Abstract
The PTPN22 1858T genetic variant (and its gene product Lyp620W) is associated with multiple autoimmune disorders characterized by autoantibody production. Because B cell receptor (BCR) signal strength is tightly coupled to central and peripheral tolerance, and Lyp620W results in blunted B cell receptorsignaling in memory B cells, we tested whether Lyp620W may directly promote a loss of B cell tolerance. We found that healthy individuals heterozygous for the PTPN22 1858T variant display significant alterations in the peripheral B cell pool, including an expansion of both the transitional and anergic IgD+IgMnegCD27neg B cell subsets. Further, the PTPN22 1858T variant was associated with significantly diminished BCR signaling and a resistance to apoptosis in transitional and naïve B cells. Strikingly, parallel changes in BCR signaling and composition of the peripheral B cell compartment were observed in type I diabetic (T1D) subjects, irrespective of PTPN22 genotype; revealing a novel immune phenotype and likely shared mechanisms leading to a loss of B cell tolerance. Our combined findings suggest that Lyp620W-mediated alterations in BCR signaling contribute to a breakdown of peripheral tolerance and implicate parallel, proximal signaling deficits in altered B cell tolerance in T1D.
Introduction
Human autoimmune diseases are genetically complex, arising from the combined impact of environmental factors and multiple polymorphic alleles with varying disease risk (Concannon et al., 2009). Among the genetic variants associated with autoimmunity, the PTPN22 1858C/T coding variant is associated with multiple autoimmune diseases including rheumatoid arthritis (RA), systemic lupus erythmatosus (SLE), T1D, Graves disease, and myasthenia gravis (Stanford et al., 2010; Gregersen and Olsson, 2009). PTPN22 encodes the lymphoid tyrosine phosphatase, Lyp, expressed in multiple hematopoietic cell types. The disease-associated SNP is a missense mutation at position 1858 (C→T; Arg620→Trp), which results in a gain-of-function manifest by blunting of T and BCR signaling (Stanford et al., 2010; Vang et al., 2005). In previous studies, we demonstrated that healthy individuals who carry the PTPN22 1858T allele exhibit alterations in BCR signal transduction characterized by diminished phosphorylation of proximal signaling effectors, impaired BCR-driven proliferation, and a decrease in the size of the memory B cell compartment (Arechiga et al., 2009; Rieck et al., 2007).
Multiple tolerance checkpoints censor autoreactive B cells during maturation in the bone marrow and the periphery (Chung et al., 2003). Each of these checkpoints relies, in part, on antigen-receptor controlled mechanisms for counterselection of autoreactive cells and include clonal deletion, receptor editing, or anergy (Nemazee, 2006; Tussiwand et al., 2009; von Boehmer, and Melchers, 2010). The BCR signaling threshold is pivotal to the ultimate fate of autoreactive B cells and aberrations in proximal BCR signaling during B cell development have the potential to result in a loss of tolerance, and increased survival of autoreactive cells (King and Monroe, 2000; Niiro and Clark, 2002; Norvell et al., 1995; Su et al., 2004). In individuals with SLE and RA, the development of autoantibodies and perturbations in the B cell compartment parallel an increased frequency of polyreactive new emigrant/transitional B cells and the accumulation of self-reactive cells in the mature naïve compartment, implying defects in central and peripheral tolerance in these diseases (Dorner et al., 2010; Jacobi et al., 2009; Samuels et al., 2005; Yurasov et al., 2005). However, the mechanisms that permit escape of autoreactive B cells during human B cell development and the role for such changes in human autoimmunity remain unclear.
In this study, we sought to address the role of Lyp620W in B cell tolerance with respect to the composition of the transitional and naïve B cell compartments and its direct impact on BCR signaling and B cell survival. We also extended our studies to T1D, a human autoimmune disease strongly associated with the PTPN22 1858T variant. We demonstrate that Lyp620W is associated with signaling defects in both transitional and naïve B cells in healthy subjects, and an increased resistance to BCR-driven apoptosis in these cell populations. In addition, PTPN22 1858C/T subjects displayed alterations in the composition of the developing B cell pool including an increasedfrequency of both transitional cells and, IgD +IgMnegCD27neg (BND) anergic B cells, a recently defined peripheral reservoir of autoreactive cells (Duty et al., 2009). Further, we observed nearly identical alterations in T1D subjects. Our combined findings suggest a mechanism by which Lyp620W contributes to a loss of B cell tolerance and implicate a range of as yet uncharacterized, analogous B cell signaling deficits in T1D.
