Abstract
Abnormal phosphorylation of the microtubule-associated protein tau develops in selected brain regions in normal aging and becomes widespread throughout the brain in Alzheimer’s disease (AD). Braak and others have described the distribution of neurofibrillary tangles and deposition of abnormally phosphorylated tau (p-tau) and correlated this with the progressive cognitive dysfunction in AD. However, to date there have been no comprehensive studies examining abnormally phosphorylated tau deposition in the spinal cord as part of normal aging or AD. We investigated, using immunohistochemical methods, the presence of p-tau in the spinal cord of 46 cases with a clinicopathological diagnosis of AD as well as 37 non-demented (ND) individuals lacking any defined central nervous system-related clinicopathological diagnosis. We found the cervical cord segments to be the most frequently affected subdivision (96% AD vs. 43% ND), followed by thoracic (69% AD vs. 37% ND), lumbar (65% AD vs. 27% ND) and sacral (53% AD vs. 13% ND). The spinal cord was often affected at early-stage brain disease, with p-tau spinal cord immunoreactivity in 40% of subjects at Braak neurofibrillary stage I, however, there were no cases having spinal cord p-tau that did not have p-tau within the brain. As p-tau immunoreactivity is present within the spinal cords of ND as well as AD subjects, it is likely that the phosphorylation of spinal cord tau occurs in the preclinical stage of AD, prior to dementia. The presence of significant spinal cord p-tau-immunoreactive pathology has important implications for both the pathogenesis and clinical manifestations of AD.
Keywords: pathology, autopsy, senile dementia, neurofibrillary tangle, aging, systemic disorder, peripheral nervous system
Introduction
The most common neurodegenerative diseases of the elderly primarily affect the brain but it is becoming increasingly apparent the spinal cord and peripheral nervous system are often affected. Mapping the extent and severity of the characteristic histopathological lesions provides an anatomical basis from which to better understand both pathogenesis and clinical syndromes. For both Alzheimer’s disease (AD) and Parkinson’s disease (PD), it has been proposed the pathological process may spread from neuron to neuron along defined pathways. This is most simply explained as trans-synaptic degeneration, but an “infectious” process has also been postulated, with an initiating event triggered by an exogenous pathogen [1–3] or an endogenous molecular change [4] followed by propagation of the same change through inter-neuronal contact. For both AD and PD, the intranasal olfactory epithelium and/or olfactory bulb have been considered as possible locations of the initiating event, based on their early and consistent involvement, in terms of both histopathology and olfactory dysfunction [3, 5]. For PD, the realization that non-motor signs and symptoms, such as constipation, may precede the classical motor syndrome [6], became a stimulus to continue and extend earlier investigations of the distribution of microscopic peripheral nervous system lesions [5]. This led to the confirmation of widespread α-synuclein histopathology in the peripheral nervous system [5] and the proposal that the upper gastrointestinal tract may be a second disease-initiating site [1].
The spinal cord is in communication with the brain as well as the peripheral nerves and therefore is the logical place to look for disease processes that manifest in both. An increasing body of literature indicates the presence of disease-specific spinal cord histopathology in multiple neurodegenerative diseases including not only amyotrophic lateral sclerosis and other motor neuron diseases but also progressive supranuclear palsy [7], corticobasal degeneration [8], multiple system atrophy [9] and PD [5, 10]. Surprisingly, for AD, the most common neurodegenerative disease, there have been relatively few published works on spinal cord histopathology.
Tau is a microtubule associated protein whose abnormal phosphorylation in the brains of AD subjects is thought to lead to the formation of neuropil threads, dystrophic neurites and neurofibrillary tangles, all pathological hallmarks of the disease [11]. The topographical progression of these lesions within the brain has been used as the basis of a staging scheme proposed by Braak and Braak [12]. Early reports documented the presence of neurofibrillary tangles in the spinal cord of centenarians and subjects with pre-senile and senile dementia [13, 14]. After the discovery that tangles were composed largely of p-tau, it was reported to be widely expressed throughout the spinal cord in utero but largely undetectable in adults [3, 15, 16]. To our knowledge there are only five published studies on tau expression in the spinal cords of AD and normal aging subjects [13, 14, 17–19] and these lack the necessary data with which to determine the consistency, severity and topography of p-tau histopathology as well as the relationship of spinal cord histopathology to brain pathology stage. We therefore undertook the present study in order to provide a more comprehensive documentation of the spinal cord occurrence, distribution, and relationship to brain deposits (i.e. Braak staging) of abnormally phosphorylated tau protein, the major component of neurofibrillary tangles and neuropil threads, in a large set of subjects with AD, in comparison with age- similar normal subjects.
