Abstract
Bone grafting procedures have become common due in part to a global trend of population aging. Native bone graft is a popular choice when compared to various synthetic bone graft substitutes, owing to superior biological activity. Nonetheless, the insufficient ability of bone allograft to induce new bone formation and the insufficient remodeling of native bone grafts call for osteoinductive factors during bone repair, exemplified by recombinant human bone morphogenetic protein 2 (rhBMP2). We previously developed a modular bone morphogenetic peptide (mBMP) to address complications associated with the clinical use of rhBMP2 as a bone graft substitute. The mBMP is designed to strongly bind to hydroxyapatite, the main inorganic component of bone and teeth, and to provide pro-osteogenic properties analogous to rhBMP2. Our previous in vivo animal studies showed that mBMP bound to hydroxyapatite-coated orthopedic implants with high affinity and stimulated new bone formation. In this study, we demonstrate specific binding of mBMP to native bone grafts. The results show that mBMP binds with high affinity to both cortical and trabecular bones, and that the binding is dependent on the mBMP concentration and incubation time. Importantly, efficient mBMP binding is achieved in an ex vivo bone bioreactor where bone tissue is maintained viable for several weeks. In addition, mBMP binding can be localized with spatial control on native bone tissue via simple methods, such as dip-coating, spotting and direct writing. Taken together with the pro-osteogenic activity of mBMP established in previous bone repair models, these results suggest that mBMP may promote bone healing when coated on native bone grafts in a clinically compatible manner.
Keywords: bone morphogenetic protein, synthetic peptide, bone graft, binding
Bone grafting is a common procedure to repair skeletal defects caused by trauma, cancer and congenital deformation. Nearly 1.5 million bone grafting procedures are performed annually in United States and approximately 2.2 million worldwide.1, 2 The number of bone grafting procedures is rapidly increasing with a growth of almost 13% per year due to population aging.3 This trend results in a growing demand for bone grafts. Even though several synthetic bone graft substitutes have been developed, native bone grafts including autologous bone graft (autograft) and allogenic bone graft (allograft) still remain a popular choice in current clinical settings.4 They typically offer superior biological healing activity and structural strength when compared to synthetic counterparts.5
Bone autograft is the gold standard for bone grafting transplantation and most commonly used to treat bone defects, as it is histocompatible and non-immunogenic as well as osteoconductive and pro-osteogenic.6, 7 However its clinical use is often limited by the availability of autograft and potential donor site morbidity from bone graft harvesting. Bone allograft has become an attractive alternative to avoid those problems, because it is harvested from cadaver bone and readily available off-the-shelf with various shape and size. Allograft, although osteoconductive, generally lacks the ability to actively direct skeletal tissue repair to an extent depending on the preparation protocols.4, 5 In response to this drawback, osteoinductive factors and osteogenic cells have been incorporated with bone allografts to accelerate and promote bone healing.8–10
Recombinant human bone morphogenetic protein 2 (rhBMP2) is among the most potent and clinically relevant osteoinductive factors, which recruits mesenchymal stem cells and stimulates osteoblastic differentiation.11, 12 Currently, rhBMP2 is clinically used in spinal fusion and other bone repair applications with approximately $900 million in annual sales.13 However serious concerns have been raised in a series of recent studies regarding adverse side effects such as soft tissue edema, heterotopic bone formation and male infertility.14 Those potential safety issues are likely due in part to bolus delivery at a supraphysiological dose, and thus strategies for controlled delivery are required for the safe use and improved therapeutic efficacy of osteoinductive molecules including rhBMP2.
