Abstract
Objective
The focus of tissue engineering of neocartilage has traditionally been on enhancing extracellular matrix and thus biomechanical properties. Emphasis has been placed on the enhancement of collagen type and quantity, and, concomitantly, tensile properties. The objective of this study was to improve crosslinking of the collagen network by testing the hypothesis that hypoxia could promote pyridinoline (PYR) crosslinks and, thus, improve neocartilage’s tensile properties.
Methods
Chondrocyte expression of lysyl oxidase (LOX), an enzyme responsible for the formation of collagen PYR crosslinks, was first assessed pre- and post- hypoxia application. Then, the mechanical properties of self-assembled neocartilage constructs were measured, after 4 weeks of culture, for groups exposed to 4% O2 at different initiation times and durations, i.e., during the 1st and 3rd weeks, 3rd and 4th weeks, 4th week only, continuously after cell seeding, or never.
Results
Results showed that LOX gene expression was upregulated ~20-fold in chondrocytes in response to hypoxia. Hypoxia applied during the 3rd and 4th weeks significantly increased PYR crosslinks without affecting collagen content. Excitingly, neocartilage tensile properties were increased ~2-fold. It should be noted that these properties exhibited a distinct temporal dependence to hypoxia exposure, since upregulation of these properties was due to hypoxia applied only during the 3rd and 4th weeks.
Conclusion
These data elucidate the role of hypoxia-mediated upregulation of LOX and subsequent increases in PYR crosslinks in engineered cartilage. These results hold promise toward applying hypoxia at precise time points to promote tensile integrity and direct construct maturation.
Keywords: Articular cartilage tissue engineering, Collagen crosslinking, Hypoxia, Pyridinoline crosslinks, Mechanical properties
Introduction
Articular cartilage (AC) pathologies, resulting from injury/trauma or age-related degeneration, are major health problems in the developed world. Osteoarthritis (OA) is the most frequent chronic musculoskeletal disease, limiting daily activities of the elderly1. Over the past two decades, progress has been made on the development of therapeutic approaches for the treatment of early stage cartilaginous defects, thus slowing down their progression to OA. However, current therapeutic procedures including washing, shaving and debridement, stem cell stimulation-based procedures, and explant grafts have thus far proved to be incapable to effect long-term repair2. Tissue engineering has the potential to generate tissue with biomechanically competent extracellular matrix (ECM) in vitro3. However, limitations in the development of neotissues that mimic the structural composition and, hence, biomechanical behavior of native tissues present a great challenge4. To generate neotissues capable of bearing physiological loads, various exogenous stimuli during neocartilage culture have been examined.
In engineering cartilage, hypoxia has been proposed for both stem cells and differentiated chondrocytes. For stem cells, hypoxia has been reported to promote chondro-differentiation5-7 and to impede hypertrophy8. Hypoxia inducible factor 1 (HIF-1) has been hypothesized as critical for hypoxic induction of chondrogenesis8. For engineering AC, however, hypoxia has shown mixed results. For instance, some studies have suggested that Sox-9 expression is largely independent of hypoxia9, while others have shown that this transcription factor increases with reduced oxygen levels10. Likewise, ECM components (e.g., aggrecan, collagen type II, proteoglycan 4) have been shown to be upregulated with hypoxia in certain cases10,11, while others have shown that cartilaginous ECM production is indifferent to hypoxia12 or even suppressed by it in certain instances13,14. In brief, though the majority of evidence supports hypoxia as an effective method for enhancing engineered cartilage constructs, mechanisms behind such improvements have largely remained elusive. For hypoxia to be used efficiently and reliably in cartilage tissue engineering, a robust mechanistic connection between hypoxia and mechanical properties must be determined.
In the human body, normal oxygen tension, or normoxia, is between 5% and 13%15. Oxygen levels lower than 5% are considered hypoxic16, while the atmospheric oxygen tension of 21% should be considered hyperoxic17. For differentiated chondrocytes, oxygen tension can be as low as 1–2.5%, thus qualifying cartilage as functioning under a hypoxic environment14. Thus, oxygen concentration during in vitro culture likely needs to be below 5% to induce significant HIF-1α stabilization and its subsequent upregulation of various matrix-associated genes.
The anisotropic, structural and mechanical properties of AC, including its well-developed, collagen fiber architecture, determine tissue functionality and mechanical integrity18. Apart from collagen content and organization, the load-bearing capacity of collagen is related to other ECM components. Two features that directly influence native AC stiffness and determine collagen maturity are the type and quantity of collagen crosslinks19,20. Thus far, these critical features have not been sufficiently mimicked in the ECM of engineered tissues; lack of progress on this front may be a factor for the current limitations in tissue modulus and strength19,21. An opportunity exists in the modulation of collagen crosslinking in engineered neocartilage to enhance tissue structural and functional integrity. While hypoxia has been associated with collagen cross-linking in several other tissues22-24, its use for inducing crosslinks in engineered cartilage remains unexplored.
In this study, engineered cartilage is formed using a scaffoldless, self-assembling process that removes the potentially confounding effects of scaffolds. The objectives of this study are to examine two hypotheses: (1) that hypoxia (4% oxygen) will enhance the mechanical properties of engineered AC by mediating collagen crosslinking via lysyl oxidase (LOX), and (2) this specific response to hypoxia is dependent on application time.
Methods
Chondrocyte isolation
AC was sterilely harvested from the distal femur of knee joints obtained from 1-week-old calves (Research 87, Boston, MA, USA). Tissue was minced into 1 mm pieces and digested in 0.2% collagenase type II (Worthington, Lakewood, NJ, USA) for 18 h in cell culture medium (Dulbecco’s modified Eagle’s medium (DMEM) with low glucose (1 g/L)) (Life Technologies corp., Carlsbad, CA, USA), 10% fetal bovine serum (FBS) (Atlanta Biologicals), 1% non-essential amino acids (NEAA) (Life Technologies corp., Carlsbad, CA, USA), 25 mg of l-ascorbic acid (Sigma–Aldrich, St. Louis, MO, USA), and 1% penicillin/streptomycin/fungizone (PSF) (BioWhittaker Inc., Walkersville, MD, USA). After digestion, articular chondrocytes were washed three times in PBS with centrifugation and filtered through a 70-μm filter. Cells then were counted and were frozen at −80°C in culture medium supplemented with 10% FBS and 10% dimethyl sulfoxide (Fischer Scientific, Pittsburgh, PA, USA). Cells were stored in liquid nitrogen until used.
