Abstract
Objective
To assess the survival impact of initial disease distribution on patients with stage III epithelial ovarian cancer (EOC) cytoreduced to microscopic residual.
Methods
We reviewed data from 417 stage III EOC patients cytoreduced to microscopic disease and given adjuvant intravenous platinum/paclitaxel on one of three randomized Gynecologic Oncology Group (GOG) trials. We subdivided patients into three groups based on preoperative disease burden: (1) minimal disease (MD) defined by pelvic tumor and retroperitoneal metastasis (2) abdominal peritoneal disease (APD) with disease limited to the pelvis, retroperitoneum, lower abdomen and omentum; and (3) upper abdominal disease (UAD) with disease affecting the diaphragm, spleen, liver or pancreas. We assessed the survival impact of potential prognostic factors, focusing on initial disease distribution using a proportional hazards model and estimated Kaplan–Meier survival curves.
Results
The study groups had similar clinicopathologic characteristics. Median overall survival (OS) was not reached in MD patients compared to 80 and 56 months in the APD and UAD groups (P < 0.05). The five-year survival percentages for MD, APD, and UAD were 67%, 63%, and 45%. In multivariate analysis, the UAD group had a significantly worse prognosis than MD and APD both individually and combined (Progression Free Survival (PFS) Hazards Ratio (HR) 1.44; P = 0.008 and OS HR 1.77; P = 0.0004 compared to MD + APD).
Conclusion
Stage III EOC patients with initial disease in the upper abdomen have a worse prognosis despite cytoreductive surgery to microscopic residual implying that factors beyond cytoreductive effort are important in predicting survival.
Keywords: Epithelial ovarian cancer, Microscopic residual, Cytoreduction
Introduction
The American Cancer Society (Atlanta, Georgia) estimates that there will be 13,850 ovarian cancer disease-related deaths in 2010, which is more than all other female genital cancers combined [1]. Since the 1970s, multiple studies demonstrate that surgical reduction of bulky disease improves survival [2–4]. Because disease residual after primary surgery is one of the only modifiable prognostic factors and inversely related to prognosis, investigators have attempted to identify a threshold of “optimally” debulked disease [5]. The Gynecologic Oncology Group (GOG) progressively reduced their definition from ≤ 3 cm in greatest diameter in the 1970s to the more recent standard of ≤ 1 cm [6]. Even with these standards, evidence indicates that complete resection of disease results in the best prognosis [7]. In the past decade, an increasing number of groups extended the traditional boundaries of gynecologic oncology surgery and through extensive or radical resections, rendered many previously “unresectable” patients free of macroscopic disease [5,8,9]. With institutional outcomes in such patients approaching that of patients with less extensive disease, many now regard “optimal” as microscopic residual disease only [10–12].
Additional factors, intrinsic to both the patient and the tumor may significantly impact prognosis. A recent review of the GOG experience found that age, performance status, and tumor histology independently impacted prognosis in stage III ovarian cancer [7]. Other data suggest that particularly aggressive tumors may manifest as extensive disease or carcinomatosis [13,14]. Many of these factors are collectively referred to as aggressive, adverse, or bad tumor biology [15].
Controversy remains whether preoperative extent of disease, in addition to being a greater surgical challenge, is a marker for aggressive tumor biology. The extension of this argument fundamentally questions the relative importance of biology versus surgery [15]. Practical, ethical, and scientific considerations preclude conducting a definitive prospective randomized trial to directly address the benefit of cytoreductive surgery and therefore investigators can only draw limited conclusions based on retrospective experiences and smaller institutional trials. As such, many of the questions of biology versus surgery remain unanswered. In this study, we probed one aspect of the above controversy. Specifically, our study addresses whether a patient with extensive upper abdominal disease who is cytoreduced to microscopic residual will achieve similar outcomes as a patient with advanced, but more limited initial disease burden.
Patients and methods
We reviewed data from International Federation of Gynecology and Obstetrics (FIGO) stage III epithelial ovarian cancer (EOC) patients who participated in one of three prospective randomized GOG chemotherapy protocols (GOG protocol 114, 158, and 172) conducted between 1992 and 2001. All patients included in these protocols were diagnosed with primary, histologically confirmed, epithelial ovarian or primary peritoneal cancer with no residual mass greater than 1 cm after staging laparotomy. Investigators then initiated platinum and paclitaxel based chemotherapy per specific protocol. The primary endpoints in each of the studies were progression free (PFS) and overall survival (OS). Further details of eligibility criteria and results for the original studies have been published previously [16–18].
