Abstract
Veterinarians and veterinary medicine have been integral to the development of stem cell therapies. The contributions of large animal experimental models to the development and refinement of modern hematopoietic stem cell transplantation were noted nearly five decades ago. More recent advances in adult stem cell/regenerative cell therapies continue to expand knowledge of the basic biology and clinical applications of stem cells. A relatively liberal legal and ethical regulation of stem cell research in veterinary medicine has facilitated the development and in some instances clinical translation of a variety of cell-based therapies involving hematopoietic (HSC) and mesenchymal stem cells (MSC) as well as other adult regenerative cells and recently embryonic stem cells (ESC) and induced pluripotent stem cells (iPSC). In fact, many of the pioneering developments in these fields of stem cell research have been achieved through collaborations of veterinary and human scientists. This review aims to provide an overview of the contribution of large animal veterinary models in advancing stem cell therapies for both human and clinical veterinary applications. Moreover, in the context of the “One Health Initiative”, the role veterinary patients may play in the future evolution of stem cell therapies for both human and animal patients will be explored.
Keywords: Veterinary, stem cells, cell-based therapies, regenerative medicine
INTRODUCTION
Several centuries ago, prior to the development of medical specialization, physicians treated both human patients and their animals. More recently, synergy between veterinarians, physicians and other scientific health and environmental professionals has been promoted in an initiative known as “One Health” to improve the lives of all species through the integration of human and veterinary medicine.2 The importance of “One Health” world-wide strategy is highlighted by its endorsement by such organizations as the American Medical Association (AMA), the American Veterinary Medical Association (AVMA), the American Society of Tropical Medicine and Hygiene, the Centers for Disease Control and Prevention (CDC), the United States Department of Agriculture (USDA), and the U.S. National Environmental Health Association (NEHA), as well as similar international organizations.3 This “One Health” initiative promises to efficiently transform medicine for all species, particularly the field of regenerative medicine which aims to restore cell, tissue or organ function following damage or loss secondary to injury, disease or aging. Harnessing the potential of stem cells to efficiently heal injured tissues, while minimizing untoward side effects of their application, is an area of intense investigation in both veterinary and human medicine. The fewer regulatory hurdles that exist for veterinary regenerative medical products, compared to human therapeutics,4 has encouraged rapid translation of cell-based therapies into clinical veterinary practice for a variety of pathologic conditions.
The American Veterinary Medical Association monitors both legal and regulatory issues relating to the practice of veterinary medicine, including regenerative medicine, at both the state and federal levels. The products administered during the course of such practice, however, are regulated by the US Food and Drug Administration (FDA).4 Although regulations on the use of stem cells in humans have been promulgated by the FDA, such regulations regarding use in veterinary patients have yet to be instituted. It is likely that a veterinary regulatory scheme will be modeled after that established for human medicine in the future. However in the meantime, the popular appeal of cell-based therapies, and their widespread commercialization, has led to their application for many conditions in veterinary patients for which there are little to no evidence-based preclinical animal studies or even supporting in vitro data. Therefore, enthusiasm for stem cell therapies as a powerful treatment strategy for the repair and regeneration of tissue injury and disease must be tempered until experimental evidence is sufficient to supersede anecdotal reports. Thus, evidenced-based clinical trials of stem cell therapies in veterinary patients provide tremendous opportunities for efficient advancement of regenerative medicine for all species.
Use of companion animal species as translational models
Veterinary patients, including companion (dogs, cats, and horses) and farm animals (cows, sheep, goats and pigs), are increasingly recognized as critical translational models of human diseases. Compared to rodents, all are considered large animal models of human disease. It should be noted that this nomenclature regarding companion animals, such as dogs and cats, can be confusing because as veterinary patients these animals are considered “small animal” species (compared to horses, cows and other ruminants). As the focus of this review is in their utility as translational models, dogs and cats will be referred to as large animal models whether they are used in studies as clinical (client-owned) patients or in the research setting.
Although the utility of rodents, particularly genetically altered murine models in the elucidation of pathophysiology and response to therapy for various disease states is profound, naturally occurring pathologies in large animal models caused by single gene defects or due to complex interactions between multiple genes and environmental factors promise to play an important role in the development of clinical advances for a number of serious diseases. For example, some 292 canine, 163 feline, 142 bovine and 109 equine genetic diseases are homologous with human genetic defects,5 although in some cases the pathophysiology and resulting phenotype of such mutations may be undefined or may vary from that in humans. In addition, the basic biochemical and physiological processes in these large animal models more closely resemble those in humans, compared to rodents.6
Unlike laboratory rodents, companion animal species live longer, are outbred and in a non-laboratory setting are exposed to external and environmental factors underlying various disease states, such as obesity, diabetes and cancer. They are also susceptible to traumatic injuries like those sustained by human patients. Similar to humans and unlike small animal models, many dogs and horses are expected to resume an athletic career (sport horses and agility dogs, for example) or a working career (service dogs). As well, imaging and repeated biologic sampling that are difficult or impossible in rodent models increase the ability to detect untoward side effects of novel therapies and minimize both veterinary and human patient risk.
Furthermore, the increase in demand for sophisticated, state of the art care for animal companions has led to a surge in clinical trials in veterinary patients. With improved design of trials to include appropriate controls and outcome assessments, these should provide a unique opportunity for assessing both efficacy and safety of human adult stem cell therapies that can be translated to human medicine.7 Given the value of companion animal models for translational studies to advance human medicine, as well as the obvious impact on cutting edge veterinary therapies, a thorough knowledge of the state of stem cell therapies in veterinary practice and in translational studies is critical for those interested in advancing the fields of tissue engineering and regenerative medicine.
STEM CELLS IN VETERINARY MEDICINE
For nearly half a century, companion animals have played a key role in advancing stem cell therapies. Adult stem cells are prime targets for cell-based therapies as they escape the ethical issues associated with embryonic stem cells (ESC). The cell types having received the most attention are hematopoietic stem cells (HSC) and mesenchymal stem cells (MSC). Although the clinical impact of HSC therapy in veterinary medicine pales in comparison to its impact in human medicine, the characterization of MSC from various tissues in multiple species, followed by an increasing number of preclinical translational studies has led to the relatively widespread use of MSC-based therapies in clinical veterinary medicine. In addition, other cell-based therapies such as those using adipose derived-stromal vascular fraction (Ad-SVF) are now routinely used in clinical veterinary patients. Future translational studies and clinical trials in large animal models (both experimental and clinical veterinary patients) will continue to yield a significant impact on similar future trials in humans and the optimization of such therapies for both veterinary and human patients. Finally, the development and application of ESC and induced pluripotent stem cells (iPSC) from large animals remains in its infancy but is a rapidly expanding focus which will help define the safety and efficacy of such cells in regenerative medicine.
ADULT STEM CELL APPLICATIONS IN LARGE ANIMAL MODELS AND CLINICAL VETERINARY PATIENTS
Hematopoietic stem cells
HSC give rise to all the cellular components of blood. These cells are found in high numbers within the bone marrow but also can be isolated from umbilical cord blood as well as in lower numbers from peripheral blood in a mobilized cytokine-induced state. HSC therapies have become routine for acquired or inherited bone marrow and hematologic disorders in man, as well as following high dose chemotherapy. Critical to the development of these therapies are translational studies performed in the preclinical canine model, starting in the 1960s.8 The canine model has continued to be used for the refinement of techniques for optimizing HSC transplantation in people over the last four decades, with post-transplantation outcome assessments in dogs accurately predicting outcome in human patients.9 Studies in random-bred dogs have led to improved long-term engraftment and diminished graft-versus-host disease through the elucidation of the role of histocompatibility barriers in these processes, as well as improved methods for graft collection and conditioning regimens for the recipient. The latter have allowed for increased success in expanded patient populations of increasing age and with multiple comorbidities.10,11 Despite the large body of literature describing HSC transplantation in the preclinical canine model, only recently have HSC-based therapies been used for clinical canine patients with cancer.10,11
Mesenchymal stem cells
MSC therapies have been used to treat a variety of clinical conditions in companion animals and in pre-translational models of human and companion animal diseases (detailed below). Extensive efforts have been dedicated to isolation and phenotypic characterization of MSC from companion animals as well as other farm animal species such as cattle, pigs and sheep used as preclinical research models. For veterinary applications, the most common harvest sites for MSC are bone marrow (BM),12–23 adipose (Ad) tissue, 14,17,19,19,20,23–26 and umbilical cord/placenta,16,27–34 although MSC from other sites including peripheral blood,17 muscle,35 periosteum,20,35,36 and teeth/periodontal ligament37 have also been characterized and/or used therapeutically (see Table 1). As with MSC harvested from laboratory animals or humans, MSC isolated from companion animals are characterized by their cell surface markers, multipotency and capacity to adhere to plastic. Bone marrow is harvested from humerus, iliac crest or tibia in dogs and cats while it is most commonly aspirated from the sternum and tuber coxae in horses. Adipose tissue samples are easily harvested from inguinal, abdominal, lateral thoracic or caudal scapular regions in dogs and cats, whereas the pericoccygeal, inguinal, and sternal regions are more common in horses. Site-specific differences in numbers and differentiation capacity have been noted for both Ad- and BM-MSC,12,24 which may help direct optimal donor site selection for clinical trials. Furthermore, species-specific differences have been identified in differentiation conditions,38 which may have important implications for defining the most appropriate preclinical models to develop tissue engineering strategies for man.
Table 1. Stem cells in large animal veterinary species.
