Abstract
Introduction
The α2-adrenoceptor agonist dexmedetomidine is an effective postoperative sedative without clear advantages over midazolam or propofol. We hypothesized that routine use of dexmedetomidine allows early extubation in cardiac surgery patients. Secondary outcomes included the use of narcotic and non-narcotic analgesics during the first 48 hours, early postoperative functional status, and the incidence of bradycardia or hypotension.
Methods
We retrospectively analyzed patients admitted to a cardiothoracic intensive care unit after cardiac surgery. Patient charts and the Society of Thoracic Surgery National database were reviewed. Patients who received no sedation were compared to those who received dexmedetomidine.
Results
Ninety-nine patients (52 receiving no sedation and 47 receiving dexmedetomidine) were included in this study. The median time to extubation was 3.9 (2.8-5.4) hours in the control group versus 4.7 (3.45-6.52) hours in the dexmedetomidine (P=.16). The incidence of bradycardia, hypotension, the ability to ambulate, and Glascow Coma Scores = 15 on postoperative day 0 did not differ significantly. Acetaminophen was used more frequently in the first 48 hours postoperatively in dexmedetomidine patients (P=.02) and a trend toward higher opioid (P=.09) and ketorolac use (P=.30) over the first 48 hours was noted.
Conclusions
The use of dexmedetomidine did not allow earlier extubation or less use of analgesics when compared to no sedation. Bradycardia and hypotension were not a problem with the use of dexmedetomidine.
Keywords: cardiothoracic surgery, cardiac surgery, cardiovascular anesthesia, post operative sedation, dexmedetomidine, time to extubation
INTRODUCTION
The use of sedatives in the post-cardiac surgery patient minimizes anxiety, agitation, pain, and delirium [1]. Often, short acting medications such as midazolam or propofol are used with the goal of early extubation in these patients. These GABA-mimetics have no analgesic properties, and the use of opioids or other medications is required for pain control [2]. Titrating sedatives in individual patients may be problematic for critical care staff. Studies have pointed out that continuous intravenous sedation, for example, can extend a patient’s stay in the intensive care unit (ICU) [3], cause vasodilatory effects, and result in respiratory depression, which may be exacerbated by opioids [4].
In 1999, the Food and Drug Administration (FDA) approved the use of dexmedetomidine as an alternative to GABA-mimetic drugs for ICU sedation [5]. Dexmedetomidine is an α2-adrenoceptor agonist, which has been shown to provide sedation and analgesia with minimal respiratory depression. This occurs via central nervous system receptors, particularly in the locus coeruleus, regulating memory, affect, awareness, and nociception [6].
Stimulation of these receptors also inhibits the release of norepinephrine, which may contribute to bradycardia and hypotension [7].
To date, investigators have not shown a consistent reduction in time to extubation for patients receiving dexmedetomidine. When compared to propofol in post-cardiac surgery patients, dexmedetomidine had no impact on time to extubation according to Barletta et al. [2].
Conversely, the SEDCOM (Safety and Efficacy of Dexmedetomidine Compared with Midazolam) trial noted that the median time to extubation was 1.9 days shorter in dexmedetomidine versus midazolam-treated medical ICU patients requiring mechanical ventilation for more than 24 hours [8]. If earlier extubation of cardiac surgery patients could be demonstrated, there might be a justification for the additional expense of dexmedetomidine. Dexmedetomidine has analgesic properties and one could reasonably expect that the use of narcotics and non-narcotic analgesics would be lower in dexmedetomidine treated patients. If patients had less pain and received fewer narcotics, earlier mobility and improved cognition might occur immediately after surgery.
We hypothesized that routine use of dexmedetomidine after cardiac surgery would decrease time to extubation, reduce analgesic use and improve early postoperative functional status.
METHODS
A retrospective analysis was conducted at Geisinger-Community Medical Center (GCMC) reviewing records of 99 patients admitted to the Cardiothoracic Intensive Care Unit (CTICU) for post-cardiac surgery care. The patients that did not receive dexmedetomidine were treated between January and March 2011, and the dexmedetomidine treated patients were operated on between February and May 2012. There were no other changes in surgeons, anesthesia staff, protocols or treatment strategies, other than the introduction of intravenous acetaminophen, between these two time periods. Intravenous acetaminophen replaced the oral route of administration at our site in late 2011. Patients with infective endocarditis, cardiogenic shock, prolonged ICU stay secondary to stroke or major intraoperative complication, and those who died during the index hospitalization were excluded. Charts were reviewed for patient characteristics, procedure type, postoperative complications, analgesic usage, and early postoperative functional status assessed by their ability to get out of bed. The Wright Center for Graduate Medical Education Institutional Review Board (WCGME-IRB: SRC032612) approved this study.