With the exception of the experimental arm of GOG 158, patients included in this reanalysis were treated with primary surgical cytoreduction removing all macroscopic disease followed by six cycles of a 24-hour infusion of intravenous paclitaxel (135 mg/m2) followed by intravenous cisplatin (75 mg/m2), the control arm of the three trials. We included patients with microscopic residual from the experimental arm of GOG 158 because this trial demonstrated statistically equivalent clinical outcomes between the control and experimental arms (Table 1). Patients with primary peritoneal cancer were excluded. From GOG databases, we abstracted patient and tumor characteristics including age, race, performance status, tumor grade, and histology. Patient charts were then individually reviewed to specifically annotate surgical procedures and intraoperative extent of disease prior to debulking based on 56 anatomic locations specified in GOG surgical reporting forms and diagrams. Data were verified using operative notes and pathology reports. When discrepancies occurred, the latter were accepted in nearly all cases.
Table 1.
GOG protocols and study population.
| GOG protocol no. | Original study eligibility | Current study eligibility | Treatment regimen | No. of patients eligible |
|---|---|---|---|---|
| 114 | Optimal (≤ 1-cm residual), stage III EOC | Microscopic residual, stage III EOC | IV paclitaxel 135 mg/m2, cisplatin 75 mg/m2, × six cycles | 81 |
| 158 | Optimal (≤ 1-cm residual), stage III EOC | Microscopic residual, stage III EOC | IV paclitaxel 135 mg/m2, cisplatin 75 mg/m2, × six cycles or IV paclitaxel 175 mg/m2(3 h), carboplatin AUC 7.5, × six cycles | 281 |
| 172 | Optimal (≤ 1-cm residual), stage III EOC/PPC | Microscopic residual, stage III EOC | IV paclitaxel 135 mg/m2, cisplatin 75 mg/m2, × six cycles | 75 |
Abbreviations: GOG, Gynecologic Oncology Group; EOC, epithelial ovarian cancer; IV, intravenously; AUC, area under the curve; PPC, primary peritoneal carcinoma.
We divided all patients meeting our inclusion criteria into three groups based on preoperative extent of disease. The minimal disease (MD) group (n = 59) had tumor limited to the pelvis and retroperitoneal (nodal) metastasis. The abdominal peritoneal disease (APD) group (n = 260) had disease limited to the pelvis and abdomen but excluding the liver, spleen, gallbladder, pancreas, or diaphragm, with or without retroperitoneal spread. The upper abdominal disease (UAD) group (n = 98) had disease affecting the pelvis with or without lower abdominal and retroperitoneal disease, plus involvement of at least one of the following: liver, spleen, gallbladder, pancreas, or diaphragm. Baseline performance status (PS) before initiating chemotherapy was defined according to GOG criteria as 0 for normal activity, 1 for symptomatic and fully ambulatory, and 2 for symptomatic and in bed less than 50% of the time.
OS was calculated from the date of randomization in the initial protocol to either death or date of last contact. PFS was calculated from the time of randomization to progression, death, or date of last contact. PFS and OS curves were generated using the Kaplan–Meier methods and compared using the log-rank test [19,20]. Variables significant in univariate analysis were subsequently entered into a multivariate analysis using a Cox proportional hazards model [21].
Results
The GOG assessed 1669 stage III patients on protocols 114, 158, and 172. 1229 patients received intravenous platinum and paclitaxel chemotherapy. Of these, 429 patients had microscopic residual disease and received at least one cycle of adjuvant chemotherapy. Twelve patients had primary peritoneal disease sparing the pelvis or no initial pelvic disease on further audit. After excluding these patients, 417 remained for this analysis. Table 2 shows the clinical and pathologic characteristics of these patients stratified by initial distribution of disease. The three study groups were balanced for prognostic factors including age, race, performance status, tumor grade, and histology. Investigators from the GOG staged and cytoreduced patients per protocol. Surgeons performed radical hysterectomies or radical pelvic resections and/or large-bowel resections (P < 0.05) more commonly on patients with UAD. They were more likely to perform lymph node dissections in MD patients (P < 0.05) (Table S1).
Table 2.
