in ozone-treated rats rendered leukopenic with cyclophosphamide, and in silica-treated rats depleted of alveolar macrophages via clodronate liposomes (75, 76). Similarly, macrophage accumulation in the lung and toxicity induced by ozone are inhibited by pretreatment of rats with gadolinium chloride, which, as indicated above, blocks M1 macrophage activation (7, 60). Acute lung injury and expression of inducible nitric oxide synthase (NOS-2) induced by bleomycin are also abrogated in CCR4-deficient mice, which do not generate M1 macrophages (4). Similar protective effects have been described in IL-18 knockout mice, which also display defective M1 development (77). Production of inflammatory mediators and lung injury induced by endotoxin are also reduced in knockout mice lacking the gene for CD40, a cell surface receptor required for M1 macrophage activation and NOS-2 expression (78). Conversely, mice lacking CCR2 are hypersensitive to hyperoxia-induced acute lung injury due to defects in M2 macrophage recruitment and tissue repair (79). The importance of classically activated M1 macrophages in the pathogenesis of lung injury is also supported by findings that direct activation of these cells can augment tissue damage. Hence, pretreatment of rats with macrophage activators such as Bacillus Calmette-Guérin or LPS enhances acute lung injury induced by endotoxin or radiation, as well as bleomycin-induced tissue damage and consequent pulmonary fibrosis (67, 80, 81).
Pulmonary injury that is induced following inhalation of mineral dusts such as asbestos and silica is characterized by acute injury followed by fibrosis. Lung macrophages are known to produce mediators involved in initiation and perpetuation of inflammation, as well as in mesenchymal cell transformation and fibrosis induced by these agents (5, 9, 82). Inhaled silica particles and asbestos fibers are phagocytized by alveolar macrophages via the MARCO (macrophage receptor with a collagenous structure) receptor (83). However, because these inert particles cannot be digested, macrophages rupture, releasing chemokines and proteases that amplify the inflammatory response and tissue injury. Recent data suggest that this response is dependent on the Nalp3 inflammasome, a cytosolic multiprotein platform responsible for activation of the inflammatory response (84). Alveolar macrophages activated by silica particles have also been reported to release chemotactic factors and mitogens for lung fibroblasts and to release TGFβ, thus promoting the development of lung fibrosis (85). A similar contribution of macrophages and inflammatory mediators to lung fibrosis has been described following exposure of animals to bleomycin and radiation (86, 87).
In the lung, as in the liver, macrophage phenotype appears to be important in terms of the precise contribution of these cells to disease pathogenesis. Whereas acute lung injury involves a prolonged or exaggerated response of classically activated M1 cells and defective M2 macrophage–mediated repair (described above), the development of chronic diseases such as fibrosis and cancer is a consequence of hyperresponsive, alternatively activated M2 cells. For example, in an experimental model of silicosis, markers of alternatively activated M2 macrophages including arginase-1, Fizz1, Ym1/2, and mannose receptor are upregulated in the lung (88, 89). Interestingly, this occurred within three days of silica exposure, suggesting that these cells participate in early events leading to fibrosis. A skewing toward M2 macrophage phenotype has also been described in patients with idiopathic pulmonary fibrosis, COPD, and cystic fibrosis, and in experimental models of bleomycin-induced pulmonary fibrosis (86, 90). A role of M2 macrophages in the development of chronic lung disease is most clearly evident from findings that pulmonary fibrosis is exacerbated in animals that overexpress IL-10 or IL-13 (91, 92) but reduced in animals that are (a) treated with serum amyloid P, which inhibits the development of M2 macrophages in the lung (86); (b) deficient in the M2 chemokine CCR2 (93) or deficient in IL-4 (89); or (c) administered anti-CCL2/MCP-1 gene therapy (94). Interestingly, the M1 macrophage marker NOS-2 and the M1-associated chemokine CXCL10/IP10 increase in M2-suppressed animals, demonstrating that alternatively activated macrophages are important in suppressing M1 activity and that a balance in the activity of these cells dictates the outcome of the toxic response. These findings are in accord with reports that the development of fibrosis in a granulomatous lung disease model is associated with upregulation of the M2 marker arginase, and that administration of IL-12 results in overexpression of NOS-2 and reduced fibrosis (95).
MACROPHAGE-DERIVED PROINFLAMMATORY/CYTOTOXIC MEDIATORS IMPLICATED IN TISSUE INJURY
Macrophages classically activated by LPS and Th1 cytokines are known to synthesize and release a myriad of molecules with proinflammatory and cytotoxic activity. Among the more prominent mediators that have been implicated in tissue injury are ROS and RNS, proteases, and proinflammatory lipid mediators and cytokines. These most likely act in concert to promote tissue damage.
Reactive Oxygen and Nitrogen Species
ROS and RNS, including superoxide anion, hydrogen peroxide, hydroxyl radical, nitric oxide, and peroxynitrite, are generated via enzyme-catalyzed reactions and during mitochondrial respiration. At low levels and under physiologic conditions, ROS and RNS function to regulate cell signaling molecules important in maintaining tissue homeostasis. In contrast, ROS and RNS produced in larger quantities by activated macrophages during acute inflammatory responses are key to the destruction of invading pathogens and foreign materials. However, when ROS and RNS are generated in excessive quantities or without control, oxidative and nitrosative stress can ensue, leading to tissue injury. Lipids, proteins, and DNA are targets for modification by ROS/RNS with diverse pathologic consequences, including altered functioning, necrosis, and apoptosis. Oxidative/nitrosative stress can also lead to activation of redox-sensitive transcription factors such as nuclear factor κB (NF-κB) and activator protein 1 (AP-1), amplifying the inflammatory response and tissue injury.
Macrophages generate superoxide anion via membrane-associated NADPH oxidases. This radical rapidly dismutates to hydrogen peroxide, which, in the presence of transition metals, forms hydroxyl radicals. These macrophage-derived ROS have been directly implicated in the pathogenesis of tissue injury induced by a number of diverse pulmonary and hepatic toxicants (Table 1). Macrophages that accumulate in target tissues following toxicant exposure produce increased amounts of ROS (13, 15, 17, 29, 59–61, 65, 66, 96, 97). Moreover, stimulation of these cells to produce additional oxidants augments tissue injury. For example, in rats administered large doses of vitamin A, which activates macrophages in the liver to produce ROS, tissue injury induced by hepatotoxicants such as endotoxin and carbon tetrachloride is exacerbated (98, 99). Conversely, hepatotoxicity induced by galactosamine and 1,2-dichlorobenzene, as well as carbon tetrachloride and vitamin A, is abrogated by methyl palmitate, an effective inhibitor of oxidative metabolism in liver macrophages (33, 98, 100). Additionally, increasing levels of antioxidants by administration of macrophage activators such as LPS or IL-1 protect against lung injury induced by hyperoxia, and ozone and liver injury induced by acetaminophen and carbon tetrachloride (11, 62, 101, 102). Protection is also observed after systemic administration of antioxidants to rodents, as well as in mice overexpressing antioxidant enzymes such as superoxide dismutase (SOD) or catalase, which reduce oxidative stress in macrophages (38, 63, 103–107).
RNS are generated in macrophages via NOS-2, an enzyme that catalyzes the oxidation of L-arginine to nitric oxide and citrulline. Nitric oxide reacts rapidly with superoxide anion to form peroxynitrite, a relatively long-lived cytotoxic oxidant (108). NOS-2 is upregulated in macrophages in response to inflammatory mediators such as LPS and Th1 cytokines and is highly expressed by classically activated M1 macrophages (2, 7, 109). Excessive production of RNS by macrophages has been described in the liver and lung after exposure to diverse hepatic and pulmonary toxicants (Table 1). Experimentally, administration of NOS-2 inhibitors or peroxynitrite scavengers reduces tissue damage induced by many of these toxicants (12, 110–112). Moreover, mice with a targeted disruption of the NOS-2 gene or mice that overexpress SOD, which cannot generate peroxynitrite, are protected from tissue injury induced by toxicants such as ozone, particulate matter, asbestos, silica, bleomycin, nitrogen mustard, acetaminophen, carbon tetrachloride, and alcohol (37, 59, 111, 113–115). These findings demonstrate that RNS derived from NOS-2 can contribute directly to toxicity.
Proteases
Classically activated macrophages release a variety of proteolytic enzymes that can act directly on cells and tissues and induce damage. These include neutral proteases (e.g., elastase, gelatinase, matrix metalloproteinases, collagenase, and plasminogen activator) and acid hydrolases (e.g., proteases, lipases, cathepsins, ribonucleases, phosphatases, and glycosidases) as well as lysozyme. The extent of injury induced by proteases depends on the amounts of antiproteases generated in the tissue that counteract their activity. Pulmonary diseases such as emphysema are characterized by excessive macrophage protease activity, and protease inhibitors have been used therapeutically to mitigate disease pathology (116). Toxicant exposure is associated with increased activity of various proteases in both the liver and the lung (117–120). Administration of protease inhibitors such as ilomastat or doxycyclin abrogates tissue injury in a number of experimental models, demonstrating the importance of these proteolytic enzymes in the pathogenic response (121–123).