Prior to February 2012, patients typically received no sedation, or rarely propofol or midazolam, beyond the short-acting narcotic based general anesthesia they received in the operating room. Starting in February 2012, cardiac surgical patients were given dexmedetomidine HCl infusion (loading dose of 1 mcg/kg over 10 minutes followed by maintenance doses ranging from 0.2 to 1.0 mcg/kg/hr) and benzodiazepine or propofol were no longer used as first-line sedatives. Thus, the study was retrospective and involved sequential groups of patients.
The time to extubation was measured as the interval between arrival in the CTICU and removal from mechanical ventilation, based on the Society of Thoracic Surgeons (STS) National Database. The use of opioid analgesics during the first 48 hours was recorded as equi-analgesic doses of morphine. Thus, narcotics such as fentanyl and oxycodone were converted to equal potency. Non-narcotics such as ketorolac and acetaminophen were recorded as milligrams administered to patients during the first 48 hours postoperatively.
Instances of bradycardia were defined as a heart rate lower than 55 beats per minute and hypotension was defined as a systolic blood pressure lower than 80 mmHg during the first 3 hours in CTICU, as documented in the care flow sheets. Glasgow Coma Scores over the first 48 hours postoperatively were also derived from the flow sheets. Functional status over the first 48 hours was determined by whether or not the patient was able to get out of bed and ambulate. Data collection took place at GCMC in the medical records department and via query of the STS computerized database. Patient identifiers except for a record number were removed from the Microsoft Excel Spreadsheet used to gather information. Results were summarized as mean ± standard deviation (SD) or as median (interquartile range) when appropriate. Statistical comparisons included Student’s t-test, or median test and chi squared tests for continuous and categorical data, respectively, using spreadsheet software (Microsoft® Office Excel® 2007; Microsoft® Corporation; Redmond, WA). All P values are 2-sided and a significance level (α) of 0.05 was selected.
RESULTS
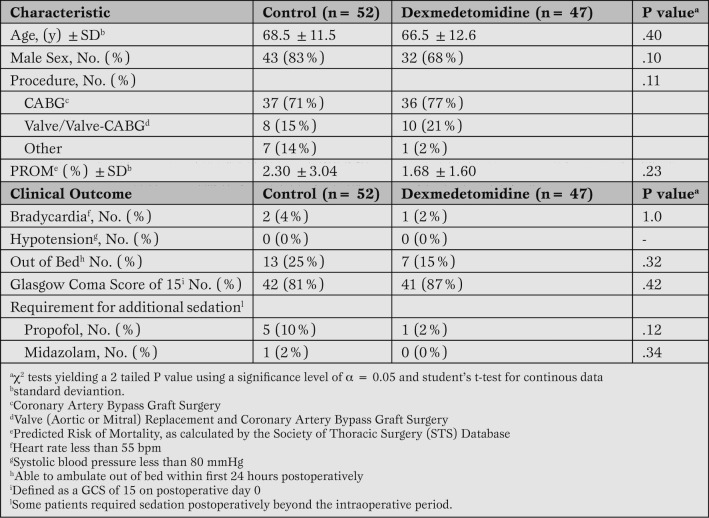
We reviewed 52 records of patients treated prior to the introduction of dexmedetomidine in February 2012 (control group) and 47 records in the dexmedetomidine treated group. There were no self extubations or reintubations, and groups were similar with regards to need for additional sedatives (Table 1).
Table 1.
Patient characteristics and safety profile.
The two groups were similar with respect to age, gender, surgery type, and predicted risk of mortality (PROM) (Table 1).
