Abstract
Background/Objective
HIV lipodystrophy - characterized by peripheral lipoatrophy, with or without central fat accumulation - confers increased metabolic risk. However, the functional activity of HIV lipodystrophic tissue in relation to metabolic risk has yet to be fully explored in vivo through the use of non-invasive imaging techniques. This study assesses the relationship between FDG uptake in various fat depots and metabolic/immune parameters among subjects with HIV lipodystrophy.
Design/Methods
Lipodystrophic men on anti-retroviral therapy (ART) underwent whole-body 18F-fluorodeoxyglucose positron emission tomography (FDG-PET)/computed tomography (CT) scans and detailed metabolic/immune phenotyping.
Results
FDG uptake in the subcutaneous adipose tissue (SAT) of the extremities (mean standardized uptake value, or SUV, of the arm and leg SAT) was found to correlate with the degree of peripheral lipoatrophy (r = 0.7, p = 0.01). Extremity SAT FDG uptake was positively associated with HOMA-IR (r = 0.6, p = 0.02) and fasting hyperinsulinemia (r = 0.7, p 0.01), while fat percentage of extremities was not. Further, extremity SAT FDG uptake was significantly associated with CD4 count (r = 0.6, p = 0.05). In multivariate modeling for HOMA-IR, extremity SAT FDG uptake remained significant after controlling for BMI and TNF-α (R2 for model = 0.71, p = 0.02; SUV in the extremity SAT β-estimate 12.3, p = 0.009).
Conclusions
In HIV lipodystrophic patients, extremity SAT FDG uptake is increased in association with reduced extremity fat and may contribute to insulin resistance. Noninvasive assessments of in situ inflammation using FDG-PET may usefully complement histological and gene expression analyses of metabolic dysregulation in peripheral fat among HIV+ patients.
Keywords: HIV, lipodystrophy, FDG-PET/CT, insulin resistance
BACKGROUND
HIV lipodystrophy is known to confer an increased risk of insulin resistance and diabetes, thereby predisposing to cardiovascular disease(1). Peripheral lipoatrophy - with or without central fat accumulation - is considered the hallmark of lipodystrophy(2). Affected lipoatrophic tissue, on biopsy, shows a characteristic excess of apoptotic adipocytes(3) and inflammatory cells(4) coupled with deficient and defective adipocyte mitochondria(5). These morphologic changes in atrophic subcutaneous adipose tissue (SAT) are thought to promote in situ insulin resistance and portend remote secondary pathology in muscle and liver(6–8). However, the functional activity of HIV lipodystrophic tissue in relation to metabolic risk has yet to be fully explored in vivo through the use of non-invasive imaging techniques.
Whole-body 18F-fluorodeoxyglucose positron emission tomography (FDG-PET)/computed tomography (CT) represents a promising imaging technique for non-invasively assessing metabolic activity (adipocyte glucose utilization and degree of macrophage infiltration) in fat. Our group has previously shown that patients with HIV lipodystrophy on antiretroviral therapy (ART) have increased FDG uptake in the abdominal subcutaneous adipose tissue (SAT) but not in the visceral adipose tissue (VAT) compared with healthy controls(9). Bleeker-Rovers et al. showed that in 4 HIV-infected subjects with stavudine-associated lipodystrophy, FDG uptake in extremity SAT was relatively increased compared to that in HIV-infected subjects without clinical lipodystrophy(10). In the present study, we seek to advance the discourse by assessing, among HIV lipodystrophic subjects on ART, fat-depot-specific FDG uptake in relation to degree of lipoatrophy and to metabolic and immune parameters. We show for the first time that the degree of peripheral lipoatrophy -objectively reflected by a decreased percentage of extremity fat - relates to extremity SAT FDG uptake. Extremity SAT FDG uptake, in turn, contributes to the prediction of insulin resistance in this group more so than does percentage extremity fat.