Proinflammatory Lipid Mediators
Macrophages activated by proinflammatory cytokines and LPS synthesize a variety of bioactive lipids including prostaglandins and leukotrienes, which promote inflammation and contribute to tissue injury. These two types of eicosanoids are derived from membrane-bound arachidonic acid via the actions of cyclooxygenase (COX) and lipoxygenase (LOX), respectively. Increased expression of COX-2, the major enzyme isoform mediating macrophage prostanoid production, and release of proinflammatory prostaglandins such as PGE2 have been observed in the lung following exposure of animals to pulmonary toxicants and in the liver after administration of hepatotoxicants (64, 124–127). Moreover, COX-2 inhibitors prevent injury to the lung and liver in a number of these models (128–131). Proinflammatory leukotrienes generated via LOXs are also known to be involved in the pathogenesis of immune and inflammatory diseases including asthma (132, 133). One example is leukotriene B4, which is a potent neutrophil chemoattractant that stimulates production of macrophage IL-1, TNFα, and hydrogen peroxide. Leukotriene B4 has been reported to be elevated in the lung and liver after toxicant exposure (132, 134, 135). In addition, LOX inhibitors or antagonists afford protection against injury in several experimental models; similar protection is observed in 5-LOX knockout mice (130, 132, 134, 136–139).
Another macrophage-derived lipid inflammatory mediator implicated in tissue injury is platelet activating factor (PAF) (140, 141). PAF is thought to act in a paracrine and autocrine manner to amplify and propagate early stages of the inflammatory response. PAF released from macrophages stimulates phagocyte chemotaxis and oxidative metabolism (141, 142). In the lung, PAF mediates ozone-induced airway inflammation, microvascular leakage, and edema (143, 144). In animal models, ozone inhalation upregulates functional PAF receptors on alveolar macrophages, which may represent an important mechanism by which these cells become activated and contribute to tissue injury (145). Endotoxemia is also associated with increased production of PAF in both the lung and the liver (140, 146). The findings that administration of PAF inhibitors or receptor antagonists reduce tissue injury induced by endotoxin, acetaminophen, carbon tetrachloride, and bleomycin suggest that PAF participates in pathogenic processes associated with exposure to these toxins (140, 144, 147, 148).
Proinflammatory Cytokines and Chemokines
Macrophages release a number of proinflammatory cytokines with pathogenic potential. Most notable are TNFα and IL-1. These early-response cytokines are rapidly released at sites of injury, where they function to initiate and amplify inflammatory responses. TNFα and IL-1 upregulate adhesion molecules and stimulate the endothelium to produce chemokines, thus directly promoting the accumulation of inflammatory cells in tissues. TNFα also sensitizes neutrophils and macrophages to produce ROS and RNS, and, along with IL-1, it induces the release of proinflammatory mediators including IL-6, PAF, prostaglandins, matrix metalloproteinase, and various chemokines from macrophages and other cell types (149, 150). TNFα is unique among inflammatory cytokines because it also has the capacity to directly induce cytotoxicity and apoptosis. Tissue injury induced by various lung and liver toxicants is characterized by excessive production of TNFα and/or IL-1 by macrophages (14, 37, 38, 40, 44, 46, 68, 126, 127, 151–157). Moreover, administration of antibodies to these cytokines or cytokine receptor antagonists prevents the development of pulmonary lesions and fibrosis induced by bleomycin and silica, as well as hepatotoxicity induced by acetaminophen, galactosamine, endotoxin, cadmium chloride, and carbon tetrachloride (40, 153, 154, 158–161). Similarly, protective effects against lung injury induced by radiation, ozone, or silica and liver injury as a result of exposure to carbon tetrachloride are observed in transgenic mice lacking IL-1, TNFα, or the latter’s major proinflammatory receptor, TNFR1 (40, 157, 162–164). These data demonstrate that TNFα and IL-1 are indeed critical mediators of macrophage-induced tissue injury.
Activated macrophages also release chemokines, an important group of proinflammatory cytokines that recruit leukocytes to sites of injury. Two major structurally distinct groups of chemokines have been characterized: C-C chemokines, which induce migration and activation of macrophages/monocytes and lymphocytes, and C-X-C chemokines, which are primarily neutrophil chemoattractants and activators. Continuous local release of chemokines at sites of injury is thought to mediate the ongoing migration of effector cells into inflammatory lesions. Both C-C and C-X-C chemokines have been implicated in tissue injury and disease pathogenesis, but experimental data suggest that their roles in toxicity are distinct. Thus whereas C-X-C chemokines induce neutrophilic inflammation that promotes tissue injury, C-C chemokines attract protective macrophages that exhibit anti-inflammatory and wound-healing activity. In this regard, treatment of rodents with neutralizing antibodies against CXCR2 (CINC) has been reported to protect them from acute lung and liver injury induced by toxicants, whereas toxicity is unaltered by CCL2 (MCP-1) or CCL5 (RANTES) antibodies and exacerbated in MCP-1 (CCL2) and CCR2 knockout mice (3, 6, 53, 165, 166).
MACROPHAGE-DERIVED MEDIATORS INVOLVED IN SUPPRESSING INFLAMMATION AND INITIATING WOUND REPAIR
As described above, evidence suggests that alternatively activated M2 macrophages play a role in the resolution of inflammation and in initiation of tissue repair. This activity is mediated by anti-inflammatory cytokines (e.g., IL-4, IL-10, IL-13), bioactive lipids (e.g., 15d-PGJ2, lipoxins, resolvin), and growth factors (e.g., TGFβ, VEGF) (5, 167, 168). Overproduction of these mediators may, however, contribute to increased susceptibility to infections and the development of chronic diseases.
Anti-Inflammatory Lipid Mediators
The resolution of inflammation and initiation of wound healing is an active process that involves decreased generation of proinflammatory mediators and clearance of cytotoxic/proinflammatory cells, including classically activated M1 macrophages, from sites of injury. Evidence suggests that lipid mediators, in particular lipoxins and 15d-PGJ2, are important in this process. Production of these bioactive lipids occurs at later stages of the inflammatory response and may be due, in part, to a COX-2- and PGE2-mediated phenotypic switch in the activity of macrophages from the generation of proinflammatory to anti-inflammatory mediators (169). 15d-PGJ2 is a metabolite of PGD2. In addition to stimulating apoptosis and facilitating neutrophil clearance, 15d-PGJ2 upregulates expression of cytoprotective proteins and antioxidant enzymes, including cytosolic heat-shock proteins and heme oxygenase-1 (170). The biologic activities of 15d-PGJ2 are mediated, in large part, by binding to peroxisome proliferator-activated receptor γ (PPARγ), a ligand-activated transcription factor that suppresses inflammatory mediator production (171). PPARγ has been identified in a variety of cell types, including macrophages, and it plays an important role in recruiting alternatively activated macrophages into injured tissue (171). Another group of lipid mediators with anti-inflammatory activity are lipoxins such as lipoxin A4 and lipoxin B4. Derived from arachidonic acid via 15-LOX and 5-LOX, lipoxins promote the resolution of inflammation (169). Thus they inhibit neutrophil diapedesis into inflamed tissues, as well as proinflammatory cytokine production and activation of the proinflammatory transcription factor NF-κB (172). Lipoxin A4 also stimulates macrophage chemotaxis and phagocytic activity, which are important in inflammatory neutrophil clearance. Lipoxins have been shown to play a key role in the resolution of acute lung injury, and defects in the biosynthesis of lipoxin A4 promote the development of asthma (173).
Recent studies have identified a novel group of lipid mediators derived from omega-3 polyun-saturated essential fatty acids that are also important in resolution of inflammation (169). These include E-series resolvins (RvE1, RvE2), D-series resolvins (RvD1, RvD2), the protectins neuroprotectin D1 (NPD1) and protectin D1 (PD1), and the aspirin-triggered epimeric forms of resolvins. Resolvins and protectins appear at earlier times during the inflammatory response, suppress neutrophil chemotaxis and oxidative metabolism, and stimulate macrophage phagocytosis of apoptotic neutrophils (174). In addition, PD1 has been reported to reduce production of TNFα and IFNγ, suggesting that it induces M2 macrophage activation. Studies indicate that resolvins and protectins display potent protective actions in the lung and liver against toxicants (169, 175).
Anti-Inflammatory Cytokines and Growth Factors
Anti-inflammatory cytokines such as IL-4, IL-10, and IL-13 are generated by macrophages and other cell types in the lung and liver following toxicant exposure (37, 44, 49–51, 176–178). These cytokines facilitate the recovery of tissues from acute injury, inhibiting the production of proinflammatory cytokines and stimulating the generation of extracellular matrix proteins, in part by inducing alternative M2 macrophage activation. That these cytokines are important in toxicity is supported by findings that administration of IL-13 protects mice from lethal endotoxemia and that anti-IL-13 antibodies significantly decrease survival rate (45). Similarly, in mice treated with IL-10, hepatotoxicity is ameliorated, whereas it is exacerbated in IL-10 knockout mice treated with carbon tetrachloride or acetaminophen (50, 51).
Alternatively activated macrophages also generate a variety of growth factors such as TGFβ, VEGF, and EGF, which are key to angiogenesis, tissue regeneration, and repair. However, release of these mediators must be carefully controlled, as excessive production can lead to pathologies such as fibrosis and cancer. TGFβ is a mediator of fibrosis, stimulating production of extracellular matrix proteins, and is generated in large amounts by alternatively activated macrophages following exposure to toxicants (179–181). Evidence for a role of this M2-derived growth factor in the fibrogenic process comes from findings that the development of fibrosis is prevented in CCR2 knockout mice and in mice treated with the Kupffer cell inhibitor methyl palmitate, and that this is directly correlated with decreased TGFβ production (43, 182). A profibrogenic role for M2-derived TGFβ is further supported by reports that silica-induced pulmonary fibrosis and carbon tetrachloride–induced hepatic fibrosis are significantly reduced in IL-10 knockout mice but augmented in IL-10-overexpressing animals, and that this correlates with tissue levels of TGFβ (91, 182, 183).