Materials and Methods
The study took place at Banner Sun Health Research Institute (BSHRI), with autopsies performed on elderly subjects who had volunteered for the BSHRI Brain and Body Donation Program and the Arizona Study of Aging and Neurodegenerative Disease (AZSAND), a longitudinal clinicopathological study of normal aging, dementia and parkinsonism [20]. The study was approved by the Banner Health Institutional Review Board and all participants, next of kin, or their legal representatives gave informed consent. The database was initially queried for cases between the years of 1997–2010 that had clinicopathological diagnoses of AD, lacked a concurrent diagnosis of another tauopathy (e.g. progressive supranuclear palsy or corticobasal degeneration) and had whole body autopsy with the entire spinal cord taken (AD, N=46). Control cases were obtained through a database query for subjects without dementia or a defined central nervous system-related clinicopathological diagnosis (ND, N=23). Subjects with AD met consensus clinicopathological diagnostic criteria, defined by the National Institute on Aging Reagan Institute as “intermediate” or “high” probability that dementia was due to AD [21].
Tissue processing methods and neuropathological assessment have been previously described [20]. In brief, formaldehyde-fixed paraffin-embedded (FFPE) sections were stained with hematoxylin and eosin while large-format, 40–80 μm-thick formaldehyde-fixed sections were stained for plaques, tangles and other features using Gallyas, Thioflavine-S and Campbell-Switzer methods [22]; the large-format sections were used, as in the original Braak and Braak protocol [12] to assess Braak stage for neurofibrillary degeneration. Both neurofibrillary tangle density (NFT) and neuritic plaque densities were estimated based on the templates provided by the Consortium to Establish a Registry for Alzheimer’s disease (CERAD) [23].
One to three sections (FFPE, 6 μm) of spinal cord was taken from the mid-portion of the cervical, thoracic and lumbar subdivisions while the sacral section was taken from the rostral one-third. Sections were stained for phosphorylated tau (p-tau: AT8, mouse monoclonal, 1:1000, Fitzgerald, Acton, MA, USA) using an immunoperoxidase method with an 80% formic acid pretreatment for epitope exposure. The AT8 antibody is one of the most widely used phosphorylation dependent anti-tau antibodies and requires the tau protein to be phosphorylated at both serine 202 and threonine 205 [24]. The presence or absence of p-tau neurofibrillary changes was noted by a trainee (JAH) and then confirmed by an experienced observer (BND), both blinded to clinical and pathological diagnosis. If positively stained elements were seen, their location was noted as being in the dorsal horn, ventral horn or intermediolateral region.
Statistical analysis
All histopathological scores were converted to binary variables for statistical purposes (1= presence, 0= absence). All statistical analyses were performed with Sigma Plot 12.0 (Systat Software, Inc., San Jose, CA, USA). For demographic data, group means were compared using Mann-Whitney U-tests. Gender ratios and the prevalence ratios of p-tau within the experimental groups were compared using chi-squared and Fisher’s exact tests. The significance level for all tests was set at p < 0.05. Spearman rank order correlations were conducted to note any relationship between prevalence ratios and Braak neurofibrillary stage or age.
Results
The basic characteristics of the study subjects are shown in Table 1. There were no significant differences between ND and AD groups with respect to gender distribution or age of death. As expected, Braak neurofibrillary stage and CERAD neuritic plaque densities were significantly greater in AD subjects. Table 2 demonstrates the percentage of cases in the ND and AD groups that contained immunoreactive p-tau neural elements, by spinal cord subdivision. There was a rostrocaudal gradient in that more rostral spinal cord regions were more likely to contain p-tau deposits than d more caudal regions. In all spinal cord regions AD subjects were significantly more likely to have p-tau immunoreactivity (cervical: 95.6% vs. 43.2%; thoracic: 68.9% vs. 36.7%; lumbar: 65.2% vs. 26.7%; and sacral: 53.5% vs. 12.9%). In both groups p-tau immunoreactivity was more likely to be present in the ventral horns than in the intermediolateral region or dorsal horns. Within the ventral horns, p-tau was most frequently observed within lamina VIII and IX while in the dorsal horn primarily lamina III and IV were involved. In the intermediolateral region lamina X and VII were most frequently affected. Immunoreactivity was mainly within neurites or neuropil threads and these attained focally moderate densities, while neuronal perikaryal immunoreactivity was rare to sparse, consisting mostly of granular material consistent with “pre-tangles”; NFTs were rare (Figure 1). These lesions were mostly restricted to grey matter regions although occasional neurites and even cell bodies were seen in the white matter columns. We hypothesize the perikaryal staining in the white matter to be astrocytic but are uncertain due to small size. Also showing p-tau immunoreactivity were thorn-like astrocytes located near the pial surface and around the central canal; these were present in 17% of cases both in the ND (2 cases) and AD (10 cases) groups. Diagrammatic representations of overall and average lesion densities are shown in Figure 2. With respect to age, there was a significant relationship between the prevalence of p-tau in only the cervical region of ND cases (Spearman rho=0.39, P=0.02), there were no other significant correlations with age amongst the ND or AD group.