DESIGN AND METHODS
Subjects
13 HIV-infected lipodystrophic men were recruited from March 2010 to May 2011 for FDG-PET/CT assessment of dorsocervical and other fat depots, as previously described(11). Analyses of glucose utilization in dorsocervical fat were recently published and we now report data on the abdominal visceral and subcutaneous as well as extremity fat depots not previously published. Subjects were required to have HIV lipodystrophy which developed while on ART, and to have been on a stable ART regimen for >12 months. Lipodystrophy was judged clinically by the investigator based on facial/extremity lipoatrophy or dorsocervical/abdominal lipohypertrophy. Exclusion criteria included known diabetes or thyroid disease, therapy with steroid/growth hormones or medications affecting adrenergic hormones, recent-onset opportunistic infection, or major chronic illness (e.g. liver disease)(11). The study was approved by the Partners Human Research Committee. Written informed consent was obtained, and study visits were conducted at the Massachusetts General Hospital (MGH) Clinical Research Center (CRC).
Procedures
Study procedures included fasting blood sampling, anthropometric measurements, dual-energy X-ray absorptiometry (DXA) scanning (Hologic, Bedford, MA) for determination of lean and fat mass, and whole-body FDG-PET/CT scanning for determination of glucose utilization in various fat depots.
Assays
Lipids, glucose, and insulin were assessed using standard techniques in the MGH Research Laboratory. HIV viral load was quantified (COBAS TaqMan HIV-1 Test, Roche Diagnostics). TNF-α levels were measured by ELISA (Invitrogen).
18F-Fluorodexoxyglucose positron-emission tomography with computed tomography
Methods used for FDG-PET/CT imaging and determination of FDG uptake in specific adipose tissue depots were previously described by our group(11). FDG-PET/CT data are presented as standardized uptake value (SUV) of FDG, which represents the decay-corrected tissue concentration of FDG, adjusted for injected FDG dose and body weight. SUVmean and standard deviation (SD’s) were determined in the following fat-density anatomic regions: left upper arm SAT at the level of proximal humeral metaphysis; abdominal SAT and VAT at the level of L4; left thigh SAT at the level of the proximal femoral diaphysis; and right hepatic lobe. SUV of the extremity SAT was defined as the mean SUV in the SAT of the left upper and lower extremities ([SUV left upper arm + SUV left thigh] / 2). SUVs were measured in triplicate, averaged, and normalized to liver SUV.
Body Composition
Fat percentage of extremities by DXA was defined as the proportion of limb mass composed of fat : [L arm fat (kg) + L leg fat (kg) / L arm mass (kg) + L leg mass (kg)] × 100]. The area of abdominal SAT and VAT was determined using a single CT image (obtained at the level of L4) that was extracted from the FDG-PET/CT dataset.
Statistical analysis
12 of the 13 subjects studied had an undetectable viral load. The single subject with a markedly elevated viral load was excluded from analysis. Baseline characteristics of the study group were assessed. For each subject, FDG uptake in the VAT was compared to that in the SAT. Univariate regression modeling was used to compare the FDG uptake in various fat depots to parameters of interest including age, duration of HIV, CD4 count, duration of select therapies, anthropometrics, percent lean and fat mass, insulin resistance (HOMA-IR), lipids, cytokines, and hormones. Multiple regression modeling was used to assess the contribution of FDG uptake in various fat depots to HOMA-IR after controlling for known predictive factors. All data are presented as mean ± SE unless otherwise indicated. Statistical significance was defined as p < 0.05. Analyses were performed using SAS JMP 9.0.
RESULTS
Baseline Characteristics
Subjects in this study were prospectively recruited for the presence of HIV lipodystrophy, as judged by a clinical investigator. Subjects were males with undetectable viral load on combined ART. Mean age was 47.5 years and mean duration of HIV was 14.1 years. Mean body mass index (BMI) was 23.7 kg/m2. Mean fat percentage of extremities was 16.5%, with a range of 8.2 to 27.9%. Additional baseline metabolic and immune parameters were also determined (Table 1).
Table 1.