MODEL FOR THE ROLE OF MACROPHAGES IN TISSUE INJURY AND REPAIR
On the basis of accumulated experimental data, a model for the role of macrophages in tissue injury and repair has been developed (Figure 1). According to this model, macrophages responding to tissue injury are activated by inflammatory signals in their microenvironment and develop into classically activated M1 macrophages, which release mediators important in host defense, or alternatively activated M2 macrophages, which generate products that downregulate inflammation and initiate wound repair. An imbalance in the activity of these macrophages populations, which can occur following toxicant exposure, leads to excessive production of proinflammatory mediators and cytotoxicity/tissue injury if the balance is tipped toward M1 macrophages, and fibrosis/cancer if it is tipped toward M2 macrophages.
CONCLUSIONS
As critical cellular components of nonspecific host defense, macrophages represent the primary system not only for protecting tissues from invading pathogens and toxicants but also for initiating the resolution of inflammation and wound healing. It is becoming increasingly apparent that these diverse functions are accomplished by different macrophage subpopulations, some of which are capable of cytotoxicity (classically activated macrophages), whereas others function to suppress inflammation and initiate wound repair (alternatively activated macrophages). The biological activity of macrophages is mediated by cytokines, oxidants, lipid mediators, and growth factors released by the different types of macrophages. Under homeostatic conditions, macrophage activation and release of these inflammatory mediators is carefully regulated. However, in response to toxicants, control mechanisms may be compromised, leading to disproportionate macrophage activity and excessive release of cytotoxic, proinflammatory, and fibrogenic products that promote tissue injury and chronic disease pathogenesis. Understanding the nature of these mediators and the relative contributions of differently activated macrophage populations to host defense against toxicants may lead to the development of better and more efficacious approaches for treating and/or preventing tissue injury and chronic disease.
Acknowledgments
This work was supported by National Institutes of Health grants GM034310, ES004738, CA132624, AR055073, and ES005022.
Glossary
- TNF
tumor necrosis factor
- LPS
lipopolysaccharide
- IL
interleukin
- ROS
reactive oxygen species
- RNS
reactive nitrogen species
- TGF
transforming growth factor
- SOD
superoxide dismutase
- COX
cyclooxygenase
- LOX
lipoxygenase
- PAF
platelet activating factor
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Contributor Information
Debra L. Laskin, Email: laskin@eohsi.rutgers.edu.
Vasanthi R. Sunil, Email: sunilvr@eohsi.rutgers.edu.
Carol R. Gardner, Email: cgardner@eohsi.rutgers.edu.
Jeffrey D. Laskin, Email: jlaskin@eohsi.rutgers.edu.
LITERATURE CITED
- 1.Metchnikoff E. Lectures on the Comparative Pathology of Inflammation Delivered at the Pasteur Institute in 1891. New York: Dover; 1968. [Google Scholar]
- 2.Zhang X, Mosser DM. Macrophage activation by endogenous danger signals. J Pathol. 2008;214:161–78. doi: 10.1002/path.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holt MP, Cheng L, Ju C. Identification and characterization of infiltrating macrophages in acetaminophen-induced liver injury. J Leukoc Biol. 2008;84:1410–21. doi: 10.1189/jlb.0308173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trujillo G, O’Connor EC, Kunkel SL, Hogaboam CM. A novel mechanism for CCR4 in the regulation of macrophage activation in bleomycin-induced pulmonary fibrosis. Am J Pathol. 2008;172:1209–21. doi: 10.2353/ajpath.2008.070832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–61. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 6.Dambach DM, Watson LM, Gray KR, Durham SK, Laskin DL. Role of CCR2 in macrophage migration into the liver during acetaminophen-induced hepatotoxicity in the mouse. Hepatology. 2002;35:1093–103. doi: 10.1053/jhep.2002.33162. [DOI] [PubMed] [Google Scholar]
- 7.Laskin DL. Macrophages and inflammatory mediators in chemical toxicity: a battle of forces. Chem Res Toxicol. 2009;22:1376–85. doi: 10.1021/tx900086v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Porcheray F, Viaud S, Rimaniol AC, Leone C, Samah B, et al. Macrophage activation switching: an asset for the resolution of inflammation. Clin Exp Immunol. 2005;142:481–89. doi: 10.1111/j.1365-2249.2005.02934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–69. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stout RD, Suttles J. Functional plasticity of macrophages: reversible adaptation to changing microenvironments. J Leukoc Biol. 2004;76:509–13. doi: 10.1189/jlb.0504272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laskin DL, Gardner CR. Nonparenchymal cells, inflammatory macrophages, and hepatotoxicity. In: Kaplowitz N, DeLeve LD, editors. Drug-Induced Liver Disease. 2 New York: Informa Healthcare; 2007. pp. 159–84. [Google Scholar]
- 12.Gardner CR, Heck DE, Yang CS, Thomas PE, Zhang X-J, et al. Role of nitric oxide in acetaminophen-induced hepatotoxicity in the rat. Hepatology. 1998;26:748–54. doi: 10.1002/hep.510270316. [DOI] [PubMed] [Google Scholar]
- 13.Laskin DL, Pilaro AM. Potential role of activated macrophages in acetaminophen hepatotoxicity. I Isolation and characterization of activated macrophages from rat liver. Toxicol Appl Pharmacol. 1986;86:204–15. doi: 10.1016/0041-008x(86)90051-7. [DOI] [PubMed] [Google Scholar]
- 14.Orfila C, Lepert JC, Alric L, Carrera G, Beraud M, et al. Expression of TNF-α and immunohistochemical distribution of hepatic macrophage surface markers in carbon tetrachloride–induced chronic liver injury in rats. Histochem J. 1999;31:677–85. doi: 10.1023/a:1003851821487. [DOI] [PubMed] [Google Scholar]
- 15.Laskin DL, Robertson FM, Pilaro AM, Laskin JD. Activation of liver macrophages following phenobarbital treatment of rats. Hepatology. 1988;8:1051–55. doi: 10.1002/hep.1840080512. [DOI] [PubMed] [Google Scholar]
- 16.MacDonald JR, Beckstead JH, Smuckler EA. An ultrastructural and histochemical study of the prominent inflammatory response in D+-galactosamine hepatotoxicity. Br J Exp Pathol. 1987;68:189–99. [PMC free article] [PubMed] [Google Scholar]
- 17.Pilaro AM, Laskin DL. Accumulation of activated mononuclear phagocytes in the liver following lipopolysaccharide treatment of rats. J Leukoc Biol. 1986;40:29–41. doi: 10.1002/jlb.40.1.29. [DOI] [PubMed] [Google Scholar]
- 18.McCloskey TW, Todaro JA, Laskin DL. Lipopolysaccharide treatment of rats alters antigen expression and oxidative metabolism in hepatic macrophages and endothelial cells. Hepatology. 1992;16:191–203. doi: 10.1002/hep.1840160130. [DOI] [PubMed] [Google Scholar]
- 19.Lloyd SA, Franklin MR. Modulation of carbon tetrachloride hepatotoxicity and xenobiotic-metabolizing enzymes by corticosterone pretreatment, adrenalectomy and sham surgery. Toxicol Lett. 1991;55:65–75. doi: 10.1016/0378-4274(91)90028-5. [DOI] [PubMed] [Google Scholar]
- 20.Madhu C, Maziasz T, Klaassen CD. Effect of pregnenolone-16 α-carbonitrile and dexamethasone on acetaminophen-induced hepatotoxicity in mice. Toxicol Appl Pharmacol. 1992;115:191–98. doi: 10.1016/0041-008x(92)90323-k. [DOI] [PubMed] [Google Scholar]
- 21.Laskin DL, Gardner CR, Price VF, Jollow DJ. Modulation of macrophage functioning abrogates the acute hepatotoxicity of acetaminophen. Hepatology. 1995;21:1045–50. [PubMed] [Google Scholar]
- 22.Michael SL, Pumford NR, Mayeux PR, Niesman MR, Hinson JA. Pretreatment of mice with macrophage inactivators decreases acetaminophen hepatotoxicity and the formation of reactive oxygen and nitrogen species. Hepatology. 1999;30:186–95. doi: 10.1002/hep.510300104. [DOI] [PubMed] [Google Scholar]
- 23.Muriel P, Alba N, Perez-Alvarez VM, Shibayama M, Tsutsumi VK. Kupffer cell inhibition prevents hepatic lipid peroxidation and damage induced by carbon tetrachloride. Comp Biochem Physiol C Toxicol Pharmacol. 2001;130:219–26. doi: 10.1016/s1532-0456(01)00237-x. [DOI] [PubMed] [Google Scholar]
