Abstract
Earlier, we have reported that Polychlorinated Biphenyls (PCBs) exposure in Slovak population has made differential gene expression that has linked to the possibilities of some diseases and disorder development in the studied population. Here we report that down-regulation of LEPR (Leptin receptor) gene in the 45-month children may have been following consequences in developing obesity later in life. A pilot high-throughput qRT-PCR [Taqman Low Density Array (TLDA)] study in a small population also corroborated the gene-expression results, and their pathways underlying the consequences of the diseases, amid further detailed large-scale population validation. The study shows the opportunity of predicting long-term effects of chemical exposures using selected genomic classifiers may reflect exposure effect and risk from environmental toxicants.
Keywords: PCBs, Gene Expression, Leptin Receptor, Obesity
INTRODUCTION
In utero exposure of the fetus to a chemical stressor can lead to disease in later life. Over the last several decades, the prevalence of obesity has raised sharply in most countries worldwide (Yach et al. 2006). However, there is emerging evidence that low-dose exposure to various industrial chemicals, known or presumed to disrupt endocrine systems and frequently termed endocrine disrupting chemicals (ECDs), may also be an important contributor to the obesity epidemic (Grun and Blumberg 2009; Newbold et al. 2009). There is growing evidence that perturbations of central endocrine regulatory systems by the endocrine disrupting chemicals (e.g. dioxins, PCBs, Organochlorine pesticides, etc.) established in early gestation may contribute to the development of obesity in later life (Dirinck et al. 2011). Although PCBs have not been used commercially since 1977, in the US, they can be detected in human blood and tissues even today. It is still a persistent problem causing deleterious health effects, especially in Eastern Europe, China, and worldwide. The emerging hypothesis based on data from animal and human epidemiological studies on several chemicals states that the obesity epidemic could be due to chemical exposures during vulnerable windows of development, mainly in utero and the first few years of life. Among them, polychlorinated biphenyls (PCBs) may be particularly interesting, because low dose exposure to PCBs was linked to type 2 diabetes, insulin resistance, and metabolic syndrome, in all of which obesity is believed to play a critical role (Philibert et al. 2009; Arrebola et al. 2010; Lee at al. 2012).
In the eastern Slovakia, region of Michalovce, improper disposal from the Chemko plant via release of effluent directly into the Laborec River resulted in the long-term contamination of water sediment (Kocan et al. 2001). Several surveys in eastern Slovakia found high levels of PCBs and dioxins in the locally produced food (Chovancova et al. 2005; Sonneborn et al. 2008). In the late 1980’s, concentrations in breast milk in the Michalovce district averaged 4.0–4.4 mg/kg lipids (Hertzman 1995). Sum of 15 PCB congeners was 3105 ng/g of lipid in subjects from Michalovce district versus 871 ng/g of lipid for subjects from the background districts of Svidnik and Stropkov. Relatively high PCB concentration in children from the Michalovce district, sum PCB = 766 ng/g of lipid versus 372 ng/g of lipid in children from background area, suggests ongoing exposure from environmental reservoirs and contaminated food (Petrik et al. 2006). Several studies have shown a positive association between diabetes and biomarkers of organochlorine (OC) exposure, including several polychlorinated biphenyls (PCB) congeners and chlorinated pesticides (Dirinck et al. 2011; Alonso-Magdalena et al. 2011). Published studies have also evidenced that PCB impacted heavily on the exposed Slovak population regarding thyroid disruption (Langer et al. 2009), and diabetes Ukropec et al. 2010). Our pioneering recent gene expression studies with Slovak exposed population, as well as in human PBMC in vitro model (Ghosh et al. 2011) have successfully revealed the incidences of disease and disorder development that are in accord with various reported epidemiological studies on PCB exposure (Mitra et al. 2012; Dutta et al. 2012). The results indicated the involvement of biochemical and disease pathways, which are already reported in pathophysiological conditions related to PCB exposure in epidemiological studies, and some of the signature gene/genes in the panel pathways) can act as signature biomarkers of PCB exposures (Dutta et al. 2008; Mitra et al. 2012; Dutta et al. 2012).