Characteristics of the study subjects
| Mean ± SE (unless otherwise noted) | |
|---|---|
| Demographics | |
| Age (years) | 47.5 ± 2.5 |
| Gender (male) | 12/12 (100%) |
| Immune parameters | |
| Duration HIV (years) | 14.1 ± 1.5 |
| Duration ART (years) | 9.2 ± 1.7 |
| NRTI (years) | 9.0 ± 1.6 |
| NNRTI (years) | 1.7 ± 0.9 |
| PI (years) | 7.7 ± 2.1 |
| NRTI | |
| current | 11/12 (92%) |
| past | 1/12 (8%) |
| never | 0/12 (0%) |
| NNRTI | |
| current | 2/12 (17%) |
| past | 3/12 (25%) |
| never | 7/12 (58%) |
| PI | |
| current | 5/12 (42%) |
| past | 3/12 (25%) |
| never | 4/12 (33%) |
| CD4 count (#/mm3) | 536 ± 46 |
| viral load (undetectable) | 12/12 (100%) |
| TNF-α (pg/mL) | 13 ± 3 |
| Body composition parameters | |
| BMI (kg/m2) | 23.7 ± 1.4 |
| iliac waist circumference (cm) | 87 ± 3 |
| abdominal subcutaneous adipose tissue (cm) by CT | 131 ± 24 |
| visceral adipose tissue (cm) by CT | 100 ± 21 |
| arm fat (kg) by DXA | 0.9 ± 0.1 |
| leg fat (kg) by DXA | 1.7 ± 0.2 |
| fat percentage of extremities by DXA | 16.5 ± 1.9 (range 8.2 – 27.9%) |
| Metabolic parameters | |
| Total cholesterol (mmol/l) | 4.4 ± 0.2 |
| LDL cholesterol (mmol/l) | 2.5 ± 0.2 |
| HDL cholesterol (mmol/l) | 1.2 ± 0.1 |
| Triglycerides (mmol/l) | 1.5 ± 0.3 |
| Fasting glucose (mmol/l) | 4.6 ± 0.2 |
| HOMA-IR | 0.8 ± 0.2 (range 0.1 – 3.1) |
FDG Uptake in Abdominal VAT and SAT in Relation to Metabolic Parameters
Among all subjects, there was a relatively higher SUV in the VAT compared with abdominal SAT (0.27 ± 0.04 vs. 0.14 ± 0.02, p = 0.0004). For each individual subject, the SUV in the VAT was greater than the SUV in the abdominal SAT by matched pairs analysis (p = 0.0004). Overall, the SUV in the VAT was found to relate strongly to the SUV in the abdominal SAT (r = 0.9, p < 0.0001). SUV in the abdominal SAT was inversely associated with the cross-sectional area of abdominal SAT (r = −0.85, p = 0.0004). There was a trend towards an inverse relationship between SUV in the VAT and the cross-sectional area of VAT (r = −0.54, p = 0.07). Both SUV in the abdominal SAT and VAT were inversely associated with the fat percentage of the trunk (grams of fat in trunk/mass trunk) (r = −0.8, p = 0.001 and r = −0.8, p = 0.002). The cross-sectional area of VAT was related to HOMA-IR (r = 0.6, p = 0.04) but the area of abdominal SAT, SUV in the abdominal SAT, and SUV in the VAT were not.
FDG Uptake in Extremity SAT in Relation to Metabolic and Immune Parameters
SUV in the extremity SAT was strongly, and inversely associated with the fat percentage of extremities (r = −0.7, p = 0.01) (Figure 1). Of note, neither SUV in the extremity SAT nor the fat percentage of extremities was correlated with degree of overall adiposity (BMI) or central adiposity (VAT). SUV in the extremity SAT was correlated with the VAT:TAT ratio (r = 0.64, p = 0.02).
