Atherosclerosis is a lipoprotein disorder with inflammatory, vascular and hemodynamic determinants. Total white blood cell count and sub-populations including granulocytes, monocytes, T cells and bone marrow derived precursors have been implicated in atherosclerotic cardiovascular disease (CVD)1, 2. Although these associations may inform biological processes, they have not, to date, permitted specific mechanistic understanding of subsets of leukocytes involved, nor have they advanced risk prediction and therapeutic targeting or monitoring in clinic. Although short lived in circulation, blood monocytes are proposed to play a specific role in atherosclerosis because (1) blood monocytes are the major source of tissue macrophages, (2) monocyte derived macrophages and dendritic cells are the predominant leukocytes in atherosclerotic lesion, and (3) macrophages/dendritic cells are have a variety of important pro-inflammatory (inflammatory recruitment/activation of other leukocytes and vascular cells, foam cell formation and lipid accumulation, fibrous cap erosion and plaque rupture) and anti-inflammatory (phagocytosis, efferocytosis, and leukocyte egress) actions in experimental atherosclerotic lesions1, 3, 4. Despite these mechanistic insights from human pathology and experimental studies in rodents, there have been disparate findings in studies of total monocyte counts and CVD5, 6.
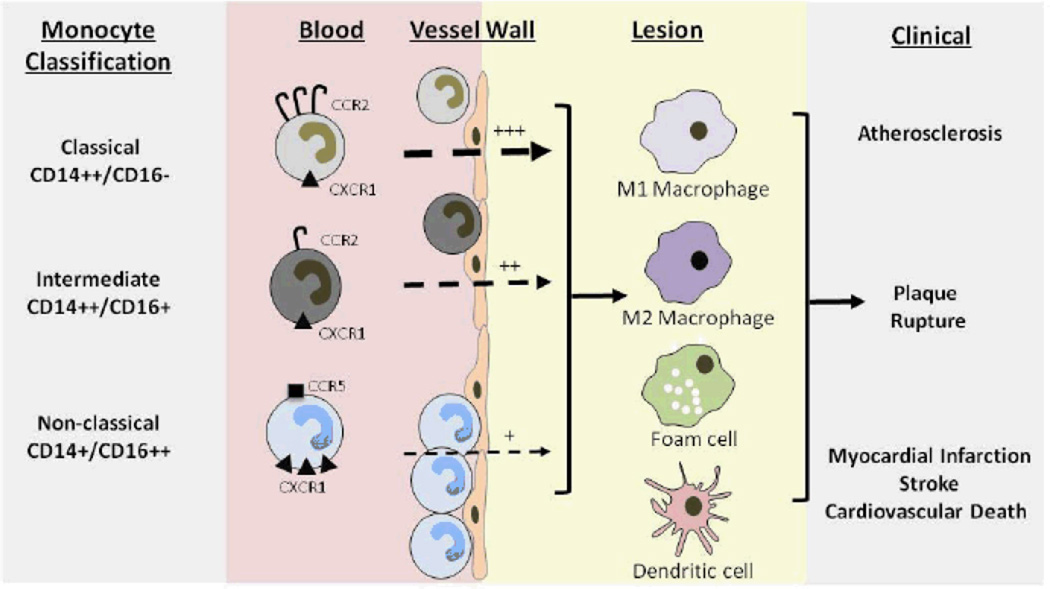
There are several challenges to our understanding of the role of monocytes in atherosclerosis7, 8. First, circulating monocytes are heterogeneous due to sub-type differentiation in bone marrow. The regulation and rate of sub-population recruitment as well as their roles in atherosclerosis are uncertain. Second, monocyte sub-populations are typically defined by differential expression of specific cell surface antigens including receptors that modulate their function e.g., chemokine receptors chemokine (C-C motif) receptor 2 (CCR2), CX3C chemokine receptor 1 (CX3CR1) and C-C chemokine receptor type 5(CCR5), as well as adhesion molecule integrin receptors Very Late Antigen-4 (VLA-4, also called integrin α4β1) and LFA-1(integrin αLβ2)7, 8. Markers of monocyte subpopulations have evolved with time and differ between human and mouse leading to a dynamic and sometimes confusing classifications as well as uncertainty in directly comparing human and mouse studies. Third, the plasticity of monocytes is well documented with differentiation of monocytes to macrophage sub-types or dendritic cells depending on local tissue cues which may differ in atherosclerosis at various stages of disease. Finally, the fate and survival of specific monocyte sub-populations after entry into plaque is poorly understood. Which monocytes and how differentiate to inflammatory macrophages, foam cell or dendritic cells is poorly understood. Indeed, the relative contribution of blood monocytes versus expansion of local macrophages and dendritic cells to these lesion cells is also uncertain. Thus, monocyte heterogeneity, plasticity and fate represent significant challenges to ascribing roles within lesions to specific monocyte sub-populations (Figure 1).