A new paradigm has evolved in recent years, which stems from the idea that environmental factors in early life and in utero can have profound influences on lifelong health (e.g., the fetal basis of adult disease; Oken and Gillman 2003). Considerable epidemiological, experimental, and clinical data have amassed that the risk of developing disease in later life is dependent upon early life conditions. Recent evidence from many laboratories has shown that a variety of environmental endocrine disrupting chemicals (commonly termed as “Obesogens”) can influence adipogenesis and obesity. Obesogens can be defined functionally as chemical agents that inappropriately regulate and promote lipid accumulation and adipogenesis (Grun and Blumberg 2009). PCBs are major chemical compounds among Persistent Organic Pollutants (POPs) that are presently considered to be major endocrine/metabolic disruptors (Casals-Casas and Desvergne 2011). Increasing evidence suggests that the commonly held causes of obesity, which are over-eating, inactivity and genetic predisposition, do not fully explain the current obesity epidemic. Interestingly, the production and use of synthetic chemicals have increased dramatically, in parallel with growing obesity, and it has been suggested that EDCs may play a key role in obesity development by altering physiological control mechanisms (Tang-Peronard et al. 2011). Leptin receptor also known as LEP-R is a protein that in humans is encoded by the LEPR gene. LEP-R functions as a receptor for the fat cell-specific hormone leptin. The protein encoded by this gene belongs to the gp130 family of cytokine receptors that are known to stimulate gene transcription via activation of cytosolic STAT proteins (Bailleul et al. 1997). This adipokineleptin has received significant interest as a potential programming factor; alterations in the profile of leptin in early life are associated with altered susceptibility to obesity and metabolic disorders in adulthood. Maintenance of a critical leptin level during early development facilitates the normal maturation of tissues and signaling pathways involved in metabolic homeostasis (Vickers et al. 2012). In advent of the obesity as serious health concern around the world, the investigation on this gene function in developing obesity is becoming more and more important.
MATERIAL AND METHODS
The gene expression studies on the selected subjects who had attained 45-months of age, from an existing mother-child cohort (see details about recruitment and characterization of the cohort described in Park et al. 2010) were conducted over Affymetrix platform using Human Genome U133 Plus 2.0 Array, and were analyzed by Partek® Genomics Suite™. Ingenuity Pathway Analysis (IPA) software was employed to evaluate the functional association of the genes and their pathways in the treated groups, underlying the consequences of developing diseases (see Ghosh et al. 2011; Dutta et al. 2012 for details). High-throughput qRT-PCR (TLDA) was done on ABI platform to further validate LEPR, through a small population (n=72; with high PCBs-exposure) for the LEPR gene expression level status. Briefly, for the present study, the TLDA card was configured into respective gene set (triplicate per assay). The TLDA array card also contained two endogenous control genes, GAPDH and 18s RNA. The RNAs were synthesized with the cDNA (5 μL) and was mixed with 45 μL of H2O and 50 μL of 2x TaqMan Universal PCR Mix (Applied Biosystems, Foster City, CA). Each sample (100 μL) was loaded into a port of the micro-fluid card, centrifuged (for 2 minutes twice with swing rotor), and run on an ABI Fast 7900HT System (ABI, CA) for 2 min at 50°C, 10 min at 94.5°C, followed by 40 cycles for 30 sec. at 97°C and 1 min at 59.7°C. Data analysis was done through SDS RQ Manager, 1.2.1.
