Abstract
Background
The current study examined the effects of recent binge drinking on cerebellar morphometry in a sample of healthy adolescents.
Methods
Participants were 106 teenagers (46 bingers and 60 controls) aged 16–19 who received a high-resolution magnetic resonance imaging (MRI) scan. FreeSurfer segmented and quantified the volume of each cerebellum. Maximum drinks during a binge in the past three months and duration since last binge were examined as predictors of cerebellar volume, after controlling for potentially confounding variables.
Results
In the 106 teens, higher peak drinks predicted smaller left hemisphere cerebellar gray (f2=.06, p = .02) and white matter (f2=.08, p = .02) and right hemisphere cerebellar gray matter (f2=.08, p = .006), and marginally predicted smaller right hemisphere cerebellar white matter (f2=.05, p = .09). Gender did not moderate these effects.
Conclusion
More intense adolescent binge drinking is linked to smaller cerebellar volumes even in healthy teens, above and beyond variability attributable to risk factors for binge drinking. Longitudinal research is needed to see if cerebellar volumes worsen with protracted drinking and recover with abstinence. Interventions aimed at improving brain structure in adolescent binge drinkers are necessary given the high prevalence of risky drinking in youth
Keywords: Adolescence, Brain Imaging, MRI, Alcohol, Binge Drinking, Cerebellum
1. INTRODUCTION
Alcohol continues to be the most widely used intoxicant among teens, with almost a third of 12th graders reporting past month binge drinking (4 standard alcohol drinks on an occasion in females and 5 drinks for males) (Johnston, O’Malley, Bachman, & Schulenberg, 2011). Despite this, relatively few studies have examined the impact of binge drinking on brain structure in teens.
Alcohol exposure appears to particularly affect the cerebellum (see Sullivan & Pfefferbaum, 2005 for review) by exerting its effects on GABAergic neurons and glutamatergic granule cells (Valenzuela, Lindquist & Zamudio-Bolcock, 2010). In rats, chronic alcohol exposure has resulted in structural abnormalities in Purkinje cells (Pierce et al., 2011). The cerebellum plays a critical role in coordination and balance, affect regulation, and executive functioning (Schmahmann & Sherman, 1998), which are often disrupted in adult alcohol dependent samples (see Sullivan & Pfefferbaum, 2005 for review).
Because adolescence is a dynamic developmental stage, adult findings might not apply to teens. Several brain regions including the cerebellum continue to undergo gray matter synaptic pruning into the mid-twenties (Lenroot & Giedd, 2006), and maturation of white matter tracts appears to continue into the early thirties (Jernigan & Gamst, 2005). More specific to the current study, the cerebellum continues to functionally (e.g., Luna et al., 2001) and structurally mature through adolescence, with most studies suggesting continued pruning into the early twenties (Castellanos et al., 2002; Hill et al., 2007). Gender differences in whole brain (Lenroot & Giedd, 2010) and cerebellar (Tiemeier et al., 2010) development have also been reported, with girls peaking earlier and having relatively smaller total cerebellar volumes.
Given this neurodevelopment, the emergence of binge drinking during the teen years may differentially influence the trajectory of brain development for boys and girls. Animal studies suggest that, compared to adults, adolescents may be particularly vulnerable to the neurotoxic effects of both chronic and binge alcohol exposure (see Spear, 2010 for reviews). Despite the much higher prevalence of binge drinking compared to alcohol use disorders (AUD), the majority of the studies conducted thus far have specifically recruited teens with a history of AUD. Studies utilizing MRI have revealed abnormalities in teens with AUD, including smaller left hippocampal (De Bellis et al., 2000; Nagel, et al., 2005; Medina et al., 2007) and prefrontal cortex (De Bellis et al., 2005; Medina et al., 2008) volumes. Only one study investigated the cerebellum, which reported smaller cerebellar volumes in boys with AUD as compared to male controls (DeBellis et al., 2005).
More recently, studies have examined the link between hazardous alcohol use (i.e., binge drinking) and brain health in youth. Using diffusion tensor imaging, our laboratory (McQueeny et al., 2009) found that adolescent binge drinkers, compared to light drinkers, had significantly reduced white matter quality in several brain regions. Although binge drinking was not directly assessed, we (Medina et al., 2010) found that increased overall use of alcohol during the past year was significantly related to smaller cerebellar vermis volumes in substance-using teens. Taken together, these studies suggest that recent binge drinking is associated with structural brain changes although effects on the cerebellum have not been examined. Therefore, the aim of the current study was to assess the dose-dependent effects of recent binge drinking on cerebellar volumes in a sample of 106 typically developing teenagers without comorbid psychiatric disorders. Based on adult findings, we hypothesized that higher peak binge drinking levels will be associated with smaller cerebellar volumes. Based on prior research (Medina et al., 2008; Squeglia et al., in press; Squeglia et al., 2009) we also hypothesized that the relationship between binge drinking and smaller cerebellar volumes would be stronger in females than males.
2. METHODS
2.1 Participants
Adolescents aged 16–19 were recruited from two projects examining youth at risk for substance use and consequences of marijuana use (McQueeny et al., 2009; Hanson et al., 2010). In both, teens were recruited via flier distribution at area schools. Studies were approved by the University of California, San Diego Human Research Protections Program and written assent and consent were obtained from each adolescent and their parent/legal guardian, respectively. Each teen and his/her parent or guardian were given an extensive screening interview to determine eligibility. Exclusionary criteria were: history of DSM-IV Axis I disorder other than mild conduct disorder; use of psychotropic medications; learning disability or mental retardation; head injury; serious medical or neurological problems; prenatal alcohol or drug exposure; complicated or premature birth; left handedness; vision or hearing problem; MRI contraindications; use of >5 joints of marijuana per month; >25 lifetime uses of all other illicit substances; and use of >10 cigarettes per month.