Construct formation via the self-assembling process
Constructs were generated by seeding articular chondrocytes into cylindrical, non-adherent wells using a technique adapted from previous work3,25. A stainless steel mold consisting of 5 mm diameter cylindrical prongs was placed into molten 2% agarose (Life Technologies corp., Carlsbad, CA, USA) in a 48-well plate. The agarose solidified at room temperature, and the mold was removed. Two changes of control medium (DMEM with GlutaMAX (Life Technologies corp., Carlsbad, CA, USA), 100 nM dexamethasone (Sigma–Aldrich, St. Louis, MO, USA), 1% NEAA, 1% PSF, 1% ITS+ premix (BD Scientific, Franklin Lakes, NJ, USA), 50 mg/mL ascorbate-2-phosphate (Sigma–Aldrich, St. Louis, MO, USA), 40 mg/mL l-proline (Sigma–Aldrich, St. Louis, MO, USA), and 100 mg/mL sodium pyruvate (Fischer Scientific, Pittsburgh, PA, USA)) were used to saturate the agarose before cell seeding. Following isolation, articular chondrocytes were thawed within 5 days of freezing. Viability assessed using trypan blue was >90%. To create each construct, 5.5 million cells in 100 μl control medium were seeded into each gelled, cylindrical agarose well, followed by addition of 400 μl control medium after 4 h. Cells coalesced into free-floating, disc-shaped constructs upon the non-adhesive agarose; t = 1 day was defined as 24 h after seeding. Constructs were cultured in the agarose wells until t = 10 days, at which point they were unconfined transferred to 48-well plates where they were unrestricted by circumferential confinement. Constructs received 500 μl medium change every 24 h and remained in culture until t = 28 days.
Hypoxia application
Following seeding, constructs were divided into five treatment groups. Constructs in the first group (control) were incubated continuously at 37°C, 5% CO2, and 21% O2. Constructs from the other groups were incubated under the same conditions but also treated with 4% O2 (hypoxia) during the following culture periods: t = 1–7 days and 15–21 days (1st and 3rd weeks), t = 15–28 days (3rd and 4th weeks), t = 22–28 days (4th week), and t = 1–28 days (continuous hypoxia). Every other day, 500 μl of medium was changed. After 4 weeks of culture, constructs were divided into parts for histological, biochemical, and biomechanical assessments.
Histology
Samples of the neotissue were cryoembedded in Histoprep (Fisher Chemical, Vernon Hills, IL, USA) and sectioned at 14 μm with orientation from top to the bottom of the construct, in all groups. Following sectioning, samples were fixed in formalin for 15 min. Qualitative evaluation of collagen and glycosaminoglycan (GAG) content of the samples was performed by using Safranin-O/fast green and Picrosirius red stains as previously described26. Phenotype maintenance of articular chondrocytes was evaluated with immunohistochemistry (IHC). Briefly, samples were fixed in chilled (4°C) acetone, rehydrated, and stained for collagen type I and II by following protocols provided by Chondrex and Vectastain as previously described26. Native bovine AC and patellar tendon were used as positive type II and type I controls, respectively.
Quantitative biochemistry
Biochemical samples were lyophilized, and dry weight was recorded. Subsequently, samples were digested using a pepsinelastase protocol as previously described26. Collagen content in the engineered tissue was evaluated using a colorimetric hydroxyproline assay27. A Blyscan Glycosaminoglycan Assay kit (Biocolor, Newtownabbey, Northern Ireland) was used for sulfated GAG content quantification28,29. Cell content in the engineered tissue was approximated using a Picogreen dsDNA reagent (Molecular Probes, Eugene, OR, USA) for DNA quantification and a conversion factor of 7.7 pg DNA/cell.
High-performance liquid chromatography (HPLC)
The abundance of pyridinoline (PYR) crosslinks in the engineered tissue was quantified using HPLC. Portions of the constructs were weighed wet, digested in 400 μl 6 N HCl, and dried in a vacuum concentrator. 50 μL of an aqueous solution containing 10 nmol pyridoxine/mL and 2.4 μmol homoarginine/mL was used for sample re-suspension and then diluted 5-fold with an aqueous solution of 0.5% heptafluorobutyric acid (HFBA) in 10% acetonitrile. Following this, 50 μL of each sample was injected into a 25 mm C18 column (Shimadzu, Columbia, MD, USA) and eluted using a solvent profile as previously described30. A calibration curve was performed using PYR standards (Quidel, San Diego, CA, USA) for crosslink quantification.
Compression testing
The compressive properties of the constructs were evaluated with creep indentation testing as previously described3. A 0.7 g (0.007 N) mass was applied through a 0.45 radius flat, porous indenter tip on each sample stabilized to a stainless steel surface. Compressive properties of the neocartilage, represented by aggregate modulus, permeability and Poisson’s ratio were calculated by adapting a semi-analytic, semi-numeric, linear biphasic model31.
Tensile testing
A uniaxial material testing apparatus (Instron, model 5565; Canton, MA, USA) (Instron Model 5565) was used to quantify the tensile properties of the constructs as previously described26. Each specimen was prepared by cutting it into a dog-bone shape with a 1-mm-long gauge length. Photographs were taken of each specimen and thickness and width were determined using ImageJ software. Uniaxial tension until failure within the gauge length at a strain rate of 1% of the gauge length per second was performed using a 5 kN load cell. Force-displacement curves were generated, and stress–strain curves were calculated by normalizing data to specimen dimension. The apparent Young’s modulus, a measure of specimen tensile stiffness, was determined by least squares fitting of the linear region of the stress–strain curve. The ultimate tensile strength (UTS) was determined as the maximum stress reached during a test.