Patient characteristics by group.
| MD | APD | UAD | P | |
|---|---|---|---|---|
|
|
|
|
||
| n = 59 | n = 260 | n = 98 | ||
| Age group (years) | 0.676 | |||
| < 50 | 20 (33.9) | 82 (31.5) | 37 (37.8) | |
| 50–59 | 14 (23.7) | 63 (24.2) | 25 (25.1) | |
| 60–69 | 26 (27.1) | 62 (23.9) | 24 (24.5) | |
| ≥ 70 | 9 (15.3) | 53 (20.4) | 12 (12.3) | |
| Median (range) | 55 (34–76) | 57.5 (20–86) | 54 (29–85) | |
| Race | 0.31 | |||
| White | 51 (86.4) | 218 (83.9) | 90 (91.8) | |
| Black | 4 (6.8) | 20 (7.7) | 2 (2.0) | |
| Other | 4 (638) | 22 (8.5) | 6 (6.1) | |
| Performance status | 0.11 | |||
| 0 | 36 (61.0) | 117 (45.0) | 40 (40.8) | |
| 1 | 20 (33.9) | 130 (50.0) | 50 (51.0) | |
| 2 | 3 (5.1) | 13 (5.0) | 8 (8.2) | |
| Tumor grade | 0.143 | |||
| 1 | 3 (5.1) | 40 (15.4) | 17 (17.4) | |
| 2 | 16 (27.1) | 82 (31.5) | 31 (31.6) | |
| 3 | 40 (67.8) | 138 (53.1) | 50 (51.0) | |
| Histology | 0.07 | |||
| 1. Serous | 33 (55.9) | 161 (61.2) | 71 (72.5) | |
| 2. Endometrioid | 6 (10.2) | 29 (11.2) | 8 (8.2) | |
| 3. Clear cell | 10 (17.0) | 14 (5.4) | 5 (5.1) | |
| 4. Mucinous | 1 (1.7) | 12 (4.6) | 3 (3.1) | |
| 5. Mixed epithelial | 3 (5.1) | 25 (9.6) | 7 (7.1) | |
| 6. Other | 6 (10.2) | 19 (7.3) | 4 (4.1) |
Abbreviations: MD, minimal disease; APD, abdominal peritoneal disease; UAD, upper abdominal disease.
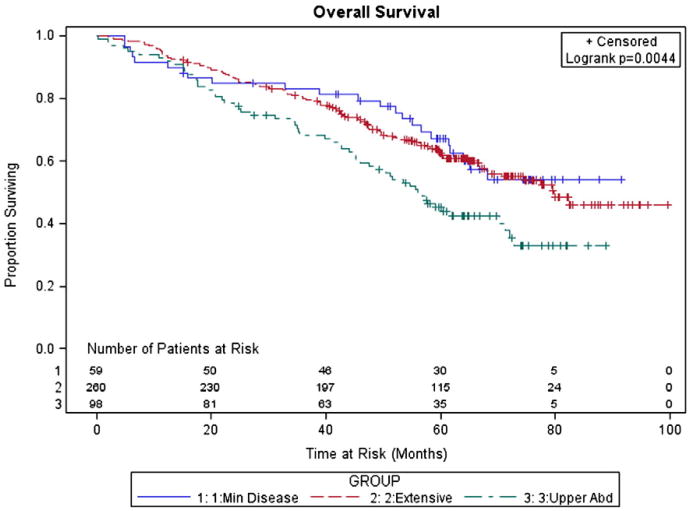
With a median patient follow-up of 64 months (range: 15–100 months), there were 258 recurrences and 188 deaths. The median PFS was 34 months and the median OS was 73 months. Table 3 shows median PFS and OS based on patient characteristics. In this univariate analysis, age less than fifty years old was associated with improved OS while better performance status correlated with improved PFS and OS (P < 0.05). Patients with endometrioid histology had improved outcomes compared with those with serous tumors, while mucinous and clear cell tumors had decreased survival (P < 0.05). Patients with minimal stage III disease as defined by this study had a median PFS of 48 months with OS not reached, compared to PFS of 35 months and OS of 80 months in those with abdominal peritoneal disease sparing the upper abdomen. When disease initially involved the diaphragm, liver, spleen, pancreas, or gallbladder, median PFS and OS were only 24 and 56 months respectively (P < 0.05). The 5-year survival percentages for MD, APD, and UAD were 67%, 63%, and 45% (Figs. 1 and 2). Race and grade were not significantly associated with PFS or OS.
Table 3.