In order to collect data from a wide range of binge drinking histories, eligible participants were classified into two groups based on pattern of substance use: (1) binge drinkers (n=46), who reported at least one binge drinking episode within the past three months, defined as ≥5 (for males) or ≥4 (for females) standard alcohol drinks consumed in one sitting (NIAAA, 2004); or (2) controls (n=60) with no binge drinking episodes within the previous three months, and no lifetime history of an AUD. All participants were requested to abstain from any alcohol or drug use for 72 hours prior to the session, which was confirmed with breathalyzer and urine toxicology.
2.2 Measures
Demographic and Psychiatric Assessment
Screening interviews were completed separately with each adolescent and their parent/guardian. The computerized DISC-IV Predictive Scales (Lucas et al., 2001) was administered to youth and parents to exclude participants with DSM-IV Axis I mood, anxiety, psychotic, and attention deficit hyperactivity disorders. Parallel modules of the computerized Diagnostic Interview Schedule (Robins et al., 1996) were used for 18- and 19-year-olds. The Family History Assessment Module (FHAM; Rice et al., 1995) assessed family history of substance use disorders. For each participant, an index of FH density was calculated: each parent with a history of AUD contributed 0.5 and each grandparent with AUD history added 0.25 to the score (range = 0 to 2) (Zucker et al., 1994). The Structured Clinical Interview measured psychosocial functioning and handedness. The Puberty Development Scale was given to assess puberty stage (Petersen et al., 1988). The Beck Depression Inventory (BDI; Beck et al., 1988) assessed past two-week depressive symptoms. Weight and height were collected to calculate body mass index (BMI).
Premorbid IQ Estimates
The Wide Range Achievement Test-3rd Edition (WRAT-3; (Wilkinson, 1993) Reading subtest was administered to estimate verbal intelligence.
Alcohol and Substance Use
Current (past 3 month) and lifetime alcohol experiences were collected using the Customary Drinking and Drug use Record, which assesses DSM-IV abuse and dependence symptoms, symptoms of withdrawal, and substance-related adverse life events (Stewart and Brown, 1995). Peak number of drinks during a binge in the past three months, typical drinks per occasion, and total lifetime alcohol use were measured. A modified Timeline Followback (Sobell and Sobell, 1992) was also administered to youth and parents, providing detailed information in a calendar format about the type, quantity, and frequency of recent use for the past 30 days covering alcohol along with several other drugs including: marijuana, nicotine, stimulants (e.g., amphetamine, methamphetamine, MDMA/ecstasy, cocaine), opiates (e.g., heroin, Vicodin), hallucinogens, barbiturates, benzodiazepines, and misuse of other prescription and over-the-counter medications.
2.3 Procedures
Trained research associates administered screening interviews to adolescents and parents to assess eligibility. If eligible, prospective participants and their parent/guardians were individually administered a detailed interview. To facilitate open and honest disclosure, confidentiality of provided information and toxicology results was ensured for youths and parents within ethical and legal guidelines. Data from adolescents with self-reported drug or alcohol use within three days, or positive urine toxicology screens or breath samples (AlcoSensor IV, Intoximeter, Inc., St. Louis, MO) at the time of evaluation were not included in these analyses. Parents and teens received financial compensation for participation upon completion of the study.
Participants completed a 1-hour brain imaging session at the UCSD Keck fMRI Center in a 3-Tesla CXK4 short bore Excite-2 MR system (General Electric, Milwaukee, WI) with an 8-channel phase-array head coil. Participants were placed comfortably on the scanner table and the head was stabilized within the head coil using foam cushions (NoMoCo Pillow, La Jolla, CA). Scan sessions involved a 10-second scout scan to assure good head placement and slice selection covering the whole brain. A sagittally-acquired high-resolution 3d T1-weighted anatomical was collected: FOV 24 cm, 256 × 256 × 192 matrix, 0.94 × 0.94 × 1 mm voxels, 176 slices, TR=20 ms, TE=4.8 ms; flip angle 12°, acquisition time 7 minutes 26 seconds.
2.4 Data Processing and Analysis
MRI Preprocessing and ICV Calculation
To obtain overall intracranial volume (ICV), a hybrid watershed and deformable surface semi-automated skull-stripping program, followed by manual editing in AFNI (Cox, 1996), was utilized to remove non-brain materials from each 3d T1-weighted anatomical dataset.