RESULTS AND DISCUSSION
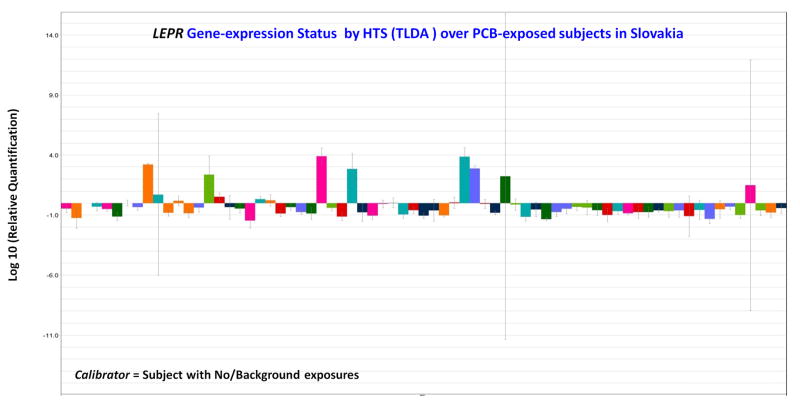
Our gene expression analysis revealed the down-regulation of LEPR gene (−2.79; p <0.05) in the studied population. The IPA analysis of the gene list from the differential gene expression of Slovak population identified the connectivity of important genes in their respective pathways leading to major disease and disorders (e.g.; Cardiovascular, Obesity, Diabetes, Cancers, Neurobehavioral deficits, etc.). The study also showed that there is significant association of LEPR (major molecule in Bio-functions) with the involvement of Leptin Signaling in Obesity as the preeminent canonical pathways, with the genes in networks. The Endocrine System Disorders in disease and disorders conditions through IPA analysis (Threshold > 1.5) have shown notable expression having a major impact in developing toxicities by altering cellular and molecular functions in these PCBs-exposed subjects. The high throughput qRT-PCR validation of LEPR gene also corroborated the gene expression study, where similar down-regulation of the gene is observed in most of the subjects (70.8%, 51 out of 72) with high-PCB exposure (Fig. 1). This TLDA allowed us for simultaneous testing of gene transcript in micro-fluidic card format with reproducibility, analyzing the gene expressions, confirming the ability of PCBs to alter the expression of genes identified initially by cDNA microarray.
Fig. 1.
Quantitative Real-time PCR (qRT-PCR) validation of the LEPR genes by Taqman Low Density Array (TLDA) in ABI platform (7900HT Fast Real-Time PCR System) after analysis by SDS RQ Manager Version 1.2.1. The relative quantification is calculated in contrast to calibrator samples, i.e.; the subjects with no /or background PCBs exposures in the population studies. The data are attenuated automatically and the reflection of triplicate observation already seated within the TLDA plates. This study represents that this high-throughput biomarker-based method will be capable of identifying high-risk individuals with specificity & selectivity, through therapeutically relevant genomic classifiers, as a measure of biological responses to environmental stressors.
The organohalogen exposure scenario in eastern Slovakia is world-wide unique by its extent and numerous exposed subjects. On the other hand, it gives us a tremendous opportunity to study concentration-effect relationship for a broad spectrum of health outcomes. The PCB blood concentrations in the area of the current studies exceed the concentrations of PCBs observed in other comparable cohorts in various parts of the world (Faroe, North Quebec, Anniston) (Govarts et al. 2012; Hertz-Picciotto et al. 2003). Several epidemiological investigations, performed earlier with the same cohort, have indicated that PCBs impacted heavily on the exposed Slovak population, regarding endocrine disruption (Langer et al. 2009), and prevalence of prediabetes and diabetes in a dose-dependent manner (Ukropec et al. 2010). Our previous gene-expression analysis have clearly showed that a discreet gene sets/pathways that can serve as “molecular signatures” can be used as a biomarker of a biological endpoint with an involvement of such disease and disorder progression in PCB exposed population (Dutta et al. 2012; Mitra et al. 2012; Ghosh et al. 2012).
In this study, the down-regulation LEPR gene is becoming more relevant as the decrease of leptin receptor gene may perturb the normal function of leptin and suggests decreased action of leptin. Particularly, with the children who had experienced high pre- and postnatal PCBs exposure, the alterations in the profile of leptin in early life may be associated with altered susceptibility to obesity and metabolic disorders in adulthood. The epidemia also suggests that the origin of the disease may occur during fetal development and early life with the concept of “developmental programming” and recent research indicate that the adipokineleptin plays a critical role in this programming, supported by experimental studies in animal models and numerous epidemiological data (Djiane and Attig 2008). Specially, maintenance of a critical leptin level during early development facilitates the normal maturation of tissues and signaling pathways involved in metabolic homeostasis (Vickers et al. 2012), and the variations in the leptin receptor