Table 1.
Subject demographics. AD cases had significantly higher Braak NFT stages and CERAD plaque scores when compared to ND individuals. SD = standard deviation.
| ND | AD | P values | |
|---|---|---|---|
| N total (M:F) | 37 (24:13) | 46 (25:21) | 0.46 |
| age at death mean ± SD | 79 ± 14 | 84± 5 | 0.15 |
| Braak NFT stage-median (range) | II (0 – IV) | V (II – VI) | <0.001 |
| CERAD NP density median (range) | None (none-frequent) | Frequent (moderate-frequent) | <0.001 |
AD= Alzheimer’s disease; ND= non-demented controls; NFT= neurofibrillary tangle; NP = neuritic plaque; CERAD= Consortium to Establish a Registry for Alzheimer’s disease.
Table 2.
Frequency of p-tau immunoreactive neural elements in the cervical, thoracic, lumbar, and sacral portions of the spinal cord of non-demented controls (ND) and Alzheimer’s disease (AD) cases.
| spinal cord region | total Ns (ND, AD) | ND | AD | P values |
|---|---|---|---|---|
| Cervical: | 37,45 | 43.2% | 95.6% | <0.001 |
| ventral | 40.5% | 71.1% | 0.01 | |
| dorsal | 2.7% | 24.4% | 0.01 | |
| intermediolateral | 35.1% | 28.9% | 0.71 | |
|
| ||||
| Thoracic: | 30,45 | 36.7% | 68.9% | 0.01 |
| ventral | 33.3% | 62.2% | 0.03 | |
| dorsal | 6.7% | 6.7% | 1.0 | |
| intermediolateral | 3.3% | 11.1% | 0.39 | |
|
| ||||
| Lumbar: | 30,46 | 26.7% | 65.2% | 0.002 |
| ventral | 23.3% | 50.0% | 0.04 | |
| dorsal | 6.7% | 10.9% | 0.70 | |
| intermediolateral | 6.7% | 15.2% | 0.47 | |
|
| ||||
| Sacral: | 31,43 | 12.9% | 53.5% | <0.001 |
|
| ||||
| Overall positivity (all regions) | 23,46 | 51.4 % | 95.7% | <0.001 |
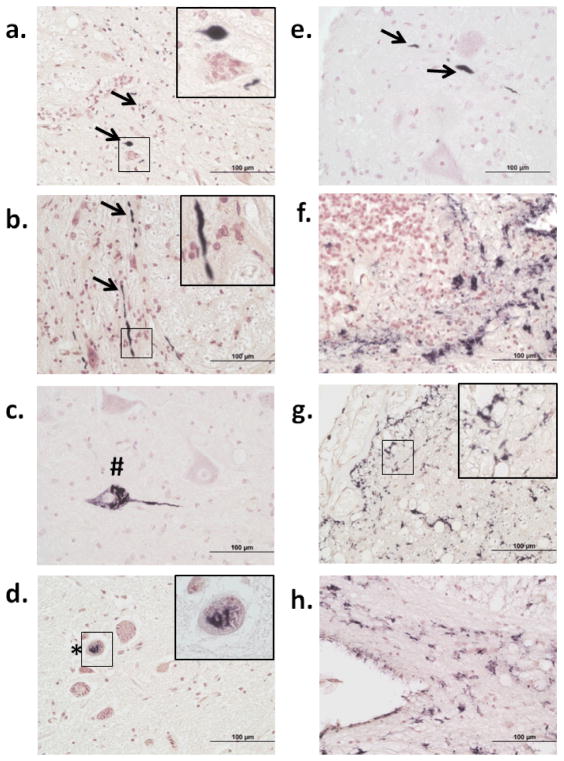
Fig 1.