Progression free and overall survival by patient characteristics.
| Characteristic | No. of patients | PFS | OS | ||
|---|---|---|---|---|---|
|
|
|
||||
| Median (months) | P | Median (months) | P | ||
| Age group, years | |||||
| < 50 | 139 | 39.3 | 0.0843 | N/A | 0.0004 |
| 50–59 | 102 | 29.5 | 70.3 | ||
| 60–69 | 102 | 34.2 | N/A | ||
| 70+ | 74 | 25.3 | 52.2 | ||
| Race | |||||
| White | 359 | 33.1 | 0.6665 | 71.2 | 0.1964 |
| Black | 26 | 27.2 | 55.1 | ||
| Other | 32 | 39.3 | 82.4 | ||
| GOG performance status | |||||
| 0 | 193 | 43.3 | 0.0037 | N/A | 0.0002 |
| 1 | 200 | 29.0 | 64 | ||
| 2 | 24 | 27.4 | 55.1 | ||
| Histology | |||||
| Serous | 265 | 32.9 | 0.0376 | 71.2 | < .0001 |
| Endometrioid | 43 | 37.2 | N/A | ||
| Clear cell | 29 | 26.2 | 43.3 | ||
| Mucinous | 16 | 12.9 | 19.6 | ||
| Mixed epithelial | 35 | 56.7 | 79.8 | ||
| Other | 29 | 40.4 | 65.1 | ||
| Tumor grade | |||||
| 1 | 60 | 54.4 | 0.1311 | N/A | 0.2444 |
| 2 | 129 | 32.1 | 66.9 | ||
| 3 | 228 | 31.9 | 70.3 | ||
| Initial disease distribution | |||||
| MD | 59 | 47.6 | 0.0139 | N/A | 0.0044 |
| APD | 260 | 34.8 | 79.8 | ||
| UAD | 98 | 23.9 | 56.1 | ||
Abbreviations: PFS, progression free survival; OS, overall survival; GOG, Gynecologic Oncology Group; MD, minimal disease; APD, abdominal peritoneal disease; UAD, upper abdominal disease.
Fig. 1.
Kaplan–Meier estimate of progression free survival (PFS).
Fig. 2.
Kaplan–Meier estimate of overall survival (OS).
All variables considered as potential prognostic factors (age, GOG performance status, histology, and initial disease distribution) were included in a Cox proportional hazards regression model to identify independent prognostic factors. Compared to the MD (reference) group, the UAD patients had significantly decreased PFS (HR = 1.79, P = 0.007) and OS (HR = 1.97, P = 0.0081) (Table 4). We performed a further pairwise analysis of the UAD group compared to reference groups MD and APD both individually and combined. Tests of significance were computed using the log-rank test from Kaplan–Meier methods and then Hazard Ratios controlling for age, GOG performance status and histology were computed and tested for significance. In all comparisons, the UAD group had a significantly worse prognosis (Table S2).
Table 4.
Multivariate analysis of disease distribution.
| Prognostic factor | PFS | OS | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Events/patients | HR | 95% CI | P | Events/patients | HR | 95% CI | P | |
| MD | 33/59 | Ref | 23/59 | Ref | ||||
| APD | 170/260 | 1.34 | 0.92 to 1.97 | 0.133 | 107/260 | 1.17 | 0.74 to 1.87 | 0.50 |
| UAD | 74/98 | 1.85 | 1.21 to 2.82 | 0.004 | 58/98 | 2.03 | 1.24 to 3.35 | 0.005 |
Abbreviations: PFS, progression free survival; OS, overall survival; MD, minimal disease; APD, abdominal peritoneal disease; UAD, upper abdominal disease.
Discussion
Based on this analysis, stage III EOC patients with disease involving the upper abdomen that were debulked to microscopic residual had a poorer prognosis than similarly staged patients whose initial disease burden did not involve the upper abdomen. These findings suggest that upper abdominal disease may be one of several factors indicating a biologically less favorable patient subgroup and extensive surgery does not render a prognosis equivalent to more consolidated disease completely cytoreduced.
These findings mirror those of Hoskins et al. in their review of GOG Protocol 52. In this study, patients with large-volume extra-pelvic disease who were cytoreduced to “optimal” 1 cm or less residual were compared to patients who initially had extra-pelvic disease of 1 cm or less. The volume of initial extra-pelvic disease remained a significant predictor of decreased survival failing to prove the hypothesis that initial cytoreductive surgery would result in equal survival between someone with large-volume disease rendered optimal versus someone with initial small-volume disease. This study did not address if further cytoreduction to no gross visible disease could overcome these other factors important in predicting survival and it is important to note that 40.5% of patients in the small-volume group had complete gross resection compared to only 17.1% in the large-volume group [14].