Selected publications of the characterization and/or therapeutic application of Mesenchymal Stem cells (MSC) derived from bone marrow (BM), adipose (Ad), placenta/umbilical cord (P/UC), peripheral blood and other sources, embryonic stem cells (ESC) or putative ESC, and induced pluripotent stem cells (iPSC) in common companion and farm animals used in translational research.
| Dog | Cat | Rabbit | Horse | Pig | Cow | Small Ruminants | |
|---|---|---|---|---|---|---|---|
| BM-MSC | 12,35,38 | 13,14 | 15,16 | 17–19,29,39 | 20 | 21 | 22,23 |
| Ad-MSC | 24,35 | 14 | 25 | 17–19,39 | 20 | 26 | 23 |
| P/UC- MSC | 27 | 28 | 16 | 29,30,39 | 31 | 32 | 33,34 |
| Peripheral blood/Other- MSC | 35 | 36 | 17 | 20 | |||
| ESC/ESC-like | 100 | 101 | 102 | 103,104 | 105,106,109 | 107,108 | 109 |
| iPSC | 115–117 | 125 | 118,119 | 120–122,126 | 123 | 124 |
Despite an increasing knowledge about the basic biology of MSC, it remains unclear which cell source is most appropriate for individual therapeutic applications. Certainly, the ease of collection and the increased number of MSC in fat makes Ad-MSC potentially more attractive compared to BM-MSC; however, most research suggests tissue harvest site influences MSC differentiation capacity. In particular, in the majority of studies Ad-MSC are inferior in their ability to differentiate along multiple lineages compared to BM-MSC given the current understanding of ex vivo inducing (differentiation) conditions.17,39,40 Although these comparisons have largely focused on differentiation capacity, the ability of cells harvested from these different tissues to produce reparative trophic mediators and, more importantly, to exert biologic effects in vivo is largely unknown.
Given that ex vivo expansion protocols to achieve clinically relevant numbers of BM- and Ad-MSC are required following harvest, the use of the Ad-SVF has been advocated to enable immediate use or use following short-term processing in clinical veterinary patients. In addition to MSC, SVF also contains a mixture of endothelial progenitor cells and pericytes known to be involved in angiogenesis, monocytes and macrophages, as well as fibroblasts and preadipocytes. It is unclear whether this mixture of cells may have a positive effect and coordinately contribute to therapeutic effects of the SVF, or whether a heterogeneous mixture of cells may be deleterious therapeutically. Despite the fact that the majority of basic research and preclinical studies have focused on BM-MSC and to a lesser extent Ad-MSC, cellular therapies utilizing Ad-SVF represent the major proportion of what is offered for clinical use by American veterinary practitioners (see Table 2), reflecting a proactive and successful publicity campaign on the part of its commercial providers. According to its website, Vet-stem (one commercial provider) has processed SVF from Ad samples for more than 8,000 companion animal patients since 2004. Although Ad-SVF is commercially available from multiple vendors and is used by veterinarians for a variety of clinical applications, to date only two evidenced-based studies defining its effects on osteoarthritis in canine patients appear in the literature.41,42
Table 2.
Commercial sources of “regenerative cells” available for companion animals in North America.
| Company/Institution | Website | Species | Product | Processing time from patient tissue harvest |
|---|---|---|---|---|
| Advanced Regenerative Therapies | http://www.art4dvm.com/ | Equine, Canine | BM-MSC | 2–3 week expansion protocol; cryostorage available |
| Celavet | http://www.celavet.com | Equine | ESC (allogeneic) | |
| Equstem | http://www.equstem.com | Equine | UCB-MSC, BM-MSC | 2 week expansion protocol; cryostorage available |
| Lifelife labs | http://www.lifelinelabsllc.com | Equine | UCB-MSC | |
| Medivet | http://www.medivet-america.com/ | Equine, Canine, Feline | Ad-SVF | 2–3 days for processing/shipment; in-practice processing kits available |
| Renovocyte | http://renovocyte.com/ | Equine, Canine, Feline, Lapine | Teeth, reproductive organ or placenta- derived MSC | week expansion protocol; cryostorage available |
| Rood and Riddle Stem Cell laboratory | http://www.roodandriddle.com/stemcell.html | Equine | UCB-MSC, BM-MSC | 2–3 week expansion protocol |
| Stemlogix | https://www.stemlogix.com/ | Equine, Canine, Feline | Ad-SVF and BM- derived stem cells; Ad- and BM-MSC | In-practice processing; culture expanded processing; cryostorage available |
| UC Davis Regenerative Medicine Laboratory | http://www.vetmed.ucdavis.edu/vmth/regen_med | Equine, Canine | Ad-SVF, BM-MSC, UCB/Cord tissue-MSC (equine) | Ad-SVF: 2–3 days for processing/shipment; cryostorage available |
| VetCell | http://www.vetcell.com/ | Equine | BM-MSC | 2–3 week expansion protocol; cryostorage available |
| VetStem | https://www.vet-stem.com | Equine, Canine, Feline | Ad-SVF | 2–3 days for processing/shipment; cryostorage available |
Musculoskeletal applications
Tendon
Injuries to the soft tissues of the musculoskeletal system are common in both humans and animals, particularly athletes, and lead to considerable morbidity in both patient populations.43,44 Healing of damaged tendons and ligaments occurs with the formation of fibrous scar tissue of inferior biomechanical properties, leading to reduced performance as well as increased risk of reinjury. Horses and dogs both suffer from clinically significant, naturally occurring tendinopathies with similar histopathologic, MRI and ultrasonographic characteristics to those seen in human tendinopathies.43,44 Equine superficial digital flexor tendon (SDFT) strain injuries are common in race and other sport horses.43 Given the high rate of injury recurrence, coupled with evidence that MSC can improve the regenerative response in models such as the rabbit,45 cell-based therapies have been investigated in both experimental models of and naturally occurring SDFT in the horse to develop improved therapies.
Preclinical studies to examine efficacy of MSC-based therapy for equine tendinopathy have primarily focused on in vitro and experimentally induced models. These studies suggest that MSC ameliorate tissue composition and organization while improving biomechanics of injured tendons and ligaments. Initial studies examining efficacy of BM-MSC and Ad-SVF, alone and as vehicles for gene delivery, have shown significant improvement in healing, compared to controls, at both the gross and histologic levels. 46,47 In vivo models show that administered cells persist in lesions, albeit at low numbers, for up to 3 months50 and that both BM-MSC and Ad-SVF exert a significant anti-inflammatory effect that likely contributes to improved healing of the tendon.46,47 While extensive post-mortem examinations to rule out neoplasia at distant sites are lacking, these experimental models do provide preliminary evidence that MSC-based therapies do not lead to neoplastic transformation of transplanted cells.
Clinical studies conducted in equine athletes support the therapeutic efficacy and safety of MSC for tendon injuries. Initial work by Pacini et al.48 showed significant clinical recovery in 9 of 11 racehorses with SDFT injury treated with BM-MSC, compared to none of 15 horses treated with conventional therapy, as assessed by ultrasonography and ability to return to racing without re-injury. In a recent analysis of 113 racehorses treated with BM-MSC, the re-injury rate was significantly lower than that seen with other treatment modalities.49 To date, no controlled studies examining Ad-MSC or Ad-SVF in equine patients with tendinopathy are available; however, a report on the use of allogeneic Ad-MSC to treat tendonitis in a small group of horses (N=16) suggests that allogeneic culture expanded Ad-MSC in conjunction with platelet rich plasma (PRP) are also capable of improving healing of equine tendonitis and allow return to pre-injury function in the majority (14/16) of horses.53 Despite, the lack of case-controlled clinical trials examining efficacy of MSC and SVF for equine tendinopathy, improvement in outcome measures such as return to pre-injury function and reduction in re-injury rates compared to success rates with conventional therapy suggests that these therapies hold promise.
Bone
There is significant clinical need for cell-based therapies to improve bone healing and outcome for fracture or segmental defect repair, incorporation of implants for total joint replacement, and arthrodesis in both humans and companion animals, particularly the dog. Both BM- and Ad-MSC, delivered in a variety of carriers, are effective in improving healing of segmental defects of long and craniofacial bones as well as of non-union fractures in experimental large animal models.54–58 Allogeneic BM-MSC were also found to enhance repair of canine femoral critical-sized segmental defects in a manner equivalent to that seen with autologous cells, while inducing no significant immune reaction.59 Additional studies in experimental canine models support the potential for clinical application of MSC-based tissue engineering strategies for mandibular reconstruction as well as to reverse alveolar bone resorption secondary to periodontal disease.60,61 Furthermore, MSC-based therapies to enhance osseous healing effectively promote arthrodesis,62 as required for both spinal fusions and salvage procedures for joint pathology. Finally, MSC-based strategies have also shown promise for a minimally invasive approach to reverse bone loss associated with Legg-Calves-Perthes disease, a degenerative condition of the hip joint that affects both children and young, small breed dogs.54 Together, these studies suggest that MSC-based therapies can improve bone formation in a variety of clinically relevant conditions for translation to both human and veterinary applications. Companion animal models, whether clinical patients with naturally occurring conditions or research subjects with surgically induced defects, may serve as excellent translational models for the optimization of stem cell-based therapies for bone repair using improved bioscaffolds and/or gene therapy-mediated enhancement of the effects of delivered stem cells in randomized, controlled clinical trials.63
Intraarticular disease
Lameness associated with developmental, acute/traumatic and chronic acquired intraarticular pathologies is a frequent condition seen by veterinary practitioners. Canine orthopaedic disorders such as anterior cruciate ligament rupture, meniscal injury, osteochondrosis, cartilage defects and osteoarthritis (OA) are common spontaneously occurring clinical conditions. Similarly, young equine athletes and geriatric leisure horses suffer from intraarticular pathology such as cartilage defects, osteochondrosis and OA. Similar to humans, current standard of care for canine or equine patients includes a combination of physical therapy, exercise modification, as well as medical and surgical therapies. The same regimes can be applied to experimental/research animals of these species. Clinical trials of disease modifying agents for joint pathology in veterinary patients present a unique opportunity to improve treatment options of naturally occurring OA and other intraarticular pathologies in both veterinary and human patients.
Initial interest in the use of MSC for cartilage resurfacing and tissue engineering strategies for meniscal regeneration stemmed from their ability to differentiate into various mesenchymal lineages, including chondrocytes. More recently, there is greater appreciation for the ability of MSC to modulate joint pathology through their ability to secrete various trophic mediators and to modulate the inflammatory response. Experimental studies performed in large animal models suggest MSC-based therapies improve reparative responses in cartilage64,65 as well as promote meniscal regeneration,66 although evidence for clinical efficacy has been limited. In the horse, early improvements in histologic and biochemical parameters of healing in BM-MSC treated full-thickness articular cartilage defects have been shown; however, improvements are lost in longer term (8 month) evaluation.64 Comparing the ability of culture expanded BM-MSC and Ad-SVF to alter progression of early stage OA in an experimental equine model, Frisbie et al showed that BM-MSC treated joints showed significantly less synovial fluid effusion and prostaglandin E2 concentrations compared to those treated with Ad-SVF, although neither cell therapy yielded significant improvements in cartilage biochemistry, histology, synovial fluid cytology and analysis, or other clinical parameters.18 Clinicopathologic assessment of intraarticular response to allogeneic and autologous placental-derived MSC also suggests safety of intraarticular injection of allogeneic MSC.30 It should be noted that in contrast to humans in which tissue engineered constructs are being sought, it is not possible to prevent weight bearing to protect sites of equine tissue engineered cartilage in the post-operative period due to the development of life-threatening laminitis on the other limbs, thereby making the development of such therapeutic strategies an even greater challenge in the horse.