P-tau immunoreactivity in the spinal cord. Ventral part of the cervical horn of a non-demented control (ND) case (a); near the border of ventral horn and intermediolateral region of the lumbar subdivision of an Alzheimer’s disease (AD) case (b); ventral horn of the lumbar subdivision of an AD case (c); intermediolateral region of the lumbar subdivision of an AD case (d); ventral region of the cervical subdivision of an AD case (e); central canal region of the thoracic subdivision of an AD case (f); subpial region of the lumbar subdivision of an AD case (g); and anterior median fissure region of the thoracic subdivision of an AD case (h). Arrows indicate neuropil threads; # indicates neurofibrillary tangles; * indicates pre-tangles; thorned shaped astrocytes are present in (g–h). Insets in a, b, d, g represent higher magnification of small boxed areas. All photos taken at 40x magnification.
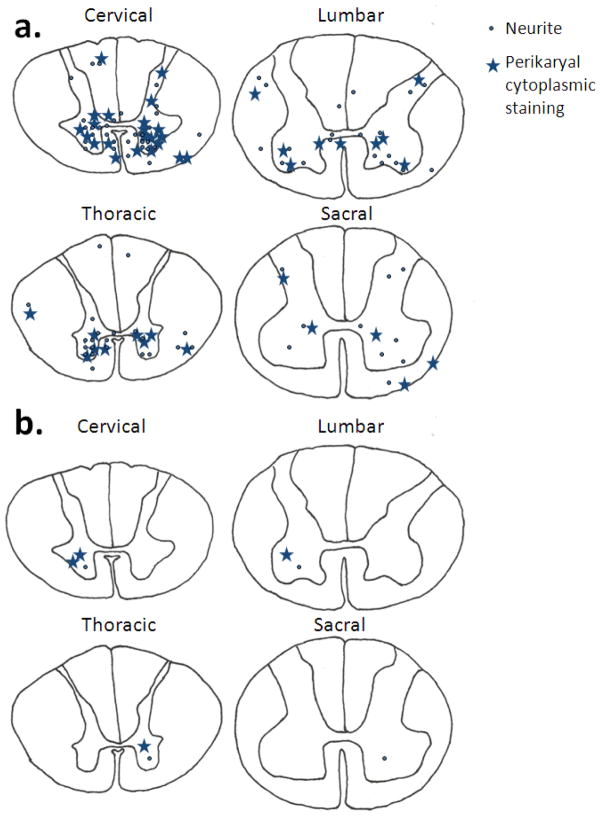
Fig 2.
Location and densities of all neurites and perikaryal cytoplasmic staining seen across all analyzed Alzheimer’s disease cases at each level of the spinal cord (a). A representation of the typical distribution and density of neurites and perikaryal cytoplasmic staining on 5μm sections of an individual Alzheimer’s disease case at each level of the spinal cord (b). Each neurite is represented by dot while perikaryal cytoplasmic staining by a star.