- 24.Muriel P, Escobar Y. Kupffer cells are responsible for liver cirrhosis induced by carbon tetrachloride. J Appl Toxicol. 2003;23:103–8. doi: 10.1002/jat.892. [DOI] [PubMed] [Google Scholar]
- 25.Abdel-Zaher AO, Abdel-Rahman MM, Hafez MM, Omran FM. Role of nitric oxide and reduced glutathione in the protective effects of aminoguanidine, gadolinium chloride and oleanolic acid against acetaminophen-induced hepatic and renal damage. Toxicology. 2007;234:124–34. doi: 10.1016/j.tox.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 26.Goldin RD, Ratnayaka ID, Breach CS, Brown IN, Wickramasinghe SN. Role of macrophages in acetaminophen (paracetamol)-induced hepatotoxicity. J Pathol. 1996;179:432–35. doi: 10.1002/(SICI)1096-9896(199608)179:4<432::AID-PATH609>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 27.Przybocki JM, Reuhl KR, Thurman RG, Kauffman FC. Involvement of nonparenchymal cells in oxygen-dependent hepatic injury by allyl alcohol. Toxicol Appl Pharmacol. 1992;115:57–63. doi: 10.1016/0041-008x(92)90367-2. [DOI] [PubMed] [Google Scholar]
- 28.Iimuro Y, Yamamoto M, Kohno H, Itakura J, Fujii H, Matsumoto Y. Blockade of liver macrophages by gadolinium chloride reduces lethality in endotoxemic rats—analysis of mechanisms of lethality in endotoxemia. J Leukoc Biol. 1994;55:723–28. doi: 10.1002/jlb.55.6.723. [DOI] [PubMed] [Google Scholar]
- 29.Ishiyama H, Ogino K, Hobara T. Role of Kupffer cells in rat liver injury induced by diethyldithio-carbamate. Eur J Pharmacol. 1995;292:135–41. doi: 10.1016/0926-6917(95)90005-5. [DOI] [PubMed] [Google Scholar]
- 30.Koop DR, Klopfenstein B, Iimuro Y, Thurman RG. Gadolinium chloride blocks alcohol-dependent liver toxicity in rats treated chronically with intragastric alcohol despite the induction of CYP2E1. Mol Pharmacol. 1997;51:944–50. doi: 10.1124/mol.51.6.944. [DOI] [PubMed] [Google Scholar]
- 31.Harstad EB, Klaassen CD. Gadolinium chloride pretreatment prevents cadmium chloride–induced liver damage in both wild-type and MT-null mice. Toxicol Appl Pharmacol. 2002;180:178–85. doi: 10.1006/taap.2002.9385. [DOI] [PubMed] [Google Scholar]
- 32.Andres D, Sanchez-Reus I, Bautista M, Cascales M. Depletion of Kupffer cell function by gadolinium chloride attenuates thioacetamide-induced hepatotoxicity: expression of metallothionein and HSP70. Biochem Pharmacol. 2003;66:917–26. doi: 10.1016/s0006-2952(03)00443-x. [DOI] [PubMed] [Google Scholar]
- 33.Al-Tuwaijri A, Akdamar K, Di Luzio NR. Modification of galactosamine-induced liver injury in rats by reticuloendothelial system stimulation or depression. Hepatology. 1981;1:107–13. doi: 10.1002/hep.1840010204. [DOI] [PubMed] [Google Scholar]
- 34.Kalabis GM, Wells PG. Biphasic modulation of acetaminophen bioactivation and hepatotoxicity by pretreatment with the interferon inducer polyinosinic-polycytidylic acid. J Pharmacol Exp Ther. 1990;255:1408–19. [PubMed] [Google Scholar]
- 35.Maddox JF, Amuzie CJ, Li M, Newport SW, Sparkenbaugh E, et al. Bacterial- and viral-induced inflammation increases sensitivity to acetaminophen hepatotoxicity. J Toxicol Environ Health A. 2010;73:58–73. doi: 10.1080/15287390903249057. [DOI] [PubMed] [Google Scholar]
- 36.Masubuchi Y, Sugiyama S, Horie T. Th1/Th2 cytokine balance as a determinant of acetaminophen-induced liver injury. Chem Biol Interact. 2009;179:273–79. doi: 10.1016/j.cbi.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 37.Gardner CR, Laskin JD, Dambach DM, Sacco M, Durham SK, et al. Reduced hepatotoxicity of acetaminophen in mice lacking inducible nitric oxide synthase: potential role of tumor necrosis factor-α and interleukin-10. Toxicol Appl Pharmacol. 2002;184:27–36. [PubMed] [Google Scholar]
- 38.Dambach DM, Durham SK, Laskin JD, Laskin DL. Distinct roles of NF-κB p50 in the regulation of acetaminophen-induced inflammatory mediator production and hepatotoxicity. Toxicol Appl Pharmacol. 2006;211:157–65. doi: 10.1016/j.taap.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 39.Pritchard MT, Cohen JI, Roychowdhury S, Pratt BT, Nagy LE. Early growth response-1 attenuates liver injury and promotes hepatoprotection after carbon tetrachloride exposure in mice. J Hepatol. 2010;53:655–62. doi: 10.1016/j.jhep.2010.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morio LA, Chiu H, Sprowles KA, Zhou P, Heck DE, et al. Distinct roles of tumor necrosis factor-α and nitric oxide in acute liver injury induced by carbon tetrachloride in mice. Toxicol Appl Pharmacol. 2001;172:44–51. doi: 10.1006/taap.2000.9133. [DOI] [PubMed] [Google Scholar]
- 41.Vollmar B, Ruttinger D, Wanner GA, Leiderer R, Menger MD. Modulation of Kupffer cell activity by gadolinium chloride in endotoxemic rats. Shock. 1996;6:434–41. doi: 10.1097/00024382-199612000-00008. [DOI] [PubMed] [Google Scholar]
- 42.Hatano M, Sasaki S, Ohata S, Shiratsuchi Y, Yamazaki T, et al. Effects of Kupffer cell-depletion on concanavalin A–induced hepatitis. Cell Immunol. 2008;251:25–30. doi: 10.1016/j.cellimm.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 43.Karlmark KR, Weiskirchen R, Zimmermann HW, Gassler N, Ginhoux F, et al. Hepatic recruitment of the inflammatory Gr1+ monocyte subset upon liver injury promotes hepatic fibrosis. Hepatology. 2009;50:261–74. doi: 10.1002/hep.22950. [DOI] [PubMed] [Google Scholar]
- 44.Gardner CR, Laskin JD, Dambach DM, Chiu H, Durham SK, et al. Exaggerated hepatotoxicity of acetaminophen in mice lacking tumor necrosis factor receptor-1: potential role of inflammatory mediators. Toxicol Appl Pharmacol. 2003;192:119–30. doi: 10.1016/s0041-008x(03)00273-4. [DOI] [PubMed] [Google Scholar]
- 45.Matsukawa A, Hogaboam CM, Lukacs NW, Lincoln PM, Evanoff HL, et al. Expression and contribution of endogenous IL-13 in an experimental model of sepsis. J Immunol. 2000;164:2738–44. doi: 10.4049/jimmunol.164.5.2738. [DOI] [PubMed] [Google Scholar]
- 46.Yee SB, Bourdi M, Masson MJ, Pohl LR. Hepatoprotective role of endogenous interleukin-13 in a murine model of acetaminophen-induced liver disease. Chem Res Toxicol. 2007;20:734–44. doi: 10.1021/tx600349f. [DOI] [PubMed] [Google Scholar]
- 47.Laskin DL, Chen L, Hankey PA, Laskin JD. Role of STK in mouse liver macrophage and endothelial cell responsiveness during acute endotoxemia. J Leukoc Biol. 2010;88:373–82. doi: 10.1189/jlb.0210113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu Y, Gardner CR, Laskin JD, Laskin DL. Classical and alternative activation of rat liver Kupffer and endothelial cells: impact of acetaminophen (APAP) The Toxicologist. 2010;114:158. (Abstr.) [Google Scholar]
- 49.Ju C, Reilly TP, Bourdi M, Radonovich MF, Brady JN, et al. Protective role of Kupffer cells in acetaminophen-induced hepatic injury in mice. Chem Res Toxicol. 2002;15:1504–13. doi: 10.1021/tx0255976. [DOI] [PubMed] [Google Scholar]
- 50.Bourdi M, Masubuchi Y, Reilly TP, Amouzadeh HR, Martin JL, et al. Protection against acetaminophen-induced liver injury and lethality by interleukin 10: role of inducible nitric oxide synthase. Hepatology. 2002;35:289–98. doi: 10.1053/jhep.2002.30956. [DOI] [PubMed] [Google Scholar]
- 51.Louis H, Van Laethem JL, Wu W, Quertinmont E, Degraef C, et al. Interleukin-10 controls neutrophilic infiltration, hepatocyte proliferation, and liver fibrosis induced by carbon tetrachloride in mice. Hepatology. 1998;28:1607–15. doi: 10.1002/hep.510280621. [DOI] [PubMed] [Google Scholar]
- 52.Donahower B, McCullough SS, Kurten R, Lamps LW, Simpson P, et al. Vascular endothelial growth factor and hepatocyte regeneration in acetaminophen toxicity. Am J Physiol Gastrointest Liver Physiol. 2006;291:G102–9. doi: 10.1152/ajpgi.00575.2005. [DOI] [PubMed] [Google Scholar]
- 53.Hogaboam CM, Bone-Larson CL, Steinhauser ML, Matsukawa A, Gosling J, et al. Exaggerated hepatic injury due to acetaminophen challenge in mice lacking C-C chemokine receptor 2. Am J Pathol. 2000;156:1245–52. doi: 10.1016/S0002-9440(10)64995-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heymann F, Trautwein C, Tacke F. Monocytes and macrophages as cellular targets in liver fibrosis. Inflamm Allergy Drug Targets. 2009;8:307–18. doi: 10.2174/187152809789352230. [DOI] [PubMed] [Google Scholar]