The effect of stem cell therapy for late stage/chronic OA in canine patients has been assessed in two separate studies. The first, a randomized, blinded, placebo-controlled clinical trial, examined the effectiveness of Ad-SVF therapy for the treatment of chronic hip OA.42 Dogs treated with Ad-SVF had significant improvement in lameness and pain scores, as well as range of motion compared to control dogs. A subsequent uncontrolled study by the same authors examining efficacy of these cells for use in chronic OA of the canine humeroradial (elbow) joint also documented similar significant improvements in those clinical parameters at six months follow-up.41 Although these results are promising, it is clear that larger studies, which optimize the therapeutic efficacy demonstrated in these initial studies, are needed. Furthermore, it remains unclear how the many different commercially available “stem cell” products (see Table 2) compare in efficacy for treatment of canine OA.
Soft tissue applications
Neuromuscular injury
Spinal cord disease, leading to functional motor and sensory dysfunction, is a significant clinical problem in canine patients. Similar to humans, spinal cord injury in dogs occurs secondary to intervertebral disc disease (IVDD), as well as other degenerative causes, or traumatic injury and negatively impacts the quality of life of affected dogs and the owners who must care for them. Several studies suggest that MSC transplantation may improve functional neurologic recovery in experimental models of canine spinal cord injury thus prompting further investigation of cell-based approaches for the treatment of naturally occurring spinal cord disease in human and veterinary patients. Functional neurologic recovery of dogs with experimentally induced spinal cord injury is significantly improved following therapy with both autologous and allogeneic MSC harvested from either BM or umbilical cord.27,67 Park et al identified optimal timing of MSC delivery on clinical outcome following spinal cord injury. They established that MSC transplantation one week after spinal cord injury is superior in ability to improve clinical neurologic examination scores and neuronal regeneration (reduced fibrosis on histologic examination) 8 weeks post-transplantation, compared to either 12 hour or 2 week post-injury administration.68
Cell-based interventions to inhibit progression of IVDD, a major cause of degenerative spinal cord disease in humans and dogs, have shown promise in experimentally induced canine models of IVDD.69,70 These studies suggest that both BM-MSC and Ad-SVF directly injected into the intervertebral disc space may be effective in preserving intervertebral disc morphology and enhancing disc matrix production, although direct comparisons of efficacy of these two cell types have yet to be performed. Optimal BM-MSC dosing (1×106 UC-MSC), established under experimental conditions, provides critical information for future clinical trials for this use in naturally occurring disease models.69
In contrast to MSC-induced clinical improvement in experimental studies, a clinical case series examining efficacy of intralesional BM-MSC injection in dogs with severe, acute spinal cord injury secondary to fracture and luxation treated with surgical stabilization failed to reveal improvement in sensory function in any of the 7 dogs treated.71 It is however important to note that an absence of complications (including infection, neuropathic pain, worsening of neurologic function) along with long-term follow-up (29–62 months), suggests feasibility and safety of such therapy in this preliminary study. Nonetheless, given the small sample size and severity of injury in this clinical assessment, it is prudent that further examination of the ability of MSC therapies to mediate recovery following spinal cord injury be performed in a larger cohort of canine patients before conclusions regarding experimental efficacy are drawn, given the significant improvement in experimental animals and preliminary safety data.
In addition to their potential for treatment of spinal cord injury, data suggests MSC may also be effective in the treatment of other neuromuscular pathologies. BM-MSC have also shown promise for promoting peripheral nerve regeneration and functional recovery in sciatic nerve gaps of dogs.72 Several canine studies also suggest that MSC may prove effective for both the treatment of muscular pathologies, such as Duchenne muscular dystrophy.73 Furthermore, clinical use for acute traumatic muscular injuries in working dogs has met with anecdotal success.74
Cardiac
Another major target for cell-based therapies in humans is cardiovascular disease, given the fact that heart disease and vascular dysfunction are the leading cause of death in developed countries.75 A meta-analysis of studies examining cell-based therapies for ischemic heart disease in large animal experimental models (pig, sheep and dog) confirmed the validity of such models to predict the outcome of clinical trials.76 In addition, approximately 10% of pet dogs develop cardiac disease,77 including chronic valvular disease as well as dilated cardiomyopathy, which underscores the need to develop alternative cell-based strategies to treat these veterinary patients. Although the ability of MSC to transdifferentiate into cardiomyocytes is controversial, MSC have been shown to significantly improve cardiac function and survival in several experimental animal models including the dog.78,79 Additional studies suggest MSC may do this through the production of trophic mediators which protect resident cells and promote neovascularization of ischemic tissues.80 Subsequent studies have sought to optimize MSC-based therapies by establishing superior pre-delivery conditioning (such as pre-treatment with growth factors to induce cardiomyogenic specification prior to transplant)81 as well as delivery methods.82 Newer data suggest that MSC are not only capable of improving myocardial dysfunction, but also conduction deficits. Preliminary studies using a canine model suggest that MSC-based therapies may be a promising new strategy to improve care in patients with cardiac arrhythmias (complete atrioventricular block), as evidenced by their ability to promote atrial and ventricular myocardial fusion and conductivity in conjunction with surgery, over surgery alone.83
Cutaneous
Rejuvenation of healing in impaired and chronic wounds by therapeutic application of adult stem cells has received considerable attention, particularly in murine models of impaired wound healing as well as in clinical human use.84 Despite the utility of rodent models in the initial dissection of mechanisms of action and advantages of mice and rats with respect to strains available, cost, and ethics compared to experimental large animal models, accurate assessment of translatability is of concern since naturally-occurring chronic wounds do not exist in these species. While chronic wounds in client-owned companion animals (dogs, cats and horses) occur naturally, these clinical models have yet to be utilized in studies examining adult stem cell therapies. A single case report detailing the treatment of severe decubital ulcers in a septic neonatal foal with MSC plus platelet rich plasma (PRP), PRP alone or aloe gel showed that healing was fastest in the wound treated with MSC plus PRP.85 Although this case report in itself fails to contribute definitive evidence for the use of stem cells for decubital ulcers in horses, it does offer one example of MSC use in clinical veterinary wound management.
Other animal models have been used to examine the ability of MSC-based therapies to improve quality of repair as well as rejuvenate impaired cutaneous wound healing. Autologous BM-MSC have been shown to improve healing of excisional wounds in a rabbit model86,87, with effects similar to those seen using allogeneic BM-MSC.87 MSC isolated from Wharton’s jelly of caprine umbilical cord also improved quality of repair of excisional wounds in goats by accelerating reepithelialization and diminishing scar tissue formation.88 The ischemic rabbit ear model has been used to establish efficacy of both BM-MSC and Ad-MSC in a model of impaired wound healing.15,25 BM-MSC were shown to improve reepithelialization, neovascularization and granulation tissue formation in this model15, while in another study Ad-MSC positively influenced granulation tissue formation but did not improve reepithelialization.25 Finally, Ad-MSC have been shown to reduce scarring of excisional wounds in Yorkshire pigs89, while several studies suggest autologous BM- and Ad-MSC positively influence healing in a minipig model of cutaneous radiation syndrome.90,91 However their effects in healing excisional wounds in irradiated skin appear less robust since autologous Ad-MSC were found to improve healing only when delivered with PRP while allogeneic Ad-MSC showed no effect.92
Urologic
Several translational studies have investigated the use of MSC to improve health of the urinary system in companion animals. Because of the commonality that exists between canine, feline and human renal disease, translational studies aimed at improving clinical outcomes in these animal models have important implications for similar therapies in human patients. As chronic kidney disease is a significant cause of morbidity and mortality in cats, Quimby et al93 examined the effect of autologous intrarenal MSC therapy in a small number of cats with chronic renal disease. The authors concluded that, while feasible and moderately effective in improving renal function in two of the four cats, alternative cell sources and delivery strategies require optimization prior to recommendation of their further use in a clinical setting. Although cats with chronic renal failure did not show any immediate or late adverse effects to intrarenal MSC therapy, delivery of BM-MSC into the renal artery of dogs induced inflammation, tubular necrosis and mineralization in two healthy dogs, prompting caution in widespread use until additional safety studies are pursued.94 Given the tremendous potential to impact both veterinary and human renal patient care, further investigation in larger case controlled clinical trials is warranted to determine safety and efficacy of stem cell based therapies for chronic and acute renal disease. In addition to its application for renal disease, tissue engineering strategies to promote bladder regeneration using BM-MSC-seeded small intestinal submucosal scaffolds in a canine cystectomy model suggest alternative clinical urologic applications for veterinary and human patients.95
FUTURE DIRECTIONS: EMBRYONIC STEM CELL AND INDUCED PLURIPOTENT STEM CELL APPLICATIONS IN LARGE ANIMAL MODELS AND THEIR ROLE IN TRANSLATIONAL MEDICINE
While cell-based therapies possess great potential for the treatment of several degenerative diseases, the ideal cell type has yet to be determined. Depending on the differentiation potential, stem cells can be classified as toti-, pluri-, multi-, and uni- potent cells. The multipotent adult mesenchymal stem cells discussed thus far have the ability to differentiate into osteoblasts, adipocytes and chondrocytes. Embryonic stem cells (ESC), on the other hand, are considered by some to have superior potential in regenerative medicine due not only to their capacity for self-renewal but also to their ability to differentiate into cells of all three germ layers (endoderm, mesoderm and ectoderm). As such, they theoretically can develop into any cell in the body. In addition, ESC represent a powerful tool for studying cell fate determination, epigenetic regulation, and disease. Pluripotent ESC were established from mice in 1981,96 in non-human primates by veterinarian James Thomson in 199597 and finally, in humans (also by Thomson) in 1998.98 However, ethical and legislative issues associated with the harvest from embryonic sources have hindered investigations for human applications. Moreover, ESC present several problems for cell transplant therapy including the possibility of contamination from mouse feeder layers or animal serum-supplemented medium with pathogens or xenogens that might trigger host immune reactions; the possibility of teratoma formation because current protocols produce differentiated cell populations that are no more than 80% pure; and epigenetic instability due to a vulnerability to environmental or culture conditions.99 Therefore, translational studies which successfully capitalize on the advantages of ESC while establishing strategies to promote their safe use in large animal models have tremendous potential to improve both human and veterinary health.