Results and Discussion
Altered B cell compartment in healthy subjects heterozygous for PTPN22 1858T
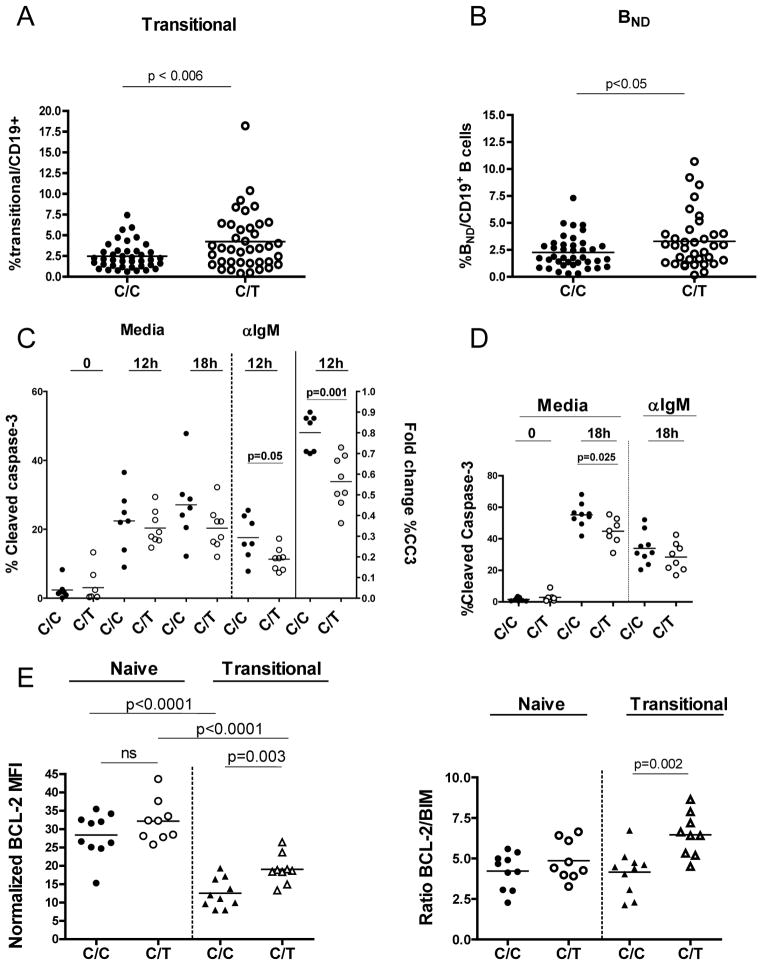
In order to address whether the PTPN22 variant directly impacts B cell development and early B cell tolerance checkpoints, we performed detailed flow cytometric analyses of the B cell compartment in healthy subjects based on PTPN22 genotype. While the frequency and absolute number of peripheral CD19+B cells did not differ between the two groups (data not shown), the percentage of transitional B cells, defined as CD19+ CD27negCD10+CD24hiCD38hi cells (Sims et al., 2005; Suryani et al., 2010), was significantly increased in individuals heterozygous for the PTPN22 1858T, as compared to age-matched C/C healthy adult control subjects (p<0.006) (Figure 1A). We also determined the frequency of anergic B cells defined as CD19+CD27negIgD+ IgMneg (BND) B cells. Prior characterization of BND B cells has revealed anti-DNA and anti-HEp-2 reactivity, without evidence for somatic mutation or class switch recombination, consistent with derivation from naïve, naturally autoreactive cells (Duty et al., 2009). The BND B cell population was increased among individuals heterozygous for the PTPN22 1858T variant (p<0.05) (Figure 1B). Thus, Lyp620W expression is associated with altered B cell homeostasis leading to an expansion of the transitional and anergic B cells; findings that suggest that larger numbers of autoreactive B cells enter and/or survive within the peripheral Bcell compartment in individuals who carry the risk variant.
Figure 1. Altered B cell homeostasis in healthy PTPN22 1858C/T subjects is associated with reduced apoptosis of naïve and transitional B cells.
Previously frozen PBMC from age-matched control 1858C/C and C/T subjects (ages 19–46 years) were stained with antibodies to CD19, CD27, CD10, CD24, CD38, IgM and IgD and analyzed by FACS for the relative frequencies of transitional, naïve, memory and anergic B ND B cells. (A) Transitional B cells (CD19+CD27negCD10+CD24hiCD38hi) are increased in individuals who carry the PTPN22T risk allele (p<0.006; n=40 1858C/C subjects, n=39 1858C/T subjects). (B) Increased frequency of BND B cells (CD19+CD27negIgD+IgMneg) in PTPN22 1858T individuals (p<0.05; n=40 1858C/C subjects, mean 2.26% ±0.24 SEM; n=36 1858C/T subjects, mean 3.3% ±0.42 SEM). BND B cells are also CD10loCD24loCD38int. Bars represent the mean frequency relative to total CD19+ B cells and each symbol represents an individual. (C) Naïve B cells from healthy 1858C/C (n=7) and C/T (n=8) subjects were sorted from fresh PBMC and cultured in the presence or absence of anti-IgM F(ab′)2 for 0, 12, and 18 h. The percentage of active caspase -3+ cells (left axis) is indicated for each condition (media vs. anti-IgM). Right axis values depict fold change in the percentage of caspase3+ naïve B cells (anti-IgM-treated/media) after 12 h. (D) Transitional B cells from healthy 1858C/C (n=9) and C/T (n=7, media; n=8, anti -IgM-treated) were sorted from fresh PBMC and cultured as in (B) for 0 and 18 h and apoptosis measured as in (B). (E) Previously frozen PBMCs were stained with anti-CD20, CD27, -CD38, and –CD24 mAbs to identify transitional, naïve, and memory B cell subsets. Fixed and permeabilized cells were stained with anti-BCL-2, anti-Bim, or the corresponding isotype control antibodies and analyzed by flow cytometry. Left panel, BCL-2 expression in gated naïve and transitional B cells (n=10 C/C, n=9 C/T); Right panel, ratio BCL-2/Bim expression for subjects depicted in top panel. Each symbol represents a unique individual and bars represent the mean.