In this context, we developed a modular bone morphogenetic peptide (mBMP) with high affinity for hydroxyapatite (HAP), the main inorganic component of bone and teeth. This mBMP consists of an osteocalcin-inspired HAP-binding sequence at the C-terminus (γEPRRγEVAγEL) and a biologically active, BMP2-mimetic sequence at the N-terminus (KIPKASSVPTELSAISTLYL) (Table 1). We previously showed that mBMP binds strongly to synthetic HAP with a controllable affinity, and that mBMP induces osteogenic differentiation of human mesenchymal stem cells in vitro.15, 16 Furthermore, sheep studies indicated that, when incorporated onto the surface of HAP-coated medical devices such as fixation screws and metallic implants, mBMP can accelerate and improve bone repair.17, 18 In light of the expanding demand for native bone tissue as a bone graft, and the pro-osteogenic properties of mBMP demonstrated on synthetic HAP implants, here we investigated the binding of mBMP to natural bone tissue in multiple experimental formats. Specifically, we explored mBMP binding to native bone - either as cadaver bone (allograft model) or in a living bone bioreactor (autograft model). The peptides used in this study were conjugated with rhodamine to quantify their binding to bone in terms of fluorescence intensity. In addition, taking into consideration a variety of morphological structure of native bones, we included sheep cortical (compact) bone and bovine trabecular (cancellous, spongy) bone in the study.
Table 1.
Amino Acid Sequence of Natural Templates and Modular Peptides.
| Amino acid sequence | |
|---|---|
| BMP2 template | KIPKACCVPTELSAISMLYL |
| Osteocalcin template | γEPRRγEVCγEL |
| mBMP | KIPKASSVPTELSAISTLYL-AAAA-γEPRRγEVAγEL |
| mBMP-mut | KIPKASSVPTELSAISTLYL-AAAA-EPRREVAEL |
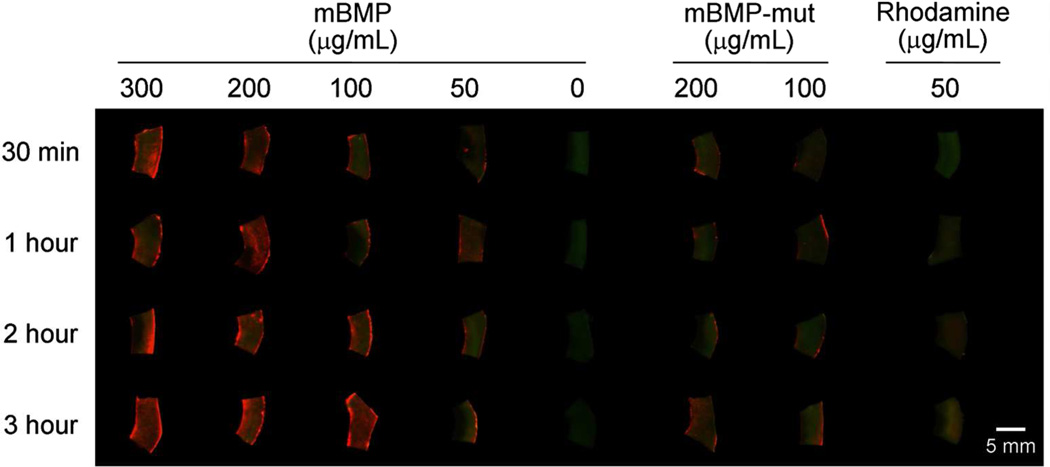
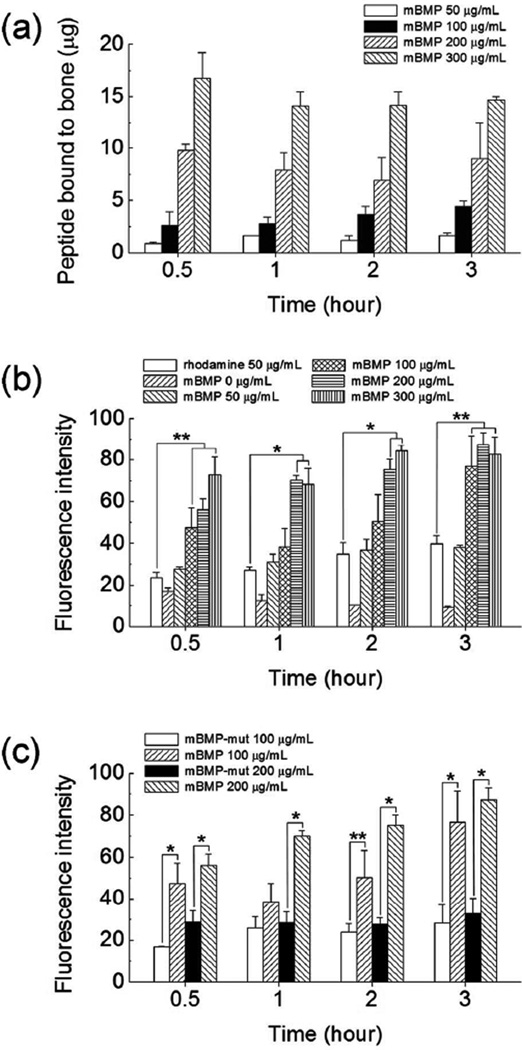
We first examined mBMP binding to periosteum-removed cortical bone (ca. 4 mm × 7 mm × 1.5 mm) from the shaft of a sheep tibia by incubating the bone tissue in mBMP solution in phosphate buffered saline (PBS) (Figure 1). The binding of mBMP was quantified from the concentration change of mBMP solution after binding (Figure 2a) and the fluorescence intensity of mBMP bound to the bone (Figure 2b). The binding was concentration-dependent for each incubation time tested. The amount of binding was ranging from 0.7 to 19.3 microgram peptide per each bone sample (Figure 2a). The quantity of mBMP binding was proportional to the solution concentration, which was clearly observed through all concentrations at 0.5 hour. However, this dependence was not apparent at higher concentrations at later time points. Specifically, fluorescence intensity analysis revealed that the