RT-PCR
Real-time PCR analysis was performed to investigate hypoxia-mediated gene expression of the target genes. Dynamic changes in LOX expression can occur during the first 18 h of a culture’s introduction to hypoxia17,32. In order to observe LOX expression at steady state, measurements within the short time period immediately after hypoxia exposure were avoided. On the other hand, since LOX-induced crosslink formation can require weeks to mature, it was desirable to assess for LOX expression as early as possible. Thus, hypoxia was applied in 3 week-engineered neocartilage for 2 days and the gene expression of LOX was quantified as previously described17. S18 (housekeeping gene) and LOX primers were purchased from US Biological. RT was performed by incubating 500 ng of RNA with SuperScript III (Life Technologies corp., Carlsbad, CA, USA) as recommended by the manufacturer. Real-time PCR was done using SYBR Green mastermix and 1 μm primers on a Rotor-gene 3000 real-time PCR machine (Corbett Research, Bath, UK). A 10 min denaturing step was employed, followed by 45 cycles of 95°C (15 s) and 60°C (60 s). The take-off cycle (CT) for each gene of interest (GOI) was compared to the housekeeping gene GAPDH. Relative gene expressions were calculated DD using the 2−ΔΔCT method.
Statistical analysis
All biochemical and mechanical assessments in this study were performed using n = 5–8 samples per group. Numerical data are represented as mean ± 95% confidence interval for the mean (95% CI). The presence of outliers was determined using either Grubb’s test and/or by noting testing abnormalities (e.g., failure at the grips during tensile testing). Prior to performing statistical tests, the data were checked for both normality and equal-variance using Levene’s test. To compare among treatment groups, one-way analysis of variance (ANOVA) was performed using StatView software (StaView inc Cary, Nesbit, MS, USA). If significance was identified, Fisher’s post hoc testing was applied. Unequal sample sizes were addressed by non-parametric all-pairs multiple comparisons based on pairwise rankings in the one-way design with the Steel–Dwass procedure. P-values less than 0.05 were considered significant.
Results
Gross morphology and histology
At the end of the culture period the morphological properties and composition of the engineered constructs were assessed via gross inspection and histological evaluation. All constructs presented with similar flat surfaces without abnormalities and no significant differences were detected in morphology among the groups. Table I describes morphological characteristics (diameter, thickness, and wet weight (WW)) of the constructs. Histology showed that all constructs stained uniformly positive for both GAG and collagen content (Fig. 1). Additionally, IHC showed that neocartilage from all groups stained positive for collagen type II and negatively for collagen type I proving normal AC phenotype maintenance in all groups (Fig. 1).
Table I.
Growth metrics and biochemical content of 4 weeks neocartilage constructs*
| Groups | Diameter (mm) (P = 0.4645) |
Thickness (mm) (P = 0.2384) |
Wet weight (mg) (P = 0.4566) |
Water content (%) (P = 0.7472) |
Cell number (millions) (P = 0.2669) |
|---|---|---|---|---|---|
| Control | 5.21 ± 0.22 | 1.53 ± 0.11 | 29.87 ± 4.93 | 88.75 ± 13.23 | 3.01 ± 2.86 |
| 1st & 3rd weeks | 5.17 ± 0.33 | 1.35 ± 0.25 | 29.65 ± 8.80 | 90.50 ± 12.11 | 4.03 ± 3.26 |
| 3rd & 4th weeks | 5.11 ± 0.23 | 1.40 ± 0.22 | 26.45 ± 2.06 | 87.46 ± 7.93 | 4.24 ± 5.22 |
| 4th week | 5.09 ± 0.33 | 1.48 ± 0.42 | 28.30 ± 7.42 | 87.81 ± 9.52 | 4.46 ± 4.39 |
| Continuous | 5.20 ± 0.24 | 1.45 ± 0.35 | 28.09 ± 10.95 | 88.19 ± 5.43 | 2.64 ± 2.22 |
The effects of hypoxia as a function of application time during the self-assembly of AC (control, 1st & 3rd weeks, 3rd & 4th weeks, 4th week, and continuous hypoxia groups) were examined on gross morphology (diameter, thickness, and WW) and biochemical properties (water content and cell number) after 4 weeks of culture. Values are mean ± 95% CI. No significant differences were detected among controls and hypoxia-treated groups. See Results section for details. Col = collagen.
Fig. 1.
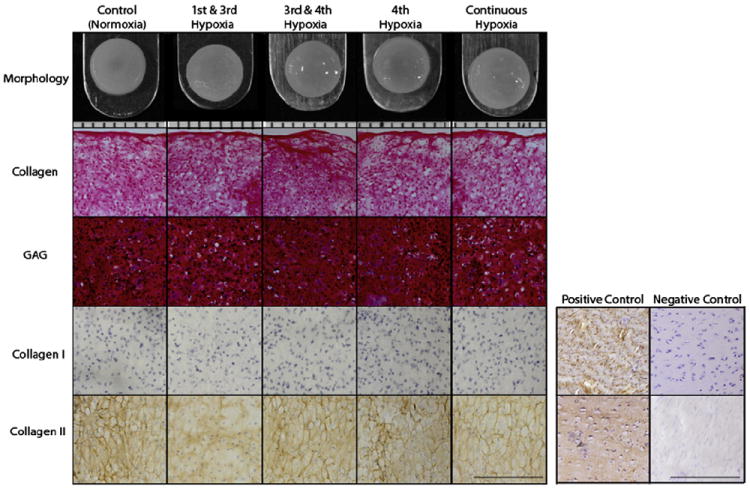
Gross morphology, histology, and IHC of self-assembled neocartilage. Both controls and hypoxia-treated constructs formed uniform neocartilage constructs with similar flat surfaces without physical abnormalities. The orientation of cryosectioning was from top to bottom in all groups (14 μm sections). Safranin-O/fast green staining for GAGs and Picrosirius red staining for collagen showed that constructs produced these matrix components uniformly for all groups. IHC illustrated that all groups were positive stained for collagen type II but not for collagen type I, suggesting AC phenotype. For gross morphology (top row), each notch represent 1 mm. For histology, bar represents 200 μm.