with the obesity have also been indicated, where leptin-receptor polymorphisms relate to obesity through blunted leptin-mediated sympathetic nerve activation in a Caucasian male population (Masuo et al. 2008). It is to be noted that the heritable changes in gene expression or cellular phenotypes caused by changes in DNA sequence (epigenetic) might have played an important role to the changes in gene expression undergone by adipose tissue during obesity. Importantly, epigenetic marks may be reprogrammed in response to both stochastic and environmental stimuli, such as changes in diet and the in utero environment (Jaenisch and Bird 2003). Our study also corroborates the other epidemiological findings, where a recent prospective study (Lee et al. 2011) among young adults reported that low dose exposure to p, p′-DDE (a persistent lipophilic metabolite of DDT), p, p′-DDT, and PCBs with more chlorine atoms predicted future body mass index (BMI). In a recent prospective investigation of the vasculature in Uppsala seniors (PIVUS) study using both a cross-sectional and a prospective approach, demonstrated that the less chlorinated PCBs, some OC pesticides including p, p′-DDE, and dioxin showed an increased risk of abdominal obesity (Lee at al. 2012). This has also been reflected while investigating the cord blood PCB and DDE concentrations which were associated with increased BMI or change in BMI from ages 1 to 3 years in a Belgian prospective study (Verhulst et al. 2009). In a cross-sectional study of adolescents, 3 serum PCB congeners (138, 153, and 180) were associated with decreased BMI but a fourth (118) was positively associated with BMI among 14- and 15-year olds (Meeker 2012). Our study also ascertains the fact that high PCBs exposure is linked with the development of obesity in the developing children, and might find a link that environmental chemical exposures during development (or other windows of sensitivity) can lead to weight gain, altered glucose/insulin sensitivity and altered lipid metabolism that led to the development of obesity, where over-eating, inactivity and genetic pre-disposition, do not fully explain the current obesity epidemic. It is anticipated that the most sensitive time for exposures to affect the disease outcomes will be during development, e.g. in utero and/or neonatal or early childhood.
Given the premises that the relevant gene information and the pathways (genes in panel) are predictive for the respective disease pathways’ process, the results we stated above do show promise that large-scale evaluation of changes in gene expression using microarrays, combined with a primary validation through high-throughput TLDA, may become an useful tool for toxicity evaluation, biomarker validation, and will empower us to study the process of development of diseases and aid to our understanding the potential health risk of PCBs. We will be able to identify all related genes that are significant at the specific pathway level leading to diseases, before the clinical symptoms arise amid downstream application to measure the biological responses to environmental stressors. Obesity is notoriously difficult to treat; however, the present investigation provides a possible clue to the exposure response to the etiology of obesity, and could be a hint which is critical to developing primary prevention strategies. The optimistic view is that if the chemicals that seem to be related to the obesity epidemic are removed from products which are the primary contributors to human exposures, there would be reason to hope that the current trend of increasing obesity epidemic worldwide can be controlled, even reversed.
Acknowledgments
This study is supported by the 1UO1ES016127-01 from the National Institute of Environmental Health Sciences (NIEHS/NIH), Howard University Education Program project R200174 to SKD, and from the European Commission through the 7FP project OBELIX (No. 227391). The contents of this report are solely the responsibility of the authors.
References
- Alonso-Magdalena P, Quesada I, Nadal A. Endocrine disruptors in the etiology of type 2 diabetes mellitus. Nat Rev Endocrinol. 2011;7:346–353. doi: 10.1038/nrendo.2011.56. [DOI] [PubMed] [Google Scholar]