concentration of 200 and 300 µg/mL resulted in similar mBMP binding at 1 and 2 hours, and no significant difference was detected among 100, 200 and 300 µg/mL at the 3 hour time point. The detailed statistical analyses can be found in Tables S1 to S3 in Supporting Information. Interestingly, a statistically significant dependence of mBMP binding on the incubation time was not observed. The insignificant dependence on the incubation time is likely attributed to the rapid binding of mBMP to native bone tissue. The higher fluorescence intensity from mBMP-treated bones when compared with rhodamine-treated group confirms the specific affinity of mBMP to cadaver bones.
Figure 1.
Fluorescence images of cortical bones after incubating in rhodamine-labeled modular peptide solution with different concentrations for different time periods. Green and red fluorescence were emitted from native bone and rhodamine, respectively.
Figure 2.
(a) Amount of rhodamine-labeled mBMP bond to cortical bone after incubating in various conditions. Quantification of fluorescence intensity of (b) rhodamine-labeled mBMP bound to cortical bone by incubating in various conditions, and (c) rhodamine-labeled mBMP and mBMP-mut bound to cortical bone by incubating in 100 µg/mL peptide solution for different time periods. Data are shown as mean ± standard deviation. * p < 0.01 and ** p < 0.05.
To verify the origin of the high affinity binding of mBMP to bones, we compared the binding of mBMP and a mutated version of mBMP - in which Gla residues were replaced with Glu residues (mBMP-mut). We previously demonstrated that mBMP-mut has an altered HAP-binding motif and substantially decreased HAP binding affinity15, 16 Specifically, the HAP-binding motif of mBMP includes three γ-carboxylic glutamic acid (γE, Gla) residues that are known to play a critical role in the binding to HAP.19 In mBMP-mut, the γE residues were replaced by glutamic acid (E, Glu) (Table 1), which led to a reduced binding affinity to synthetic HAP particles and coatings in previous studies.15, 16 As expected, the amount of mBMP-mut bound was significantly less than that of mBMP in each experimental condition tested (Figure 1 and 2c). The fluorescence intensity level of mBMP-mut treated bones was similar to that of the rhodmamine-treated group. Additionally, unlike mBMP binding results, the mBMP-mut binding was independent of incubation time and peptide concentration (see Table S2 and S3 in Supporting Information for statistical analysis). The comparison of the binding of mBMP and mBMP-mut confirmed that the high level of bone binding was primarily mediated by γE residues in the HAP-binding motif of mBMP, analogous to the bone binding mechanism of natural OCN protein.