- 55.Seki E, de Minicis S, Inokuchi S, Taura K, Miyai K, et al. CCR2 promotes hepatic fibrosis in mice. Hepatology. 2009;50:185–97. doi: 10.1002/hep.22952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Investig. 2005;115:56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Laskin DL, Weinberger B, Laskin JD. Functional heterogeneity in liver and lung macrophages. J Leukoc Biol. 2001;70:163–70. [PubMed] [Google Scholar]
- 58.Tsushima K, King LS, Aggarwal NR, De Gorordo A, D’Alessio FR, Kubo K. Acute lung injury review. Intern Med. 2009;48:621–30. doi: 10.2169/internalmedicine.48.1741. [DOI] [PubMed] [Google Scholar]
- 59.Fakhrzadeh L, Laskin JD, Laskin DL. Deficiency in inducible nitric oxide synthase protects mice from ozone-induced lung inflammation and tissue injury. Am J Respir Cell Mol Biol. 2002;26:413–19. doi: 10.1165/ajrcmb.26.4.4516. [DOI] [PubMed] [Google Scholar]
- 60.Pendino KJ, Meidhof TM, Heck DE, Laskin JD, Laskin DL. Inhibition of macrophages with gadolinium chloride abrogates ozone-induced pulmonary injury and inflammatory mediator production. Am J Respir Cell Mol Biol. 1995;13:125–32. doi: 10.1165/ajrcmb.13.2.7542894. [DOI] [PubMed] [Google Scholar]
- 61.Pendino KJ, Laskin JD, Shuler RL, Punjabi CJ, Laskin DL. Enhanced production of nitric oxide by rat alveolar macrophages after inhalation of a pulmonary irritant is associated with increased expression of nitric oxide synthase. J Immunol. 1993;151:7196–205. [PubMed] [Google Scholar]
- 62.Pendino KJ, Gardner CR, Shuler RL, Laskin JD, Durham SK, et al. Inhibition of ozone-induced nitric oxide synthase expression in the lung by endotoxin. Am J Respir Cell Mol Biol. 1996;14:516–25. doi: 10.1165/ajrcmb.14.6.8652180. [DOI] [PubMed] [Google Scholar]
- 63.Fakhrzadeh L, Laskin JD, Gardner CR, Laskin DL. Superoxide dismutase–overexpressing mice are resistant to ozone-induced tissue injury and increases in nitric oxide and tumor necrosis factor-α. Am J Respir Cell Mol Biol. 2004;30:280–87. doi: 10.1165/rcmb.2003-0044OC. [DOI] [PubMed] [Google Scholar]
- 64.Fakhrzadeh L, Laskin JD, Laskin DL. Ozone-induced production of nitric oxide and TNF-α and tissue injury are dependent on NF-κB p50. Am J Physiol Lung Cell Mol Physiol. 2004;287:L279–85. doi: 10.1152/ajplung.00348.2003. [DOI] [PubMed] [Google Scholar]
- 65.Tasat DR, Mancuso R, Evelson P, Polo JM, Llesuy S, Molinari B. Radiation effects on oxidative metabolism in young and aged rat alveolar macrophages. Cell Mol Biol. 2002;48:529–35. [PubMed] [Google Scholar]
- 66.Inghilleri S, Morbini P, Oggionni T, Barni S, Fenoglio C. In situ assessment of oxidant and nitrogenic stress in bleomycin pulmonary fibrosis. Histochem Cell Biol. 2006;125:661–69. doi: 10.1007/s00418-005-0116-7. [DOI] [PubMed] [Google Scholar]
- 67.Johnston CJ, Williams JP, Elder A, Hernady E, Finkelstein JN. Inflammatory cell recruitment following thoracic irradiation. Exp Lung Res. 2004;30:369–82. doi: 10.1080/01902140490438915. [DOI] [PubMed] [Google Scholar]
- 68.Pendino KJ, Shuler RL, Laskin JD, Laskin DL. Enhanced production of interleukin-1, tumor necrosis factor-α, and fibronectin by rat lung phagocytes following inhalation of a pulmonary irritant. Am J Respir Cell Mol Biol. 1994;11:279–86. doi: 10.1165/ajrcmb.11.3.8086166. [DOI] [PubMed] [Google Scholar]
- 69.Malaviya R, Sunil VR, Cervelli J, Anderson DR, Holmes WW, et al. Inflammatory effects of inhaled sulfur mustard in rat lung. Toxicol Appl Pharmacol. 2010;248:89–99. doi: 10.1016/j.taap.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wigenstam E, Rocksen D, Ekstrand-Hammarstrom B, Bucht A. Treatment with dexamethasone or liposome-encapsuled vitamin E provides beneficial effects after chemical-induced lung injury. Inhal Toxicol. 2009;21:958–64. doi: 10.1080/08958370802596298. [DOI] [PubMed] [Google Scholar]
- 71.Haddad EB, Liu SF, Salmon M, Robichaud A, Barnes PJ, Chung KF. Expression of inducible nitric oxide synthase mRNA in Brown Norway rats exposed to ozone: effect of dexamethasone. Eur J Pharmacol. 1995;293:287–90. doi: 10.1016/0926-6917(95)00032-1. [DOI] [PubMed] [Google Scholar]
- 72.DiMatteo M, Reasor MJ. Modulation of silica-induced pulmonary toxicity by dexamethasone-containing liposomes. Toxicol Appl Pharmacol. 1997;142:411–21. doi: 10.1006/taap.1996.8057. [DOI] [PubMed] [Google Scholar]
- 73.Chen F, Gong L, Zhang L, Wang H, Qi X, et al. Short courses of low dose dexamethasone delay bleomycin-induced lung fibrosis in rats. Eur J Pharmacol. 2006;536:287–95. doi: 10.1016/j.ejphar.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 74.Giri SN, Benson J, Siegel DM, Rice SA, Schiedt M. Effects of pretreatment with anti-inflammatory drugs on ozone-induced lung damage in rats. Proc Soc Exp Biol Med. 1975;150:810–14. doi: 10.3181/00379727-150-39130. [DOI] [PubMed] [Google Scholar]
- 75.Bhalla DK, Daniels DS, Luu NT. Attenuation of ozone-induced airway permeability in rats by pretreatment with cyclophosphamide, FPL 55712, and indomethacin. Am J Respir Cell Mol Biol. 1992;7:73–80. doi: 10.1165/ajrcmb/7.1.73. [DOI] [PubMed] [Google Scholar]
- 76.Elder AC, Gelein R, Oberdorster G, Finkelstein J, Notter R, Wang Z. Efficient depletion of alveolar macrophages using intratracheally inhaled aerosols of liposome-encapsulated clodronate. Exp Lung Res. 2004;30:105–20. doi: 10.1080/01902140490266510. [DOI] [PubMed] [Google Scholar]
- 77.Hoshino T, Okamoto M, Sakazaki Y, Kato S, Young HA, Aizawa H. Role of proinflammatory cytokines IL-18 and IL-1β in bleomycin-induced lung injury in humans and mice. Am J Respir Cell Mol Biol. 2009;41:661–70. doi: 10.1165/rcmb.2008-0182OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hashimoto N, Kawabe T, Imaizumi K, Hara T, Okamoto M, et al. CD40 plays a crucial role in lipopolysaccharide-induced acute lung injury. Am J Respir Cell Mol Biol. 2004;30:808–15. doi: 10.1165/rcmb.2003-0197OC. [DOI] [PubMed] [Google Scholar]
- 79.Okuma T, Terasaki Y, Sakashita N, Kaikita K, Kobayashi H, et al. MCP-1/CCR2 signalling pathway regulates hyperoxia-induced acute lung injury via nitric oxide production. Int J Exp Pathol. 2006;87:475–83. doi: 10.1111/j.1365-2613.2006.00502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chyczewska E, Chyczewski L, Bankowski E, Sulkowski S, Niklinski J. Stimulation of alveolar macrophages by BCG vaccine enhances the process of lung fibrosis induced by bleomycin. Folia Histochem Cytobiol. 1993;31:113–16. [PubMed] [Google Scholar]
- 81.Tasaka S, Ishizaka A, Urano T, Sayama K, Sakamaki F, et al. BCG priming enhances endotoxin-induced acute lung injury independent of neutrophils. Am J Respir Crit Care Med. 1995;152:1041–49. doi: 10.1164/ajrccm.152.3.7663781. [DOI] [PubMed] [Google Scholar]
- 82.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 83.Hamilton RF, Jr, Thakur SA, Mayfair JK, Holian A. MARCO mediates silica uptake and toxicity in alveolar macrophages from C57BL/6 mice. J Biol Chem. 2006;281:34218–26. doi: 10.1074/jbc.M605229200. [DOI] [PubMed] [Google Scholar]
- 84.Cassel SL, Eisenbarth SC, Iyer SS, Sadler JJ, Colegio OR, et al. The Nalp3 inflammasome is essential for the development of silicosis. Proc Natl Acad Sci USA. 2008;105:9035–40. doi: 10.1073/pnas.0803933105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kuhlmann UC, Chwieralski CE, Van Den Brule S, Rocken C, Reinhold D, et al. Modulation of cytokine production and silica-induced lung fibrosis by inhibitors of aminopeptidase N and of dipeptidyl peptidase-IV-related proteases. Life Sci. 2009;84:1–11. doi: 10.1016/j.lfs.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 86.Murray LA, Rosada R, Moreira AP, Joshi A, Kramer MS, et al. Serum amyloid P therapeutically attenuates murine bleomycin-induced pulmonary fibrosis via its effects on macrophages. PLoS One. 2010;5:e9683. doi: 10.1371/journal.pone.0009683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Buttner C, Skupin A, Reimann T, Rieber EP, Unteregger G, et al. Local production of interleukin-4 during radiation-induced pneumonitis and pulmonary fibrosis in rats: macrophages as a prominent source of interleukin-4. Am J Respir Cell Mol Biol. 1997;17:315–25. doi: 10.1165/ajrcmb.17.3.2279. [DOI] [PubMed] [Google Scholar]
- 88.Misson P, Van Den Brule S, Barbarin V, Lison D, Huaux F. Markers of macrophage differentiation in experimental silicosis. J Leukoc Biol. 2004;76:926–32. doi: 10.1189/jlb.0104019. [DOI] [PubMed] [Google Scholar]