- Arrebola JP, Fernandez MF, Porta M, Rosell J, de la Ossa RM, Olea N, Martin-Olmedo P. Multivariate models to predict human adipose tissue PCB concentrations in Southern Spain. Environ Intl. 2010;36:705–13. doi: 10.1016/j.envint.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Bailleul B, Akerblom I, Strosberg AD. The leptin receptor promoter controls expression of a second distinct protein. Nucleic Acids Res. 1997;25:2752–2758. doi: 10.1093/nar/25.14.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casals-Casas C, Desvergne B. Endocrine disruptors: from endocrine to metabolic disruption. Annu Rev Physiol. 2011;73:135–162. doi: 10.1146/annurev-physiol-012110-142200. [DOI] [PubMed] [Google Scholar]
- Chovancova J, Kocan A, Jursa S. PCDDs, PCDFs and dioxin-like PCBs in food of animal origin (Slovakia) Chemosphere. 2005;61:1305–1311. doi: 10.1016/j.chemosphere.2005.03.057. [DOI] [PubMed] [Google Scholar]
- Dirinck E, Jorens PG, Covaci A, Geens T, Roosens L, Neels H, Mertens I, Van Gaal L. Obesity and persistent organic pollutants: possible obesogenic effect of organochlorine pesticides and polychlorinated biphenyls. Obesity (Silver Spring) 2011;19:709–14. doi: 10.1038/oby.2010.133. [DOI] [PubMed] [Google Scholar]
- Djiane J, Attig L. Role of leptin during perinatal metabolic programming and obesity. J Physiol Pharmacol. 2008;59 (Suppl 1):55–63. [PubMed] [Google Scholar]
- Dutta SK, Ghosh S, De S, Hoffman EP. CYP1A1 and MT1K are congener specific biomarker genes for liver diseases induced by PCBs. Environ Toxicol Pharmacol. 2008;25:218–221. doi: 10.1016/j.etap.2007.10.018. [DOI] [PubMed] [Google Scholar]
- Dutta SK, Mitra PS, Ghosh S, Zang S, Sonneborn D, Hertz-Picciotto I, Trnovec T, Palkovicova L, Sovcikova E, Ghimbovschi S, Hoffman EP. Differential Gene Expression and Functional Analysis of PCB-exposed Children: Understanding Disease and Disorder Development. Environ Intl. 2012;40:143–154. doi: 10.1016/j.envint.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Zang S, Mitra PS, Ghimbovschi S, Hoffman EP, Dutta SK. Global gene expression and Ingenuity biological functions analysis on PCBs 153 and 138 induced human PBMC in vitro reveals differential mode(s) of action in developing toxicities. Environ Intl. 2011;37:838–857. doi: 10.1016/j.envint.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govarts E, Nieuwenhuijsen M, Schoeters G, Ballester F, Bloemen K, de Boer M, Chevrier C, Eggesbo M, Guxens M, Kramer U, Legler J, Martínez D, Palkovicova L, Patelarou E, Ranft U, Rautio A, Petersen MS, Slama R, Stigum H, Toft G, Trnovec T, Vandentorren S, Weihe P, Kuperus NW, Wilhelm M, Wittsiepe J, Bonde JP OBELIX: ENRIECO . Birth weight and prenatal exposure to polychlorinated biphenyls (PCBs) and dichlorodiphenyldichloroethylene (DDE): a meta-analysis within 12 European Birth Cohorts. Environ Health Perspect. 2012;120:162–170. doi: 10.1289/ehp.1103767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grun F, Blumberg B. Perturbed nuclear receptor signaling by environmental obesogens as emerging factors in the obesity crisis. Rev Endocr Metab Disord. 2007;8:161–171. doi: 10.1007/s11154-007-9049-x. [DOI] [PubMed] [Google Scholar]
- Hertzman C. A report for the Environmental Action Programme for Central and Eastern Europe. World Bank; 1995. Environment and Health in Eastern Europe. [Google Scholar]
- Hertz-Picciotto I, Trnovec T, Kocan A, Charles MJ, Ciznar P, Langer P, Sovcikova E, James R. PCBs and early childhood development in Slovakia: Study design and background. Fresen Environ Bull. 2003;12:208–214. [Google Scholar]
- Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nature Genetics. 2003;33(Suppl):245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- Kocan A, Petrik J, Jursa S, Chovancova J, Drobna B. Environmental contamination with polychlorinated biphenyls in the area of their former manufacture in Slovakia. Chemosphere. 2001;43:595–600. doi: 10.1016/s0045-6535(00)00411-2. [DOI] [PubMed] [Google Scholar]
- Langer P, Kocan A, Tajtakova M, Susienkova K, Radikova Z, Koska J, Ksinantova L, Imrich R, Huckova M, Drobna B, Gasperikova D, Trnovec T, Klimes I. Multiple adverse thyroid and metabolic health signs in the population from the area heavily polluted by organochlorine cocktail (PCB, DDE, HCB, dioxin) Thyroid Research. 2009;2:3. doi: 10.1186/1756-6614-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Lind L, Jacobs DR, Jr, Salihovic S, van Bavel B, Lind PM. Associations of persistent organic pollutants with abdominal obesity in the elderly: The Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study. Environ Intl. 2012;40:170–178. doi: 10.1016/j.envint.2011.07.010. [DOI] [PubMed] [Google Scholar]