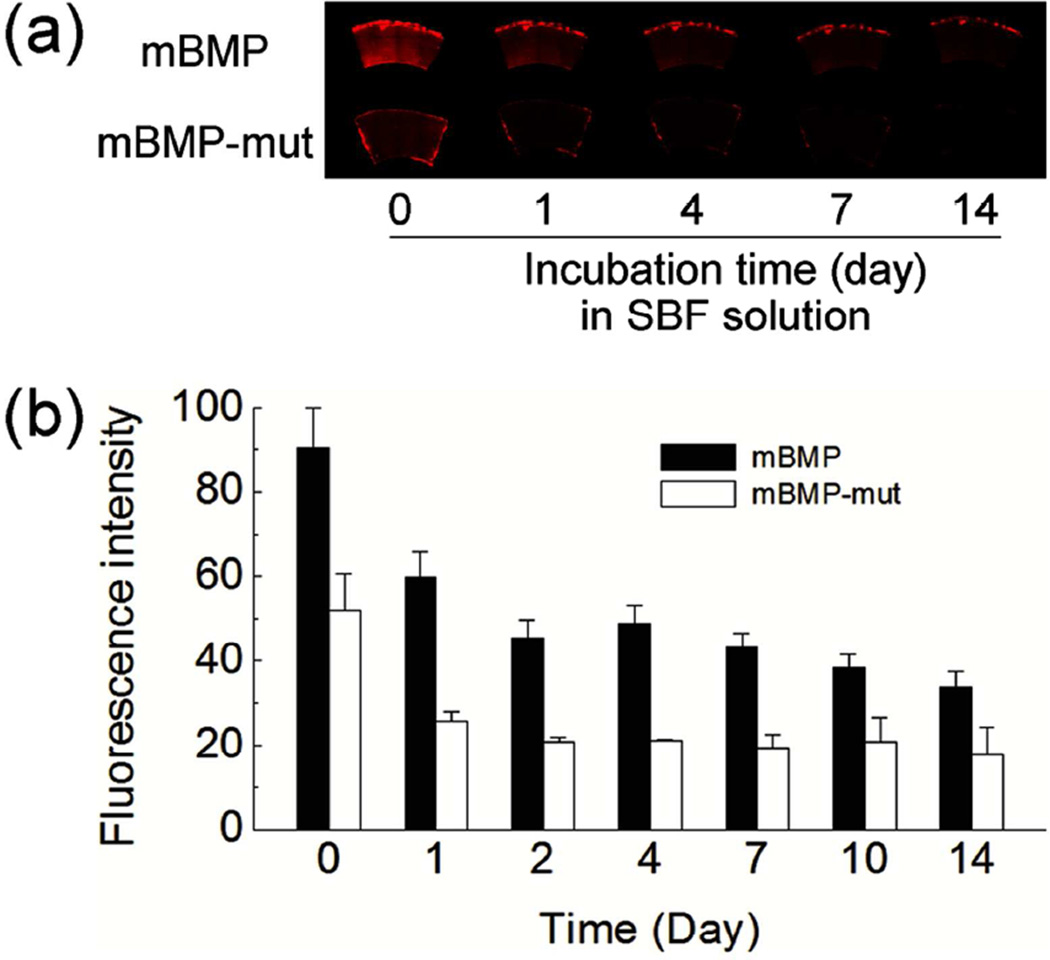
To gain insight into the duration of modular peptide on native bone tissue, the fluorescence intensity of peptide bound to cortical bone was monitored when incubated in a simulated body fluid (SBF) which contains the ionic concentration similar to human blood plasma (Figure 3). After 2-week incubation in SBF, the peptide-bound bone still maintained significant fluorescence intensity with over 30 % of the initial intensity. The fluorescence intensity was dropped rapidly for the first one or two days, and the intensity decrease was slowed down afterwards. In addition, we found that mBMP bound to bone was mostly retained upon the exposure to sodium hydroxide or sodium dodecyl sulfate (Figure S1 in Supporting Information). These data imply that mBMP on native bone can remain for an extended time period.
Figure 3.
(a) Fluorescence images and (b) fluorescence intensity of modular peptide-bound cortical bones after incubating in simulated body fluid for different time periods. Data are shown as mean ± standard deviation.