- 89.Migliaccio CT, Buford MC, Jessop F, Holian A. The IL-4Rα pathway in macrophages and its potential role in silica-induced pulmonary fibrosis. J Leukoc Biol. 2008;83:630–39. doi: 10.1189/jlb.0807533. [DOI] [PubMed] [Google Scholar]
- 90.Hancock A, Armstrong L, Gama R, Millar A. Production of interleukin 13 by alveolar macrophages from normal and fibrotic lung. Am J Respir Cell Mol Biol. 1998;18:60–65. doi: 10.1165/ajrcmb.18.1.2627. [DOI] [PubMed] [Google Scholar]
- 91.Barbarin V, Xing Z, Delos M, Lison D, Huaux F. Pulmonary overexpression of IL-10 augments lung fibrosis and Th2 responses induced by silica particles. Am J Physiol Lung Cell Mol Physiol. 2005;288:L841–48. doi: 10.1152/ajplung.00329.2004. [DOI] [PubMed] [Google Scholar]
- 92.Lee CG, Homer RJ, Zhu Z, Lanone S, Wang X, et al. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor β1. J Exp Med. 2001;194:809–21. doi: 10.1084/jem.194.6.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Okuma T, Terasaki Y, Kaikita K, Kobayashi H, Kuziel WA, et al. C-C chemokine receptor 2 (CCR2) deficiency improves bleomycin-induced pulmonary fibrosis by attenuation of both macrophage infiltration and production of macrophage-derived matrix metalloproteinases. J Pathol. 2004;204:594–604. doi: 10.1002/path.1667. [DOI] [PubMed] [Google Scholar]
- 94.Inoshima I, Kuwano K, Hamada N, Hagimoto N, Yoshimi M, et al. Anti-monocyte chemoattractant protein-1 gene therapy attenuates pulmonary fibrosis in mice. Am J Physiol Lung Cell Mol Physiol. 2004;286:L1038–44. doi: 10.1152/ajplung.00167.2003. [DOI] [PubMed] [Google Scholar]
- 95.Hesse M, Cheever AW, Jankovic D, Wynn TA. NOS-2 mediates the protective anti-inflammatory and antifibrotic effects of the Th1-inducing adjuvant, IL-12, in a Th2 model of granulomatous disease. Am J Pathol. 2000;157:945–55. doi: 10.1016/S0002-9440(10)64607-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Arthur MJP, Bentley IS, Tanner AR, Saunders PK, Millward-Sadler GH, Wright R. Oxygen-derived free radicals promote hepatic injury in the rat. Gastroenterology. 1985;89:1114–22. doi: 10.1016/0016-5085(85)90218-5. [DOI] [PubMed] [Google Scholar]
- 97.Alric L, Orfila C, Carrere N, Beraud M, Carrera G, et al. Reactive oxygen intermediates and eicosanoid production by Kupffer cells and infiltrated macrophages in acute and chronic liver injury induced in rats by CCl4. Inflamm Res. 2000;49:700–7. doi: 10.1007/s000110050649. [DOI] [PubMed] [Google Scholar]
- 98.elSisi AE, Hall P, Sim WL, Earnest DL, Sipes IG. Characterization of vitamin A potentiation of carbon tetrachloride–induced liver injury. Toxicol Appl Pharmacol. 1993;119:280–88. doi: 10.1006/taap.1993.1070. [DOI] [PubMed] [Google Scholar]
- 99.Hendriks HF, Horan MA, Durham SK, Earnest DL, Brouwer A, et al. Endotoxin-induced liver injury in aged and subacutely hypervitaminotic A rats. Mech Ageing Dev. 1987;41:241–50. doi: 10.1016/0047-6374(87)90044-3. [DOI] [PubMed] [Google Scholar]
- 100.Guanawardhana L, Mobley SA, Sipes IG. Modulation of 1,2-dichlorobenzene hepatotoxicity in the Fischer-344 rat by a scavenger of superoxide anions and an inhibitor of Kupffer cells. Toxicol Appl Pharmacol. 1993;119:205–13. doi: 10.1006/taap.1993.1061. [DOI] [PubMed] [Google Scholar]
- 101.Rahman I, Massaro D. Endotoxin treatment protects rats against ozone-induced lung edema: with evidence for the role of manganese superoxide dismutase. Toxicol Appl Pharmacol. 1992;113:13–18. doi: 10.1016/0041-008x(92)90003-b. [DOI] [PubMed] [Google Scholar]
- 102.Tsan MF, Lee CY, White JE. Interleukin 1 protects rats against oxygen toxicity. J Appl Physiol. 1991;71:688–97. doi: 10.1152/jappl.1991.71.2.688. [DOI] [PubMed] [Google Scholar]
- 103.Mikawa K, Nishina K, Maekawa N, Obara H. Attenuation of hyperoxic lung injury in rabbits with superoxide dismutase: effects on inflammatory mediators. Acta Anaesthesiol Scand. 1995;39:317–22. doi: 10.1111/j.1399-6576.1995.tb04069.x. [DOI] [PubMed] [Google Scholar]
- 104.Nakae D, Yamamoto K, Yoshiji H, Kinugasa T, Maruyama H, et al. Liposome-encapsulated superoxide dismutase prevents liver necrosis induced by acetaminophen. Am J Pathol. 1990;136:787–95. [PMC free article] [PubMed] [Google Scholar]
- 105.Shiratori Y, Kawase T, Shiina S, Okano K, Sugimoto T, et al. Modulation of hepatotoxicity by macrophages in the liver. Hepatology. 1988;8:815–21. doi: 10.1002/hep.1840080420. [DOI] [PubMed] [Google Scholar]
- 106.Nakahira K, Takahashi T, Shimizu H, Maeshima K, Uehara K, et al. Protective role of heme oxygenase-1 induction in carbon tetrachloride–induced hepatotoxicity. Biochem Pharmacol. 2003;66:1091–105. doi: 10.1016/s0006-2952(03)00444-1. [DOI] [PubMed] [Google Scholar]
- 107.Rabbani ZN, Anscher MS, Folz RJ, Archer E, Huang H, et al. Overexpression of extracellular superoxide dismutase reduces acute radiation-induced lung toxicity. BMC Cancer. 2005;5:59–71. doi: 10.1186/1471-2407-5-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Laskin JD, Heck DE, Laskin DL. Nitric oxide pathways in toxic responses. In: Ballantyne B, Marrs T, Syversen T, editors. General and Applied Toxicology. Hoboken, NJ: Wiley; 2009. pp. 425–38. [Google Scholar]
- 109.Edwards JP, Zhang X, Frauwirth KA, Mosser DM. Biochemical and functional characterization of three activated macrophage populations. J Leukoc Biol. 2006;80:1298–307. doi: 10.1189/jlb.0406249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yeh DY, Feng NH, Chen CF, Lin HI, Wang D. Inducible nitric oxide synthase expressions in different lung injury models and the protective effect of aminoguanidine. Transplant Proc. 2008;40:2178–81. doi: 10.1016/j.transproceed.2008.07.087. [DOI] [PubMed] [Google Scholar]
- 111.Genovese T, Cuzzocrea S, Di Paola R, Failla M, Mazzon E, et al. Inhibition or knock out of inducible nitric oxide synthase result in resistance to bleomycin-induced lung injury. Respir Res. 2005;6:58. doi: 10.1186/1465-9921-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nozaki Y, Hasegawa Y, Takeuchi A, Fan ZH, Isobe KI, et al. Nitric oxide as an inflammatory mediator of radiation pneumonitis in rats. Am J Physiol Lung Cell Mol Physiol. 1997;272:L651–58. doi: 10.1152/ajplung.1997.272.4.L651. [DOI] [PubMed] [Google Scholar]
- 113.Becher R, Bucht A, Ovrevik J, Hongslo JK, Dahlman HJ, et al. Involvement of NADPH oxidase and iNOS in rodent pulmonary cytokine responses to urban air and mineral particles. Inhal Toxicol. 2007;19:645–55. doi: 10.1080/08958370701353528. [DOI] [PubMed] [Google Scholar]
- 114.Venkatraman A, Shiva S, Wigley A, Ulasova E, Chhieng D, et al. The role of iNOS in alcohol-dependent hepatotoxicity and mitochondrial dysfunction in mice. Hepatology. 2004;40:565–73. doi: 10.1002/hep.20326. [DOI] [PubMed] [Google Scholar]
- 115.Zeidler P, Hubbs A, Battelli L, Castranova V. Role of inducible nitric oxide synthase–derived nitric oxide in silica-induced pulmonary inflammation and fibrosis. J Toxicol Environ Health A. 2004;67:1001–26. doi: 10.1080/15287390490447296. [DOI] [PubMed] [Google Scholar]
- 116.Owen CA. Roles for proteinases in the pathogenesis of chronic obstructive pulmonary disease. Int J Chronic Obstr Pulm Dis. 2008;3:253–68. doi: 10.2147/copd.s2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Adamson IY, Vincent R, Bakowska J. Differential production of metalloproteinases after instilling various urban air particle samples to rat lung. Exp Lung Res. 2003;29:375–88. doi: 10.1080/01902140303753. [DOI] [PubMed] [Google Scholar]
- 118.Tanner A, Keyhani A, Reiner R, Holdstock G, Wright R. Proteolytic enzymes released by liver macrophages may promote injury in a rat model of hepatic damage. Gastroenterology. 1981;80:647–54. [PubMed] [Google Scholar]
- 119.Yan C, Zhou L, Han YP. Contribution of hepatic stellate cells and matrix metalloproteinase 9 in acute liver failure. Liver Int. 2008;28:959–71. doi: 10.1111/j.1478-3231.2008.01775.x. [DOI] [PubMed] [Google Scholar]
- 120.Park SY, Shin HW, Lee KB, Lee MJ, Jang JJ. Differential expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in thioacetamide-induced chronic liver injury. J Korean Med Sci. 2010;25:570–76. doi: 10.3346/jkms.2010.25.4.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Anderson DR, Taylor SL, Fetterer DP, Holmes WW. Evaluation of protease inhibitors and an antioxidant for treatment of sulfur mustard–induced toxic lung injury. Toxicology. 2009;263:41–46. doi: 10.1016/j.tox.2008.08.025. [DOI] [PubMed] [Google Scholar]