Figure.
Monocyte subsets in atherosclerosis. Human monocytes are classified as classical (CD14++CD16-; high CCR2 and low CX3CR1 expression), intermediate (CD14++CD16+), and non-classical (CD14+CD16++; no CCR2 and high CX3CR1 expression). Classical CD14++CD16- monocytes are increased by high fat diet and are rapidly recruited to atherosclerosis (via CCR2 and CX3CR1) whereas non classical CD14+CD16++ patrol the endothelium and are more slowly recruited to lesions (via CX3CR1 and CCR5). Whether specific monocyte subsets preferentially differentiate to macrophage subtypes, foam cells or dendritic cells is uncertain. Thus their specific roles in atherosclerosis progression, lesion stability and plaque rupture, and ultimately clinical events are uncertain. Berg et al19 report that the classical CD14++CD16- subset are independent predictors of incident cardiovascular events
Recent classifications of monocytes (classical, intermediate, and non-classical)8 strive to use morphology and immunomarker profiles without inferring function but much literature has ascribed, perhaps incorrectly, specific “pro-inflammatory” or “anti-inflammatory” functions to monocyte subsets. Human monocytes have been defined on the basis of morphology, cytochemistry and, more recently, by flow cytometry particularly using antibodies to cell-surface markers such as CD14 (endotoxin co-receptor) and CD16 (FcγIII receptor). This technology enables the identification of monocyte subsets and their classification, based on differential expression of CD14 and CD16, as classical (CD14++CD16-; cells with high CCR2 and low CX3CR1 expression), intermediate (CD14++CD16+cells), and non-classical (CD14+CD16++; cells with no CCR2 and high CX3CR1 expression). The latter two subsets are sometimes combined as CD16+ monocytes and account for 10–20% of all circulating monocytes. Although different cell-surface markers are required, a parallel classification of mouse monocytes based on LyC6 and CD43 expression with Ly6C++CD43+ considered equivalent to CD14++CD16- and Ly6C+CD43++ thought to be equivalent to CD14+CD16++.
CD16+ monocytes have been designated “pro-inflammatory” in some literature because they are efficient producers of inflammatory cytokines whereas they secrete little anti-inflammatory interleukin (IL)-109. Further, CD16+ monocyte counts are elevated in numerous inflammatory conditions such as sepsis, asthma and inflammatory bowel disease, and are reported as associated with atherosclerosis as well as CVD and stroke10–13. Indeed, many properties support the relevance of CD16+ monocytes in atherosclerosis and CVD7, 8: (1) a high affinity for the endothelium due to surface expression of chemokine receptors and adhesion molecules such as CX3CR1, CCR5 and VLA-4; (2) residence in the marginal pool where they can be rapidly mobilized; (3) homing to endothelium in a CX3CR1 dependent manner and ability to recruit T-lymphocytes and additional monocytes; and (4) the relevance of CX3CL1 and CX3CR1 in atherosclerosis14–16. As noted, however, recent classifications tend to avoid ascribing specific pro- or anti-inflammatory function to subsets because properties defined in vitro or even in vivo do not necessarily reflect their actions and functions in atherosclerosis and disease. For example, classical monocytes (CD14++CD16-) respond acutely to inflammation and infection, are increased substantially by high fat diet17, express high levels of CCR2 (implicated in both human and mouse atherosclerosis), also express low levels of functional CX3CR118, and in rodent are more rapidly recruited to atherosclerotic lesions than the Ly6C+CD43++ (human CD14+CD16++) subpopulation. Further, blood levels of non-classical monocytes (CD14+CD16++, Ly6C+CD43++ in mouse) are less responsive to high-fat diet and have been ascribed “patrolling”, “resident” or “homeostatic” actions in rodents7. It is not surprising given these challenges that convincing data implicating specific monocyte sub-populations in human CVD is lacking. To date, small studies support a role for CD16+ monocytes in human atherosclerosis and CVD10–13, but there has been an absence of data indicating that the classical CD16- monocytes contribute to CVD.
Now, a large prospective epidemiological study demonstrates for the first time that classical CD14++CD16- monocytes predict incident cardiovascular events independently of other risk factors. Berg and colleagues19 randomly selected a nested case (n=123)-control (n=536) sample from the cardiovascular arm of the large Malmo Diet and Cancer study and utilized mononuclear leukocytes frozen at baseline to enumerate monocyte subsets based on CD14 and CD16 expression at flow cytometry. They found that the percentage and number of classical CD14++CD16- monocytes were increased at baseline in the group that developed cardiovascular-events compared to those without an event, e.g., hazard ratio of 1.66 after adjusting for traditional risk factors for the top tertile of CD14++CD16- monocyte numbers. Notably, however, the CD14++CD16- monocyte subset did not associate with the extent of carotid atherosclerosis measured by intimal-media thickness (IMT) at baseline. Surprisingly, although CD16+ monocytes were not significant predictors of CVD events after adjusting for risk factors, the percentage of CD14++CD16+ monocytes was inversely associated with baseline carotid IMT Finally, they also found that there was no difference in CCR2, CX3CR1 and CCR5 chemokine receptor expression intensity on any of the monocyte subsets between incident cases and controls.