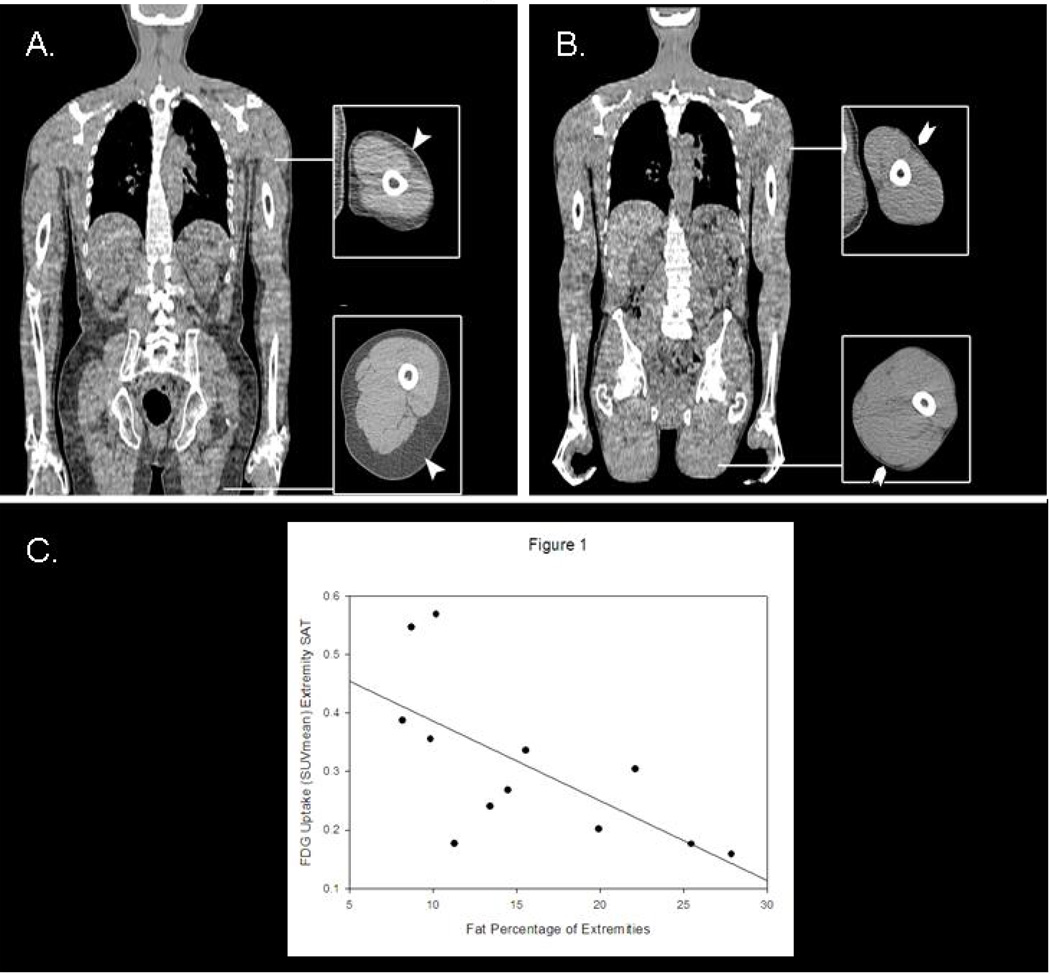
Figure 1.
Panels 1A and 1B are representative CT images from FDG-PET/CT studies in two subjects with varying degrees of HIV lipodystrophy. Both Subjects A and B are on combined ART, and have undetectable viral loads. Despite having relatively similar external body habitus (BMI 20.9 kg/m2 for Subject A and BMI 18.6 kg/m2 for Subject B), the two subjects differ markedly in the degree of extremity lipoatrophy (27.9% extremity fat for Subject A and 8.2% extremity fat for Subject B) and in the degree of extremity SAT FDG uptake (not shown). Panel C shows the relationship between fat percentage of extremities by DXA and extremity SAT FDG uptake (SUVmean) by FDG-PET/CT among all subjects studied (r = −0.7, p= 0.01).
On univariate regression, SUV in the extremity SAT was positively associated with HOMA-IR (r = 0.6, p = 0.02) and fasting hyperinsulinemia (r = 0.7, p 0.01) while the fat percentage of extremities was not. Further, SUV in the extremity SAT, but not the fat percentage of extremities, was significantly associated with CD4 count (r = 0.6, p = 0.05). SUV in the extremity SAT was not found to relate to age, fasting lipids, or TNF-α levels. In multivariate modeling for HOMA-IR, SUV in the extremity SAT remained significant even after controlling for BMI and TNF-α (systemic inflammation) (R2 for model = 0.71, p = 0.02; SUV in the extremity SAT β-estimate 12.3 ± SE 3.6, p = 0.009).
DISCUSSION
In this report, we demonstrate for the first time that FDG uptake in extremity SAT is significantly associated with the degree of peripheral lipoatrophy among patients with HIV lipodystrophy. We further demonstrate that FDG uptake in extremity SAT relates to insulin resistance more strongly than does the fat percentage of extremities. Taken together, our data suggest that among HIV+ patients, markedly increased metabolic activity is seen in lipoatrophic extremity fat, in association with abnormal systemic metabolic parameters. Our group previously demonstrated that among HIV+ patients, FDG uptake in the abdominal SAT exceeds that in non-HIV controls, while FDG uptake in the VAT is not different between the two groups(9). The present study is the first to assess extremity SAT FDG uptake among HIV+ patients in relation to critical metabolic and immune parameters.