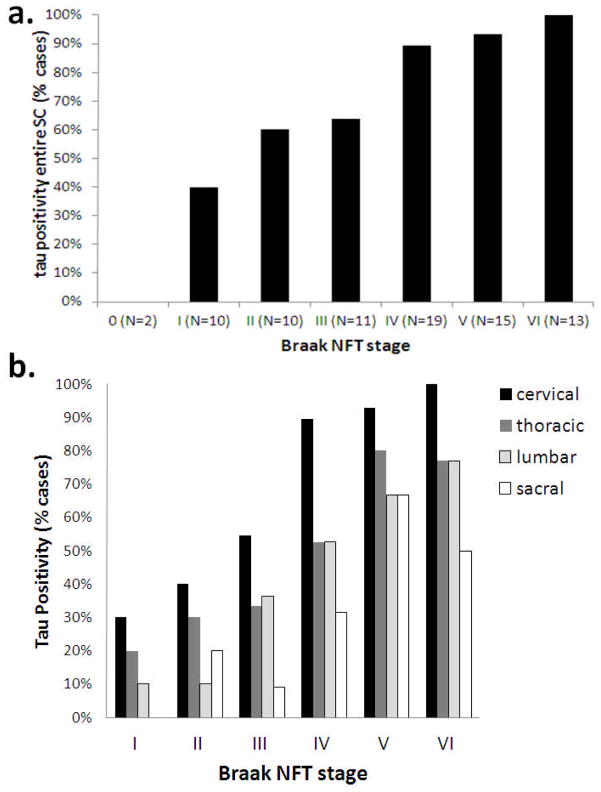
There was a significant relationship between Braak neurofibrillary (brain) stage and the prevalence ratio of p-tau spinal cord immunoreactivity (Spearman rho=0.49, P<0.001, Figure 3). In Braak stage 0, 0/2 (0%) cases contained p-tau while this rose to 4/10 (40%) at Braak stage I, 6/10 (60%) at Braak stage II, 7/11 (64%) at Braak stage III, 17/19 (89%) at Braak stage IV, 14/15 (93%) at Braak stage V, and 13/13 (100%) at Braak stage VI (Figure 3A). Figure 3B demonstrates the percentages of cases showing p-tau immunoreactivity by spinal cord subdivision across Braak stages. Cervical and thoracic regions were more likely to demonstrate p-tau immunoreactivity with advancing age in the entire series of AD and ND (rho=0.39 0.24; P<0.001, P=0.04, respectively). Amongst ND subjects, only cervical regions were more likely to demonstrate p-tau immunoreactivity with advancing age (rho=0.386, P<0.02), and no significant age correlations were present within the AD group. There were no relationships between presence of p-tau in any spinal cord region in either the AD or ND group with respect to gender.
Fig 3.
Prevalence (percentage) of p-tau immunoreactivity in subjects by Braak neurofibrillary stage (a) and spinal cord subdivision (b). Both Alzheimer’s disease and non-demented control subjects are included.
Discussion
The tau protein develops abnormally phosphorylated sites within particular neuronal populations as part of normal aging and this phenomenon becomes accentuated and widespread during the progression of AD. Tau was discovered to be a major constituent of neurofibrillary tangles in the late 1980s and shortly afterwards its abnormal phosphorylation in AD was documented and explored [11]. The cerebral and brainstem distribution of NFTs and p-tau was subsequently described in great detail, particularly by Braak and co-workers [12].
Our results demonstrate p-tau to be very common in the spinal cords of normal elderly subjects, and significantly more common in the spinal cords of subjects with a clinicopathologic diagnosis of AD. Immunoreactivity was present in at least one section of spinal cord in 59/68 cases. Immunoreactivity was nearly universal (45/47) in cases meeting NIA-Reagan intermediate or high criteria for AD and all Braak stage VI cases were positive. Of ND individuals 19/37 were positive. When considering all spinal cord subdivisions in the rostrocaudal extent, the cervical region had the highest frequency of p-tau positivity in both ND and AD, while in the dorsal to ventral orientation the anterior horn had the highest rate of positivity. Furthermore, p-tau positivity had an increased frequency and distribution with increasing Braak neurofibrillary brain stage. The lack of p-tau in 2 cases at Braak stage 0 indicated spinal cord involvement probably does not precede that of the transentorhinal cortex or other brain regions. However, the presence of p-tau in 4 of 10 cases at Braak stage I indicates the disease may spread early to the spinal cord. The presence of p-tau in ND individuals suggests it is deposited within the spinal cord before the onset of dementia, although whether or not any particular individual would have progressed to dementia is not possible to know.
We note numerous studies have recently suggested the locus ceruleus as well as other brainstem nuclei may be affected with NFTs and p-tau even prior to the transentorhinal stage and hence prior to Braak stage I and may even start in early adult hood [25–29]. In our study, we found the cervical region, the spinal cord region most proximal to the brain and brainstem, to be most affected; with no case with spinal cord p-tau lacking brain deposits of tau; furthermore, with respect to increasing age in only the ND cervical region did the frequency of p-tau deposition increase. A hypothetical progression of tau could start within the brainstem, as studies suggest, and then spread to cortex as well as descend through the spinal cord. This is further supported by our finding that 40% of cases at Braak stage I had p-tau deposits, with progressively higher percentages as Braak stage increases.