Similarly Crawford et al. evaluated the impact of surgery in the Scottish Randomized Trial in Ovarian Cancer (SCOTROC), a large, international prospective, randomized trial comparing docetaxel–carboplatin with paclitaxel–carboplatin as first-line chemotherapy for stage IC to IV epithelial ovarian cancer. The authors computed a prognostic score based on stage, histology, CA-125, and presence of an omental cake. They divided the study group into quartiles based on this score and found that debulking (to ≤ 2 cm) had much less impact on PFS in patients with greater extent of disease before surgery [13]. In response to valid concerns regarding the conclusions of this study [22,23], the authors performed a subsequent analysis and found the same trends when the analysis included microscopic residual disease and was restricted to Stage III and IV patients [24].
It is important to note that our findings do not evaluate the impact of surgical effort to resect extensive disease versus more conservative surgery in the face of similar disease spread. Numerous reports support the hypothesis that extensive or radical surgery improves survival, particularly when no gross residual can be achieved [8,9,25,26]. Indeed, our group's review of GOG data from stage IV EOC patients found that a significant survival advantage was achieved only in the microscopic residual group, with patients having 0.1 to 1 cm and 1.1 to 5 cm residual having similar outcomes [27]. In a detailed study of preoperative tumor burden and cytoreductive outcome in stage IIIC EOC patients, Eisenkop et al. demonstrated that though the total extent of intra-abdominal tumor burden did independently influence survival, complete surgical resection to microscopic residual had a more significant independent influence on survival [9]. An International collaborative group recently corroborated these findings on a larger scale in an exploratory analysis of three prospective randomized multicenter trials [28].
Large institutional experiences support these findings. Aletti et al. found that aggressive surgical effort minimizing residual disease significantly benefitted patients with carcinomatosis [8]. Extensive initial disease affected survival largely by limiting surgical cytoreduction, but after adjusting for residual disease, initial tumor burden became a minor determinant of survival. In the subset of patients with carcinomatosis, patients who had radical surgery had equivalent survival to those who were rendered optimal (residual < 1 cm) by less aggressive surgery (5-year disease specific overall survival 46% versus 47%; P = 0.80) [8]. Eisenhauer et al. reported similar findings based on the Memorial Sloan-Kettering experience. In their study, patients requiring extensive upper abdominal procedures to achieve optimal cytoreduction (residual ≤ 1 cm) had equivalent survival to patients optimally cytoreduced by standard surgical techniques. Survival in these groups was significantly better than patients who were suboptimally cytoreduced [29]. These findings strongly support the assertion that maximal cytoreductive effort including extensive or radical procedures positively affects survival even in the setting of carcinomatosis or extensive upper abdominal disease. Furthermore, in these institutional experiences, aggressive surgery appears to significantly offset the prognostic significance of preoperative disease burden with survival approaching or equaling that of patients without extensive disease.
Significantly, in an update and analysis of the Memorial Sloan-Kettering cytoreductive experience, investigators analyzed separately 93 patients who underwent a complete gross resection of stage IIIC EOC. Patients found to have no visible or palpable disease cephalad to the greater omentum at the beginning of surgery had significantly improved PFS of 33 months compared to 22 months in patients with disease cephalad to the greater omentum (P = 0.03). The authors hypothesized that the latter group represented “patients with more aggressive ovarian cancer phenotypes or patients who may have had their disease for a longer period of time, allowing for advance growth and implantation [30].” The findings from this large single institution series mirror our data from across the GOG.
Despite the overwhelming retrospective evidence that residual tumor after surgery is a significant independent prognostic factor, no evidence-based data (randomized trial) answers the question, “Does the molecular profile of the tumor allow complete cytoreduction or is the surgical procedure itself responsible for improved outcome independent of the tumor biology?” Data suggest that gene expression patterns are associated with, and may predict, optimal versus suboptimal cytoreduction [31]. In this context, some urge caution in moving towards more radical surgery considering its time, effort, and morbidity as well as the surgical and institutional expertise required [32,33]. Because of the perceived relative importance of tumor biology and morbidity of extensive debulking in a primary setting, others advocate further exploring neoadjvant chemotherapeutic approaches to bulky or extensive EOC [34].
A limitation of this study is our focus on the subgroup of patients with microscopic residual only. As such, we were unable to directly assess the effects of varying degrees of macroscopic residual disease at the conclusion of surgery. Therefore, despite demonstrating decreased survival of 56 months in patients with upper abdominal disease compared to those with more limited disease, our study findings do not preclude a benefit from attempts to microscopically debulk these patients. Winter and colleagues provide context for the impact of macroscopic residual in their study of similarly treated patients. In six GOG prospective trials, including the three in this analysis, they found a median survival of 42 months in patients with 0.1–1 cm residual and only 35 months with > 1 cm residual [7]. These findings provide a reasonable reflection of expected survival in our study population if they had been left with macroscopic residual.