Biochemical properties
Growth of the constructs was evaluated through assessment of the amount of collagen, GAG, DNA, and PYR of neotissue at the end of the culture period. Table II describes the biochemical characteristics of the constructs. No significant differences were detected in GAG per wet weight (GAG/WW) among the groups (P = 0.718). For collagen per wet weight (Col/WW), no significant differences were detected among control, and late hypoxia application groups (3rd and 4th weeks, and 4th week group) (P = 0.2716 and P = 0.9919, respectively). Interestingly, constructs treated with hypoxia at an early stage (1st and 3rd weeks and all weeks groups) presented with significantly lower amount of collagen over control and late treated groups (P = 0.004 and P = 0.004).
Table II.
Biochemical properties of self-assembled neocartilage*
| Groups | Collagen/WW % (P < 0.0001) |
GAG/WW % (P = 0.718) |
PYR/WW (nmol/g) (P = 0.0036) |
PYR/Col (nmol/mg) (P < 0.0001) |
|---|---|---|---|---|
| Control | 3.03 ± 0.61A | 4.39 ± 2.10 | 4.99 ± 1.86B | 0.16 ± 0.030D |
| 1st & 3rd weeks | 1.93 ± 1.27B | 3.84 ± 3.85 | 5.68 ± 1.00A,B | 0.30 ± 0.169A |
| 3rd & 4th weeks | 3.33 ± 1.23A | 4.96 ± 3.44 | 6.63 ± 1.90A | 0.20 ± 0.034C |
| 4th week | 3.03 ± 1.50A | 4.91 ± 3.50 | 5.19 ± 1.06B | 0.17 ± 0.105C,D |
| Continuous | 1.91 ± 0.44B | 4.44 ± 2.96 | 5.08 ± 1.88B | 0.26 ± 0.063B |
Biochemical properties of the engineered neocartilage as represented by Col/WW, GAG/WW, PYR/WW and PYR/Col. Values are mean ± 95% CI. Fisher’s post hoc testing was applied if P < 0.05. Groups not connected by the same letter are significantly different. See Results section for details. Col = collagen.
Hypoxia applied during 3rd and 4th weeks significantly increased the amount of PYR/WW over controls (34% increase) (P = 0.003), while no significant effects were detected on the other applied groups over controls (P = 0.4160, P = 0.9753, and P = 0.9991 for 1st and 3rd weeks, 4th week and continuous groups, respectively) (Table II). In contrast, when PYR content was normalized to collagen content (PYR/Col), early application of hypoxia (1st and 3rd weeks, continuous groups) exhibited the highest amount of collagen crosslinks (P < 0.001 and P = 0.001, respectively) (Table II). For the 3rd and 4th weeks group, the amount of PYR/Col was also significantly higher over controls (P = 0.0499), while in the 4th week group no significant differences were detected (P = 0.4583).
Biomechanical properties
To quantify the influence of low oxygen tension applied at different time periods on the biomechanics of self-assembled neocartilage, compressive properties represented by the aggregate modulus, permeability, and poison’s ratio, and tensile properties represented by Young’s modulus (EY) and UTS and were determined. No significant difference was detected for compressive properties among groups (P = 0.4017, P = 0.1101, and P = 0.3124 for aggregate modulus, permeability, and Poisson’s ratio, respectively) (Table III). Low oxygen tension applied during 3rd and 4th weeks of the self-assembling process significantly increased the tensile stiffness of the constructs over controls (~80% increase) (P < 0.0001). Similar trends were observed concerning the UTS of the constructs (Table III). Enhanced mechanical properties reflected the observed changes in the PYR content.
Table III.
Biomechanical properties of the engineered neocartilage*
| Group | Aggregate modulus (MPa) (P = 0.4017) |
Permeability (10−15 m4/N’s) (P = 0.1101) |
Poisson’s ratio (P = 0.0773) |
Young’s modulus (MPa) (P < 0.0001) |
UTS (MPa) (P = 0.4600) |
|---|---|---|---|---|---|
| Control | 0.11 ± 0.06 | 18.50 ± 15.69 | 0.09 ± 0.22 | 0.45 ± 0.23B,C | 0.07 ± 0.04 |
| 1st & 3rd weeks | 0.10 ± 0.06 | 16.85 ± 16.89 | 0.08 ± 0.02 | 0.19 ± 0.23C | 0.11 ± 0.07 |
| 3rd & 4th weeks | 0.12 ± 0.05 | 11.62 ± 9.68 | 0.05 ± 0.11 | 0.82 ± 0.53A | 0.06 ± 0.09 |
| 4th week | 0.13 ± 0.04 | 16.04 ± 8.70 | 0.01 ± 0.02 | 0.53 ± 0.54A,B | 0.11 ± 0.11 |
| Continuous | 0.11 ± 0.06 | 24.98 ± 28.31 | 0.06 ± 0.12 | 0.25 ± 0.19B,C | 0.18 ± 0.18 |
Compression properties of tissue engineered neocartilage constructs represented by aggregate modulus, permeability, and Poisson’s ratio, and tensile properties represented by Young’s modulus and UTS. Values are mean ± 95% CI. Fisher’s post hoc testing was applied if P < 0.05. Groups not connected by the same letter are significantly different. See Results section for details.
RT-PCR
Real-time PCR was employed to investigate the effects of hypoxia in the engineered neocartilage using the self-assembly method. Application of hypoxia in the neotissue promoted an 18-fold increase in the gene expression of LOX over controls cultured under normoxic conditions.