Paramount to the development of such studies is the ability to establish ESC from non-rodent or non-human primate veterinary species; however, this milestone has proven elusive in spite of decades of intense trials. Putative ESC lines have been derived from both companion and farm animals (Table 1) including the dog,100 cat,101 rabbit,102 horse,103,104 pig,105,106 cow,107,108 and sheep;109 however, the common problem faced by laboratories worldwide is loss of pluripotency over a relatively short number of passages and in vivo. Moreover, none of these ES-like cell lines has been definitively proven to be ESC, because of the paucity of appropriate species-specific markers.110 However, researchers working with large animal species now have new resources (e.g. gene banks, BAC libraries, microarrays and quantitative real-time PCR) which, along with the rapid advances being made in mouse and human stem cell biology, should aid in identifying specific markers of pluripotency.111 Finally, no ES-like cell line from farm animals has been used successfully as a biological reagent in a manner similar to the use of human, monkey or mouse ESC (i.e. directed pluripotent in vitro differentiation or as a means of genetically engineering a mammal through embyonic chimera formation).111 The identification of appropriate stem cell markers, functional cytokine pathways, and key pluripotency-maintaining factors along with the release of more comprehensive genomes of veterinary species, provide encouragement for establishment of nonmurine, non-primate ESC lines in the near future.111
Clinical use of these cells is currently being investigated as a superior cell-based therapy for the treatment of experimentally-induced equine tendon lesions.50,51 In a small blinded, placebo-controlled, short-term study, Watts and colleagues showed clinical improvement following intra-lesional injection of fetal-derived ES-like cells into an experimental model of SDFT injury, providing compelling evidence for further development of pluripotent cell-based therapies for equine tendon injuries.51 Anecdotal evidence from several hundred equine patients treated with ES-like cells suggests that this is a promising and safe therapy for equine tendinopathy, although there currently are no published data in such a population.52 Randomized, controlled studies comparing clinical efficacy of Ad-SVF, BM- and Ad-MSC and putative ESC in naturally occurring tendon injuries is warranted to determine superior cell source and timing of delivery for maximal clinical benefit.
The pioneering work by the Yamanaka group which identified four transcription factors capable of reprogramming somatic mouse cells into induced pluripotent stem cells (iPSC) allowed for generation of pluripotent cells while circumventing the controversial use of embryos.112 The potential value of iPSC derives from their characteristics of infinite expansion, clonal isolation, differentiation into any cell type in the body in vitro and in vivo, and incorporation into the germline of chimeric animals.1 These features allow the knock-in or knock-out of numerous genes, the creation of homogeneous populations of altered cells, and the engineering of cell lines and animals that have desirable characteristics previously obtainable only in the mouse. Because iPSC can be developed from a patient’s own somatic cells, it is theorized that this will prevent any immunogenic responses, though Zhao et al have recently challenged this assumption113.
Breakthroughs in the generation of iPSC raise the hope that it will one day be possible to screen patients for a genetic cause of disease, develop autologous cell lines, reprogram them back to iPSC and finally differentiate them into the cell types that develop the disease, to study its process. That being said, a number of challenges must be addressed in order to effectively use these cell lines for disease modeling. These include the low efficiency of iPSC generation without genetic alterations, the possibility of tumor formation in vivo, the random integration of retroviral-based delivery vectors into the genome and unregulated growth of the remaining cells that are partially reprogrammed and refractory to differentiation.114 Moreover, to one day apply iPSC-based therapies to the clinical setting, preclinical evaluations using large animal models are indispensable for evaluation of feasibility and safety.
Fundamental groundwork for translational studies of iPSC applications in large animal models (both companion and farm animals) include the development of canine, equine, porcine, ovine and bovine iPSC. Canine iPSC were first generated from canine embryonic fibroblasts115 and shortly thereafter, using canine adult fibroblasts116 and adipose stromal cells,117 with validation of their in vivo fate following autologous transplantation into canine hearts. The clinical potential of these canine iPSC was demonstrated by generating endothelial cells from the iPSC which were then used to treat immunodeficient murine models of myocardial infarction and hindlimb ischemia.117 To date, there are no published reports characterizing feline iPSC. In contrast, two groups have reported successful generation of equine iPSC, including Khodadadi et al who generated equine iPSC by retroviral-mediated transduction of adult equine fibroblasts.118,119 Canine and equine iPSC lines generated from adult cells hold the promise of developing a whole new range of autologous stem cell-based regenerative therapies in companion animal medicine as well as aiding the development of preclinical models for human applications. The successful generation of iPSC from pigs,120–122 cows,123,124 and sheep127 has also paved the way for the use of iPSC for biotechnological and agricultural purposes.
OPTIMIZATION OF STEM CELL THERAPIES IN COMPANION ANIMALS
As detailed above, both experimental studies in large animal models and clinical trials in companion animals provide evidence that cell-based treatments represent an effective alternative to traditional medical options. Considering the mainstream media coverage and the hype associated with the real, and perceived, hope of veterinary stem cell therapies, thousands of pet owners and their veterinarians have turned to such treatment options. In contrast to the slower translation of such therapies in human medicine, cell-based veterinary therapies have been rapidly translated from experimental models (in the same or different species) to mainstream clinical practice due to the current, comparatively liberal legal and regulatory environment for such therapies. Unfortunately, this rapid commercialization and utilization of veterinary cell-based therapies has occurred without appropriate evidence-based clinical veterinary studies.
It is clear that regenerative medicine has immense potential for the treatment of a variety of naturally occurring diseases shared by both companion animals and humans, however, additional veterinary clinical trials are needed to provide evidence for purported benefits that have yet to be substantiated, as well as optimization of therapies for which evidence of use has already been supported through studies. Continued basic research focused on stem cells of companion animals, particularly ESC and iPSC is needed. Furthermore, blinded, randomized and controlled clinical trials to determine efficacy of cell-based therapies in clinical veterinary patients are needed, although challenges with enrollment in such randomized, placebo controlled studies may slow such progress. Such studies remain critical to determining efficacy for unsubstantiated therapeutic applications as well as optimizing therapies for which stem cells have already been proven efficacious.
Optimization of therapies is required at several levels, including defining donor cell characteristics (such as age of donor or site of harvest), pre-delivery strategies (isolation, enrichment or pre-delivery conditioning) and delivery (timing of and route of delivery, biomaterial delivery and recipient characteristics) associated with maximal therapeutic response (see Figure 1). Future studies and collaborative efforts of investigators to promote evidence-based practice of regenerative medical therapies will be critical to establish which interventions should be promoted and optimized. Critical to achieving these goals has been the formation of several key organizations, including the North American Veterinary Regenerative Medicine Association (NAVRMA) and the International Veterinary Regenerative Medicine Society (IVRMS) that aim to foster exchange and distribution of scientific information and its clinical applications between research investigators, veterinary practitioners and industry.
Figure 1. Schematic illustration of strategies for optimization of cell-based therapies in veterinary regenerative medicine.
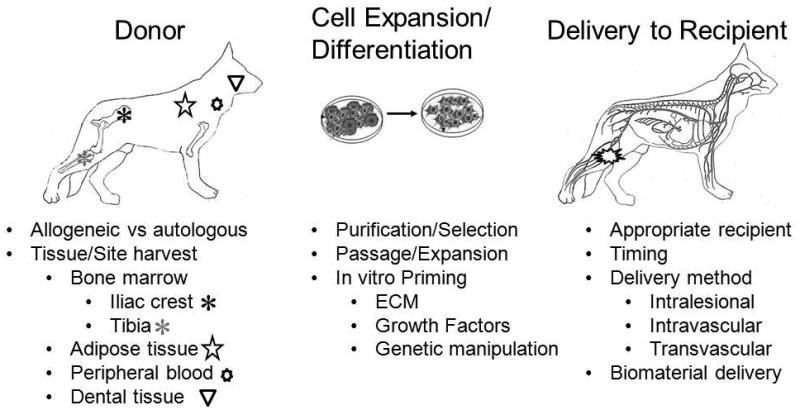
Optimization of stem cell therapies to maximize therapeutic potential may involve identification of superior donor cell populations and characteristics, pre-delivery cell expansion/differentiation protocols, and methods for delivery. Clinical trials involving companion animals as large animal translational models will provide important contributions in identifying under what circumstances allogenic or autologous MSC-based therapies are preferred as well as the optimal site from which to harvest these cells. Elucidation of pre-delivery strategies which improve isolation and enrichment of cells or “prime” desired responses of delivered cells through in vitro manipulation with extracellular matrix components (ECM), growth factors or genetic manipulation may further enhance therapeutic efficacy. Finally, the full potential of cell based therapies can only be realized with identification of appropriate recipient populations, optimal timing and route of delivery, as well as biomaterials that may enhance proregenerative activities of delivered stem cells.
CONCLUSIONS
It is an extremely exciting time for veterinary regenerative medicine, as companion animals are able to receive cutting edge therapies that are often not yet available to their human counterparts. However, in the current state, it is unclear whether some therapies offered clinically are truly efficacious. Companion animals suffer from a wide variety of naturally occurring pathologic conditions that lack effective therapies and for which regenerative medicine may provide superior options to those that currently exist. Adult stem cell therapies are currently being used by primary care veterinarians, particularly for orthopaedic conditions. A large proportion of the pet owning population is looking towards regenerative medicine and these veterinary patients will be a critical component of future clinical trials. Establishment of confirmed ESC and iPSC in companion animals is required to provide insight into their potential clinical application in veterinary and human medicine. Although the use of stem cell-based therapies has tremendous potential for advancing treatment options for companion animals, further evidence-based studies in clinical patients are warranted to substantiate their efficacy. Such clinical trials will also be critical in predicting efficacy and optimizing therapy for human patients, when appropriate and clinically relevant veterinary models have been identified.