Previous literature on NFTs and abnormal p-tau within the human spinal cord is relatively sparse but has spanned the human life history from fetus to adult, included demented and normal tissues, and used a variety of methods for abnormal tau detection ranging from a variety of silver stains to multiple anti-tau antibodies [3, 13–19]. In 1978, one of the first modern-era reports of spinal cord tau pathology described spinal cord NFTs in 3/15 AD subjects and 0/10 normal elderly controls, using the von Braumuhl silver method; this series was relatively young (average age of both groups was about 64 years) [13]. Wang et al., using the Gallyas silver stain, reported that of a series of centenarians, spinal cord NFTs and/or neuropil threads were present in 9/10 cases with a clinicopathologic diagnosis of AD while 7/9 non-demented cases were positive for these findings, predominantly within the cervical subdivision [14]. A study employing multiple anti-tau antibodies as well as Gallyas silver staining, revealed tau- and/or Gallyas positive structures within the spinal cord of all 11 AD subjects, as well as 7/10 normal controls; densities were higher in the anterior horn and there was a rostrocaudal gradient of decreasing lesion density, consistent with the results we report here [17]. Of several p-tau antibodies used, AT8 was found to be the most sensitive for these changes [17]. Other, less-extensive studies have demonstrated generally similar results [18, 19]. With respect to age, an early report on p-tau immunoreactivity using the Alz-50 antibody found widespread and dense fibers and puncta at all spinal cord levels and throughout subjects spanning the human lifespan but this was judged to represent normal tau [15]. Subsequent groups have emphasized the nearly-complete developmental loss of p-tau within the spinal cord by adulthood [3, 16]. The presence, within approximately equal percentages of AD and ND subjects, of p-tau-immunoreactive thorn-shaped astrocytes, mostly restricted to subpial and subependymal locations, suggests that these are an age-related variant morphology, similar or perhaps identical to that reported by Schultz et al within the aged medial temporal lobe [30]. Although our study and others have demonstrated p-tau within the spinal cord of AD, clinically, spinal cord symptoms are not prominent in AD. There have been reports of slower movements and reduced postural control being related to an increased risk of being cognitively impaired [31–36]. Peripheral nervous system (PNS) or spinal cord-originating signs and symptoms such as urinary retention and constipation are common in demented subjects [37] and while many of these may represent spinal cord or PNS Lewy body disease [5] it is possible normal elderly subjects and subjects with AD may develop similar disturbances based on spinal cord neuronal p-tau-based dysfunction, but there is no direct evidence for or against this. Further detailed clinicopathological studies may shed light on exact mechanisms.
Acknowledgments
The Brain and Body Donation Program is supported by the National Institute of Neurological Disorders and Stroke (U24 NS072026 National Brain and Tissue Resource for Parkinson’s Disease and Related Disorders), the National Institute on Aging (P30 AG19610 Arizona Alzheimer’s Disease Core Center), the Arizona Department of Health Services (contract 211002, Arizona Alzheimer’s Research Center), the Arizona Biomedical Research Commission (contracts 4001, 0011, 05-901 and 1001 to the Arizona Parkinson’s Disease Consortium) and the Michael J. Fox Foundation for Parkinson’s Research.
References
- 1.Hawkes CH, Del Tredici K, Braak H. Parkinson’s disease: a dual-hit hypothesis. Neuropathol Appl Neurobiol. 2007;33:599–614. doi: 10.1111/j.1365-2990.2007.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roberts E. Alzheimer’s disease may begin in the nose and may be caused by aluminosilicates. Neurobiol Aging. 1986;7:561–567. doi: 10.1016/0197-4580(86)90119-3. [DOI] [PubMed] [Google Scholar]