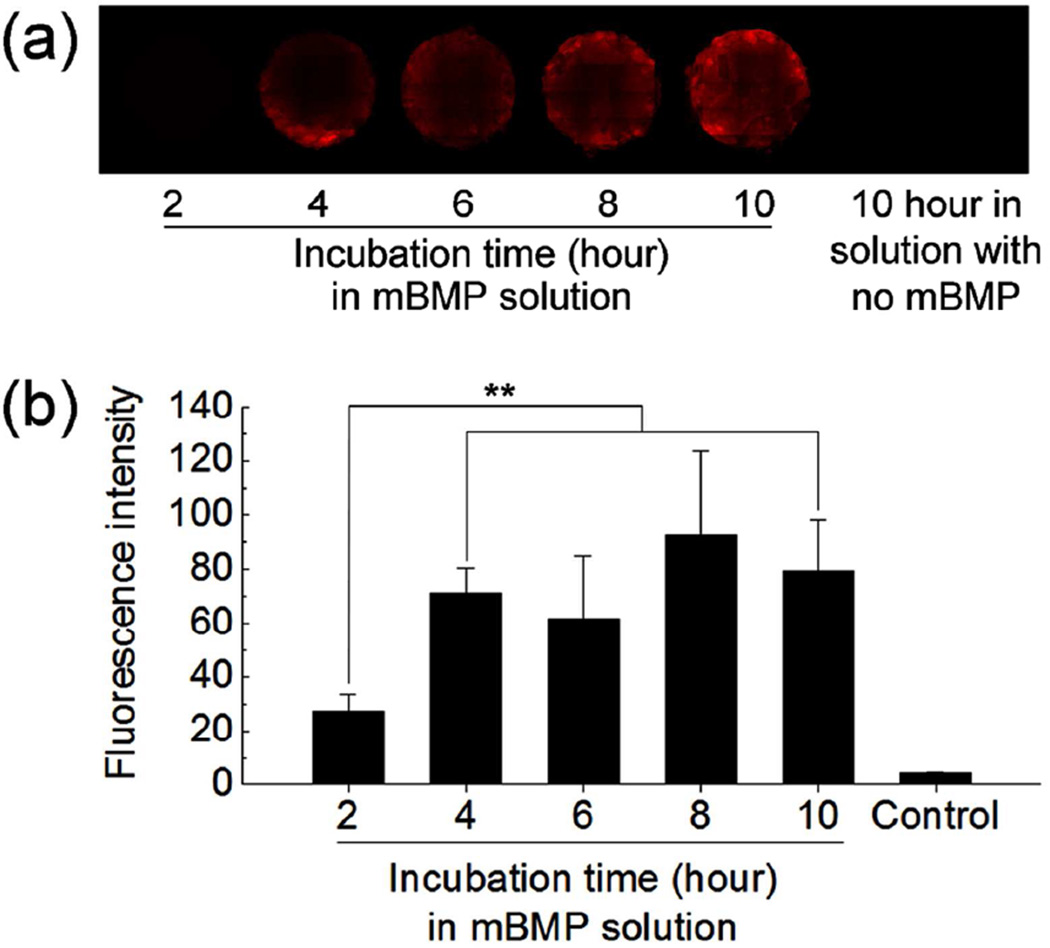
We next assessed mBMP binding to living trabecular bone cores (1 cm in diameter and 0.5 cm in thickness) harvested from bovine sternum. For this set of experiments, modular peptide binding occurred in a bone bioreactor where bone cores were kept alive, and a 100 µg/mL mBMP solution in Dulbecco’s modified Eagle medium (DMEM) was continuously circulated through the bone perfusion chamber. This bone culture system allows for ex vivo culture of three dimensional trabecular bone explants which included about one million osteocytes, marrow cells and extracellular matrix for several weeks.20, 21 This experimental platform was used to provide insights into mBMP binding to living bone tissue in a context that may mimic some aspects of direct mBMP injection into bone tissue in vivo. After incubating for various time periods, the binding was measured in terms of fluorescence intensity from rhodamine labeled mBMP. The binding was gradually increased until 4 hours and reached a plateau afterwards (Figure 4). Specifically, the binding at longer time points (4, 6, 8 and 10 hours) was significantly higher than binding at 2 hours. However no statistical difference in binding was observed when comparing incubation times longer than 4 hours (Figure 4b and Table S4 in Supporting Information). This result indicates that the mBMP can be incorporated into living trabecular bone during circulation.
Figure 4.