Acknowledgments
No external funding was received for this perspective review. SWV would like to acknowledge support for translational stem cell work in her laboratory from NIAMS (K08AR053945) and the Canine Health Foundation (CHF00970 and CHF01876-A). The authors wish to thank Laura Parnell for the original idea of this mini-review. This work was presented at the 2012 annual meeting of the Wound Healing Society in Atlanta GA.
Footnotes
The authors report no conflict of interest.
References
- 1.West F, Stice S. Progress toward generating informative porcine biomedical models using induced pluripotent stem cells. Ann New York Academy Sci. 2011;1245(1):21–3. doi: 10.1111/j.1749-6632.2011.06337.x. [DOI] [PubMed] [Google Scholar]
- 2.Kahn LH, Kaplan B, Monath TP, Steele JH. Teaching “one medicine, one health”. Am J Med. 2008;121:169–70. doi: 10.1016/j.amjmed.2007.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. [accessed 12-12-0012];One Health Initiative. 2012 www.onehealthinitiative.com/
- 4.Nobert KM. The regulation of veterinary regenerative medicine and the potential impact of such regulation on clinicians and firms commercializing these treatments. Vet Clin Equine. 2011;27:383–91. doi: 10.1016/j.cveq.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Online Mendelian Inheritance of Animals. Faculty of Veterinary Science. University of Sydney and Australian National Genomic Information Service; 2007. [Google Scholar]
- 6.Parker HG, Shearin AL, Ostrander EA. Man’s best friend becomes biology’s best in show: Genome analyses in the domestic dog. Annu Rev Genet. 2010;44:309–36. doi: 10.1146/annurev-genet-102808-115200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown DC. Control of selection bias in parallel-group controlled clinical trials in dogs and cats: 97 trials (2000–2005) J Am Vet Med Assoc. 2006;229(6):990–3. doi: 10.2460/javma.229.6.990. [DOI] [PubMed] [Google Scholar]
- 8.Thomas ED, Storb R. The development of the scientific foundation of hematopoietic cell transplantation based on animal and human studies. In: Thomas ED, Blume KG, Forman SJ, editors. Hematopoietic Cell Transplantation. Boston: Blackwell Science; 1999. pp. 1–11. [Google Scholar]
- 9.Deeg HJ, Storb R. Canine marrow transplantation models. Curr Topics Vet Res. 1994;1:103– 14. [Google Scholar]
- 10.Suter SE. Collection of peripheral blood CD34+ progenitor cells from healthy dogs and dogs diagnosed with lymphoproliferative diseases using a Baxter-Fenwal CS-3000 Plus Blood Cell Separator. J Vet Intern Med. 2011;25:1406–13. doi: 10.1111/j.1939-1676.2011.00827.x. [DOI] [PubMed] [Google Scholar]
- 11.Escobar C, Grindem C, Neel JA, Suter SE. Hematologic changes after total body irradiation and autologous transplantation of hematopoietic peripheral blood progenitor cells in dogs with lymphoma. Vet Pathol. 2012;49(2):341–3. doi: 10.1177/0300985811410721. [DOI] [PubMed] [Google Scholar]
- 12.Volk SW, Wang Y, Hankenson KD. Effects of donor characteristics and ex vivo expansion on canine mesenchymal stem cell properties: Implications for MSC-based therapies. Cell Transplant. 2012 doi: 10.3727/096368912X636821. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin DR, Cox NR, Hathcock TL, Niemeyer GP, Baker HJ. Isolation and characterization of multipotential mesenchymal stem cells from feline bone marrow. Exp Hematol. 2002;30:879–86. doi: 10.1016/s0301-472x(02)00864-0. [DOI] [PubMed] [Google Scholar]
- 14.Webb TL, Quimby JM, Dow SW. In vitro comparison of feline bone marrow-derived and adipose tissue-derived mesenchymal stem cells. J Feline Med Surg. 2012;14:165–8. doi: 10.1177/1098612X11429224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Volk SW, Radu A, Zhang L, Liechty KW. Stromal progenitor cell therapy corrects the wound healing defect in the ischemic rabbit ear model of chronic wound repair. Wound Rep Reg. 2007;15:736–47. doi: 10.1111/j.1524-475X.2007.00277.x. [DOI] [PubMed] [Google Scholar]
- 16.Fan ZX, Lu Y, Deng L, Li XQ, Zhi W, Li-Ling J, Yang ZM, Xie HQ. Placenta- versus bone-marrow-derived mesenchymal cells for the repair of segmental bone defects in a rabbit model. FEBS J. 2012;279(13):2455–65. doi: 10.1111/j.1742-4658.2012.08625.x. [DOI] [PubMed] [Google Scholar]
- 17.Ahren BJ, Schaer TP, Terkhorn SP, Jackson KV, Mason NJ, Hankenson KD. Evaluation of equine peripheral blood apheresis product, bone marrow, and adipose tissue as sources of mesenchymal stem cells and their differentiation potential. Am J Vet Res. 2011;72:127–33. doi: 10.2460/ajvr.72.1.127. [DOI] [PubMed] [Google Scholar]
- 18.Frisbie DD, Kisiday JD, Kawcak CE, Werpy NM, McIlwraith CW. Evaluation of adipose-derived stromal vascular fraction or bone marrow-derived mesenchymal stem cells for treatment of osteoarthritis. J Orthop Res. 2009;27:1675–80. doi: 10.1002/jor.20933. [DOI] [PubMed] [Google Scholar]
- 19.Vidal MA, Robinson SO, Lopez MJ, Paulsen DB, Borkhsenious O, Johnson JR, Moore RM, Gimble JM. Comparison of chondrogenic potential in equine mesenchymal stromal cells derived from adipose tissue and bone marrow. Vet Surg. 2008;37:713–24. doi: 10.1111/j.1532-950X.2008.00462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stockmann P, Park J, von Wilmowsky C, Nkenke E, Felszeghy E, Dehner JF, Schmitt C, Tudor C, Schlegel KA. Guided bone regeneration in pig calvarial bone defects using autologous mesenchymal stem/progenitor cells: A comparison of different tissue sources. J Craniomaxillofac Surg. 2012;40(4):310–20. doi: 10.1016/j.jcms.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Erickson I, van Veen S, Sengupta S, Kestle S, Mauck R. Cartilage matrix formation by bovine mesenchymal stem cells in three-dimensional culture is age-dependent. Clin Orthop Rel Res. 2011;469(10):2744–53. doi: 10.1007/s11999-011-1869-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cao L, Liu G, Gan Y, Fan Q, Yang F, Zhang X, Tang T, Dai K. The use of autologous enriched bone marrow MSCs to enhance osteoporotic bone defect repair in long-term estrogen deficient goats. Biomaterials. 2012;33(20):5076–84. doi: 10.1016/j.biomaterials.2012.03.069. [DOI] [PubMed] [Google Scholar]
- 23.Niemeyer P, Fechner K, Milz S, Richter W, Suedkamp NP, Mehlhorn AT, Pearce S, Kasten P. Comparison of mesenchymal stem cells from bone marrow and adipose tissue for bone regeneration in a critical size defect of the sheep tibia and the influence of platelet-rich plasma. Biomaterials. 2010;31(13):3572–9. doi: 10.1016/j.biomaterials.2010.01.085. [DOI] [PubMed] [Google Scholar]
- 24.Neupane M, Chang C-C, Kiupel M, Yuzbasiyan-Gurkan V. Isolation and characterization of canine adipose-derived mesenchymal stem cells. Tissue Eng. 2008;14(6):1007–15. doi: 10.1089/ten.tea.2007.0207. [DOI] [PubMed] [Google Scholar]
- 25.Steinberg JP, Hong SJ, Geringer MR, Galiano RD, Mustoe TA. Equivalent effects of topically-delivered adipose-derived stem cells and dermal fibroblasts in the ischemic rabbit ear model for chronic wounds. Aesthet Surg J. 2012;32(4):504–19. doi: 10.1177/1090820X12442679. [DOI] [PubMed] [Google Scholar]
- 26.Zhao Y, Waldman SD, Flynn LE. The effect of serial passaging on the proliferation and differentiation of bovine adipose-derived stem cells. Cells Tissues Organs. 2012;195(5):414–27. doi: 10.1159/000329254. [DOI] [PubMed] [Google Scholar]
- 27.Lim J-H, Byeon H-H, Jeong YH, Lee Y-W, Kim WH, Kang K-S, Kweon O-K. Transplantation of canine umbilical cord-blood-derived mesenchymal stem cells in experimentally induced spinal cord injured dogs. J Vet Sci. 2007;8(3):275–82. doi: 10.4142/jvs.2007.8.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin GZ, Yin XJ, Yu XF, Cho SJ, Choi EG, Lee YS, Jeon JT, Yee ST, Kong IK. Generation of neuronal-like cells from umbilical cord blood-derived mesenchymal stem cells of a RFP-transgenic cloned cat. J Vet Med Sc. 2008;70(7):723–6. doi: 10.1292/jvms.70.723. [DOI] [PubMed] [Google Scholar]
- 29.Vidal MA, Walker NJ, Napoli E, Borjesson DL. Evaluation of senescence in mesenchymal stem cells isolated from equine bone marrow, adipose tissue, and umbilical cord tissue. Stem Cells Dev. 2012;21(2):273–83. doi: 10.1089/scd.2010.0589. [DOI] [PubMed] [Google Scholar]
- 30.Carrade DD, Owens SD, Galuppo LD, Vidal MA, Ferraro GL, Librach F, Buerchler S, Friedman MS, Walker NJ, Borjesson DL. Clinicopathologic findings following intra-articular injection of autologous and allogeneic placentally derived equine mesenchymal stem cells in horses. Cytotherapy. 2010;13(4):419–30. doi: 10.3109/14653249.2010.536213. [DOI] [PubMed] [Google Scholar]
- 31.Kumar BM, Yoo JG, Ock SA, Lim JG, Song HJ, Kang EJ, Cho SK, Lee SL, Cho JH, Balasubramanian S, Rho GJ. In vitro differentiation of mesenchymal progenitor cells derived from porcine umbilical cord blood. Mol Cells. 2007;24(3):343–50. [PubMed] [Google Scholar]