Discussion
This is the first study to demonstrate that hypoxia’s effect on enhancing engineered cartilage’s tensile properties is through collagen crosslinking. Experimental data proved both hypotheses motivating the study, showing (1) hypoxia is a viable method for promoting collagen crosslinking in engineered tissue through LOX gene expression and (2) distinctly different responses can be elicited by this stimulus by manipulating application time and duration. Hypoxia can be restricted to influence only the tensile properties of the constructs, while other neocartilage properties such as gross morphology, collagen and GAG biochemistry, and compressive stiffness remain unaltered. By determining a method wherein hypoxia can be isolated to act only through collagen crosslinks, this study provides a robust method for improving the tensile properties of engineered cartilage that has the potential to be extended to other engineered tissues.
In this study, hypoxia was applied in tissue engineered AC to enhance crosslinking by promoting gene expression of LOX. Low oxygen tension (4% O2) applied during the 3rd and 4th weeks of self-assembly of neocartilage significantly increased the amount of PYR molecules (PYR/WW and PYR/Col) over controls and the other treated groups with concomitant increase in the gene expression of LOX (18-fold) over controls. In contrast, no significant differences were observed among the 3rd and 4th weeks, the 4th week and control groups for collagen, GAG and DNA content per WW. Additionally, hypoxia applied during the 3rd and 4th weeks increased the tensile stiffness of neocartilage by approximately 80% over controls (P < 0.0001). These results demonstrate that application of hypoxia can be directed to promote collagen crosslinking and enhance the tensile properties in engineered cartilage, independently of other biochemical and biomechanical properties.
In contrast, hypoxia applied during times other than the 3rd and 4th weeks did not result in improved properties. At times, these properties were diminished, shedding light onto the mixed results seen in the cartilage engineering literature that employ hypoxia. Early application of hypoxia (1st and 3rd weeks and continuous application groups) significantly increased the PYR/Col content, but collagen content was diminished simultaneously. Early hypoxia application allowed for a more time for collagen crosslinking to form. For reference, the characteristic time constants for the formation of immature (difunctional) and mature (trifunctional) PYR crosslinks have been reported to be 1–2 and 7–30 days, respectively33. However, early hypoxia application also significantly decreased the collagen content of neotissue at these groups. The net effect is that early hypoxia application did not increase PYR/ WW, and the mechanical properties of neotissue remained unchanged. Biochemical evaluation further showed no significant differences in terms of the number of cells per construct and GAG/WW in these groups. Thus, it appears that early hypoxia application is actually detrimental to engineering self-assembled cartilage when it is applied during a time previously identified as the collagen synthesis phase25.
These results are similar to a previous study investigating the effects of 5% and 20% O2 applied continuously for 2, 4 and 6 weeks in a scaffold-free chondrocyte culture13. In this study, engineered cartilage from scaffold-free cultured chondrocytes at 20% O2 produced better ECM than that at 5% O2. Thus, the present study offers an examination on the conflicting results currently seen with hypoxia; hypoxia applied during the collagen synthesis phase of engineered cartilage can diminish collagen production and therefore adversely affect construct biomechanical properties. Use of hypoxia to induce crosslinking should instead be employed after collagen is already present. This time dependence issue should be further investigated in other cartilage engineering approaches.
In three-dimensional cultures or engineered tissues, work on studying the effects of different oxygen concentrations might be impaired by poor oxygen diffusion that creates an oxygen gradient within tissue34. Additionally, HIF-1α protein is very unstable at the environmental oxygen level while its mRNA is not affected by hypoxic conditions16. This makes it difficult to demonstrate, experimentally, that the HIF-1α protein increases in response to hypoxia. However, the link between hypoxia, LOX gene expression, and LOX-mediated collagen crosslinking has been explored in many other tissues17,23,25,35. Specifically, growth of porcine aortic endothelium cells in 0% or 2% oxygen tension resulted in little change in cell numbers or cell protein, but a fall in collagen synthesis and in proline and lysine hydroxylases, as well as a rise in LOX gene expression, were observed35. Similarly, the crosslink pattern and the gene expression of lysyl hydroxylase 2 (LH2) were investigated using skin cells from systemic scleroderma, cultured under low oxygen tension conditions. Prolonged hypoxia induced a marked increase of the mRNA level of LH2 in relation to collagen I22. Thus, the use of hypoxia to promote LOX- and LH-mediated collagen crosslinking can be considered as a robust mechanism and this experiment showed not only that hypoxia can be used in engineering cartilage, but that its effects can be isolated to tensile properties only.
Further proof of the robustness of the hypoxia-tensile relationship can be seen in engineering other tissues17,34,36. It has been reported that 7% O2 applied in human vascular-derived myofibroblasts seeded onto a biodegradable scaffold similarly increases construct properties as seen in this study36. In another case, 4% O2 enhanced gene expression of LOX and LH217. It is, thus, conceivable that the use of hypoxia can be translated to other musculoskeletal tissues. The ECM of native ligaments, tendons, bone, and other musculoskeletal tissues is composed of proteoglycans and fibrillar proteins, such as elastin and collagen. The load-bearing capacity of collagen is, apart from collagen content and organization, highly dependent on collagen crosslinking, which stabilizes the collagen fibrils37. The in vitro formation of these highly important ECM components can be affected by mechanical, biochemical, and environmental stimuli, such as oxygen concentration36,38,39. As with this experiment, hypoxia could potentially improve the mechanical properties of other engineered musculoskeletal tissues through collagen crosslinking enhancement. However, application time for these engineered tissues must be optimized as to restrict its effects on crosslinks only.
Due to HIF-1α’s link with other ECM gene expression, this study provides strong support for further development of other hypoxia duty cycles. Specifically, hypoxia may be applied during certain times to encourage proper differentiation, switching to normoxia to induce collagen synthesis, and returning to hypoxia to promote crosslinking. The motivation that underlies such a duty cycle can be found in multiple studies. For stem cells, hypoxia, through HIF-1α overexpression, has been shown to be effective and sufficient in inducing a chondrocytic phenotype on human bone marrow stem cells without use of exogenous growth factors40. Similar results have been obtained with adipose-derived stem cells41. While hypoxia seems beneficial for chondrodifferentation, early application during neocartilage tissue engineering does not result in improved properties as demonstrated by this and prior work13. Thus, the present study provides strong support for further development of other hypoxia duty cycles, suggesting that, until robust mechanisms can be determined for hypoxia’s effects on ECM synthesis, this potent stimulus should only be applied for differentiation and for collagen crosslinking.