Cerebellar Region of Interest (ROI)
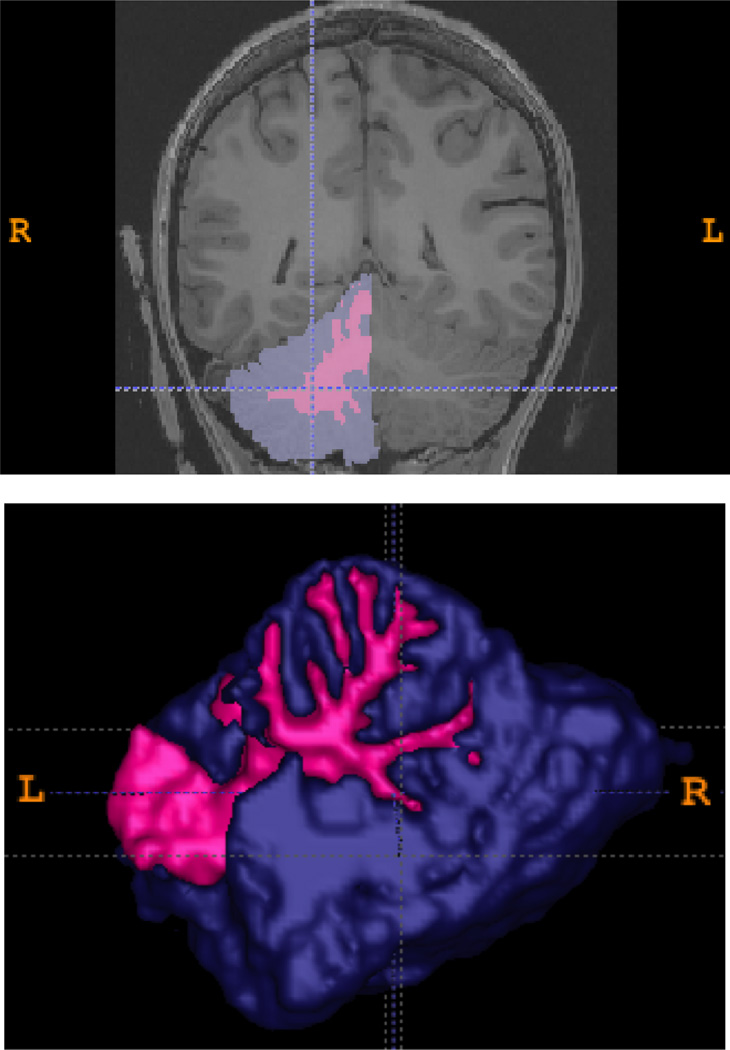
All 3d T1-weighted datasets were aligned and transformed into standard (Talairach) space, corrected for inhomogeneities, and right and left gray and white matter cerebellar volumes were automatically parcellated using FreeSurfer (v5.0.0) software (http://surfer.nmr.mgh.harvard.edu; Fischl et al., 2002). Briefly, each image was rigid-body registered and non-linearly morphed to a probabilistic brain atlas, then each voxel was labeled as a distinct anatomical area based on Bayesian modeling derived from manually segmented images. This morphometric technique has shown reliability and validity against manual tracing (Fischl et al., 2002). Each individual’s cerebellar segmentation were visually-inspected and hand-edited if necessary by KL, who was blind to group status and demonstrated reliability in cerebellar tracing (Medina et al., 2010). Both right and left hemisphere gray and white matter volumes were measured (see Figure 1).
Figure 1.
Two-dimensional coronal slice and three-dimensional rendering of right cerebellar gray matter (purple) and white matter (pink) regions of interest.
Statistical Analyses
Statistical analyses were conducted in SPSS 18.0. ANOVAs and chi-square tests compared groups on demographic and drug use variables (see Table 1). Interpretations of statistical significance were made if p< .05. To examine whether binge drinking was related to cerebellar morphometry, a series of linear regressions were run. Peak drinks in the past three months was used to predict right and left gray and white matter cerebellar volumes after controlling for potentially confounding variables (ICV, gender, recent tobacco use, lifetime marijuana use, lifetime other drug use, depressive symptoms, conduct disorder diagnosis, and family history of substance use disorder; N=106). Next, gender-by-peak binge interactions were examined in regressions similar as the above.
Table 1.
Demographic, substance use, and cerebellar volume variables by group
| Binge Drinkers (n=46) % or M(SD) |
Controls (n=60) % or M(SD) |
|
|---|---|---|
| Age (range: 16–19 years) | 18.0 (0.8) | 17.7 (1.0) |
| Puberty Development Scale (females) | 19.0 (0.8) | 19.1 (1.0) |
| Puberty Development Scale (males) | 17.7 (1.7) | 17.0 (2.3) |
| Gender (% male) | 67% | 58% |
| Ethnicity (% Caucasian) | 67% | 73% |
| Parent socioeconomic status (Hollingshead) | 27.6 (15.2) | 26.1 (14.1) |
| % Completed 12th grade | 54% | 37% |
| % Familial history of substance use disorder * | 39% | 15% |
| % Conduct disorder (mild only) * | 11% | 0% |
| WRAT-3 Reading standard score | 109.2 (7.6) | 108.1 (8.8) |
| Body mass index | 23.1 (3.5) | 22.4 (4.1) |
| Beck Depression Inventory total * | 3.3 (3.9) | 2.0 (2.5) |
| Peak drinks, past 3 months * | 9.2 (3.7) | 0.3 (.7) |
| Peak BAC, past 3 months * | .24 (.09) | .01 (.02) |
| Total binge drinking days, past month * | 2.5 (5.4) | 0.0 (0) |
| % Lifetime history of alcohol use disorder | 8% | 0% |
| Days since last alcohol a * | 26.7 (28.6) | 211.7 (319.7) |
| Days since last binge drinking episode a * | 28.7 (18.9) | 167.9 (67.5) |
| Lifetime marijuana use days * | 7.6 (19.3) | 0.7 (1.8) |
| Days since last marijuana use a * | 161.6 (259.5) | 483.2 (436.4) |
| Lifetime other drug use episodes* | 2.5 (5.3) | 0.0 (0) |
| Past month cigarettes smoked * | 0.8 (2.2) | 0.0 (0) |
| Intracranial volume (cc3) | 1544.8 (142.7) | 1525.3 (140.4) |
| Right hemisphere cerebellar gray matter (cc3) | 54.8 (6.1) | 56.4 (6.6) |
| Right hemisphere cerebellar white matter (cc3) | 14.7 (2.0) | 14.9 (1.9) |
| Left hemisphere cerebellar gray matter (cc3) | 55.4 (5.5) | 55.7 (6.7) |
| Left hemisphere cerebellar white matter (cc3) | 14.8 (2.1) | 14.9 (1.8) |
Calculated only for those who used the substance within their lifetime.
p<.05.