- 32.Raoufi MF, Tajik P, Dehghan MM, Eini F, Barin A. Isolation and differentiation of mesenchymal stem cells from bovine umbilical cord blood. Reprod Domest Anim. 2011;46(1):95–9. doi: 10.1111/j.1439-0531.2010.01594.x. [DOI] [PubMed] [Google Scholar]
- 33.Jäger M, Bachmann R, Scharfstädt A, Krauspe Ovine cord blood accommodates multipotent mesenchymal progenitor cells. In Vivo. 2006;20(2):205–14. [PubMed] [Google Scholar]
- 34.Qiu P, Bai Y, Liu C, He X, Cao H, Li M, Zhu H, Hua J. A dose-dependent function of follicular fluid on the proliferation and differentiation of umbilical cord mesenchymal stem cells (MSCs) of goat. Histochem Cell Biol. 2012:1–11. doi: 10.1007/s00418-012-0975-7. [DOI] [PubMed] [Google Scholar]
- 35.Kisiel AH, McDuffee LA, Masaoud E, Bailey TR, Esparza Gonzalez BP, Nino-Fong R. Isolation, characterization, and in vitro proliferation of canine mesenchymal stem cells derived from bone marrow, adipose tissue, muscle, and periosteum. Am J Vet Res. 2012;73(8):1305–17. doi: 10.2460/ajvr.73.8.1305. [DOI] [PubMed] [Google Scholar]
- 36.Hui JH, Li L, Teo YH, Ouyang HW, Lee EH. Comparative study of the ability of mesenchymal stem cells derived from bone marrow, periosteum, and adipose tissue in treatment of partial growth arrest in rabbit. Tissue Eng. 2005;11(5–6):904–12. doi: 10.1089/ten.2005.11.904. [DOI] [PubMed] [Google Scholar]
- 37.Mrozik KM, Zilm PS, Bagley CJ, Hack S, Hoffman P, Gronthos S, Bartold PM. Proteomic characterization of mesenchymal stem cell-like populations derived from ovine periodontal ligament, dental pulp, and bone marrow: analysis of differentially expressed proteins. Stem Cells Dev. 2010;19(10):1485–99. doi: 10.1089/scd.2009.0446. [DOI] [PubMed] [Google Scholar]
- 38.Volk SW, Diefenderfer DL, Christopher SA, Haskins ME, Leboy PS. Effects of osteogenic inducers on cultures of canine mesenchymal stem cells. Am J Vet Res. 2005;66(10):1729–37. doi: 10.2460/ajvr.2005.66.1729. [DOI] [PubMed] [Google Scholar]
- 39.Toupadakis CA, Wong A, Genetos DC, Cheung WK, Borjesson DL, Ferraro GL, Galuppo LD, Leach JK, Owens SD, Yelloley CE. Comparison of the osteogenic potential of equine mesenchymal stem cells from bone marrow, adipose tissue, umbilical cord blood, and umbilical tissue. Am J Vet Res. 2010;71:1237–45. doi: 10.2460/ajvr.71.10.1237. [DOI] [PubMed] [Google Scholar]
- 40.Liu TM, Martina M, Hutmacher DW, Hui JH, Lee EH, Lim B. Identification of common pathways mediating differentiation of bone marrow- and adipose tissue-derived human mesenchymal stem cells into three mesenchymal lineages. Stem Cells. 2007;25:750–60. doi: 10.1634/stemcells.2006-0394. [DOI] [PubMed] [Google Scholar]
- 41.Black LL, Gaynor J, Adams C, Dhupa S, Sams AE, Taylor R, Harmon S, Gingerich DA, Harmon R. Effect of intraarticular injection of autologous adipose-derived mesenchymal stem and regenerative cells on clinical signs of chronic osteoarthritis of the elbow joint in dogs. Vet Ther. 2008;9(3):192–200. [PubMed] [Google Scholar]
- 42.Black LL, Gaynor J, Gahring D, Adams C, Aron D, Harman S, Gingerich DA, Harmon R. Effect of adipose-derived mesenchymal stem and regenerative cells on lameness in dogs with chronic osteoarthritis of the coxofemoral joints: a randomized, double-blinded, multicenter, controlled trial. Vet Ther. 2007;8(4):272–84. [PubMed] [Google Scholar]
- 43.Dowling BA, Dart AJ, Hodgson DR, Smith RKW. Superficial digital flexor tendonitis in the horse. Equine Vet J. 2000;32(5):369–78. doi: 10.2746/042516400777591138. [DOI] [PubMed] [Google Scholar]
- 44.Bergenhuyzen AL, Vermote KA, van Bree H, Van Ryssen B. Long-term follow-up after arthroscopic tenotomy for partial rupture of the biceps brachii tendon. Vet Comp Orthop Traumatol. 2010;23(1):51–3. doi: 10.3415/VCOT-09-01-0005. [DOI] [PubMed] [Google Scholar]
- 45.Awad HA, Butler DL, Boivin GP, Smith FN, Malaviya P, Huibregtse B, Caplan AI. Autologous mesenchymal stem cell-mediated repair of tendon. Tissue Eng. 1999;5(3):267–77. doi: 10.1089/ten.1999.5.267. [DOI] [PubMed] [Google Scholar]
- 46.Nixon AJ, Dahlgren LA, Haupt JL, Yeager AE, Ward DL. Effect of adipose-derived nucleated cell fractions on tendon repair in horses with collagenase-induced tendonitis. Am J Vet Res. 2008;69:928–37. doi: 10.2460/ajvr.69.7.928. [DOI] [PubMed] [Google Scholar]
- 47.Schnabel LV, Lynch ME, van der Meulen MC, Yeager AE, Kornatowski MA, Nixon AJ. Mesenchymal stem cells and insulin-like growth factor-I gene-enhanced mesenchymal stem cells. J Orthop Res. 2009;27:1392–8. doi: 10.1002/jor.20887. [DOI] [PubMed] [Google Scholar]
- 48.Pacini S, Spinabella S, Trombi L, Fazzi R, Galimberti S, Dini F, Carlucci F, Petrini M. Suspension of bone marrow-derived undifferentiated mesenchymal stromal cells for repair of superficial digital flexor tendon in race horses. Tiss Eng. 2007;13(12):2949–55. doi: 10.1089/ten.2007.0108. [DOI] [PubMed] [Google Scholar]
- 49.Godwin EE, Young NJ, Dudhia J, Beamish IC, Smith RKW. Implantation of bone marrow-derived mesenchymal stem cells demonstrates improved outcome in horses with overstrain injury of the superficial digital flexor tendon. Equine Vet J. 2011;44:25–32. doi: 10.1111/j.2042-3306.2011.00363.x. [DOI] [PubMed] [Google Scholar]
- 50.Guest DJ, Smith MRW, Allen WR. Equine embryonic stem-like cells and mesenchymal stromal cells have different survival rates and migration patterns following their injection into damaged superficial digital flexor tendons. Equine Vet J. 2010;42(7):636–42. doi: 10.1111/j.2042-3306.2010.00112.x. [DOI] [PubMed] [Google Scholar]
- 51.Watts AE, Yeager AE, Kopyov OV, Nixon AJ. Fetal derived embryonic-like stem cells improve healing in a large animal flexor tendonitis model. Stem Cell Res & Ther. 2011;2(4):4–16. doi: 10.1186/scrt45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nixon AJ, Watts AE, Schnabel LV. Cell- and gene-based approaches to tendon regeneration. J Shoulder Elbow Surg. 2012;21:278–94. doi: 10.1016/j.jse.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 53.Del Bue M, Ricco S, Ramoni R, Conti V, Gnudi G, Grolli S. Equine adipose-tissue derived mesenchymal stem cells and platelet concentrates: their association in vitro and in vivo. Vet Res Comm. 2008;32(Suppl 1):51–5. doi: 10.1007/s11259-008-9093-3. [DOI] [PubMed] [Google Scholar]
- 54.Crovace A, Favia A, Lacitignola L, Di Comite MS, Staffieri F, Francioso E. Use of autologous bone marrow mononuclear cells and cultured bone marrow stromal cells in dogs with orthopaedic lesions. Vet Res Comm. 2008;32(Suppl 1):39–44. doi: 10.1007/s11259-008-9095-1. [DOI] [PubMed] [Google Scholar]
- 55.Bruder SP, Jaiswal N, Ricalton NS, Mosca JD, Kraus KH, Kadiyala S. Mesenchymal stem cells in osteobiology and applied bone regeneration. Clin Orthop Rel Res. 1998;355:247–56. doi: 10.1097/00003086-199810001-00025. [DOI] [PubMed] [Google Scholar]
- 56.Cui L, Liu B, Liu G, Zhang W, Cen L, Sun J, Yin S, Liu W, Cao Y. Repair of cranial bone defects with adipose derived stem cells and coral scaffold in a canine model. Biomaterials. 2007;28:5477–86. doi: 10.1016/j.biomaterials.2007.08.042. [DOI] [PubMed] [Google Scholar]
- 57.Kon E, Muraglia A, Corsi A, Bianco P, Marcacci M, Martin I, Boyde A, Ruspantini I, Chistolini P, Rocca M, Giardino R, Cancedda R, Quarto R. Autologous bone marrow stromal cells loaded onto porous hydroxyapatite ceramic accelerate bone repair in critical-size defects of sheep long bones. J Biomed Mater Res. 2000;49(3):328–37. doi: 10.1002/(sici)1097-4636(20000305)49:3<328::aid-jbm5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 58.Umeda H, Kanemaru SI, Yamashita M, Ohno T, Suehiro A, Tamura Y, Hirano S, Nakamura T, Omori K, Ito J. In situ tissue engineering of canine skull with guided bone regeneration. Acta Otolaryngol. 2009;129(12):1509–18. doi: 10.3109/00016480902801212. [DOI] [PubMed] [Google Scholar]
- 59.Arinzeh T, Peter S, Archambault M, van den Bos C, Gordon S, Kraus K, Smith A, Kadiyala S. Allogeneic mesenchymal stem cells regenerate bone in a critical-sized canine segmental defect. J Bone Joint Surg. 2003;85-A:1927–35. doi: 10.2106/00004623-200310000-00010. [DOI] [PubMed] [Google Scholar]
- 60.Yuan J, Zhang WJ, Liu G, Qi ZL, Liu W, Cui L, Cao YL. Repair of canine mandibular bone defects with bone marrow stromal cells and coral. Tissue Eng Pt A. 2010;16(4):1385–94. doi: 10.1089/ten.TEA.2009.0472. [DOI] [PubMed] [Google Scholar]
- 61.Weng Y, Wang M, Liu W, Hu X, Chai G, Yan Q, Zhu L, Cui L, Cao Y. Repair of experimental alveolar bone defects by tissue-engineered bone. Tiss Eng. 2006;12(6):1503–13. doi: 10.1089/ten.2006.12.1503. [DOI] [PubMed] [Google Scholar]