A second limitation results from the time periods in which the analyzed trials accrued. A clear trend has evolved in the past decade in the United States pushing the boundaries of what may have previously been considered unresectable disease [10]. Though the GOG trials in our analysis form the basis of current adjuvant treatment of EOC, the patient accrual period predates much of this trend toward extensive or radical surgery. As such, fewer patients undergoing extensive resections were available for evaluation during this era. A third limitation is our selection of involvement of a particular anatomic site to correlate with survival. This was chosen as the most consistent, reproducible variable in the data sets. Others have explored relationships between biology and extent of disease by correlating survival and other surrogates for disease burden such as CA-125 levels, ascites volume, largest tumor diameter, number of metastatic implants, necessity to use certain procedures, or more complex scoring systems [5,9,13,35]. Inconsistencies exist in observations related to these variables and none has been proven more valid than others. The strength of our study compared with previous reports is the size and similarity of the population which represents a broad cross-section of institutions and surgical practices yet adheres to strict protocol regimens of primary surgery and postoperative therapy.
Based on our findings placed in the context of the broader body of literature regarding cytoreduction, we confidently assert that biology versus surgery is hardly the zero-sum argument which is often portrayed. In this multi-institutional study group despite extensive surgery, patients with upper abdominal disease burden had a poorer prognosis than patients with more consolidated disease even when surgery resulted in no gross residual disease. These findings support that other factors and perhaps tumor biology are important in predicting survival, but the importance of these factors do not preclude benefit from aggressive cytoreductive surgery.
Footnotes
Presented in part at the Society of Gynecologic Oncologists 2010 Annual Meeting on Women's Cancer, March 14–17, 2010, San Francisco, CA.
This study was supported by the National Cancer Institute grants to the Gynecologic Oncology Group Administrative Office (CA 27469) and the Gynecologic Oncology Group Statistical Office (CA 37517). The following Gynecologic Oncology Group member institutions participated in the primary treatment studies: University of Alabama at Birmingham, Oregon Health Sciences University, Duke University Medical Center, Abington Memorial Hospital, University of Rochester Medical Center, Walter Reed Army Medical Center, Wayne State University, University of Minnesota Medical School, University of Southern California at Los Angeles, University of Mississippi Medical Center, Colorado Gynecologic Oncology Group P.C., University of California at Los Angeles, University of Washington, University of Pennsylvania Cancer Center, University of Miami School of Medicine, Milton S. Hershey Medical Center, Georgetown University Hospital, University of Cincinnati, University of North Carolina School of Medicine, University of Iowa Hospitals and Clinics, University of Texas Southwestern Medical Center at Dallas, Indiana University School of Medicine, Wake Forest University School of Medicine, Albany Medical College, University of California Medical Center at Irvine, Tufts-New England Medical Center, Rush-Presbyterian-St. Luke's Medical Center, University of Kentucky, Eastern Virginia Medical School, The Cleveland Clinic Foundation, Johns Hopkins Oncology Center, State University of New York at Stony Brook, Eastern Pennsylvania GYN/ONC Center, P.C., Southwestern Oncology Group, Washington University School of Medicine, Memorial Sloan-Kettering Cancer Center, Columbus Cancer Council, University of Massachusetts Medical School, Fox Chase Cancer Center, Medical University of South Carolina, Women's Cancer Center, University of Oklahoma, University of Virginia Health Sciences Center, University of Chicago, University of Arizona Health Science Center, Tacoma General Hospital, Eastern Collaborative Oncology Group, Thomas Jefferson University Hospital, Case Western Reserve University, and Tampa Bay Cancer Consortium.
The opinions or assertions expressed in this article represent the private views of the authors and should not be construed as reflecting the official views of the Department of the Air Force, Department of the Navy, the Department of the Army or the Department of Defense.
Supplementary materials related to this article can be found online at doi:10.1016/j.ygyno.2011.04.041.