(a) Fluorescence images and (b) fluorescence intensity of trabecular bone cores after incubating in rhodamine-labeled mBMP solution for different time periods in a bone bioreactor. Data are shown as mean ± standard deviation. ** p < 0.05.
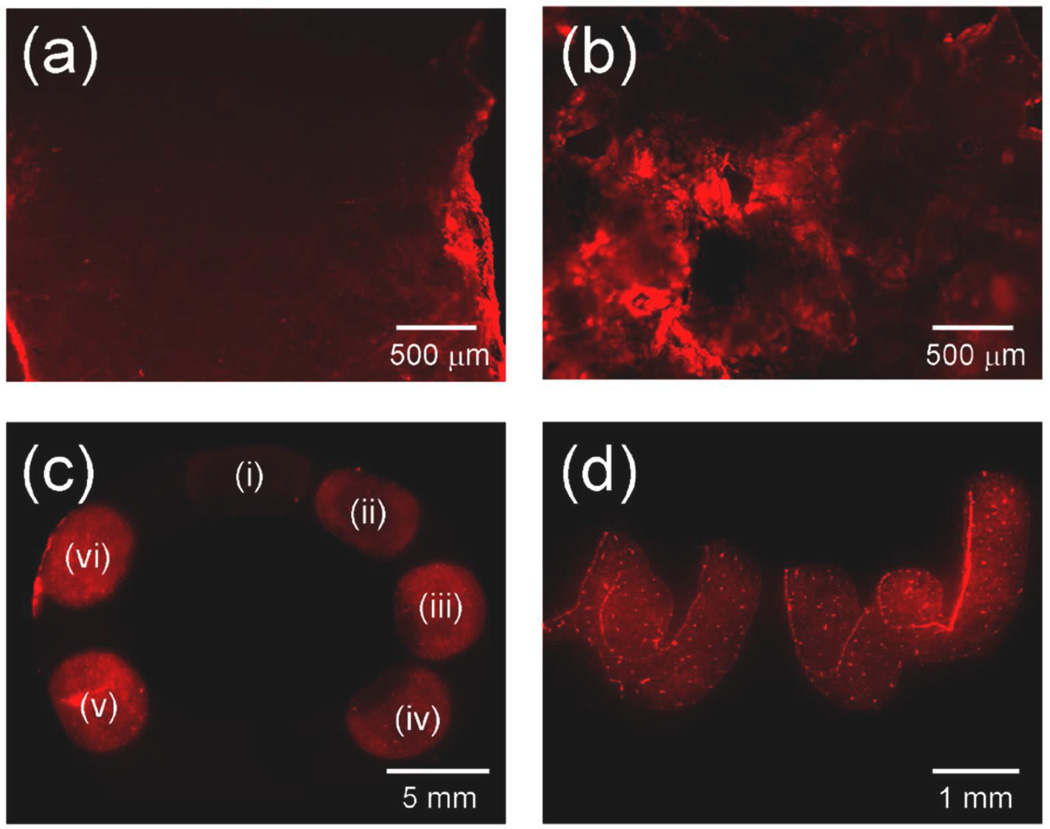
In summary, we showed that mBMP could bind to native bone tissues having different microstructure and porosity. The quantity and kinetics of mBMP binding was dependent on the concentration of mBMP solution and incubation time. The γE moieties in the HAP-binding, OCN-inspired motif in mBMP were responsible for the high binding affinity to bone tissue. We also demonstrated that the mBMP could be incorporated into the bone using an ex vivo bone bioreactor. It is noteworthy that localized mBMP binding on cortical bone tissue was also possible using mBMP. When a bone piece was “dip-coated” in mBMP solution, a significant amount of mBMP was found to bind to the bone surface (Figure 5a), which indicated that the binding occurred quickly upon contact. Furthermore, the mBMP could be incorporated in a spatially controlled manner by spotting, or direct writing with peptide solution (Figures 5b and 5c). This result suggested that mBMP can be loaded onto the bone with a robust spatial control using simple methods that may be easily applied to clinical practice.
Figure 5.