There are several strengths of this paper. A relatively large sample, nested in a prospective cohort study which included both CVD outcomes and a measure of atherosclerosis burden was utilized. Furthermore, lab techniques were robust, including use of multiple controls with rigorous quality checks of monocyte markers and receptor expression by flow cytometry, as well as experiments to validate use of frozen materials. The statistical analytical approach considered multiple confounders of CVD including traditional risk factors and circulating CRP. Finally, the combination of carotid IMT and CVD event data within the same study population is a unique strength raising novel hypotheses. However, there are several weaknesses and unanswered questions. First, the study sample may have been underpowered. The effect sizes for the number of CD14++CD16+ (1.66, 95% confidence interval 1.02 to 2.72), CD16+ (1.44; 0.87 to 2.39) and total monocytes (1.61, 0.98 to 2.66) on CVD events were very similar raising concern for power to detect and ascribe unique risk to specific monocyte subsets, particularly less abundant CD16+ monocytes. Further, the association of percent, but not number, of CD16+ cells with carotid IMT may have arisen due to statistical artifact and requires replication. Combining data for intermediate and non-classical CD16+ cells may not be supported by their functional roles in vivo. The lack of association with CVD events of CCR2, CX3CR1 and CCR5 expression on monocyte sub-populations raises specific concerns. Last, the heterogeneity of clinical outcomes (MI, stroke, and CVD death) without subgroup data limits interpretation of these associations. Overall, however, these prospective data begin to clarify a complex question and provide the foundation for addressing unanswered hypotheses relating to monocyte subsets in atherosclerotic CVD.
What are the biological implications of the study? First, these novel clinical data suggest that classical CD14+CD16- monocytes play a causal role in human CVD. Conflicting findings for CD16+ relative to prior work10–13 might point to modulation of monocyte functions in established renal and vascular disease states although they may also reflect study design differences and biases. The lack of association between monocyte CCR2, CX3CR1and CCR5 expression with CVD outcomes raises important questions: does this represent true biology or is this a technical artifact due to lack of sensitivity of the antibodies for the receptors? Are there other chemokines/receptors and cytokines/effectors of greater importance for monocyte function in clinical CVD? The apparent distinct associations with clinical events vs. IMT for CD16- vs. CD16+ raise intriguing questions regarding the role of specific monocytes in progression of atherosclerosis vs. distinct roles in plaque rupture and clinical complications. This pattern might relate to differential functions of specific chemokines in chronic atherosclerosis relative to acute CVD, e.g., CXCL12 may relate positively to carotid IMT20 but inversely to acute unstable angina21.
Although provocative, the findings of Berg et al19 have limited clinical applications. For clinical prediction, larger studies are required that replicate and extend these findings and to establish monocyte sub-types as independent predictors of CVD events and in reclassification and discrimination of those at risk. Future studies particularly of novel therapies may establish value for monocyte sub-population targeting in directly modulating cells that cause CVD or as biomarkers of efficacy of anti-inflammatory therapeutics in CVD. Basic and translational progress are required to better define monocyte subsets (within and across species), their relationship to macrophage, foam cells and dendritic cells in lesions, and their specific functions in atherosclerosis and its clinical complications. Future clinical studies of monocyte subsets in CVD should focus on very large prospective samples with homogeneous outcomes, subgroup analyses and multiple measures of subclinical atherosclerosis. These studies should examine each monocyte sub-type separately, apply novel markers as available, and compare findings to those for total monocytes, neutrophils, T-cells, bone marrow progenitors as well as inflammatory biomarkers and traditional risk factors. Integration with human genetic data and pathway analyses should provide the greatest opportunity for mechanistic insight in teasing out the effect of specific monocyte subsets and their molecular effectors in human atherosclerotic CVD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures: None
References