- 122.Guignabert C, Taysse L, Calvet JH, Planus E, Delamanche S, et al. Effect of doxycycline on sulfur mustard–induced respiratory lesions in guinea pigs. Am J Physiol Lung Cell Mol Physiol. 2005;289:L67–74. doi: 10.1152/ajplung.00475.2004. [DOI] [PubMed] [Google Scholar]
- 123.Suzuki T, Chow CW, Downey GP. Role of innate immune cells and their products in lung immunopathology. Int J Biochem Cell Biol. 2008;40:1348–61. doi: 10.1016/j.biocel.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 124.Kuhn DC, Griffith JW, Stauffer JL, Riling S, Demers LM. Characterization of alveolar macrophage eicosanoid production in a non-human primate model of mineral dust exposure. Prostaglandins. 1993;46:207–20. doi: 10.1016/0090-6980(93)90004-q. [DOI] [PubMed] [Google Scholar]
- 125.Mohr C, Davis GS, Graebner C, Hemenway DR, Gemsa D. Enhanced release of prostaglandin E2 from macrophages of rats with silicosis. Am J Respir Cell Mol Biol. 1992;6:390–96. doi: 10.1165/ajrcmb/6.4.390. [DOI] [PubMed] [Google Scholar]
- 126.Luckey SW, Petersen DR. Activation of Kupffer cells during the course of carbon tetrachloride–induced liver injury and fibrosis in rats. Exp Mol Pathol. 2001;71:226–40. doi: 10.1006/exmp.2001.2399. [DOI] [PubMed] [Google Scholar]
- 127.Gardner CR, Gray JP, Joseph LB, Cervelli J, Bremer N, et al. Potential role of caveolin-1 in acetaminophen-induced hepatotoxicity. Toxicol Appl Pharmacol. 2010;245:36–46. doi: 10.1016/j.taap.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Reilly TP, Brady JN, Marchick MR, Bourdi M, George JW, et al. A protective role for cyclooxygenase-2 in drug-induced liver injury in mice. Chem Res Toxicol. 2001;14:1620–28. doi: 10.1021/tx0155505. [DOI] [PubMed] [Google Scholar]
- 129.Begay CK, Gandolfi AJ. Late administration of COX-2 inhibitors minimize hepatic necrosis in chloroform-induced liver injury. Toxicology. 2003;185:79–87. doi: 10.1016/s0300-483x(02)00594-2. [DOI] [PubMed] [Google Scholar]
- 130.Horrillo R, Planaguma A, Gonzalez-Periz A, Ferre N, Titos E, et al. Comparative protection against liver inflammation and fibrosis by a selective cyclooxygenase-2 inhibitor and a nonredox-type 5-lipoxygenase inhibitor. J Pharmacol Exp Ther. 2007;323:778–86. doi: 10.1124/jpet.107.128264. [DOI] [PubMed] [Google Scholar]
- 131.Nakano H, Aizawa H, Matsumoto K, Fukuyama S, Inoue H, Hara N. Cyclooxygenase-2 participates in the late phase of airway hyperresponsiveness after ozone exposure in guinea pigs. Eur J Pharmacol. 2000;403:267–75. doi: 10.1016/s0014-2999(00)00524-0. [DOI] [PubMed] [Google Scholar]
- 132.Keppler D, Hagmann W, Rapp S, Denzlinger C, Koch HK. The relation of leukotrienes to liver injury. Hepatology. 1985;5:883–91. doi: 10.1002/hep.1840050530. [DOI] [PubMed] [Google Scholar]
- 133.Montuschi P. Leukotrienes, antileukotrienes and asthma. Mini Rev Med Chem. 2008;8:647–56. doi: 10.2174/138955708784567395. [DOI] [PubMed] [Google Scholar]
- 134.Titos E, Claria J, Planaguma A, Lopez-Parra M, Gonzalez-Periz A, et al. Inhibition of 5-lipoxygenase-activating protein abrogates experimental liver injury: role of Kupffer cells. J Leukoc Biol. 2005;78:871–78. doi: 10.1189/jlb.1204747. [DOI] [PubMed] [Google Scholar]
- 135.Shimbori C, Shiota N, Okunishi H. Involvement of leukotrienes in the pathogenesis of silica-induced pulmonary fibrosis in mice. Exp Lung Res. 2010;36:292–301. doi: 10.3109/01902140903585517. [DOI] [PubMed] [Google Scholar]
- 136.Shiratori Y, Tanaka M, Umihara J, Kawase T, Shiina S, Sugimoto T. Leukotriene inhibitors modulate hepatic injury induced by lipopolysaccharide-activated macrophages. J Hepatol. 1990;10:51–61. doi: 10.1016/0168-8278(90)90073-z. [DOI] [PubMed] [Google Scholar]
- 137.Ishizaka A, Hasegawa N, Sakamaki F, Tasaka S, Nakamura H, et al. Effects of ONO-1078, a peptide leukotriene antagonist, on endotoxin-induced acute lung injury. Am J Respir Crit Care Med. 1994;150:1325–31. doi: 10.1164/ajrccm.150.5.7952560. [DOI] [PubMed] [Google Scholar]
- 138.Stevens WH, Vanderheyden C, Wattie J, Lane CG, Smith W, O’Byrne PM. Effect of a leukotriene B4 receptor antagonist SC-53228 on ozone-induced airway hyperresponsiveness and inflammation in dogs. Am J Respir Crit Care Med. 1995;152:1443–48. doi: 10.1164/ajrccm.152.5.7582275. [DOI] [PubMed] [Google Scholar]
- 139.Failla M, Genovese T, Mazzon E, Gili E, Muia C, et al. Pharmacological inhibition of leukotrienes in an animal model of bleomycin-induced acute lung injury. Respir Res. 2006;7:137. doi: 10.1186/1465-9921-7-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Karidis NP, Kouraklis G, Theocharis SE. Platelet-activating factor in liver injury: a relational scope. World J Gastroenterol. 2006;12:3695–706. doi: 10.3748/wjg.v12.i23.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yost CC, Weyrich AS, Zimmerman GA. The platelet activating factor (PAF) signaling cascade in systemic inflammatory responses. Biochimie. 2010;92:692–97. doi: 10.1016/j.biochi.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gardner CR, Laskin JD, Laskin DL. Platelet-activating factor–induced calcium mobilization and oxidative metabolism in hepatic macrophages and endothelial cells. J Leukoc Biol. 1993;53:190–96. doi: 10.1002/jlb.53.2.190. [DOI] [PubMed] [Google Scholar]
- 143.Kaneko T, Ikeda H, Fu L, Nishiyama H, Okubo T. Platelet-activating factor mediates the ozone-induced increase in airway microvascular leakage in guinea pigs. Eur J Pharmacol. 1995;292:251–55. doi: 10.1016/0926-6917(95)90029-2. [DOI] [PubMed] [Google Scholar]
- 144.Tan WC, Bethel RA. The effect of platelet activating factor antagonist on ozone-induced airway inflammation and bronchial hyperresponsiveness in guinea pigs. Am Rev Respir Dis. 1992;146:916–22. doi: 10.1164/ajrccm/146.4.916. [DOI] [PubMed] [Google Scholar]
- 145.Pendino KJ, Gardner CR, Laskin JD, Laskin DL. Induction of functionally active platelet-activating factor receptors in rat alveolar macrophages. J Biol Chem. 1993;268:19165–68. [PubMed] [Google Scholar]
- 146.Rabinovici R, Esser KM, Lysko PG, Yue TL, Griswold DE, et al. Priming by platelet-activating factor of endotoxin-induced lung injury and cardiovascular shock. Circ Res. 1991;69:12–25. doi: 10.1161/01.res.69.1.12. [DOI] [PubMed] [Google Scholar]
- 147.Yue TL, Farhat M, Rabinovici R, Perera PY, Vogel SN, Feuerstein G. Protective effect of BN 50739, a new platelet-activating factor antagonist, in endotoxin-treated rabbits. J Pharmacol Exp Ther. 1990;254:976–81. [PubMed] [Google Scholar]
- 148.Yang YP, Ma XM, Wang CP, Han J, Lu YY, et al. Effect of increased hepatic platelet activating factor and its receptor portal hypertension in CCl4-induced liver cirrhosis. World J Gastroenterol. 2006;12:709–15. doi: 10.3748/wjg.v12.i5.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bradley JR. TNF-mediated inflammatory disease. J Pathol. 2008;214:149–60. doi: 10.1002/path.2287. [DOI] [PubMed] [Google Scholar]
- 150.Mukhopadhyay S, Hoidal JR, Mukherjee TK. Role of TNFα in pulmonary pathophysiology. Respir Res. 2006;7:125. doi: 10.1186/1465-9921-7-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chiu H, Gardner CR, Dambach DM, Durham SK, Brittingham JA, et al. Role of tumor necrosis factor receptor 1 (p55) in hepatocyte proliferation during acetaminophen-induced toxicity in mice. Toxicol Appl Pharmacol. 2003;193:218–27. doi: 10.1016/j.taap.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 152.Chiu H, Gardner CR, Dambach DM, Brittingham JA, Durham SK, et al. Role of p55 tumor necrosis factor receptor 1 in acetaminophen-induced antioxidant defense. Am J Physiol Gastrointest Liver Physiol. 2003;285:G959–66. doi: 10.1152/ajpgi.00219.2003. [DOI] [PubMed] [Google Scholar]
- 153.Hishinuma I, Nagakawa J, Hirota K, Miyamoto K, Tsukidate K, et al. Involvement of tumor necrosis factor-α in development of hepatic injury in galactosamine-sensitized mice. Hepatology. 1990;12:1187–91. doi: 10.1002/hep.1840120518. [DOI] [PubMed] [Google Scholar]