Conflict of interest statement:The authors have no conflicts of interest to declare.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010. CA Cancer J Clin. 2010 doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20:1248–59. doi: 10.1200/JCO.2002.20.5.1248. [DOI] [PubMed] [Google Scholar]
- 3.Fader AN, Rose PG. Role of surgery in ovarian carcinoma. J Clin Oncol. 2007;25:2873–83. doi: 10.1200/JCO.2007.11.0932. [DOI] [PubMed] [Google Scholar]
- 4.Griffiths CT. Surgical resection of tumor bulk in the primary treatment of ovarian carcinoma. Natl Cancer Inst Monogr. 1975;42:101–4. [PubMed] [Google Scholar]
- 5.Eisenkop SM, Spirtos NM, Lin WC. “Optimal” cytoreduction for advanced epithelial ovarian cancer: a commentary. Gynecol Oncol. 2006;103:329–35. doi: 10.1016/j.ygyno.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Omura GA. Progress in gynecologic cancer research: the Gynecologic Oncology Group experience. Semin Oncol. 2008;35:507–21. doi: 10.1053/j.seminoncol.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winter WE, 3rd, Maxwell GL, Tian C, Carlson JW, Ozols RF, Rose PG, et al. Prognostic factors for stage III epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:3621–7. doi: 10.1200/JCO.2006.10.2517. [DOI] [PubMed] [Google Scholar]
- 8.Aletti GD, Dowdy SC, Gostout BS, Jones MB, Stanhope CR, Wilson TO, et al. Aggressive surgical effort and improved survival in advanced-stage ovarian cancer. Obstet Gynecol. 2006;107:77–85. doi: 10.1097/01.AOG.0000192407.04428.bb. [DOI] [PubMed] [Google Scholar]
- 9.Eisenkop SM, Spirtos NM, Friedman RL, Lin WC, Pisani AL, Perticucci S. Relative influences of tumor volume before surgery and the cytoreductive outcome on survival for patients with advanced ovarian cancer: a prospective study. Gynecol Oncol. 2003;90:390–6. doi: 10.1016/s0090-8258(03)00278-6. [DOI] [PubMed] [Google Scholar]
- 10.Chi DS, Eisenhauer EL, Lang J, Huh J, Haddad L, Abu-Rustum NR, et al. What is the optimal goal of primary cytoreductive surgery for bulky stage IIIC epithelial ovarian carcinoma (EOC)? Gynecol Oncol. 2006;103:559–64. doi: 10.1016/j.ygyno.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 11.Kommoss S, Rochon J, Harter P, Heitz F, Grabowski JP, Ewald-Riegler N, et al. Prognostic impact of additional extended surgical procedures in advanced-stage primary ovarian cancer. Ann Surg Oncol. 2010;17:279–86. doi: 10.1245/s10434-009-0787-8. [DOI] [PubMed] [Google Scholar]
- 12.Harter P, Hilpert F, Mahner S, Kommoss S, Heitz F, Pfisterer J, et al. Prognostic factors for complete debulking in first- and second-line ovarian cancer. Int J Gynecol Cancer. 2009;19(Suppl 2):S14–7. doi: 10.1111/IGC.0b013e3181bffb3f. [DOI] [PubMed] [Google Scholar]
- 13.Crawford SC, Vasey PA, Paul J, Hay A, Davis JA, Kaye SB. Does aggressive surgery only benefit patients with less advanced ovarian cancer? Results from an international comparison within the SCOTROC-1 Trial. J Clin Oncol. 2005;23:8802–11. doi: 10.1200/JCO.2005.02.1287. [DOI] [PubMed] [Google Scholar]
- 14.Hoskins WJ, Bundy BN, Thigpen JT, Omura GA. The influence of cytoreductive surgery on recurrence-free interval and survival in small-volume stage III epithelial ovarian cancer: a Gynecologic Oncology Group study. Gynecol Oncol. 1992;47:159–66. doi: 10.1016/0090-8258(92)90100-w. [DOI] [PubMed] [Google Scholar]
- 15.Chi DS, Schwartz PE. Cytoreduction vs neoadjuvant chemotherapy for ovarian cancer. Gynecol Oncol. 2008;111:391–9. doi: 10.1016/j.ygyno.2008.07.058. [DOI] [PubMed] [Google Scholar]
- 16.Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 17.Markman M, Bundy BN, Alberts DS, Fowler JM, Clarke-Pearson DL, Carson LF, et al. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol. 2001;19:1001–7. doi: 10.1200/JCO.2001.19.4.1001. [DOI] [PubMed] [Google Scholar]
- 18.Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21:3194–200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 20.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–70. [PubMed] [Google Scholar]