- 1.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12:204–212. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 2.Weber C, Zernecke A, Libby P. The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models. Nat Rev Immunol. 2008;8:802–815. doi: 10.1038/nri2415. [DOI] [PubMed] [Google Scholar]
- 3.Feig JE, Pineda-Torra I, Sanson M, Bradley MN, Vengrenyuk Y, Bogunovic D, et al. LXR promotes the maximal egress of monocyte-derived cells from mouse aortic plaques during atherosclerosis regression. J Clin Invest. 2010;120:4415–4424. doi: 10.1172/JCI38911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thorp E, Tabas I. Mechanisms and consequences of efferocytosis in advanced atherosclerosis. J Leukoc Biol. 2009;86:1089–1095. doi: 10.1189/jlb.0209115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnsen SH, Fosse E, Joakimsen O, Mathiesen EB, Stensland-Bugge E, Njolstad I, et al. Monocyte count is a predictor of novel plaque formation: a 7-year follow-up study of 2610 persons without carotid plaque at baseline the Tromso Study. Stroke. 2005;36:715–719. doi: 10.1161/01.STR.0000158909.07634.83. [DOI] [PubMed] [Google Scholar]
- 6.Grau AJ, Boddy AW, Dukovic DA, Buggle F, Lichy C, Brandt T, et al. Leukocyte count as an independent predictor of recurrent ischemic events. Stroke. 2004;35:1147–1152. doi: 10.1161/01.STR.0000124122.71702.64. [DOI] [PubMed] [Google Scholar]
- 7.Woollard KJ, Geissmann F. Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol. 2010;7:77–86. doi: 10.1038/nrcardio.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, et al. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116:e74–e80. doi: 10.1182/blood-2010-02-258558. [DOI] [PubMed] [Google Scholar]
- 9.Ziegler-Heitbrock L. The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J Leukoc Biol. 2007;81:584–592. doi: 10.1189/jlb.0806510. [DOI] [PubMed] [Google Scholar]
- 10.Rogacev KS, Ulrich C, Blomer L, Hornof F, Oster K, Ziegelin M, et al. Monocyte heterogeneity in obesity and subclinical atherosclerosis. Eur Heart J. 2010;31:369–376. doi: 10.1093/eurheartj/ehp308. [DOI] [PubMed] [Google Scholar]
- 11.Heine GH, Ulrich C, Seibert E, Seiler S, Marell J, Reichart B, et al. CD14(++)CD16+monocytes but not total monocyte numbers predict cardiovascular events in dialysis patients. Kidney Int. 2008;73:622–629. doi: 10.1038/sj.ki.5002744. [DOI] [PubMed] [Google Scholar]
- 12.Rogacev KS, Seiler S, Zawada AM, Reichart B, Herath E, Roth D, et al. CD14++CD16+monocytes and cardiovascular outcome in patients with chronic kidney disease. Eur Heart J. 2011;32:84–92. doi: 10.1093/eurheartj/ehq371. [DOI] [PubMed] [Google Scholar]
- 13.Urra X, Villamor N, Amaro S, Gomez-Choco M, Obach V, Oleaga L, et al. Monocyte subtypes predict clinical course and prognosis in human stroke. J Cereb Blood Flow Metab. 2009;29:994–1002. doi: 10.1038/jcbfm.2009.25. [DOI] [PubMed] [Google Scholar]
- 14.Combadiere C, Potteaux S, Rodero M, Simon T, Pezard A, Esposito B, et al. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6C(hi) and Ly6C(lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation. 2008;117:1649–1657. doi: 10.1161/CIRCULATIONAHA.107.745091. [DOI] [PubMed] [Google Scholar]
- 15.Saederup N, Chan L, Lira SA, Charo IF. Fractalkine deficiency markedly reduces macrophage accumulation and atherosclerotic lesion formation in CCR2𢈒/− mice: evidence for independent chemokine functions in atherogenesis. Circulation. 2008;117:1642–1648. doi: 10.1161/CIRCULATIONAHA.107.743872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, et al. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landsman L, Bar-On L, Zernecke A, Kim KW, Krauthgamer R, Shagdarsuren E, et al. CX3CR1 is required for monocyte homeostasis and atherogenesis by promoting cell survival. Blood. 2009;113:963–972. doi: 10.1182/blood-2008-07-170787. [DOI] [PubMed] [Google Scholar]
- 19.Berg KE, Ljungcrantz I, Andersson L, Bryngelsson C, Hedblad B, Fredrikson GN, et al. Elevated CD14++CD16− Monocytes Predict Cardiovascular Events. Circ Cardiovasc Genet. 2012;5 doi: 10.1161/CIRCGENETICS.111.960385. XXX-XXX. [DOI] [PubMed] [Google Scholar]
- 20.Kiechl S, Laxton RC, Xiao Q, Hernesniemi JA, Raitakari OT, Kahonen M, et al. Coronary artery disease-related genetic variant on chromosome 10q11 is associated with carotid intima-media thickness and atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30:2678–2683. doi: 10.1161/ATVBAHA.110.213785. [DOI] [PubMed] [Google Scholar]
- 21.Damas JK, Waehre T, Yndestad A, Ueland T, Muller F, Eiken HG, et al. Stromal cell-derived factor-1alpha in unstable angina: potential antiinflammatory and matrix-stabilizing effects. Circulation. 2002;106:36–42. doi: 10.1161/01.cir.0000020001.09990.90. [DOI] [PubMed] [Google Scholar]