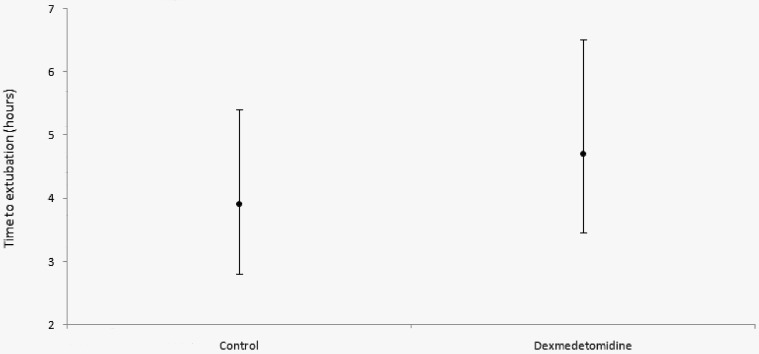
There was no statistically significant difference in time to extubation between groups. Among the control group, the median time to extubation was 3.9 (2.8-5.4) hours. In dexmedetomidine treated patients, median time was 4.7 (3.45-6.52) hours (P=.16) (Figure 1).
Figure 1.
Median (interquartile range) of time to extubation in hours.
The safety profile did not differ significantly for patients who did or did not receive dexmedetomidine. The incidence of bradycardia in the control group was 3.8% while dexmedetomidine patients had an incidence of 2.1% (P=1.0). Hypotension did not occur in any patients in either cohort. For control patients, 25.0% were able to ambulate out of bed in the first 24 hours, and 14.9% of dexmedetomidine treated patients were out of bed during this time (P=.32). Glasgow Coma Scores of 15 on the first postoperative day occurred in 80.8% of patients in the control group and 87.2% of patients receiving dexmedetomidine (P=.42) (Table 1).
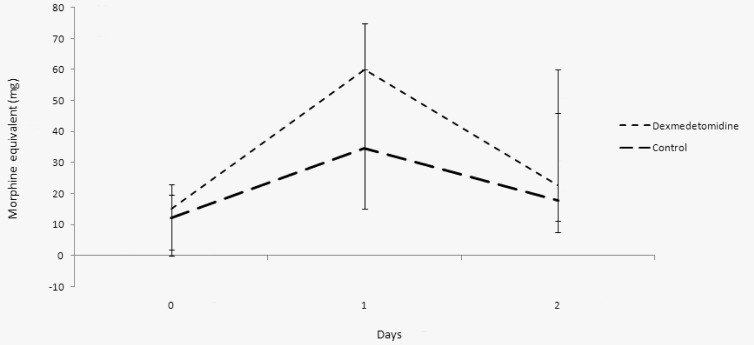
There was no significant difference between control and dexmedetomidine patients with respect to opioid analgesic use. The median opioid use in morphine equivalents over the first 48 hours was 71 ( 46-105 ) mg in the control group and 102 ( 37-151 ) mg in dexmedetomidine patients (P=.09). On the day of surgery, control patients used 12 ( 0-19 ) mg of morphine equivalents while dexmedetomidine patients used 15 ( 2-23 ) mg (P=.90). On postoperative day 1, control patients used 34 ( 15-60 ) mg of morphine equivalents, while dexmedetomidine patients received 60 ( 15-75 ) mg (P=.05). Finally, on postoperative day 2, controls received 18 (7.5-46) mg, while dexmedetomidne patients received 22 ( 11-60 ) mg (P=.19) (Figure 2).
Figure 2.
Median of opioid use expressed as morphine equivalents (mg).
The usage of the nonsteroidal anti-inflammatory drug ketorolac was not significantly different. Over the first 48 hours postoperatively, patients not receiving dexmedetomidine had a median of 30 ( 0-90 ) mg versus 45 ( 0-105 ) mg in the dexmedetomidine group (P=.30). On postoperative day 0, control patients received a median of 7.5 ( 0-15 ) mg compared to dexmedetomidine patients receiving a median of 0 ( 0-30 ) mg (P=.90). On postoperative day 1, control patients received 30 ( 0-60 ) mg compared to 0 ( 0-15 ) mg in dexmedetomidine patients (P= .20). On postoperative day 1, control patients received 30 ( 0-60 ) mg compared to dexmedetomidine patients 0 ( 0-15 ) mg (P=.20). Finally, on postoperative day 2, control median of ketorolac was 0 ( 0-0 ) mg, while dexmedetomidine patients received 0 ( 0-15 ) mg (P=.20). There was a significant difference between acetaminophen use in our two groups. Median acetaminophen usage within the first 48 hours postoperatively was 3575 ( 2518-5206 ) mg in the control group and 5400 ( 3275-6700 ) mg in the dexmedetomidine group (P=.02). On postoperative day 0, control acetaminophen dose was 650 ( 0-650 ) mg versus a median of 1000 ( 650-1650 ) mg (P<.001) in the dexmedetomidine cohort. On postoperative day 1, control patients received 1300 ( 650-2600 ) mg, while dexmedetomidine patients received 2600 ( 812-3250 ) mg of acetaminophen (P=.05). Lastly, on postoperative day 2, control patients received 1300 ( 650-2066 ) mg, whereas dexmedetomidine patients received 1625 ( 625-2600 ) mg of acetaminophen (P=.50).