Aside from PYR, other cartilage crosslinks such as arginoline and advanced glycation end products (AGEs) might similarly be manipulated to influence neocartilage stiffness. Arginoline makes up for half of the mature crosslinks in native cartilage42 but cannot be measured via HPLC, necessitating future studies on its characterization in engineered tissues. AGEs occur over a time-scale of decades as sugars react with the lysine and arginine residues during aging. Examples of common AGEs include crosslinks, such as pentosidine43-45, methylglyoxal-lysine dimer (MOLD)44, and threosidine46, as well as Nε-(carboxymethyl)lysine (CML)47 and Nε-(carboxyethyl)lysine (CEL)48. Though past work has correlated AGEs with stiffening of the collagen matrix49, AGEs have also been implicated in the progression of age-related cartilage degeneration such as OA50. AGEs may thus be an inferior candidate when compared to PYR and arginoline for enhancing neocartilage mechanical properties.
In this experiment, low oxygen tension (4% O2) was investigated at various application times during the self-assembling process both to identify optimal regimens for hypoxia application and to elucidate a mechanism through which hypoxia-induced increases in cartilage mechanical properties occur. Hypoxia applied during the collagen synthesis phase resulted in decreased collagen content. However, hypoxia applied later in culture served to induce LOX expression, crosslink formation, and increased tensile properties. Strong evidence from both this study and a plethora of prior studies suggest that this occurs through the robust mechanism of HIF-1α-induced upregulation of LOX. The results shown here are promising as they show that hypoxia can be used to tissue engineer neocartilage with robust tensile properties. In the future, while one may consider the possibility of employing hypoxia to achieve similar biomechanical improvements in cartilage just showing signs of mechanical degradation, a different regimen will have to be identified for intra-articular regulation of oxygen tension. Additionally, hypoxia may be beneficial for enhancing the mechanical properties of autografts or allografts.
Acknowledgments
The authors acknowledge funding support from R01AR053286 and R01DE019666. The funding sources did not have a role in the collection, analysis and interpretation of data.
Footnotes
Author contributions
All authors have contributed to the conception and design of the study, and acquisition, analysis and interpretation of data. The manuscript has been drafted, revised and finally approved by all authors. All authors take responsibility for the integrity of the work.
Conflict of interest
The authors declare no conflict of interest.
Contributor Information
E.A. Makris, Email: emakris@ucdavis.edu.
J.C. Hu, Email: jcyhu@ucdavis.edu.
K.A. Athanasiou, Email: athanasiou@ucdavis.edu.
References
- 1.Verbrugge LM, Patrick DL. Seven chronic conditions: their impact on US adults’ activity levels and use of medical services. Am J Public Health. 1995 Feb;85(2):173–82. doi: 10.2105/ajph.85.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clouet J, Vinatier C, Merceron C, Pot-vaucel M, Maugars Y, Weiss P, et al. From osteoarthritis treatments to future regenerative therapies for cartilage. Drug Discov Today. 2009 Oct;14(19–20):913–25. doi: 10.1016/j.drudis.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 3.Hu JC, Athanasiou KA. A self-assembling process in articular cartilage tissue engineering. Tissue Eng. 2006 Apr;12(4):969–79. doi: 10.1089/ten.2006.12.969. [DOI] [PubMed] [Google Scholar]
- 4.Schenke-Layland K. From tissue engineering to regenerative medicine – the potential and the pitfalls. Adv Drug Deliv Rev. 2011 Apr 30;63(4–5):193–4. doi: 10.1016/j.addr.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Fu WL, Jia ZQ, Wang WP, Zhang JY, Fu X, Duan XN, et al. Proliferation and apoptosis property of mesenchymal stem cells derived from peripheral blood under the culture conditions of hypoxia and serum deprivation. Chin Med J (Engl) 2011 Dec;124(23):3959–67. [PubMed] [Google Scholar]
- 6.Adesida AB, Mulet-Sierra A, Jomha NM. Hypoxia mediated isolation and expansion enhances the chondrogenic capacity of bone marrow mesenchymal stromal cells. Stem Cell Res Ther. 2012;3(2):9. doi: 10.1186/scrt100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Markway BD, Tan GK, Brooke G, Hudson JE, Cooper-White JJ, Doran MR. Enhanced chondrogenic differentiation of human bone marrow-derived mesenchymal stem cells in low oxygen environment micropellet cultures. Cell Transpl. 2010;19(1):29–42. doi: 10.3727/096368909X478560. [DOI] [PubMed] [Google Scholar]
- 8.Gawlitta D, van Rijen MH, Schrijver EJ, Alblas J, Dhert WJ. Hypoxia impedes hypertrophic chondrogenesis of human multipotent stromal cells. Tissue Eng Part A. 2012 Jun 25; doi: 10.1089/ten.TEA.2011.0657. [DOI] [PubMed] [Google Scholar]
- 9.Das RH, van Osch GJ, Kreukniet M, Oostra J, Weinans H, Jahr H. Effects of individual control of pH and hypoxia in chondrocyte culture. J Orthop Res. 2010 Apr;28(4):537–45. doi: 10.1002/jor.20994. [DOI] [PubMed] [Google Scholar]