Fluorescence images of (a) cortical bone and (b) trabecular bone dip-coated in rhodamine-labeled mBMP solution. (c) Fluorescence image of cortical bone spotted with rhodamine-labeled mBMP solution with different concentrations: (i) 6.25, (ii) 12.5, (iii) 25, (iv) 50, (v) 100 and (vi) 200 µg/mL. (d) Fluorescence image of “UW” written with rhodamine-labeled mBMP solution on cortical bone.
Experimental Section
The rhodamine-labeled peptides were prepared via solid phase peptide synthesis as previously described.16 The native bones used were harvested from sheep tibia and bovine sternum. Cortical (compact) bone slices were collected from sheep tibia with periosteum removed, and trabecular (spongy) bone cores were drilled out from bovine sternum under sterile condition. The peptide binding to native bones was tested in three different experiments. We used rhodamine-conjugated peptides to determine their binding in terms of fluorescence intensity. In the first experiment, the cortical bone slices were incubated in modular peptide solution (0.5 mL, PBS) with concentrations of 50, 100, 200 and 300 µg/mL. The incubation was continued in a static condition for a period of 0.5, 1, 2 and 3 hours. To examine the duration of modular peptide on the bone, the cortical bones treated with 200 µg/mL modular peptide solution for 3 hours were incubated in simulated body fluid at 37 °C and the change in fluorescence intensity was monitored. For another experiment, the trabecular bone cores were placed in the chamber of bone bioreactor where the peptide solution in DMEM (100 µg/mL, 6.5 mL) was continuously circulated through the chamber for a time period of 2, 4, 6, 8 and 10 hours. In the last experiment, mBMP was bound in a spatially controlled manner by dip-coating, spotting or writing with mBMP solution on native bone tissues. Dip-coating was carried out in mBMP solution (100 µg/mL), and spotting was done by dropping 10 µL of mBMP solution with different concentrations on the cortical bone surface. For writing, the letters were written with hypodermic needle which was dipped in mBMP solution (100 µg/mL). Following each experiment, the bones were rinsed with PBS to remove unbound peptide and their fluorescence images were taken using a Typhoon fluorescence scanner (GE healthcare) or a Nikon Eclipse Ti inverted microscope. The amount of mBMP bound to cortical bone was calculated from the concentration change of mBMP solution after binding, which was determined from the fluorescence intensity based on a standard curve of fluorescence intensity against mBMP concentration. To quantify the fluorescence intensity, the images were converted into 8-bit using ImageJ to present the intensity level of each pixel in the range of 0 to 255. The mean pixel intensity of a selected region of interest was considered to be proportional to the amount of peptide bound.
Supplementary Material
ACKNOWLEDGMENT
The authors acknowledge financial support from the National Institutes of Health (R01 HL093282), the National Science Foundation (DMR Collaborative Research Proposal 1106165/1105591), the AO Foundation (Exploratory Research Project), and the Shapiro Summer Research Program (to S.W.).
Footnotes
Supporting Information. Statistics on modular peptide binding to native bones. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Giannoudis PV, Dinopoulos H, Tsiridis E. Bone substitutes: an update. Injury. 2005;36:S20–S27. doi: 10.1016/j.injury.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 2.Jahangir AA, Nunley RM, Mehta S, Sharan A Washington Health Policy Fellows. Bone graft substitutes in orthopaedic surgery. AAOS Now. 2008 Jan; http://www.aaos.org/news/aaosnow/jan08/reimbursement2.asp.
- 3.Moore D. [Accessed Sep 30, 2012];Bone grafting. http://www.orthobullets.com/basic-science/9011/bone-grafting.