DISCUSSION
This study suggested no difference in the incidence of bradycardia, incidence of hypotension, or the time of mechanical ventilation in patients receiving dexmedetomidine for sedation following cardiac surgery compared to patients that received no routine postoperative sedation. Our findings are consistent with several previous studies that compared dexmedetomidine to placebo and suggested no differences in time of mechanical ventilation in patients receiving dexmedetomidine for sedation following cardiac surgery compared to patients that received no routine postoperative sedation. Our findings are consistent with several previous studies that compared dexmedetomidine to placebo and that suggested no differences in time to extubation for postsurgical patients [9, 10].
Although a prior study has suggested that the use of dexmedetomidine may even allow earlier extubation of cardiac surgery patients when compared to propofol [11], this finding was not confirmed by several studies suggesting that there was no difference in time to extubation between patients receiving dexmedetomidine or propofol in cardiac surgery patients [2, 12, 13].
So-called “fast-track” cardiac surgery anesthetic techniques aim to extubate patients within a few hours postoperatively by using short-acting narcotics and sedatives [14]. GCMC adopted these techniques years ago with the goal of reducing CTICU length of stay as well of decreasing morbidity. It is reasonable to speculate that in ICUs that do not use our fast track protocols, intubation times might be shorter in patients receiving dexmedetomidine. In the SEDCOM (Safety and Efficacy of Dexmedetomidine Compared with Midazolam) trial, the median time to extubation was 1.9 days shorter in dexmedetomidine versus midazolam for medical ICU patients who stayed on ventilators for more than 24 hours [8]. It is important to note that GCMC has consistently outperformed the national averages for cardiac surgery time to extubation. In 2010, 74.8% of GCMC post-cardiac surgery patients were extubated within 6 hours compared to the STS national average of 39.3%. Consequently, it is difficult to farther shorten time on mechanical ventilation in our population.
With regard to analgesia, we recorded more narcotic, NSAID and acetaminophen use in dexmedetomidine patients, but only acetaminophen usage met statistical significance as a result from a shift in route of administration in the study period. Our observations regarding analgesics are in contrast to previous studies comparing dexmedetomidine with placebo in noncardiac surgical patients. Triltsch et al. recorded 58% less morphine usage with dexmedetomidine [9]. Likewise, Martin et al. looked at 401 postsurgical patients, and found they required 69% less morphine when given dexmedetomidine as compared to placebo [10]. Similar results were observed when dexmedetomidine was compared to propofol after CABG surgery. Dexmedetomidine sedated patients received morphine 28% of the time, while propofol sedated patients required morphine 69% of the time. In that study, propofol treated patients required a total dose of morphine which was 4 times that of dexmedetomidine treated patients [13].
However, our observation that dexmedetomidine was not “narcotic-sparing” is consistent with several other studies. It has been suggested that dexmedetomidine increased the use of opioids for patients treated with this sedative as compared to propofol [15]. Prior work also reported that dexmedetomidine increased morphine use from 3.6% to 39.3% and ketorolac use from 3.6% to 25% over propofol in post-cardiac surgery patients in a small (n=28) population [14]. Thus, the role of dexmedetomidine in reducing narcotic and non-narcotic analgesia usage is not clearly defined yet.
We found that hypotension and bradycardia occurred very rarely in patients sedated with dexmedetomidine. The most commonly reported side effect of dexmedetomidine was hypotension [16]. Studies have also shown that when compared to placebo in ventilated postsurgical patients, dexmedetomidine is associated with lower heart rates [9]. Hypotension was more common in dexmedetomidine than propofol, 36% versus 24% (P=.11) among 300 post-CABG patients [13]. Randomized control trials, particularly the SEDCOM trial, found that dexmedetomidine treated patients were more likely to develop bradycardia than those receiving midazolam [8], with no more hypotension than with morphine [17]. In a meta-analysis of dexmedetomidine effects, a significantly increased risk of bradycardia occurred when a loading dose and a high maintenance dose (>0.7 µg kg-1 h-1) were used [18].