- Lee DH, Lind PM, Jacobs DR, Jr, Salihovic S, van Bavel B, Lind L. Polychlorinated biphenyls and organochlorine pesticides in plasma predict development of type 2 diabetes in the elderly: the prospective investigation of the vasculature in Uppsala Seniors (PIVUS) study. Diabetes Care. 2011;34:1778–1784. doi: 10.2337/dc10-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuo K, Straznicky NE, Lambert GW, Katsuya T, Sugimoto K, Rakugi H, Socratous F, Hastings J, Lambert EA, Ogihara T, Esler MD. Leptin-receptor polymorphisms relate to obesity through blunted leptin-mediated sympathetic nerve activation in a Caucasian male population. Hyperten Res. 2008;31:1093–100. doi: 10.1291/hypres.31.1093. [DOI] [PubMed] [Google Scholar]
- Meeker JD. Exposure to Environmental Endocrine Disruptors and Child Development. Arch PediatrAdolesc Med. 2012;4:E1–E7. doi: 10.1001/archpediatrics.2012.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra PS, Ghosh S, Zang S, Sonneborn D, Hertz-Picciotto I, Trnovec T, Palkovicova L, Sovcikova E, Ghimbovschi S, Hoffman EP, Dutta SK. Analysis of the toxicogenomic effects of exposure to persistent organic pollutants (POPs) in Slovakian girls: correlations between gene expression and disease risk. Environ Intl. 2012;39:188–199. doi: 10.1016/j.envint.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbold RR, Padilla-Banks E, Jefferson WN. Environmental estrogens and obesity. Mol Cell Endocrinol. 2009;304:84–89. doi: 10.1016/j.mce.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken E, Gillman MW. Fetal origins of obesity. Obesity Res. 2003;11:496–506. doi: 10.1038/oby.2003.69. [DOI] [PubMed] [Google Scholar]
- Park HY, Hertz-Picciotto I, Sovcikova E, Kocan A, Drobna B, Trnovec T. Neurodevelopmental toxicity of prenatal polychlorinated biphenyls (PCBs) by chemical structure and activity: a birth cohort study. Environ Health. 2010 Aug 23;9:51. doi: 10.1186/1476-069X-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrik J, Drobna B, Pavuk M, Jursa S, Wimmerova S, Chovancova J. Serum PCBs and organochlorine pesticides in Slovakia: age, gender, and residence as determinants of organochlorine concentrations. Chemosphere. 2006;65:410–418. doi: 10.1016/j.chemosphere.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Philibert A, Schwartz H, Mergler D. An exploratory study of diabetes in a First Nation community with respect to serum concentrations of p,p′-DDE and PCBs and fish consumption. Int J Environ Res Public Health. 2009;6:3179–3189. doi: 10.3390/ijerph6123179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonneborn D, Park HY, Babinska K, Palkovicova L, Trnovec T, Kocan A, Nguyen DV, Hertz-Picciotto I. Serum PCB concentrations in relation to locally produced food items in eastern Slovakia. J Expo Sci Environ Epidemiol. 2008;1:581–587. doi: 10.1038/jes.2008.1. [DOI] [PubMed] [Google Scholar]
- Tang-Peronard JL, Andersen HR, Jensen TK, Heitmann BL. Endocrine-disrupting chemicals and obesity development in humans: a review. Obes Rev. 2011;12:622–636. doi: 10.1111/j.1467-789X.2011.00871.x. [DOI] [PubMed] [Google Scholar]
- Ukropec J, Radikova Z, Huckova M, Koska J, Kocan A, Sebokova E, Drobna B, Trnovec T, Susienkova K, Labudova V, Gasperikova D, Langer P, Klimes I. High prevalence of prediabetes and diabetes in a population exposed to high levels of an organochlorine cocktail. Diabetologia. 2010;53:899–906. doi: 10.1007/s00125-010-1683-2. [DOI] [PubMed] [Google Scholar]
- Verhulst SL, Nelen V, Hond ED, Koppen G, Beunckens C, Vael C, Schoeters G, Desager K. Intrauterine exposure to environmental pollutants and body mass index during the first 3 years of life. Environ Health Perspect. 2009;117:122–126. doi: 10.1289/ehp.0800003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers MH, Sloboda DM. Leptin as mediator of the effects of developmental programming. Best Pract Res Clin Endocrinol Metab. 2012;26:677–687. doi: 10.1016/j.beem.2012.03.005. [DOI] [PubMed] [Google Scholar]
- Yach D, Stuckler D, Brownell KD. Epidemiologic and economic consequences of the global epidemics of obesity and diabetes. Nat Med. 2006;12:62–66. doi: 10.1038/nm0106-62. [DOI] [PubMed] [Google Scholar]