- 3.Lee JH, Goedert M, Hill WD, Lee VM, Trojanowski JQ. Tau proteins are abnormally expressed in olfactory epithelium of Alzheimer patients and developmentally regulated in human fetal spinal cord. Exp Neurol. 1993;121:93–105. doi: 10.1006/exnr.1993.1074. [DOI] [PubMed] [Google Scholar]
- 4.Olanow CW, Prusiner SB. Is Parkinson’s disease a prion disorder? Proc Natl Acad Sci U S A. 2009;106:12571–12572. doi: 10.1073/pnas.0906759106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beach TG, Adler CH, Sue LI, Vedders L, Lue L, White CL, III, Akiyama H, Caviness JN, Shill HA, Sabbagh MN, Walker DG. Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol. 2010;119:689–702. doi: 10.1007/s00401-010-0664-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abbott RD, Petrovitch H, White LR, Masaki KH, Tanner CM, Curb JD, Grandinetti A, Blanchette PL, Popper JS, Ross GW. Frequency of bowel movements and the future risk of Parkinson’s disease. Neurology. 2001;57:456–462. doi: 10.1212/wnl.57.3.456. [DOI] [PubMed] [Google Scholar]
- 7.Iwasaki Y, Yoshida M, Hashizume Y, Hattori M, Aiba I, Sobue G. Widespread spinal cord involvement in progressive supranuclear palsy. Neuropathology. 2007;27:331–340. doi: 10.1111/j.1440-1789.2007.00787.x. [DOI] [PubMed] [Google Scholar]
- 8.Iwasaki Y, Yoshida M, Hattori M, Hashizume Y, Sobue G. Widespread spinal cord involvement in corticobasal degeneration. Acta Neuropathol. 2005;109:632–638. doi: 10.1007/s00401-005-1017-5. [DOI] [PubMed] [Google Scholar]
- 9.Yoshida M. Multiple system atrophy: alpha-synuclein and neuronal degeneration. Neuropathology. 2007;27:484–493. doi: 10.1111/j.1440-1789.2007.00841.x. [DOI] [PubMed] [Google Scholar]
- 10.Del Tredici K, Braak H. Spinal cord lesions in sporadic Parkinson’s disease. Acta Neuropathol. 2012 doi: 10.1007/s00401-012-1028-y. [DOI] [PubMed] [Google Scholar]
- 11.Mandelkow EM, Mandelkow E. Biochemistry and cell biology of tau protein in neurofibrillary degeneration. Cold Spring Harb Perspect Med. 2012;2:a006247. doi: 10.1101/cshperspect.a006247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 13.Yamada M. On the distribution of senile changes in the spinal cord. Folia Psychiatr Neurol Jpn. 1978;32:249–251. doi: 10.1111/j.1440-1819.1978.tb00146.x. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Hashizume Y, Yoshida M, Inagaki T, Kameyama T. Pathological changes of the spinal cord in centenarians. Pathol Int. 1999;49:118–124. doi: 10.1046/j.1440-1827.1999.00832.x. [DOI] [PubMed] [Google Scholar]
- 15.Rye DB, Leverenz J, Greenberg SG, Davies P, Saper CB. The distribution of Alz-50 immunoreactivity in the normal human brain. Neuroscience. 1993;56:109–127. doi: 10.1016/0306-4522(93)90567-y. [DOI] [PubMed] [Google Scholar]
- 16.Liberini P, Valerio A, Moretto G, Rizzonelli P, Memo M, Rizzuto N, Spano PF. Tau protein immunolocalization in fetal and adult human spinal cord. Neurosci Res. 1995;22:197–202. doi: 10.1016/0168-0102(95)00897-2. [DOI] [PubMed] [Google Scholar]
- 17.Saito Y, Murayama S. Expression of tau immunoreactivity in the spinal motor neurons of Alzheimer’s disease. Neurology. 2000;55:1727–1729. doi: 10.1212/wnl.55.11.1727. [DOI] [PubMed] [Google Scholar]
- 18.Guo YJ, Wang LN, Zhu MW, Zhang HH, Hu YZ, Han ZT, Li JM, Wang DX. Expression of tau-related protein in spinal cord of patients with Alzheimer’s disease. Zhonghua Bing Li Xue Za Zhi. 2011;40:161–164. [PubMed] [Google Scholar]
- 19.Schmidt ML, Zhukareva V, Perl DP, Sheridan SK, Schuck T, Lee VM, Trojanowski JQ. Spinal cord neurofibrillary pathology in Alzheimer disease and Guam Parkinsonism-dementia complex. J Neuropathol Exp Neurol. 2001;60:1075–1086. doi: 10.1093/jnen/60.11.1075. [DOI] [PubMed] [Google Scholar]