Dexmedetomidine did not significantly impact the functional status of our patients as measured by the ability to get out of bed and ambulate on postoperative day 0. Other studies utilized The Richmond Agitation-Sedation Scale (RASS), the Ramsay Sedation Scale (RSS), or the Confusion Assessment Method for the ICU (CAM-ICU) to assess alertness or delirium. While the RASS is validated for assessing ICU sedation in those who are mechanically ventilated [19], we were unable to utilize these tools. Thus, we were not able to show that dexmedetomidine allowed patients to be more awake, less sedated, and more functional shortly after heart surgery.
A potential benefit of dexmedetomidine may be the avoidance of early postoperative confusion or agitation. Clinicians have described patients treated with dexmedetomidine as “calmer” and less likely to wake up suddenly and attempt to self-extubate. This could be important for patient safety and satisfaction. Dexmedetomidine treated patients are generally easily aroused as opposed to being deeply sedated. However, this “tranquilly sedated” state is difficult to quantify, and we were unable to do so in our retrospective analysis. It is possible to achieve the same level of sedation with other drugs used at the appropriate doses.
There were several limitations to this study. First, the population size for the non-dexmedetomidine and dexmedetomidine study groups were small, 52 patients and 47 patients, respectively. In addition, there are limitations related to any chart review as a consequence of variation in the charting by clinical staff. These limitations left gaps in our ability to robustly characterize some patient outcomes. Furthermore, patients with serious complications and those who died were excluded from the data-analysis.
In conclusion, in this retrospective study we found no significant difference between patients receiving and not receiving dexmedetomidine with regards to time on mechanical ventilation and to the incidence of bradycardia and hypotension.
Acknowledgments
The authors thank C. Vernon Jennings, Cardiac Data Coordinator at GCMC, for providing a list of the surgical patients that fit the criteria for this study and for delivering corresponding data from the STS National Database. Additional thanks to Maria Tagliaferri and Chris Guzzi, Medical Records Clerks at GCMC, for supplying patient charts.
Footnotes
Source of Support Nil.
Disclosures None declared.
Cite as: Chorney SR, Gooch ME, Oberdier MT, Keating D, Stahl RF. The safety and efficacy of dexmedetomidine for postoperative sedation in the cardiac surgery intensive care unit. HSR Proc Intensive Care Cardiovasc Anesth. 2013; 5 (1): 17-24.
References
- Jones G M, Murphy C V, Gerlach A T. et al. High-Dose Dexmedetomidine for Sedation in the Intensive Care Unit: An Evaluation of Clinical Efficacy and Safety. Ann Pharmacother. 2011;45:740–747. doi: 10.1345/aph.1P726. [DOI] [PubMed] [Google Scholar]
- Barletta J F, Miedema S L, Wiseman D. et al. Impact of Dexmedetomidine on Analgesic Requirements in Patients After Cardiac Surgery in a Fast-Track Recovery Room Setting. Pharmacotherapy. 2009;29:1427–1432. doi: 10.1592/phco.29.12.1427. [DOI] [PubMed] [Google Scholar]
- Kollef M H, Levy N T, Ahrens T S. et al. The use of continuous IV sedation is associated with prolongation of mechanical ventilation. Chest. 1998;114:541–548. doi: 10.1378/chest.114.2.541. [DOI] [PubMed] [Google Scholar]
- Leino K, Mildh L, Lertola K. et al. Time course of changes in breathing pattern in morphine- and oxycodone-induced respiratory depression. Anaesthesia. 1999;54:835–840. doi: 10.1046/j.1365-2044.1999.00946.x. [DOI] [PubMed] [Google Scholar]