- 62.Muschler GF, Marsukura Y, Nitto H, Boehm CA, Valdevit AD, Kambic HE, Davros WJ, Easley KA, Powell KA. Selective retention of bone marrow-derived cells to enhance spinal fusion. Clin Orthop Rel Res. 2005;432:242–51. doi: 10.1097/01.blo.0000149812.32857.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ishihara A, Bertone AL. Cell-mediated and direct gene therapy for bone regeneration. Expert Opin Biol Ther. 2012;12(4):411–23. doi: 10.1517/14712598.2012.661709. [DOI] [PubMed] [Google Scholar]
- 64.Wilke MM, Nydam DV, Nixon AJ. Enhanced early chondrogenesis in articular defects following arthroscopic mesenchymal stem cell implantation in an equine model. J Orthop Res. 2007;25(7):913–25. doi: 10.1002/jor.20382. [DOI] [PubMed] [Google Scholar]
- 65.McIlwraith CW, Frisbie DD, Rodkey WG, Kisiday JD, Werpy NM, Kawcak CE, Steadman JR. Evaluation of intra-articular mesenchymal stem cells to augment healing of microfractured chondral defects. Arthroscopy. 2011;27(11):1552–61. doi: 10.1016/j.arthro.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 66.Murphy JM, Fink DJ, Hunziker EB, Barry FP. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 2003;48(12):3464–74. doi: 10.1002/art.11365. [DOI] [PubMed] [Google Scholar]
- 67.Jung DL, Ha J, Kang BT, Kim J-W, Quan F-S, Lee J-H, Woo E-J, Park H-M. A comparison of autologous and allogeneic bone marrow-derived mesenchymal stem cell transplantation in canine spinal cord injury. J Neurolog Sci. 2009;285:67–77. doi: 10.1016/j.jns.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 68.Park S-S, Byeon Y-E, Ryu H-H, Kang B-J, Kim Y-S, Kim W-H, Kang K-S, Han H-J, Kweon O-K. Comparison of canine umbilical cord blood-derived mesenchymal stem cell transplantation times: Involvement of astrogliosis, inflammation, intracellular actin cytoskeleton pathways, and neurotropin-3. Cell Transplant. 2011;20:1867–80. doi: 10.3727/096368911X566163. [DOI] [PubMed] [Google Scholar]
- 69.Sergiano K, Sakai D, Hiyama A, Tamura F, Tanaka M, Mochida J. Effect of cell number on mesenchymal stem cell transplantation in a canine disc degeneration model. J Orthop Res. 2010;28:1267–75. doi: 10.1002/jor.21147. [DOI] [PubMed] [Google Scholar]
- 70.Ganey T, Hutton WC, Moseley T, Hedrick M, Meisel H-J. Intervertebral disc repair using adipose tissue-derived stem and regerative cells. Spine. 2009;34(21):2297–304. doi: 10.1097/BRS.0b013e3181a54157. [DOI] [PubMed] [Google Scholar]
- 71.Nishida H, Nakayama M, Tanaka H, Kitamura M, Hatoya S, Sugiura K, Harada Y, Suzuki Y, Ide C, Inaba T. Safety of autolgous bone marrow stromal cell translantation in dogs with acute spinal cord injury. Vet Surg. 2012;41:437–42. doi: 10.1111/j.1532-950X.2011.00959.x. [DOI] [PubMed] [Google Scholar]
- 72.Ding F, Wu J, Yang Y, Hu W, Zhu Q, Tang X, Liu J, Gu X. Use of tissue-engineered nerve grafts consisting of a Chitosan/Poly(lactic-co-glycolic acid)-based scaffold included with bone marrow mesenchymal cells for bridging 50-mm dog sciatic nerve gaps. Tissue Eng Pt A. 2010;16(12):3779–90. doi: 10.1089/ten.TEA.2010.0299. [DOI] [PubMed] [Google Scholar]
- 73.Nitahara-Kasahara Y, Hayashita-Kinoh H, Ohshima-Hosoyama S, Okada H, Wada-Maeda M, Nakamura A, Okada T, Takeda S. Long-term engraftment of multipotent mesenchymal stromal cells that differentiate to form myogenic cells in dogs with Duchenne muscular dystrophy. Mol Ther. 2012;20(1):168–77. doi: 10.1038/mt.2011.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brown GS, Harman RJ, Black LL. Adipose-derived stem cell therapy for severe muscle tears in working German sheperds: two case reports. Stem Cell Discovery. 2012;2:41–4. [Google Scholar]
- 75.Mendis S, Puska P, Norrving B. Global atlas on cardiovascular disease prevention and control. Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- 76.van der Spoel TIG, Jansen of Lorkeers S, Agostoni P, van Belle E, Gyöngyösi M, Sluijter JPG, Cramer MJ, Doevendans PA, Chamuleau SAJ. Human relevance of pre-clinical studies in stem cell therapy: systematic review and meta-analysis of large animal models of ischaemic heart disease. Cardiovasc Res. 2011;91(4):649–58. doi: 10.1093/cvr/cvr113. [DOI] [PubMed] [Google Scholar]
- 77.Atkins C, Bonagura J, Ettinger S, Fox P, Gordon S, Haggstrom J, Hamlin R, Keene B, Luis-Fuentes V, Stepien R. Guidelines for the diagnosis and treatment of canine chronic valvular heart disease. J Vet Intern Med. 2009;23(6):1142–50. doi: 10.1111/j.1939-1676.2009.0392.x. [DOI] [PubMed] [Google Scholar]
- 78.Silva GV, Litovsky S, Assad JAR, Sousa ALS, Martin BJ, Vela D, Coulter SC, Lin J, Ober J, Vaughn WK, Branco RVC, Oliveria EM, He R, Geng Y-J, Willerson JT, Perin EC. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation. 2005;111:150–6. doi: 10.1161/01.CIR.0000151812.86142.45. [DOI] [PubMed] [Google Scholar]
- 79.Vela DC, Silva GV, Assad JAR, Sousa ALS, Coulter S, Fernandes MR, Perin EC, Willerson JT, Buja LM. Histopathological study of healing after allogenic mesenchymal stem cell delivery in myocardial infarction in dogs. J Histochem Cytochem. 2009;57(2):167–76. doi: 10.1369/jhc.2008.952507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, Barr S, Fuchs S, Epstein SE. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109:1543–9. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 81.Bartunek J, Croissant JD, Wijns W, Gofflot S, de Lavareille A, Vanderheyden M, Kaluzhny Y, Mazouz N, Willemsen P, Penicka M, Mathieu M, Homsy C, De Bruyne B, McEntee K, Lee IW, Heyndrickx GR. Pretreatment of adult bone marrow mesenchymal stem cells with cardiomyogenic growth factors and repair of the chronically infarcted myocardium. Am J Physiol Hear Circ Physiol. 2007;292:1095–104. doi: 10.1152/ajpheart.01009.2005. [DOI] [PubMed] [Google Scholar]
- 82.Perin EC, Silva GV, Assad JAR, Vela D, Buja LM, Sousa ALS, Litovsky S, Lin J, Vaughn WK, Coulter S, Fernandes MR, Willerson JT. Comparison of intracoronary and transendocardial delivery of allogeneic mesenchymal cells in a canine model of acute myocardial infaction. J Mol Cell Cardiol. 2007;44:486–95. doi: 10.1016/j.yjmcc.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 83.Hou Y-B, Zou C-W, Wang Y, Li D-C, Li Q-B, Li H-X, Zhang H-Z, Zhang Q, Fan Q-X. Establishing a new electrical conduction pathway by anastomosis of the right auricle and right ventricle assisted by mesenchymal stem cells in a canine model. Transplan Proc. 2011;43:3980–6. doi: 10.1016/j.transproceed.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 84.Volk SW. Mesenchymal stem cells in Ischemic Wound Healing. In: Sen C, editor. Advances in Wound Care. Mary Ann Liebert, Inc; 2010. pp. 471–6. [Google Scholar]
- 85.Iacono E, Merio B, Pirrone A, Antonelli C, Brunori L, Romagnoli R, Castagnetti C. Effects of mesenchymal stem cells isolated from amnioted fluid and platelet-rich plasma gel on severe decubitus ulcers in a septic neonatal foal. Res Vet Sci. 2012 doi: 10.1016/j.rvsc.2012.04.008. epub. [DOI] [PubMed] [Google Scholar]
- 86.Borena BM, Pawde AM, Amarpal, Aithal HP, Kinjavdekar P, Rajendra S, Kumar D. Evaluation of autologous bone marrow-derived nucleated cells for healing of full-thickness skin wounds in rabbits. Int Wound J. 2010;7(4):249–60. doi: 10.1111/j.1742-481X.2010.00683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim SS, Song CK, Shon SK, Lee KY, Kim CH, Lee MJ, Wang LW. Effects of human amniotic membrane grafts combined with marrow mesenchymal stem cells on healing of full-thickness skin defects in rabbits. Cell Tissue Res. 2009;336(1):59–66. doi: 10.1007/s00441-009-0766-1. [DOI] [PubMed] [Google Scholar]
- 88.Azari O, Babaei H, Derakhshanfar A, Nematollahi-Mahani SN, Poursahebi R, Moshrefi M. Effects of transplanted mesenchymal stem cells isolated from Wharton’s jelly of caprine umbilical cord on cutaneous wound healing; histopathological evaulation. Vet Res Comm. 2011;35:211–22. doi: 10.1007/s11259-011-9464-z. [DOI] [PubMed] [Google Scholar]
- 89.Yun IS, Jeon YR, Lee WJ, Lee JW, Rah DK, Tark KC, Lew DH. Effect of human adipose derived stem cells on scar formation and remodeling in a pig model: a pilot study. Dermatol Surg. 2012:1–11. doi: 10.1111/j.1524-4725.2012.02495.x. [DOI] [PubMed] [Google Scholar]