Applying the FDG-PET technique to abdominal fat depots, we show in all subjects a higher fasting FDG uptake in VAT versus abdominal SAT, in concert with findings by Christen et al. in a non-HIV population(12). FDG uptake in fat likely represents a combination of metabolic activity (glucose utilization by adipocytes) and inflammation (glucose utilization by macrophages and other cell types in the stromovascular fraction). Our data thus extends the results from fat biopsy studies in non-HIV subjects suggesting VAT both harbors more glucose-avid adipocytes(13) and/or a denser inflammatory infiltrate(14) than abdominal SAT.
FDG uptake in the abdominal SAT and VAT of our lipodystrophic subjects decreased with increasing fat percentage of the trunk . In non-HIV subjects, increased adiposity is known to augment inflammation of both abdominal SAT and VAT in association with insulin resistance (15, 16). Thus, the trend we observed potentially reflects a counterbalancing of increased inflammatory cell glucose uptake by decreased GLUT-4-mediated adipocyte glucose uptake in the context of insulin resistance(12). A possible alternate explanation is that in patients with HIV lipodystrophy and increasing central fat accumulation, there is a progressive insulin resistance in these central fat depots, which limits glucose uptake on PET. This explanation is supported by the significant relationship we observed between VAT area and HOMA-IR.
Among our lipodystrophic cohort, FDG uptake in the extremity SAT, in contrast to abdominal VAT and SAT, showed important relationships with critical metabolic and immune parameters. With respect to body composition, FDG uptake in the extremity SAT correlated inversely with the fat percentage of extremities and thus positively with degree of peripheral lipoatrophy. Extrapolating from fat biopsy studies (4, 5), we infer that the observed increase in FDG uptake in more severe lipoatrophy likely reflects two concomitant processes: a) increased macrophage and lymphocyte glucose uptake in the setting of in situ inflammation and b) increased adipocyte GLUT-1 mediated glucose metabolism, which is known to be upregulated by mitochondrial dysfunction-related oxidative stress (17) . The increase in adipocyte GLUT-1 mediated glucose disposal contrasts with adipocyte GLUT-4 mediated glucose metabolism, which is known to be downregulated in states of inflammation (18). We further demonstrate that extremity SAT FDG uptake relates to CD4 count, suggesting a potential relationship to immune function. In this regard, higher CD4 counts may reflect a degree of immune restoration permitting increased inflammation in the fat, or, alternatively, may be a marker of ART effect.
"Given the variability in CD4 counts and ART effects, larger, stratified studies of HIV lipodystrophic subjects correlating specific ART regimens with extremity SAT FDG uptake and biopsy-proven extremity SAT pathology are needed.
Another finding in our study is that FDG uptake in the extremity SAT relates significantly on univariate and multivariate modeling to insulin resistance (as measured by HOMA-IR). Insofar as FDG uptake in the extremity SAT likely reflects inflammation and oxidative stress - causally linked to the development of insulin resistance - this observation has strong biologic plausibility. Interestingly, FDG uptake in the abdominal SAT or VAT of our studied subjects did not correlate with insulin resistance or other key metabolic or immune parameters. It is possible that in HIV lipodystrophy, inflammation in peripheral lipoatrophic fat contributes more strongly to insulin resistance than inflammation in expanded central fat depots. In light of the relatively low HOMA-IR of this group, further studies are needed to validate the relationship between extremity SAT FDG uptake and insulin resistance across a large lipodystrophic HIV+ cohort with a wide range of insulin resistance patterns.
Peripheral lipoatrophy is an important feature of lipodystrophy syndrome(2) and has been previously linked to insulin resistance(19) and cardiovascular risk(20). Here we apply FDG-PET/CT to the extremity SAT in HIV lipodystrophic patients, showing that uptake in this depot correlates with degree of lipoatrophy and with critical immune and metabolic parameters. In a recent fat biopsy study of HIV lipodystrophic patients, Hammond et al.(4) showed that switching ART regimens could reverse pathologic changes to fat morphology. Moreover, in Hammond’s work, those patients with the highest degree of subcutaneous fat inflammation were more likely to benefit from changes in ART regimens. These findings suggest subcutaneous fat inflammation may be a sensitive marker for early lipodystrophic changes. FDG-PET/CT may be a useful noninvasive measure of in situ inflammation and oxidative stress, thus representing an important technique for better understanding the metabolic risk of ART-induced lipoatrophy.