- Riker R R, Fraser G L. Altering Intensive Care Sedation Paradigms to Improve Patient Outcomes. Crit Care Clin. 2009;25:527–528. doi: 10.1016/j.ccc.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Aantaa R, Jalonen J. Perioperative use of alpha-2 adrenergic agonists and the cardiac patient. Eur J Anaesthesiol. 2006;23:361–372. doi: 10.1017/S0265021506000378. [DOI] [PubMed] [Google Scholar]
- Maze M, Scheinin M. Molecular pharmacology of alpha-2 adrenergic receptors. Anesthetic Pharmacology Review. 1993;1:233–237. [Google Scholar]
- Riker R R, Shehabi Y, Bokesh P M. et al. Dexmedetomidine vs Midazolam for Sedation of Critically Ill Patients, A Randomized Trial. JAMA. 2009;301:489–499. doi: 10.1001/jama.2009.56. [DOI] [PubMed] [Google Scholar]
- Triltsch A E, Welte M, von Homeyer P. et al. Bispectral index-guided sedation with dexmedetomidine in intensive care: A prospective, randomized, double blind, placebo-controlled phase II study. Crit Care Med. 2002;30:1007–1014. doi: 10.1097/00003246-200205000-00009. [DOI] [PubMed] [Google Scholar]
- Martin E, Ramsay G, Mantz J, Sum-Ping S T. The Role of the alpha-2 adrenergic Agonist Dexmedetomidine in Postsurgical Sedation in the Intensive Care Unit. J Intensiv Care Med. 2003;18:29–41. doi: 10.1177/0885066602239122. [DOI] [PubMed] [Google Scholar]
- Dasta J F, Jacobi J, Sesti A M, McLaughlin T P. Addition of Dexmedetomidine to Standard Sedation Regimens After Cardiac Surgery: An Outcomes Analysis. Pharmacotherapy. 2006;26:798–805. doi: 10.1592/phco.26.6.798. [DOI] [PubMed] [Google Scholar]
- Corbett S M, Rebuck J A, Greene C M. et al. Dexmedetomidine does not improve patient satisfaction when compared with propofol during mechanical ventilation. Crit Care Med. 2005;33:940–945. doi: 10.1097/01.ccm.0000162565.18193.e5. [DOI] [PubMed] [Google Scholar]
- Herr D L, Sum-Ping S T, England M. ICU Sedation After Coronary Artery Bypass Graft Surgery: Dexmedetomidine-Based Versus Propofol-Based Sedation Regimens. J Cardiothorac Vasc Anesth. 2003;17:576–584. doi: 10.1016/s1053-0770(03)00200-3. [DOI] [PubMed] [Google Scholar]
- Anger K E, Szumita P M, Baroletti S A. et al. Evaluation of Dexmedetomidine Versus Propofol-based Sedation Therapy in Mechanically Ventilated Cardiac Surgery Patients at a Tertiary Academic Medical Center. Crit Pathw Cardiol. 2010;9:221–226. doi: 10.1097/HPC.0b013e3181f4ec4a. [DOI] [PubMed] [Google Scholar]
- Reichert M G, Jones W A, Royster R L. et al. Effect of Dexmedetomidine Substitution During a Nationwide Propofol Shortage in Patients Undergoing Coronary Artery Bypass Graft Surgery. Pharmacotherapy. 2011;31:673–677. doi: 10.1592/phco.31.7.673. [DOI] [PubMed] [Google Scholar]
- Gerlach A T, Dasta J F. Dexmedetomidine: an updated review. Ann Pharmacother. 2007;41:245–254. doi: 10.1345/aph.1H314. [DOI] [PubMed] [Google Scholar]
- Shehabi Y, Grant P, Wolfenden H. et al. Prevalence of Delirium with Dexmedetomidine Compared with Morphine Based Therapy after Cardiac Surgery, A Randomized Controlled Trial (DEXmedetomidine COmpared to Morphine- DEXCOM Study). Anesthesiology. 2009;111:1075–1084. doi: 10.1097/ALN.0b013e3181b6a783. [DOI] [PubMed] [Google Scholar]
- Tan J A, Ho K M. Use of dexmedetomidine as a sedative and analgesic agent in critically ill adult patients: a meta-analysis. Intensive Care Med. 2010;36:926–939. doi: 10.1007/s00134-010-1877-6. [DOI] [PubMed] [Google Scholar]
- Ely E W, Truman B, Shintani A. et al. Monitoring Sedation Status Over Time in ICU Patients: Reliability and Validity of the Richmond Agitation-Sedation Scale (RASS). JAMA. 2003;289:2983–2991. doi: 10.1001/jama.289.22.2983. [DOI] [PubMed] [Google Scholar]