- 4.Greenwald AS, Boden SD, Goldberg VM, Khan Y, Laurencin CT, Rosier RN. Bone-graft substitutes: Facts, fictions, and applications. J. Bone Joint Surg. Am. 2001;83A:98–103. doi: 10.2106/00004623-200100022-00007. [DOI] [PubMed] [Google Scholar]
- 5.Dinopoulos H, Dimitriou R, Giannoudis PV. Bone graft substitutes: What are the options? Surg. J. R. Coll. Surg. Edinb. Irel. 2012;10:230–239. doi: 10.1016/j.surge.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Bauer TW, Muschler GF. Bone graft materials - An overview of the basic science. Clin. Orthop. Rel. Res. 2000;371:10–27. [PubMed] [Google Scholar]
- 7.Finkemeier CG. Bone-grafting and bone-graft substitutes. J. Bone Joint Surg. Am. 2002;84A:454–464. doi: 10.2106/00004623-200203000-00020. [DOI] [PubMed] [Google Scholar]
- 8.Hecht BP, Fischgrund JS, Herkowitz HN, Penman L, Toth JM, Shirkhoda A. The use of recombinant human bone morphogenetic protein 2 (rhBMP-2) to promote spinal fusion in a nonhuman primate anterior interbody fusion model. Spine. 1999;24:629–636. doi: 10.1097/00007632-199904010-00004. [DOI] [PubMed] [Google Scholar]
- 9.Nather A, David V, Teng JWH, Lee CW, Pereira BP. Effect of autologous mesenchymal stem cells on biological healing of allografts in critical-sized tibial defects simulated in adult rabbits. Ann. Acad. Med. Singapore. 2010;39:599–606. [PubMed] [Google Scholar]
- 10.Zhang XP, Xie C, Lin ASP, Ito H, Awad H, Lieberman JR, Rubery PT, Schwarz EM, O'Keefe RJ, Guldberg RE. Periosteal progenitor cell fate in segmental cortical bone graft transplantations: Implications for functional tissue engineering. J. Bone Miner. Res. 2005;20:2124–2137. doi: 10.1359/JBMR.050806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Axelrad TW, Einhorn TA. Bone morphogenetic proteins in orthopaedic surgery. Cytokine Growth Factor Rev. 2009;20:481–488. doi: 10.1016/j.cytogfr.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Termaat MF, Den Boer FC, Bakker FC, Patka P, Haarman H. Bone morphogenetic proteins - Development and clinical efficacy in the treatment of fractures and bone defects. J. Bone Joint Surg. Am. 2005;87A:1367–1378. doi: 10.2106/JBJS.D.02585. [DOI] [PubMed] [Google Scholar]
- 13.Waltz E. Spinal device cancer risk. Nat. Biotechnol. 2012;30:8. doi: 10.1038/nbt0812-737. [DOI] [PubMed] [Google Scholar]
- 14.Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 2011;11:471–491. doi: 10.1016/j.spinee.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 15.Lee JS, Lee JS, Murphy WL. Modular peptides promote human mesenchymal stem cell differentiation on biomaterial surfaces. Acta Biomater. 2010;6:21–28. doi: 10.1016/j.actbio.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JS, Lee JS, Wagoner-Johnson A, Murphy WL. Modular peptide growth factors for substrate-mediated stem cell differentiation. Angew. Chem. Int. Ed. 2009;48:6266–6269. doi: 10.1002/anie.200901618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu Y, Lee JS, Nemke B, Graf BK, Royalty K, Illgen R, Vanderby R, Markel MD, Murphy WL. Coating with a modular bone morphogenetic peptide promotes healing of a bone-implant gap in an ovine model. PLoS One. 2012;7:e50378. doi: 10.1371/journal.pone.0050378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu Y, Markel MD, Nemke B, Lee JS, Graf BK, Murphy WL. Influence of hydroxyapatite-coated and growth factor-releasing interference screws on tendon-bone healing in an ovine model. Arthroscopy. 2009;25:1427–1434. doi: 10.1016/j.arthro.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoang QQ, Sicheri F, Howard AJ, Yang DSC. Bone recognition mechanism of porcine osteocalcin from crystal structure. Nature. 2003;425:977–980. doi: 10.1038/nature02079. [DOI] [PubMed] [Google Scholar]
- 20.Davies CM, Jones DB, Stoddart MJ, Koller K, Smith E, Archer CW, Richards RG. Mechanically loaded ex vivo bone culture system 'Zetos': Systems and culture preparation. Eur. Cells Mater. 2006;11:57–75. doi: 10.22203/ecm.v011a07. [DOI] [PubMed] [Google Scholar]
- 21.Jones DB, Broeckmann E, Pohl T, Smith EL. Development of a mechanical testing and loading system for trabecular bone studies for long term culture. Eur. Cells Mater. 2003;5:48–60. doi: 10.22203/ecm.v005a05. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.