- 21.Cox DR. Regression models and life tables (with discussion) J R Stat Soc Series B. 1972;34:187–220. [Google Scholar]
- 22.Aletti GD, Cliby WA. Importance of surgical aggressiveness in advanced ovarian cancer. J Clin Oncol. 2006;24:2397. doi: 10.1200/JCO.2006.06.0111. author reply 2398-9. [DOI] [PubMed] [Google Scholar]
- 23.Chi DS, Barakat RR. Aggressive surgery and ovarian cancer. J Clin Oncol. 2006;24:2395–6. doi: 10.1200/JCO.2005.05.4890. author reply 2396-7. [DOI] [PubMed] [Google Scholar]
- 24.Crawford SC, Paul J, Kaye SB, et al. Aggressive surgery and ovarian cancer. J Clin Oncol. 2006;24:2396–7. doi: 10.1200/JCO.2005.05.4890. authors reply. [DOI] [PubMed] [Google Scholar]
- 25.Chi DS, Eisenhauer EL, Zivanovic O, Sonoda Y, Abu-Rustum NR, Levine DA, et al. Improved progression-free and overall survival in advanced ovarian cancer as a result of a change in surgical paradigm. Gynecol Oncol. 2009;114:26–31. doi: 10.1016/j.ygyno.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 26.Zivanovic O, Eisenhauer EL, Zhou Q, Iasonos A, Sabbatini P, Sonoda Y, et al. The impact of bulky upper abdominal disease cephalad to the greater omentum on surgical outcome for stage IIIC epithelial ovarian, fallopian tube, and primary peritoneal cancer. Gynecol Oncol. 2008;108:287–92. doi: 10.1016/j.ygyno.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 27.Winter WE, 3rd, Maxwell GL, Tian C, Sundborg MJ, Rose GS, Rose PG, et al. Tumor residual after surgical cytoreduction in prediction of clinical outcome in stage IV epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2008;26:83–9. doi: 10.1200/JCO.2007.13.1953. [DOI] [PubMed] [Google Scholar]
- 28.du Bois A, Reuss A, Pujade-Lauraine E, Harter P, Ray-Coquard I, Pfisterer J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d'Investigateurs Nationaux Pour les Etudes des Cancers de l'Ovaire (GINECO) Cancer. 2009;115:1234–44. doi: 10.1002/cncr.24149. [DOI] [PubMed] [Google Scholar]
- 29.Eisenhauer EL, Abu-Rustum NR, Sonoda Y, Levine DA, Poynor EA, Aghajanian C, et al. The addition of extensive upper abdominal surgery to achieve optimal cytoreduction improves survival in patients with stages IIIC–IV epithelial ovarian cancer. Gynecol Oncol. 2006;103:1083–90. doi: 10.1016/j.ygyno.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 30.Zivanovic O, Sima CS, Iasonos A, Hoskins WJ, Pingle PR, Leitao MM, Jr, et al. The effect of primary cytoreduction on outcomes of patients with FIGO stage IIIC ovarian cancer stratified by the initial tumor burden in the upper abdomen cephalad to the greater omentum. Gynecol Oncol. 2010;116:351–7. doi: 10.1016/j.ygyno.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berchuck A, Iversen ES, Lancaster JM, Dressman HK, West M, Nevins JR, et al. Prediction of optimal versus suboptimal cytoreduction of advanced-stage serous ovarian cancer with the use of microarrays. Am J Obstet Gynecol. 2004;190:910–25. doi: 10.1016/j.ajog.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 32.Covens AL. A critique of surgical cytoreduction in advanced ovarian cancer. Gynecol Oncol. 2000;78:269–74. doi: 10.1006/gyno.2000.5926. [DOI] [PubMed] [Google Scholar]
- 33.Markman M. Concept of optimal surgical cytoreduction in advanced ovarian cancer: a brief critique and a call for action. J Clin Oncol. 2007;25:4168–70. doi: 10.1200/JCO.2007.11.8992. [DOI] [PubMed] [Google Scholar]
- 34.Hou JY, Kelly MG, Yu H, McAlpine JN, Azodi M, Rutherford TJ, et al. Neoadjuvant chemotherapy lessens surgical morbidity in advanced ovarian cancer and leads to improved survival in stage IV disease. Gynecol Oncol. 2007;105:211–7. doi: 10.1016/j.ygyno.2006.11.025. [DOI] [PubMed] [Google Scholar]
- 35.Zorn KK, Tian C, McGuire WP, Hoskins WJ, Markman M, Muggia FM, et al. The prognostic value of pretreatment CA 125 in patients with advanced ovarian carcinoma: a Gynecologic Oncology Group study. Cancer. 2009;115:1028–35. doi: 10.1002/cncr.24084. [DOI] [PMC free article] [PubMed] [Google Scholar]