The current study has limitations including a cross-sectional design, inclusion of men only, and relatively small sample size, precluding stratified analysis of ART class-specific effects on fat FDG uptake. Despite these limitations, this is the first study to demonstrate, using FDG-PET, that increased metabolic activity in peripheral fat is directly proportional to the degree of lipoatrophy in HIV+ patients, and may contribute to insulin resistance and metabolic dysregulation. Further studies are necessary to understand the timing, pathophysiology, clinical significance of and potential treatments for in situ inflammation that develops in association with lipoatrophy in the context of ART. Noninvasive assessments of in situ inflammation using FDG-PET may be useful complements to histological and gene expression analyses of metabolic dysregulation in peripheral fat among HIV+ patients.
Acknowledgements
The authors would like to thank the subjects in the study for their participation and the nursing and bionutrition staffs of the MGH GCRC for their dedicated patient care.
Grant Support: This work was supported by National Institutes of Health Grants 1UL1RR025758, 2P30AI060354 to Dr. Torriani., F32 DK085969 to Dr. Zanni., K23 DK081604 to Dr. Cypess, K24 DK064545 to Dr. Grinspoon, and M01-RR-01066 and 1 UL1 RR025758-01, Harvard Clinical and Translational Science Center, from the National Center for Research Resources
Footnotes
Disclosure Statement: None of the authors has any relevant disclosures.
References
- 1.Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med. 2005 Jan 6;352(1):48–62. doi: 10.1056/NEJMra041811. [DOI] [PubMed] [Google Scholar]
- 2.Chen D, Misra A, Garg A. Clinical review 153: Lipodystrophy in human immunodeficiency virus-infected patients. J Clin Endocrinol Metab. 2002 Nov;87(11):4845–4856. doi: 10.1210/jc.2002-020794. [DOI] [PubMed] [Google Scholar]
- 3.Domingo P, Matias-Guiu X, Pujol RM, Francia E, Lagarda E, Sambeat MA, et al. Subcutaneous adipocyte apoptosis in HIV-1 protease inhibitor-associated lipodystrophy. AIDS. 1999 Nov 12;13(16):2261–2267. doi: 10.1097/00002030-199911120-00008. [DOI] [PubMed] [Google Scholar]
- 4.Hammond E, McKinnon E, Nolan D. Human immunodeficiency virus treatment-induced adipose tissue pathology and lipoatrophy: prevalence and metabolic consequences. Clin Infect Dis. 2010 Sep 1;51(5):591–599. doi: 10.1086/655765. [DOI] [PubMed] [Google Scholar]
- 5.Shikuma CM, Hu N, Milne C, Yost F, Waslien C, Shimizu S, et al. Mitochondrial DNA decrease in subcutaneous adipose tissue of HIV-infected individuals with peripheral lipoatrophy. AIDS. 2001 Sep 28;15(14):1801–1809. doi: 10.1097/00002030-200109280-00009. [DOI] [PubMed] [Google Scholar]
- 6.Giralt M, Domingo P, Villarroya F. Adipose tissue biology and HIV-infection. Best Pract Res Clin Endocrinol Metab. 2011 Jun;25(3):487–499. doi: 10.1016/j.beem.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Sevastianova K, Sutinen J, Kannisto K, Hamsten A, Ristola M, Yki-Jarvinen H. Adipose tissue inflammation and liver fat in patients with highly active antiretroviral therapy-associated lipodystrophy. Am J Physiol Endocrinol Metab. 2008 Jul;295(1):E85–E91. doi: 10.1152/ajpendo.90224.2008. [DOI] [PubMed] [Google Scholar]