Abbreviations: WRAT-3: Wide Range Achievement Test-3rd Edition; BAC: blood alcohol concentration.
3. RESULTS
3.1 Demographics
Groups did not significantly differ in age (range 16–19) [F(1,105)=2.46, p=.12], puberty stage [girls: F(1,37)=0.16, p=.69; boys: F(1,65)=1.84, p=.18], gender [x2(1)=.91, p=.34], parental socioeconomic status [F(1,105)=.21, p=.57], parental income [F(1,105)=0.25, p=.62], grades completed [x2(5)=10.1, p=.07], reading ability [F(1,105)=.41, p=.53], ethnicity [x2(7)=4.87, p=.56], Hispanic/Latino composition [x2(2)=3.7, p=.15], extent of family history of SUD (ranging 0 to 2.25) [x2(7)=6.03, p=.54], body mass index [F(1,105)=1.09, p=.30], or intracranial volume [F(1,105)=.81, p=.37]. The groups did differ in prevalence of mild conduct disorder [x2(1)=6.85, p=.009], with 5 bingers (3 males, 2 females) and no controls meeting diagnostic criteria. Of those, only 1 had elevated scores of externalizing symptoms on the Child Behavior Checklist (Achenbach, 1991). The binge group had a higher prevalence of family history of SUD (negative, mild, positive) [x2(2)=8.1, p=.02] and depressive symptoms, although only 4% of those in the binge group fell in the mildly depressed range [F(1,105)=4.76, p=.03]. All variables that differed by group were controlled for in subsequent multivariate analyses (see Table 1).
3.2 Substance use
All participants were abstinent from alcohol and any other drug for at least 3 days prior to scanning. As expected, bingers had fewer days of abstinence from any alcohol [F(1,72)=15.00, p=.001] and binge drinking [F(1,50)=134.03, p=.0001; range 108–301 days in the controls who drank in past year and 3–63 days in the bingers] than controls. Additionally, recent binge drinkers had significantly greater recent peak binge drinks [F(1,105)=325.45, p=.0001], greater maximum BAC in past 3 months [F(1,105)=355.03, p=.0001] and greater total heavy drinking days [F(1,105)=9.27, p=.003] than controls. Binge drinkers also reported greater past month tobacco use than controls [F(1,105)=8.61, p=.004], although all participants had smoked fewer than 10 cigarettes per month and no more than 1 cigarette per day. Binge drinkers had greater lifetime marijuana use [F(1,105)=7.60, p=.004] and fewer days of abstinence from marijuana [F(1,37)=8.16, p=.007] than controls. Recent bingers (8%) and controls (0%) marginally differed in their lifetime history of an alcohol use disorder [x2(2)=5.4, p=.07]. Other drug use was relatively low for binge drinkers, and the 10 bingers who had used other drugs were abstinent from them for an average of 186 days (SD=133, range 8–428 days); no control had used any other illicit drugs. Still, given these differences, past-month cigarette use, lifetime marijuana, and lifetime other drug use were controlled for in all multivariate analyses.
3.4 Cerebellar Volumes and Peak Binge Drinking
In order to describe the simple bivariate relationship between binge drinking and cerebellar volume, correlations were run between recent binge drinking and cerebellar volume in the 106 teens. It is also important to note that, as hypothesized, recent peak binge drinks was the most robust correlate (Spearman’s rank order) of total left hemisphere gray (rs(46)=−.28, p=.06) and white (rs(46)=−.36, p=.01) and right hemisphere gray (rs(46)=−.28, p=.008) and white (rs(46)=−.26, p=.02) cerebellar volumes in the binge drinkers. Correlations between age of regular alcohol use, age at first drink, hangover symptoms, and total number of drinking days were not significantly correlated with left or right cerebellar volumes in the binge drinkers. Therefore, recent binge drinking was the primary independent variable of interest.