- 90.Agay D, Scherthan H, Forcheron F, Grenier N, Hérodin F, Meineke V, Drouet M. Multipotent mesenchymal stem cell grafting to treat cutaneous radiation syndrome: Development of a new minipig model. Exp Hematol. 2010;38(10):945–56. doi: 10.1016/j.exphem.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 91.Forcheron F, Agay D, Scherthan H, Riccobono D, Herodin F, Meineke V, Drouet M. Autologous adipocyte derived stem cells favour healing in a minipig model of cutaneous radiation syndrome. PLoS ONE. 2012;7(2):e31694. doi: 10.1371/journal.pone.0031694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hadad I, Johnstone BH, Brabham JG, Blanton MW, Rogers PI, Fellers C, Solomon JL, Merfeld-Clauss S, DesRosiers CM, Dynlacht JR, Coleman JJ, March KL. Development of a porcine delayed wound-healing model and its use in testing a novel cell-based therapy. Int J radiat Oncol Biol Phys. 2010;78(3):888–96. doi: 10.1016/j.ijrobp.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 93.Quimby JM, Webb TL, Gibbons DS, Dow SW. Evaluation of intrarenal mesenchymal stem cell injection for treatment of chronic kidney disease in cats: a pilot study. J Feline Med Surg. 2011;13:418–26. doi: 10.1016/j.jfms.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yoo J-H, Park C, Jung D-I, Lim C-Y, Kang B-T, Kim J-H, Park JW, Kim J-H, Park H-M. In vivo cell tracking of canine allogenic mesenchymal stem cells administration via renal arterial catheterization and physiopathological effects on kidney in two healthy dogs. J Vet Med Sci. 2011;2:269–74. doi: 10.1292/jvms.10-0044. [DOI] [PubMed] [Google Scholar]
- 95.Zhang Y, Lin H-K, Frimberger D, Epstein RB, Kropp BP. Growth of bone marrow stromal cells on small intestinal submucosa: An alternative cell source for tissue engineered bladder. BJU Int. 2005;96(7):1120–5. doi: 10.1111/j.1464-410X.2005.05741.x. [DOI] [PubMed] [Google Scholar]
- 96.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292(154):156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 97.Thomson JA, Kalishman J, Golos TG, Durning M, Harris CP, Becker RA, Hearn JP. Isolation of a primate embryonic stem cell line. Proc Natl Acad Sci USA. 1995;92:7844–8. doi: 10.1073/pnas.92.17.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–7. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 99.Stocum DL, Zupanc GKH. Stretching the limits: Stem cells in regeneration science. Dev Dyn. 2008;237:3648–71. doi: 10.1002/dvdy.21774. [DOI] [PubMed] [Google Scholar]
- 100.Vaags AK, Rosic-Kablar S, Gartley CJ, Zheng YZ, Chesney A, Villagómez DAF, Kruth SA, Hough MR. Derivation and characterization of canine embryonic stem cell lines with in vitro and in vivo differentiation potential. Stem Cells. 2009;27(2):329–40. doi: 10.1634/stemcells.2008-0433. [DOI] [PubMed] [Google Scholar]
- 101.Gomez MC, Serrano MA, Pope CE, Jenkins JA, Biancardi MN, Lopez M, Dumas C, Galiguis J, Dresser BL. Derivation of cat embryonic stem-like cells from in vitro-produced blastocysts on homologous and heterologous feeder cells. Theriogenology. 2010;74(4):498–515. doi: 10.1016/j.theriogenology.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 102.Wang S, Tang X, Niu Y, Chen H, Li B, Li T, Zhang X, Hu Z, Zhou Q, Ji W. Generation and characterization of rabbit embryonic stem cells. Stem Cells. 2007;25(2):481–9. doi: 10.1634/stemcells.2006-0226. [DOI] [PubMed] [Google Scholar]
- 103.Li X, Zhou SG, Imreh MP, Ahrlund-Richter L, Allen WR. Horse embryonic stem cell lines from the proliferation of inner cell mass cells. Stem Cells Dev. 2006;15:523–31. doi: 10.1089/scd.2006.15.523. [DOI] [PubMed] [Google Scholar]
- 104.Desmaris J, Demers S-P, Suzuki J, Laflamme S, Vincent P, Laverty S, Smith LC. Trophoblast stem cell marker gene expression in inner cell mass-derived cells from parthenogenetic equine embryos. Reproduction. 2011;141(3):321–32. doi: 10.1530/REP-09-0536. [DOI] [PubMed] [Google Scholar]
- 105.Wheeler MB. Development and validation of swine embryonic stem cells: A review. Reprod Fertil Dev. 1994;6:563–8. doi: 10.1071/rd9940563. [DOI] [PubMed] [Google Scholar]
- 106.Li M, Li YH, Hou Y, Sun XF, Sun Q, Wang WH. Isolation and culture of pluripotent cells from in vitro produced porcine embryos. Zygote. 2004;12:43–8. doi: 10.1017/s0967199404002679. [DOI] [PubMed] [Google Scholar]
- 107.Saito S, Sawai K, Ugai H, Moriyasu S, Minamihashi A, Yamamoto Y, et al. Generation of cloned calves and transgenic chimeric embryos from bovine embryonic stem-like cells. Biochem Biophys Res Comm. 2003;309(1):104–13. doi: 10.1016/s0006-291x(03)01536-5. [DOI] [PubMed] [Google Scholar]
- 108.Wang L, Duan E, Sung LY, Jeong BS, Yang X, Tian XC. Generation and characterization of pluripotent stem cells from cloned bovine embryos. Biol Reprod. 2005;73:149–55. doi: 10.1095/biolreprod.104.037150. [DOI] [PubMed] [Google Scholar]
- 109.Notarianni E, Galli C, Laurie S, Moor RM, Evans MJ. Derivation of pluripotent, embryonic cell lines from the pig and sheep. J Reprod Fertil Suppl. 1991;43:255–60. [PubMed] [Google Scholar]
- 110.Paris DB, Stout TA. Equine embryos and embryonic stem cells: defining reliable markers of pluripotency. Theriogenology. 2010;74(4):516–24. doi: 10.1016/j.theriogenology.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 111.Keefer CL, Pant D, Blomberg L, Talbot NC. Challenges and prospects for the establishment of embryonic stem cell lines of domesticated ungulates. Anim Reprod Sci. 2007;98(147):168. doi: 10.1016/j.anireprosci.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 112.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 113.Zhao T, Xu Y. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474:212–5. doi: 10.1038/nature10135. [DOI] [PubMed] [Google Scholar]
- 114.Madonna R. Human-induced pluripotent stem cells: in quest of clinical applications. Mol Biotechnol. 2012 doi: 10.1007/s12033-012-9504-0. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 115.Shimada H, Nakada A, Hashimoto Y, Shigeno K, Shionoya Y, Nakamura T. Generation of canine induced pluripotent stem cells by retroviral transduction and chemical inhibitors. Mol Reprod Dev. 2010;77(1):2. doi: 10.1002/mrd.21117. [DOI] [PubMed] [Google Scholar]
- 116.Luo J, Suhr ST, Chang EA, Want K, Ross PJ, Nelson LL, et al. Generation of leukemia inhibitory factor and basic fibroblast growth factor-dependent induced pluripotent stem cells from canine adult somatic cells. Stem Cells Dev. 2011;20(10):1669–78. doi: 10.1089/scd.2011.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lee AS, Xu D, Plews JR, Nguyen PK, Nag D, Lyons JK, et al. Preclinical derivation and imaging of autologously translanted canine induced pluripotent stem cells. J Biol Chem. 2011;1628(37):32697–704. doi: 10.1074/jbc.M111.235739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nagy K, Sung HK, Zhang P, Laflamme S, Vincent P, Agha-Mohammadi S, et al. Induced pluripotent stem cell lines derived from equine fibroblasts. Stem Cell Rev. 2011;7(3):693–702. doi: 10.1007/s12015-011-9239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Khodadadi K, Sumer H, Pashaiasi M, Lim S, Williamson M, Verma PJ. Induction of pluripotency in adult equine fibroblasts without c-MYC. Stem Cells Int. 2012:2012429160. doi: 10.1155/2012/429160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Esteban MA, Xu J, Yang J, Peng M, Qin D, Li W, et al. Generation of induced pluripotent stem cell lines from Tibetan miniature pig. J Biol Chem. 2009;284(26):17634–40. doi: 10.1074/jbc.M109.008938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ezashi T, Telugu BPVL, Alexenki AP, Sachdev S, Sinha S, Roberts RM. Derivation of induced pluripotent stem cells from pig somatic cells. Proc Natl Acad Sci USA. 2009;106(27):10993–8. doi: 10.1073/pnas.0905284106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.West FD, Terlouw SL, Kwon DJ, Mumaw JL, Dhara SK, Hasneen K, Dobrinsky JR, Stice SL. Porcine induced pluripotent stem cells produce chimeric offspring. Stem Cells Dev. 2010;19(8):1211–20. doi: 10.1089/scd.2009.0458. [DOI] [PubMed] [Google Scholar]
- 123.Han X, Han J, Ding F, Cao S, Lim SS, Dai Y, et al. Generation of induced pluripotent stem cells from bovine embryonic fibroblast cells. Cell Res. 2011;21(10):1509–12. doi: 10.1038/cr.2011.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu J, Balehosur D, Murray B, Kelly JM, Sumer H, Verma PJ. Generation and characterization of reprogrammed sheep induced pluripotent stem cells. Theriogenology. 2012;77(2):338–46. doi: 10.1016/j.theriogenology.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 125.Honda A, Hirose M, Hatori M, Matoba S, Miyoshi H, Inoue K, et al. Generation of induced pluripotent stem cells in rabbits. J Biol Chem. 2010;285(41):31362–9. doi: 10.1074/jbc.M110.150540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhou L, Wang W, Liu Y, Fernandez de Castro J, Ezashi T, Telugu BP, et al. Differentiation of induced pluripotent stem cells of swine into rod photoreceptors and their integration into the retina. Stem Cells. 2011;29(6):972–80. doi: 10.1002/stem.637. [DOI] [PMC free article] [PubMed] [Google Scholar]