- 10.Foldager CB, Nielsen AB, Munir S, Ulrich-Vinther M, Soballe K, Bunger C, et al. Combined 3D and hypoxic culture improves cartilage-specific gene expression in human chondrocytes. Acta Orthop. 2011 Apr;82(2):234–40. doi: 10.3109/17453674.2011.566135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strobel S, Loparic M, Wendt D, Schenk AD, Candrian C, Lindberg RL, et al. Anabolic and catabolic responses of human articular chondrocytes to varying oxygen percentages. Arthritis Res Ther. 2010;12(2):R34. doi: 10.1186/ar2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mwale F, Ciobanu I, Giannitsios D, Roughley P, Steffen T, Antoniou J. Effect of oxygen levels on proteoglycan synthesis by intervertebral disc cells. Spine (Phila Pa 1976) 2011 Jan 15;36(2):E131–8. doi: 10.1097/BRS.0b013e3181d52b9e. [DOI] [PubMed] [Google Scholar]
- 13.Qu C, Lindeberg H, Ylarinne JH, Lammi MJ. Five percent oxygen tension is not beneficial for neocartilage formation in scaffold-free cell cultures. Cell Tissue Res. 2012 Apr;348(1):109–17. doi: 10.1007/s00441-012-1366-z. [DOI] [PubMed] [Google Scholar]
- 14.Buckley CT, Vinardell T, Kelly DJ. Oxygen tension differentially regulates the functional properties of cartilaginous tissues engineered from infrapatellar fat pad derived MSCs and articular chondrocytes. Osteoarthritis Cartilage. 2010 Oct;18(10):1345–54. doi: 10.1016/j.joca.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Guyton AC. HJTooacditbabfIToMPUV Respiration. New York: Elsevier; 2000. [Google Scholar]
- 16.Ke Q, Costa M. Hypoxia-inducible factor-1 (HIF-1) Mol Pharmacol. 2006 Nov;70(5):1469–80. doi: 10.1124/mol.106.027029. [DOI] [PubMed] [Google Scholar]
- 17.van Vlimmeren MA, Driessen-Mol A, van den Broek M, Bouten CV, Baaijens FP. Controlling matrix formation and cross-linking by hypoxia in cardiovascular tissue engineering. J Appl Physiol. 2010 Nov;109(5):1483–91. doi: 10.1152/japplphysiol.00571.2010. [DOI] [PubMed] [Google Scholar]
- 18.Mow VC, Holmes MH, Lai WM. Fluid transport and mechanical properties of articular cartilage: a review. J Biomech. 1984;17(5):377–94. doi: 10.1016/0021-9290(84)90031-9. [DOI] [PubMed] [Google Scholar]
- 19.Bastiaansen-Jenniskens YM, Koevoet W, de Bart AC, van der Linden JC, Zuurmond AM, Weinans H, et al. Contribution of collagen network features to functional properties of engineered cartilage. Osteoarthritis Cartilage. 2008 Mar;16(3):359–66. doi: 10.1016/j.joca.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 20.Ficklin T, Thomas G, Barthel JC, Asanbaeva A, Thonar EJ, Masuda K, et al. Articular cartilage mechanical and biochemical property relations before and after in vitro growth. J Biomech. 2007;40(16):3607–14. doi: 10.1016/j.jbiomech.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riesle J, Hollander AP, Langer R, Freed LE, Vunjak-Novakovic G. Collagen in tissue-engineered cartilage: types, structure, and crosslinks. J Cell Biochem. 1998 Dec 1;71(3):313–27. doi: 10.1002/(sici)1097-4644(19981201)71:3<313::aid-jcb1>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 22.Brinckmann J, Kim S, Wu J, Reinhardt DP, Batmunkh C, Metzen E, et al. Interleukin 4 and prolonged hypoxia induce a higher gene expression of lysyl hydroxylase 2 and an altered cross-link pattern: important pathogenetic steps in early and late stage of systemic scleroderma? Matrix Biol. 2005 Oct;24(7):459–68. doi: 10.1016/j.matbio.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Wang Z, Chesler NC. Role of collagen content and cross-linking in large pulmonary arterial stiffening after chronic hypoxia. Biomech Model Mechanobiol. 2012 Jan;11(1–2):279–89. doi: 10.1007/s10237-011-0309-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong CC, Gilkes DM, Zhang H, Chen J, Wei H, Chaturvedi P, et al. Hypoxia-inducible factor 1 is a master regulator of breast cancer metastatic niche formation. Proc Natl Acad Sci USA. 2011 Sep 27;108(39):16369–74. doi: 10.1073/pnas.1113483108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ofek G, Revell CM, Hu JC, Allison DD, Grande-Allen KJ, Athanasiou KA. Matrix development in self-assembly of articular cartilage. PLoS One. 2008;3(7):e2795. doi: 10.1371/journal.pone.0002795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Natoli RM, Responte DJ, Lu BY, Athanasiou KA. Effects of multiple chondroitinase ABC applications on tissue engineered articular cartilage. J Orthop Res. 2009 Jul;27(7):949–56. doi: 10.1002/jor.20821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woessner JF., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961 May;93:440–7. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 28.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986 Sep 4;883(2):173–7. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 29.Almarza AJ, Athanasiou KA. Seeding techniques and scaffolding choice for tissue engineering of the temporomandibular joint disk. Tissue Eng. 2004 Nov-Dec;10(11–12):1787–95. doi: 10.1089/ten.2004.10.1787. [DOI] [PubMed] [Google Scholar]
- 30.Bank RA, Beekman B, Verzijl N, de Roos JA, Sakkee AN, TeKoppele JM. Sensitive fluorimetric quantitation of pyridinium and pentosidine crosslinks in biological samples in a single high-performance liquid chromatographic run. J Chromatogr B Biomed Sci Appl. 1997 Dec 5;703(1–2):37–44. doi: 10.1016/s0378-4347(97)00391-5. [DOI] [PubMed] [Google Scholar]
- 31.Athanasiou KA, Agarwal A, Muffoletto A, Dzida FJ, Constantinides G, Clem M. Biomechanical properties of hip cartilage in experimental animal models. Clin Orthop Relat Res. 1995 Jul;316:254–66. [PubMed] [Google Scholar]