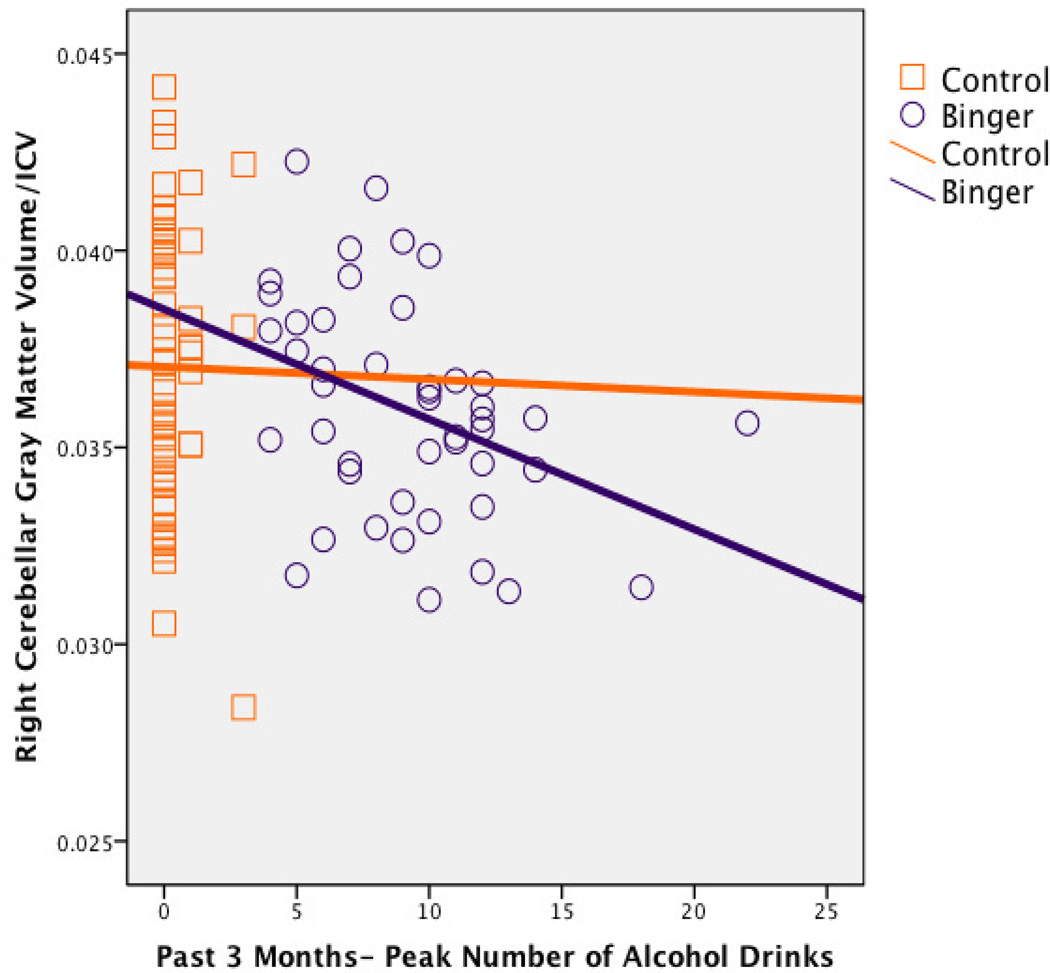
After controlling for ICV, gender, depressive symptoms, conduct disorder diagnosis, family history of substance use disorder, recent cigarette use, lifetime marijuana use, and lifetime other drug use, peak number of binge drinks in past three months was inversely associated with left [beta = −.20, p = .02, f2=.06] and right [beta = − .22, p = .006, f2=.08; see Figure 2] hemisphere gray matter, left hemisphere white matter [beta = −.20, p = .02, f2=.08], and marginally associated with right hemisphere white matter [beta = −.16, p = .09, f2=.05] cerebellar volumes in the full sample of 106 teens (see Figure 2 for simple bivariate relationships according to subgroups). Gender did not significantly interact with peak binge drinks in predicting cerebellar volumes (ps >.05).
Figure 2.
Bivariate scatterplot between peak number of alcohol drinks on an occasion in the past 3 months and right hemisphere cerebellar gray matter volume, in binge drinking (n=46; r=−.38, p=.01) and control (n=60; r=−.01, p=.96) teens.
3.5 Covariates
Males showed larger left [beta = .32, p = .001] and right [beta = .32 p = .001] cerebellar gray matter and marginally smaller right hemisphere white matter [beta = −.17, p = .10] volumes than females. As expected, greater ICV was associated with larger left [beta = .50, p = .0001] and right [beta = .49, p = .0001] cerebellar gray matter, and left [beta = .68, p = .0001] and right [beta = .63, p = .0001] cerebellar white matter volumes. Higher levels of past-month tobacco use were associated with larger left cerebellar white matter volumes [beta = .22, p = .04]. The other covariates included in the above regressions were not linked to any cerebellar compartment volume.
4. Discussion
Our first hypothesis was supported: higher peak drinks in the past three months predicted smaller cerebellar volumes, for left gray and white matter and right gray matter, and marginally for right white matter compartments in a sample of 106 teens with a range of binge drinking exposure. Differences in volume were seen after controlling for the effects of gender, intracranial volume, depressive symptoms, conduct disorder diagnosis, family history of substance use disorder, recent tobacco use, lifetime marijuana use, and lifetime other drug use. However, contrary to our second hypothesis, this relationship was of a similar magnitude for males as for females.
Alcohol exerts its effects on GABAergic neurons and glutamergic granule cells (e.g., Valenzuela, Lindquist & Zamudio-Bolcock, 2010) and may influence cerebellar structure by disrupting Purkinje neuron plasticity (Pierce et al., 2011) and upregulating inflammatory mediators (e.g., COX-2 and iNOS), resulting in neuronal death, atrophy or reduced synaptic refinement (Pascual, et al., 2007; Valles, et al., 2004). Binge drinking in adolescents may also reduce cholinergic and dopaminergic neurotransmitter gene signaling, resulting in smaller brain volumes and neuronal density (Coleman et al., 2011). Additionally, intense doses of alcohol may impact white matter functioning, as we found moderately reduced cerebellar white matter volumes in the current study and reduced white matter integrity in the superior cerebellar peduncle (McQueeny et al., 2009).
Few studies have examined the relationship between abstinence duration and brain structure in binge drinking teens. Our group recently reported that young adults who demonstrated greater durations of abstinence from alcohol and drugs at a 10-year follow-up had significantly improved executive functioning compared to recent users (Hanson, et al., 2011), providing some hope that sustained abstinence may reverse negative consequences of binge drinking in youth. Recovery in brain structure following binge drinking abstinence has been reported in binge-drinking rodents, who demonstrated relatively rapid recovery in ventricular volume and biomarkers of neuronal viability following 7 days of abstinence (Zahr et al., 2010). Future studies are needed to examine whether complete recovery of neurocognitive functioning occurs in adolescents with increasing lengths of abstinence.