- 154.Kayama F, Yoshida T, Elwell MR, Luster MI. Role of tumor necrosis factor-α in cadmium-induced hepatotoxicity. Toxicol Appl Pharmacol. 1995;131:224–34. doi: 10.1006/taap.1995.1065. [DOI] [PubMed] [Google Scholar]
- 155.Perkins RC, Scheule RK, Hamilton R, Gomes G, Freidman G, Holian A. Human alveolar macrophage cytokine release in response to in vitro and in vivo asbestos exposure. Exp Lung Res. 1993;19:55–65. doi: 10.3109/01902149309071080. [DOI] [PubMed] [Google Scholar]
- 156.Zhang Y, Lee TC, Guillemin B, Yu MC, Rom WN. Enhanced IL-1β and tumor necrosis factor-α release and messenger RNA expression in macrophages from idiopathic pulmonary fibrosis or after asbestos exposure. J Immunol. 1993;150:4188–96. [PubMed] [Google Scholar]
- 157.Fakhrzadeh L, Laskin JD, Laskin DL. Regulation of caveolin-1 expression, nitric oxide production and tissue injury by tumor necrosis factor-α following ozone inhalation. Toxicol Appl Pharmacol. 2008;227:380–89. doi: 10.1016/j.taap.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Czaja MJ, Xu J, Alt E. Prevention of carbon tetrachloride–induced rat liver injury by soluble tumor necrosis factor receptor. Gastroenterology. 1995;108:1849–54. doi: 10.1016/0016-5085(95)90149-3. [DOI] [PubMed] [Google Scholar]
- 159.Piguet PF, Collart MA, Grau GE, Kapanci Y, Vassalli P. Tumor necrosis factor/cachectin plays a key role in bleomycin-induced pneumopathy and fibrosis. J Exp Med. 1989;170:655–63. doi: 10.1084/jem.170.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Piguet PF, Collart MA, Grau GE, Sappino AP, Vassalli P. Requirement of tumour necrosis factor for development of silica-induced pulmonary fibrosis. Nature. 1990;344:245–47. doi: 10.1038/344245a0. [DOI] [PubMed] [Google Scholar]
- 161.Kruglov AA, Kuchmiy A, Grivennikov SI, Tumanov AV, Kuprash DV, Nedospasov SA. Physiological functions of tumor necrosis factor and the consequences of its pathologic overexpression or blockade: mouse models. Cytokine Growth Factor Rev. 2008;19:231–44. doi: 10.1016/j.cytogfr.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 162.Zhang M, Qian J, Xing X, Kong FM, Zhao L, et al. Inhibition of the tumor necrosis factor-α pathway is radioprotective for the lung. Clin Cancer Res. 2008;14:1868–76. doi: 10.1158/1078-0432.CCR-07-1894. [DOI] [PubMed] [Google Scholar]
- 163.Gasse P, Mary C, Guenon I, Noulin N, Charron S, et al. IL-1R1/MyD88 signaling and the inflammasome are essential in pulmonary inflammation and fibrosis in mice. J Clin Investig. 2007;117:3786–99. doi: 10.1172/JCI32285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Churg A, Zhou S, Wang X, Wang R, Wright JL. The role of interleukin-1β in murine cigarette smoke–induced emphysema and small airway remodeling. Am J Respir Cell Mol Biol. 2009;40:482–90. doi: 10.1165/rcmb.2008-0038OC. [DOI] [PubMed] [Google Scholar]
- 165.Bless NM, Huber-Lang M, Guo RF, Warner RL, Schmal H, et al. Role of CC chemokines (macrophage inflammatory protein-1β, monocyte chemoattractant protein-1, RANTES) in acute lung injury in rats. J Immunol. 2000;164:2650–59. doi: 10.4049/jimmunol.164.5.2650. [DOI] [PubMed] [Google Scholar]
- 166.Bhatia M, Brady M, Zagorski J, Christmas SE, Campbell F, et al. Treatment with neutralising antibody against cytokine induced neutrophil chemoattractant (CINC) protects rats against acute pancreatitis associated lung injury. Gut. 2000;47:838–44. doi: 10.1136/gut.47.6.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Duffield JS. The inflammatory macrophage: a story of Jekyll and Hyde. Clin Sci. 2003;104:27–38. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 168.Benoit M, Desnues B, Mege JL. Macrophage polarization in bacterial infections. J Immunol. 2008;181:3733–39. doi: 10.4049/jimmunol.181.6.3733. [DOI] [PubMed] [Google Scholar]
- 169.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–61. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Scher JU, Pillinger MH. 15d-PGJ2: the anti-inflammatory prostaglandin? Clin Immunol. 2005;114:100–9. doi: 10.1016/j.clim.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 171.Zingarelli B, Cook JA. Peroxisome proliferator-activated receptor-γ is a new therapeutic target in sepsis and inflammation. Shock. 2005;23:393–99. doi: 10.1097/01.shk.0000160521.91363.88. [DOI] [PubMed] [Google Scholar]
- 172.Chiang N, Arita M, Serhan CN. Anti-inflammatory circuitry: lipoxin, aspirin-triggered lipoxins and their receptor ALX. Prostaglandins Leukot Essent Fatty Acids. 2005;73:163–77. doi: 10.1016/j.plefa.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 173.Levy BD. Lipoxins and lipoxin analogs in asthma. Prostaglandins Leukot Essent Fatty Acids. 2005;73:231–37. doi: 10.1016/j.plefa.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 174.Serhan CN, Chiang N. Endogenous pro-resolving and anti-inflammatory lipid mediators: a new pharmacologic genus. Br J Pharmacol. 2008;153(Suppl 1):S200–15. doi: 10.1038/sj.bjp.0707489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gonzalez-Periz A, Planaguma A, Gronert K, Miquel R, Lopez-Parra M, et al. Docosahexaenoic acid (DHA) blunts liver injury by conversion to protective lipid mediators: protectin D1 and 17S-hydroxy-DHA. FASEB J. 2006;20:2537–39. doi: 10.1096/fj.06-6250fje. [DOI] [PubMed] [Google Scholar]
- 176.Gowdy K, Krantz QT, Daniels M, Linak WP, Jaspers I, Gilmour MI. Modulation of pulmonary inflammatory responses and antimicrobial defenses in mice exposed to diesel exhaust. Toxicol Appl Pharmacol. 2008;229:310–19. doi: 10.1016/j.taap.2008.01.040. [DOI] [PubMed] [Google Scholar]
- 177.Bhandari V, Choo-Wing R, Homer RJ, Elias JA. Increased hyperoxia-induced mortality and acute lung injury in IL-13 null mice. J Immunol. 2007;178:4993–5000. doi: 10.4049/jimmunol.178.8.4993. [DOI] [PubMed] [Google Scholar]
- 178.Hocke AC, Ermert M, Althoff A, Brell B, N’Guessan PD, et al. Regulation of interleukin IL-4, IL-13, IL-10, and their downstream components in lipopolysaccharide-exposed rat lungs: comparison of the constitutive expression between rats and humans. Cytokine. 2006;33:199–211. doi: 10.1016/j.cyto.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 179.Pulichino AM, Wang IM, Caron A, Mortimer J, Auger A, et al. Identification of transforming growth factor β1–driven genetic programs of acute lung fibrosis. Am J Respir Cell Mol Biol. 2008;39:324–36. doi: 10.1165/rcmb.2007-0186OC. [DOI] [PubMed] [Google Scholar]
- 180.Rube CE, Uthe D, Schmid KW, Richter KD, Wessel J, et al. Dose-dependent induction of transforming growth factor β(TGF-β) in the lung tissue of fibrosis-prone mice after thoracic irradiation. Int J Radiat Oncol Biol Phys. 2000;47:1033–42. doi: 10.1016/s0360-3016(00)00482-x. [DOI] [PubMed] [Google Scholar]
- 181.Ide M, Kuwamura M, Kotani T, Sawamoto O, Yamate J. Effects of gadolinium chloride (GdCl3) on the appearance of macrophage populations and fibrogenesis in thioacetamide-induced rat hepatic lesions. J Comp Pathol. 2005;133:92–102. doi: 10.1016/j.jcpa.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 182.Rodriguez-Rivera A, Galicia-Moreno M, Reyes-Gordillo K, Segovia J, Vergara P, et al. Methyl palmitate prevents CCl4-induced liver fibrosis. J Appl Toxicol. 2008;28:1021–26. doi: 10.1002/jat.1368. [DOI] [PubMed] [Google Scholar]
- 183.Barbarin V, Arras M, Misson P, Delos M, McGarry B, et al. Characterization of the effect of interleukin-10 on silica-induced lung fibrosis in mice. Am J Respir Cell Mol Biol. 2004;31:78–85. doi: 10.1165/rcmb.2003-0299OC. [DOI] [PubMed] [Google Scholar]
- 184.Chen LC, Gordon RE, Laskin JD, Laskin DL. Role of TLR-4 in liver macrophage and endothelial cell responsiveness during acute endotoxemia. Exp Mol Pathol. 2007;83:311–26. doi: 10.1016/j.yexmp.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zhang X, Yu WP, Gao L, Wei KB, Ju JL, Xu JZ. Effects of lipopolysaccharides stimulated Kupffer cells on activation of rat hepatic stellate cells. World J Gastroenterol. 2004;10:610–13. doi: 10.3748/wjg.v10.i4.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Nishimura Y, Nishiike-Wada T, Wada Y, Miura Y, Otsuki T, Iguchi H. Long-lasting production of TGF-β1 by alveolar macrophages exposed to low doses of asbestos without apoptosis. Int J Immunopathol Pharmacol. 2007;20:661–71. doi: 10.1177/039463200702000402. [DOI] [PubMed] [Google Scholar]