- 8.Torriani M, Thomas BJ, Barlow RB, Librizzi J, Dolan S, Grinspoon S. Increased intramyocellular lipid accumulation in HIV-infected women with fat redistribution. J Appl Physiol. 2006 Feb;100(2):609–614. doi: 10.1152/japplphysiol.00797.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hadigan C, Kamin D, Liebau J, Mazza S, Barrow S, Torriani M, et al. Depot-specific regulation of glucose uptake and insulin sensitivity in HIV-lipodystrophy. Am J Physiol Endocrinol Metab. 2006 Feb;290(2):E289–E298. doi: 10.1152/ajpendo.00273.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bleeker-Rovers CP, van der Ven AJ, Zomer B, de Geus-Oei LF, Smits P, Corstens FH, et al. F-18-fluorodeoxyglucose positron emission tomography for visualization of lipodystrophy in HIV-infected patients. AIDS. 2004 Dec 3;18(18):2430–2432. [PubMed] [Google Scholar]
- 11.Torriani M, Fitch K, Stavrou E, Bredella MA, Lim R, Sass CA, et al. Deiodinase 2 Expression Is Increased in Dorsocervical Fat of Patients with HIV-Associated Lipohypertrophy Syndrome. J Clin Endocrinol Metab. 2012 Jan 18; doi: 10.1210/jc.2011-2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christen T, Sheikine Y, Rocha VZ, Hurwitz S, Goldfine AB, Di Carli M, et al. Increased glucose uptake in visceral versus subcutaneous adipose tissue revealed by PET imaging. JACC Cardiovasc Imaging. 2010 Aug;3(8):843–851. doi: 10.1016/j.jcmg.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Westergren H, Danielsson A, Nystrom FH, Stralfors P. Glucose transport is equally sensitive to insulin stimulation, but basal and insulin-stimulated transport is higher, in human omental compared with subcutaneous adipocytes. Metabolism. 2005 Jun;54(6):781–785. doi: 10.1016/j.metabol.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 14.O'Rourke RW, Metcalf MD, White AE, Madala A, Winters BR, Maizlin II, et al. Depot-specific differences in inflammatory mediators and a role for NK cells and IFN-gamma in inflammation in human adipose tissue. Int J Obes (Lond) 2009 Sep;33(9):978–990. doi: 10.1038/ijo.2009.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klimcakova E, Roussel B, Kovacova Z, Kovacikova M, Siklova-Vitkova M, Combes M, et al. Macrophage gene expression is related to obesity and the metabolic syndrome in human subcutaneous fat as well as in visceral fat. Diabetologia. 2011 Apr;54(4):876–887. doi: 10.1007/s00125-010-2014-3. [DOI] [PubMed] [Google Scholar]
- 16.Klimcakova E, Roussel B, Marquez-Quinones A, Kovacova Z, Kovacikova M, Combes M, et al. Worsening of obesity and metabolic status yields similar molecular adaptations in human subcutaneous and visceral adipose tissue: decreased metabolism and increased immune response. J Clin Endocrinol Metab. 2011 Jan;96(1):E73–E82. doi: 10.1210/jc.2010-1575. [DOI] [PubMed] [Google Scholar]
- 17.Ebert BL, Firth JD, Ratcliffe PJ. Hypoxia and mitochondrial inhibitors regulate expression of glucose transporter-1 via distinct Cis-acting sequences. J Biol Chem. 1995 Dec 8;270(49):29083–29089. doi: 10.1074/jbc.270.49.29083. [DOI] [PubMed] [Google Scholar]
- 18.Lumeng CN, Deyoung SM, Saltiel AR. Macrophages block insulin action in adipocytes by altering expression of signaling and glucose transport proteins. Am J Physiol Endocrinol Metab. 2007 Jan;292(1):E166–E174. doi: 10.1152/ajpendo.00284.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mynarcik DC, McNurlan MA, Steigbigel RT, Fuhrer J, Gelato MC. Association of severe insulin resistance with both loss of limb fat and elevated serum tumor necrosis factor receptor levels in HIV lipodystrophy. J Acquir Immune Defic Syndr. 2000 Dec 1;25(4):312–321. doi: 10.1097/00042560-200012010-00004. [DOI] [PubMed] [Google Scholar]
- 20.Lake JE, Wohl D, Scherzer R, Grunfeld C, Tien PC, Sidney S, et al. Regional fat deposition and cardiovascular risk in HIV infection: the FRAM study. AIDS Care. 2011 Aug;23(8):929–938. doi: 10.1080/09540121.2010.543885. [DOI] [PMC free article] [PubMed] [Google Scholar]