It is important to consider that risk factors for binge drinking, such as family history of substance use disorders, conduct disorder, or subclinical symptoms of attention deficit hyperactivity or mood disorder, may lead to abnormal neurodevelopmental trajectories or abnormal cerebellar morphometry, traits that could predispose an adolescent to binge drinking (Hanson et al., 2010; Hill et al., 2007; Tapert et al., 2002). Of note, the current study statistically controlled for conduct disorder and family history of substance use disorder (neither of which were associated with cerebellar volume in this sample) and excluded individuals with premorbid psychiatric disorders such as attention deficit hyperactivity disorder. Perhaps most striking, Hill and colleagues (2007; 2011) found that family history of substance use disorder was associated with larger cerebellar volumes in youth, suggesting that our current dose-dependent findings associated with smaller cerebellar volumes may be more related to alcohol use. It is also notable that the cerebellum appears particularly vulnerable to environmental impact, as its additive genetic factor is only .49, compared to .77–.89 in cortical areas such as the prefrontal cortex (Wallace et al., 2006). Therefore, environmental factors such as substance exposure, rather than familial traits, may have a greater impact in this region. Additional studies examining the trajectory of cerebellar structure in family-history positive youth prior to and after the onset of binge drinking are necessary to confirm whether they initially demonstrate larger volumes followed by more drastic reductions in cerebellar volumes with the onset and continuation of hazardous binge drinking patterns.
In contrast to previous studies showing a higher degree of abnormality in female adolescent drinkers than male counterparts (Squeglia et al., 2009; in press), we saw no moderation effect and thus similar alcohol-cerebellum effects across the genders. A potential explanation is that gender moderates the effects of alcohol on the cerebellum in the earlier teen years, but the effects are not as evident into late adolescence and early adulthood (Tiemeier et al., 2010). Consistent with prior research (Tiemeier et al., 2010), boys (binge drinkers and controls) had larger gray but smaller white matter cerebellar volumes than girls of the same age. Although alcohol-cerebellum effects were similar for males and females, the female bingers had significantly fewer drinks per binge (females=6.73 drinks and males= 10.35 drinks on average; p=.001); therefore, it is possible that with continued drinking the female bingers will decline more rapidly than males despite less alcohol exposure. Longitudinal studies are needed to confirm this hypothesis.
Limitations of the current study include that, while we controlled for family history of substance use disorder, conduct disorder, and mood disorder, and excluded subjects with other Axis I comorbid psychiatric disorders, it is impossible to determine whether brain and cognitive abnormalities may have predated the onset of binge drinking with a cross-sectional design. Longitudinal research following teenagers from prior to the onset of alcohol use is needed to explore the influence of early binge drinking and abstinence. In addition, adolescents with recent histories of binge drinking had more tobacco, cannabis, and other drug use than controls; although we controlled statistically for these variables, it is possible that synergistic effects may still exist. Future studies examining the unique and interactive impact of these drugs on cerebellar morphometry are needed. Finally, although relatively unstudied in youth, another possible confound in this sample is thiamine deficiency associated with alcohol consumption and cerebellar structure (e.g., Baker et al., 1999; Martin et al., 2003; Pitel et al., 2011).
Given the prevalence of binge drinking and mounting suggestion of its negative impact on brain health, it is critical to disseminate these findings to high school and college students, young military enlistees, therapists, teachers, pediatricians, and parents to help minimize heavy alcohol use in teens. Providing personalized feedback about the negative effects of binge drinking to youth has shown to be effective at reducing harmful drinking patterns (Larimer & Cronce, 2007). For example, adolescents who engage in heavy drinking could be told that, “Teens who drank more than 9 alcohol drinks in one occasion had 1.8 cubic centimeters less cerebellar brain volume than teens who drank 3 or fewer drinks when drinking, on average. The cerebellum is important for coordination and thinking skills.” It could also be imparted that this deficit is less prominent in teens who have not engaged in binge drinking in the past 2 or so months. Therapists could also utilize this information during brief motivational interviewing sessions to help reduce negative effects of binge drinking on the brain.
In summary, we found that increased peak binge drinks during the past three months significantly predicted smaller bilateral cerebellar gray matter and left hemisphere cerebellar white matter volumes, and marginally predicted smaller right hemisphere white matter cerebellar volume in a sample of 106 normally developing 16 to 19-year olds. Given the high rates of binge drinking use in teens and emerging adults, these findings highlight an important public health concern. Normative feedback regarding effects of binge drinking on brain health need to be integrated into current prevention, harm-reduction, and treatment programs. More globally, interventions geared towards lowering damaging patterns of binge alcohol use in teens and young adults that have shown evidence of efficacy need to be implemented more aggressively to prevent long-term neuronal damage and ensure optimal brain health and cognitive functioning in youth.