- 32.Erler JT, Bennewith KL, Nicolau M, Dornhofer N, Kong C, Le QT, et al. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006 Apr 27;440(7088):1222–6. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- 33.Ahsan T, Harwood F, McGowan KB, Amiel D, Sah RL. Kinetics of collagen crosslinking in adult bovine articular cartilage. Osteoarthritis Cartilage. 2005 Aug;13(8):709–15. doi: 10.1016/j.joca.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 34.Brown DA, MacLellan WR, Laks H, Dunn JC, Wu BM, Beygui RE. Analysis of oxygen transport in a diffusion-limited model of engineered heart tissue. Biotechnol Bioeng. 2007 Jul 1;97(4):962–75. doi: 10.1002/bit.21295. [DOI] [PubMed] [Google Scholar]
- 35.Levene CI, Kapoor R, Heale G. The effect of hypoxia on the synthesis of collagen and glycosaminoglycans by cultured pig aortic endothelium. Atherosclerosis. 1982 Sep;44(3):327–37. doi: 10.1016/0021-9150(82)90007-7. [DOI] [PubMed] [Google Scholar]
- 36.Balguid A, Mol A, van Vlimmeren MA, Baaijens FP, Bouten CV. Hypoxia induces near-native mechanical properties in engineered heart valve tissue. Circulation. 2009 Jan 20;119(2):290–7. doi: 10.1161/CIRCULATIONAHA.107.749853. [DOI] [PubMed] [Google Scholar]
- 37.Balguid A, Rubbens MP, Mol A, Bank RA, Bogers AJ, van Kats JP, et al. The role of collagen cross-links in biomechanical behavior of human aortic heart valve leaflets – relevance for tissue engineering. Tissue Eng. 2007 Jul;13(7):1501–11. doi: 10.1089/ten.2006.0279. [DOI] [PubMed] [Google Scholar]
- 38.Mol A, Driessen NJ, Rutten MC, Hoerstrup SP, Bouten CV, Baaijens FP. Tissue engineering of human heart valve leaflets: a novel bioreactor for a strain-based conditioning approach. Ann Biomed Eng. 2005 Dec;33(12):1778–88. doi: 10.1007/s10439-005-8025-4. [DOI] [PubMed] [Google Scholar]
- 39.Rubbens MP, Mol A, Boerboom RA, Bank RA, Baaijens FP, Bouten CV. Intermittent straining accelerates the development of tissue properties in engineered heart valve tissue. Tissue Eng Part A. 2009 May;15(5):999–1008. doi: 10.1089/ten.tea.2007.0396. [DOI] [PubMed] [Google Scholar]
- 40.Duval E, Bauge C, Andriamanalijaona R, Benateau H, Leclercq S, Dutoit S, et al. Molecular mechanism of hypoxia-induced chondrogenesis and its application in in vivo cartilage tissue engineering. Biomaterials. 2012 Sep;33(26):6042–51. doi: 10.1016/j.biomaterials.2012.04.061. [DOI] [PubMed] [Google Scholar]
- 41.Jurgens WJ, Lu Z, Zandieh-Doulabi B, Kuik DJ, Ritt MJ, Helder MN. Hyperosmolarity and hypoxia induce chondrogenesis of adipose-derived stem cells in a collagen type 2 hydrogel. J Tissue Eng Regen Med. 2012 Jul;6(7):570–8. doi: 10.1002/term.464. [DOI] [PubMed] [Google Scholar]
- 42.Eyre DR, Weis MA, Wu JJ. Maturation of collagen Ketoimine cross-links by an alternative mechanism to pyridinoline formation in cartilage. J Biol Chem. 2010 May 28;285(22):16675–82. doi: 10.1074/jbc.M110.111534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sell DR, Monnier VM. Structure elucidation of a senescence cross-link from human extracellular matrix. Implication of pentoses in the aging process. J Biol Chem. 1989 Dec 25;264(36):21597–602. [PubMed] [Google Scholar]
- 44.Frye EB, Degenhardt TP, Thorpe SR, Baynes JW. Role of the Maillard reaction in aging of tissue proteins. Advanced glycation end product-dependent increase in imidazolium cross-links in human lens proteins. J Biol Chem. 1998 Jul 24;273(30):18714–9. doi: 10.1074/jbc.273.30.18714. [DOI] [PubMed] [Google Scholar]
- 45.Dyer DG, Blackledge JA, Thorpe SR, Baynes JW. Formation of pentosidine during nonenzymatic browning of proteins by glucose. Identification of glucose and other carbohydrates as possible precursors of pentosidine in vivo. J Biol Chem. 1991 Jun 25;266(18):11654–60. [PubMed] [Google Scholar]
- 46.Prabhakaram MCQ, Feather MS, Ortwerth BJ. Structural elucidation of a novel lysine-lysine crosslink generated in a glycation reaction with l-threose. Amino Acids. 1997;12:225–36. [Google Scholar]
- 47.Dunn JA, Patrick JS, Thorpe SR, Baynes JW. Oxidation of glycated proteins: age-dependent accumulation of N epsilon-(carboxymethyl)lysine in lens proteins. Biochemistry. 1989 Nov 28;28(24):9464–8. doi: 10.1021/bi00450a033. [DOI] [PubMed] [Google Scholar]
- 48.Ahmed MU, Brinkmann Frye E, Degenhardt TP, Thorpe SR, Baynes JW. N-epsilon-(carboxyethyl)lysine, a product of the chemical modification of proteins by methylglyoxal, increases with age in human lens proteins. Biochem J. 1997 Jun 1;324(Pt 2):565–70. doi: 10.1042/bj3240565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bank RA, Bayliss MT, Lafeber FP, Maroudas A, Tekoppele JM. Ageing and zonal variation in post-translational modification of collagen in normal human articular cartilage. The age-related increase in non-enzymatic glycation affects biomechanical properties of cartilage. Biochem J. 1998 Feb 15;330(Pt 1):345–51. doi: 10.1042/bj3300345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Verzijl N, DeGroot J, Thorpe SR, Bank RA, Shaw JN, Lyons TJ, et al. Effect of collagen turnover on the accumulation of advanced glycation end products. J Biol Chem. 2000 Dec 15;275(50):39027–31. doi: 10.1074/jbc.M006700200. [DOI] [PubMed] [Google Scholar]