- 20.Beach TG, Sue LI, Walker DG, Roher AE, Lue L, Vedders L, Connor DJ, Sabbagh MN, Rogers J. The Sun Health Research Institute Brain Donation Program: description and experience, 1987–2007. Cell Tissue Bank. 2008;9:229–245. doi: 10.1007/s10561-008-9067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Consensus recommendations for the postmortem diagnosis of Alzheimer’s disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer’s Disease. Neurobiol Aging. 1997;18:S1–2. [PubMed] [Google Scholar]
- 22.Braak H, Braak E. Demonstration of amyloid deposits and neurofibrillary changes in whole brain sections. Brain Pathol. 1991;1:213–216. doi: 10.1111/j.1750-3639.1991.tb00661.x. [DOI] [PubMed] [Google Scholar]
- 23.Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 24.Goedert M, Jakes R, Vanmechelen E. Monoclonal antibody AT8 recognises tau protein phosphorylated at both serine 202 and threonine 205. Neurosci Lett. 1995;189:167–169. doi: 10.1016/0304-3940(95)11484-e. [DOI] [PubMed] [Google Scholar]
- 25.Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol. 2011;70:960–969. doi: 10.1097/NEN.0b013e318232a379. [DOI] [PubMed] [Google Scholar]
- 26.Rub U, Del Tredici K, Schultz C, Thal DR, Braak E, Braak H. The evolution of Alzheimer’s disease-related cytoskeletal pathology in the human raphe nuclei. Neuropathol Appl Neurobiol. 2000;26:553–567. doi: 10.1046/j.0305-1846.2000.00291.x. [DOI] [PubMed] [Google Scholar]
- 27.Braak H, Del Tredici K. The pathological process underlying Alzheimer’s disease in individuals under thirty. Acta Neuropathol. 2011;121:171–181. doi: 10.1007/s00401-010-0789-4. [DOI] [PubMed] [Google Scholar]
- 28.Grudzien A, Shaw P, Weintraub S, Bigio E, Mash DC, Mesulam MM. Locus coeruleus neurofibrillary degeneration in aging, mild cognitive impairment and early Alzheimer’s disease. Neurobiol Aging. 2007;28:327–335. doi: 10.1016/j.neurobiolaging.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 29.Attems J, Thomas A, Jellinger K. Correlations between cortical and subcortical tau pathology. Neuropathol Appl Neurobiol. 2012;38:582–590. doi: 10.1111/j.1365-2990.2011.01244.x. [DOI] [PubMed] [Google Scholar]
- 30.Schultz C, Ghebremedhin E, Del Tredici K, Rub U, Braak H. High prevalence of thorn-shaped astrocytes in the aged human medial temporal lobe. Neurobiol Aging. 2004;25:397–405. doi: 10.1016/S0197-4580(03)00113-1. [DOI] [PubMed] [Google Scholar]
- 31.Franssen EH, Souren LE, Torossian CL, Reisberg B. Equilibrium and limb coordination in mild cognitive impairment and mild Alzheimer’s disease. J Am Geriatr Soc. 1999;47:463–469. doi: 10.1111/j.1532-5415.1999.tb07240.x. [DOI] [PubMed] [Google Scholar]
- 32.Pettersson AF, Olsson E, Wahlund LO. Motor function in subjects with mild cognitive impairment and early Alzheimer’s disease. Dement Geriatr Cogn Disord. 2005;19:299–304. doi: 10.1159/000084555. [DOI] [PubMed] [Google Scholar]
- 33.Scherder E, Eggermont L, Swaab D, van Heuvelen M, Kamsma Y, de Greef M, van Wijck R, Mulder T. Gait in ageing and associated dementias; its relationship with cognition. Neurosci Biobehav Rev. 2007;31:485–497. doi: 10.1016/j.neubiorev.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 34.Camicioli R, Wang Y, Powell C, Mitnitski A, Rockwood K. Gait and posture impairment, parkinsonism and cognitive decline in older people. J Neural Transm. 2007;114:1355–1361. doi: 10.1007/s00702-007-0778-5. [DOI] [PubMed] [Google Scholar]
- 35.Eggermont LH, Gavett BE, Volkers KM, Blankevoort CG, Scherder EJ, Jefferson AL, Steinberg E, Nair A, Green RC, Stern RA. Lower-extremity function in cognitively healthy aging, mild cognitive impairment, and Alzheimer’s disease. Arch Phys Med Rehabil. 2010;91:584–588. doi: 10.1016/j.apmr.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kluger A, Gianutsos JG, Golomb J, Ferris SH, George AE, Franssen E, Reisberg B. Patterns of motor impairement in normal aging, mild cognitive decline, and early Alzheimer’s disease. J Gerontol B Psychol Sci Soc Sci. 1997;52:P28–39. doi: 10.1093/geronb/52b.1.p28. [DOI] [PubMed] [Google Scholar]
- 37.Idiaquez J, Roman GC. Autonomic dysfunction in neurodegenerative dementias. J Neurol Sci. 2011;305:22–27. doi: 10.1016/j.jns.2011.02.033. [DOI] [PubMed] [Google Scholar]