ACKNOWLEDGEMENTS
This manuscript and much of the research presented within it was made possible through funding by the National Institute on Alcohol Abuse and Alcoholism grant R01 AA13419 and National Institute on Drug Abuse grant R01 DA021182 (PI: Tapert). During manuscript preparation, Dr. Lisdahl was supported by NIDA-funded grants R03DA027457 and R01DA030354. We would like to thank Anthony Scarlett, Diane Goldenberg, and Alejandra Infante for their assistance in establishing intracranial volumes in this sample. Finally, the authors would like to acknowledge the vast scientific contributions of our collaborators and graduate and undergraduate research assistants in the UWM Brain Imaging and Neuropsychology (BraIN) Laboratory and UCSD Adolescent Brain Imaging Project (ABIP), our research participants, and participating schools.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Achenbach TM. Manual for the Child Behavior Checklist/4-18 and 1991 Profile. Burlington, VT: University of Vermont, Department of Psychiatry; 1991. [Google Scholar]
- Baker KG, Harding AJ, Halliday GM, Kril JJ, Harper CG. Neuronal loss infunctional zones of the cerebellum of chronic alcoholics with and without Wernicke's encephalopathy. Neuroscience. 1999;91(2):429–438. doi: 10.1016/s0306-4522(98)90664-9. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical Psychology Review. 1988;8(1):77–100. [Google Scholar]
- Castellanos FX, Lee PP, Sharp W, Jeffries NO, Greenstein DK, Clasen LS, Blumenthal JD, James RS, Ebens CL, Walter JM, Zijdenbos A, Evans AC, Giedd JN, Rapoport JL. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA. 2002;288(14):1740–1748. doi: 10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
- Coleman LG, Jr, He J, Lee J, Styner M, Crews FT. Adolescent binge drinking alters adult brain neurotransmitter gene expression, behavior, brain regional volumes, and neurochemistry in mice. Alcohol Clin Exp Res. 2011;35(4):671–688. doi: 10.1111/j.1530-0277.2010.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis MD, Clark DB, Beers SR, Soloff PH, Boring AM, Hall J, Kersh A, Keshavan MS. Hippocampal volume in adolescent-onset alcohol use disorders. American Journal of Psychiatry. 2000;157(5):737–744. doi: 10.1176/appi.ajp.157.5.737. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Narasimhan A, Thatcher DL, Keshavan MS, Soloff P, Clark DB. Prefrontal cortex, thalamus and cerebellar volumes in adolescents and young adults with adolescent onset alcohol use disorders and co-morbid mental disorders. Alcoholism: Clinical and Experimental Research. 2005;29(9):1590–1600. doi: 10.1097/01.alc.0000179368.87886.76. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Hanson KL, Medina KL, Nagel BJ, Spadoni AD, Gorlick A, Tapert SF. Hippocampal volumes in adolescents with and without a family history of alcoholism. The American Journal of Drug and Alcohol Abuse. 2010;36:161–167. doi: 10.3109/00952991003736397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson KL, Medina KL, Padula CB, Tapert SF, Brown SA. How does adolescent alcohol and drug use affect neuropsychological functioning in young adulthood?: 10-year outcomes. Journal of Child & Adolescent Substance Abuse. 2011;20:135–154. doi: 10.1080/1067828X.2011.555272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Muddasani S, Prasad K, Nutche J, Steinhauer SR, Scanlon J, McDermott M, Keshavan M. Cerebellar volume in offspring from multiplex alcohol dependence families. Biol Psychiatry. 2007;61(1):41–47. doi: 10.1016/j.biopsych.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Wang S, Carter H, Tessner K, Holmes B, McDermott M, Zezza N, Stiffler S. Cerebellum volume in high-risk offspring from multiplex alcohol dependence families: association with allelic variation in GABRA2 and BDNF. Psychiatry Res. 2011;30; 194(3):304–313. doi: 10.1016/j.pscychresns.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan T, Gamst A. Changes in volume with age: Consistency and interpretation of observed effects. Neurobiology of Aging. 2005;26(9):1271–1274. doi: 10.1016/j.neurobiolaging.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national results on adolescent drug use: Overview of key findings, 2010 (NIH Publication No. 10-7583) Bethesda, MD: National Institute on Drug Abuse; 2011. [Google Scholar]
- Larimer ME, Cronce JM. Identification, prevention, and treatment revisited: individual-focused college drinking prevention strategies 1999–2006. Addictive Behaviors. 2007;32(11):2439–2468. doi: 10.1016/j.addbeh.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: Insights from anatomical magnetic resonance imaging. Neuroscience and Biobehavioral Reviews. 2006;30:718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Sex differences in the adolescent brain. Brain and Cognition. 2010;72(1):46–55. doi: 10.1016/j.bandc.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas CP, Zhang H, Fisher PW, Shaffer D, Regier DA, Narrow WE, Bourdon K, Dulcan MK, Canino G, Rubio-Stipec M, Lahey BB, Friman P. The DISC Predictive Scales (DPS): efficiently screening for diagnoses. J Am Acad Child Adolesc Psychiatry. 2001;40(4):443–449. doi: 10.1097/00004583-200104000-00013. [DOI] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, Keshavan MS, Genovese CR, Eddy WF, Sweeney JA. Maturation of widely distributed brain function subserves cognitive development. Neuroimage. 2001;13(5):786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- Martin PR, Singleton CK, Hiller-Sturmhöfel S. The role of thiamine deficiency in alcoholic brain disease. Alcohol Res Health. 2003;27(2):134–142. [PMC free article] [PubMed] [Google Scholar]
- McQueeny T, Schweinsburg BC, Schweinsburg AD, Jacobus J, Bava S, Frank LR, Tapert SF. Altered white matter integrity in adolescent binge drinkers. Alcoholism: Clinical and Experimental Research. 2009;33(7):1278–1285. doi: 10.1111/j.1530-0277.2009.00953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, McQueeny T, Nagel BJ, Hanson K, Schweinsburg AD, Tapert SF. Prefrontal cortex volumes in adolescents with alcohol use disorders: Unique gender effects. Alcoholism: Clinical and Experimental Research. 2008;32:386–394. doi: 10.1111/j.1530-0277.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Nagel BJ, Tapert SF. Cerebellar vermis abnormality in adolescent marijuana users. Psychiatry Research: Neuroimaging. 2010;182(2):152–159. doi: 10.1016/j.pscychresns.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Effects of alcohol and combined marijuana and alcohol use during adolescence on hippocampal asymmetry. Neurotoxicology and Teratology. 2007;29:141–152. doi: 10.1016/j.ntt.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel BJ, Schweinsburg AD, Phan V, Tapert SF. Reduced hippocampal volume among adolescents with alcohol use disorders without psychiatric comorbidity. Psychiatry Research. 2005;139(3):181–190. doi: 10.1016/j.pscychresns.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Blanco AM, Cauli O, Minarro J, Guerri C. Intermittent ethanol exposure induces inflammatory brain damage and causes long-term behavioural alterations in adolescent rats. Eur J Neurosci. 2007;25(2):541–550. doi: 10.1111/j.1460-9568.2006.05298.x. [DOI] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Pierce DR, Hayar A, Williams DK, Light KE. Olivary climbing fiber alterations in PN40 rat cerebellum following postnatal ethanol exposure. Brain Res. 2011;1378:54–65. doi: 10.1016/j.brainres.2011.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitel AL, Zahr NM, Jackson K, Sassoon SA, Rosenbloom MJ, Pfefferbaum A, Sullivan EV. Signs of preclinical Wernicke's encephalopathy and thiamine levels as predictors of neuropsychological deficits in alcoholism without Korsakoff's syndrome. Neuropsychopharmacology. 2011 Feb;36(3):580–588. doi: 10.1038/npp.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Hesselbrock VM, Nurnberger JI, Jr, Schuckit MA, Begleiter H. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcoholism: Clinical and Experimental Research. 1995;19:1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Robins L, Cottler L, Bucholz K, Compton W. The Diagnostic Interview Schedule, Version 4.0. (DIS 4.0) 1996 [Google Scholar]
- Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. 1998 doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring alcohol consumption: Psychosocial and biochemical methods. Totowa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- Spear LP. The Behavioral Neuroscience of Adolescence. Neuroscience and Biobehavioral Reviews. 2010;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Squeglia LM, Dager Schweinsburg A, Pulido C, Tapert SF . Adolescent binge drinking linked to abnormal spatial working memory brain activation: differential gender effects. Alcoholism: Clinical & Experimental Research. doi: 10.1111/j.1530-0277.2011.01527.x. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Spadoni AD, Infante MA, Myers MG, Tapert SF. Initiating moderate to heavy alcohol use predicts changes in neuropsychological functioning for adolescent girls and boys. Psychology of Addictive Behaviors. 2009;23(4):715–722. doi: 10.1037/a0016516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart DG, Brown SA. Withdrawal and dependency symptoms among adolescent alcohol and drug abusers. Addiction. 1995;90:627–635. doi: 10.1046/j.1360-0443.1995.9056274.x. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Neurocircuitry in alcoholism: A substrate of disruption and repair. Psychopharmacology. 2005;180:583–594. doi: 10.1007/s00213-005-2267-6. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Baratta MV, Abrantes AM, Brown SA. Attention dysfunction predicts substance involvement in community youths. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41(6):680–686. doi: 10.1097/00004583-200206000-00007. [DOI] [PubMed] [Google Scholar]
- Tiemeier H, Lenroot RK, Greenstein DK, Tran L, Pierson R, Giedd JN. Cerebellum development during childhood and adolescence: a longitudinal morphometric MRI study. Neuroimage. 2010 Jan 1;49(1):63–70. doi: 10.1016/j.neuroimage.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela CF, Lindquist B, Zamudio-Bulcock PA. A review of synaptic plasticity at Purkinje neurons with a focus on ethanol-induced cerebellar dysfunction. Int Rev Neurobiol. 2010;91:339–372. doi: 10.1016/S0074-7742(10)91011-8. [DOI] [PubMed] [Google Scholar]
- Valles SL, Blanco AM, Pascual M, Guerri C. Chronic ethanol treatment enhances inflammatory mediators and cell death in the brain and in astrocytes. Brain Pathol. 2004;14(4):365–371. doi: 10.1111/j.1750-3639.2004.tb00079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace GL, Eric Schmitt J, Lenroot R, Viding E, Ordaz S, Rosenthal MA, Molloy EA, Clasen LS, Kendler KS, Neale MC, Giedd JN. A pediatric twin study of brain morphometry. J Child Psychol Psychiatry. 2006;47(10):987–993. doi: 10.1111/j.1469-7610.2006.01676.x. [DOI] [PubMed] [Google Scholar]
- Wilkinson GS. The Wide Range Achievement Test-3 Administration Manual. Wilmington, DE: Jastak Associates; 1993. [Google Scholar]
- Zahr NM, Mayer D, Rohlfing T, Hasak MP, Hsu O, Vinco S, Orduna J, Luong R, Sullivan EV, Pfefferbaum A. Brain injury and recovery following binge ethanol: evidence from in vivo magnetic resonance spectroscopy. Biol Psychiatry. 2010;67(9):846–854. doi: 10.1016/j.biopsych.2009.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RA, Ellis DA, Fitzgerald HE. Developmental evidence for at least two alcoholisms: I. Biopsychosocial variation among pathways into symptomatic difficulty. In: Babor TF, Hesselbrock V, Meyer RE, Shoemaker W, editors. Types of Alcoholics: Evidence from Clinical, Experimental, and Genetic Research. New York: The New York Academy of Sciences; 1994. pp. 134–146. [DOI] [PubMed] [Google Scholar]