PTPN22 1858C/T mature naïve B cells exhibit intrinsic signaling defects and reduced BCR-triggered apoptotic signaling
In previous studies, we demonstrated that LypR620W expression results in altered B cell signal transduction in mature B cells following anti-IgM/IgG crosslinking (Arechiga et al., 2009. We hypothesized that if diminished BCR signaling extends to the immature and naïve B cell compartments, then censoring of autoreactive B cells might be inefficient in individuals who carry the PTPN22 1858T variant. We first tested whether Lyp620W altered the BCR signaling threshold in naïve mature B cells following crosslinking with anti-κ F(ab′)2antibodies, in an effort to simulate low affinity self-antigen engagement. We observed a significant decrease in phospho-PLCγ2 (P-PLCγ2) in naïve CD20+CD27neg B cells upo n activation withanti -κ (Supplemental Figure 1A). Treatment of 1858C/T B cells with the Lyp-specific inhibitor I-C11 (Arechiga et al., 2009; Yu et al., 2007) prior to BCR stimulation lead to a rescue in anti-κ mediated P-PLCγ2 (p = 0.01) compared with paired vehicle-treated B cells (Supplemental Figure 1B), resulting in a mean fold change in P-PLCγ2 equivalent to that of I-C11-treated 1858C/C control B cells. Of note, the more modest blunting of P-PLCγ2 in naïveB cells following stimulation with anti-IgM/IgG (Arechiga et al., 2009) compared with anti-κ cross linking suggests that this latter approach may more effectively detect intrinsic alterations in the BCR signaling threshold. Together, these data identify a clear association between the variant and altered BCR signaling in naïve B cells.
The attenuated BCR signal in Lyp620W-expressing naïve B cells suggested that apoptotic pathways coupled to the Ag receptor might be altered. Because the pro-apoptotic signal is dependent upon caspase-3 activation (Berard et al., 1999). we directly measured caspase-3 activity in response to BCR crosslinking in PTPN22 1858C/T vs. C/C subjects. Sorted, purified naïve B cells (Supplemental Figure 2A) were cultured in the presence or absence of anti-IgM, and the frequency of B cells positive for active caspase-3 quantified by intracellular flow cytometry. In the absence of BCR engagement, we observed a trend toward fewer active caspase-3+ cells in 1858C/T as compared to 1858C/C subjects (Figure 1C, left panel). Most notably, the percentage of active caspase-3+ cells was significantly reduced following anti-IgM crosslinking in 1858C/T cells (12h, Figure 1C, middle panel). Similarly, the mean fold change in active caspase-3 was also reduced in unstimulated vs. stimulated 1858C/T cells (Figure 1C, right panel). Of note, in previous studies we were unable to identify alterations in anti-apoptotic signaling in memory and naïve 1858C/T B cells (Arechiga et al., 2009); a difference that likely reflects our use of less robust BCR signal and shorter assay conditions in the current study. Thus, our phenotypic and functional data suggest that alterations in proximal BCR signaling may lead to increased survival of 1858C/T naïve B cells, thereby modulating peripheral B cell selection.
PTPN22 1858C/T transitional B cells exhibit reduced basal apoptotic signaling and increased BCL2 expression
Because maturation of transitional cells is influenced by BCR signal strength, we also determined whether the Lyp variant impacted transitional B cell signaling and survival. Biochemical studies using primary immature human B cells pose a technical challenge due to their low frequency in the peripheral circulation. Therefore, we carried out initial signaling studies using a human immature B cell line. Ramos B cells were infected with lentiviral (LV) constructs containing either wild-type (WT) or mutant phosphatase in association with a cis-linked, internal ribosomal entry site (IRES)-mCherry marker gene (Supplemental Figure 1C). Anti-IgM triggered calcium (Ca2+) flux was assessed in cells expressing either HA-tagged WT or R619W mutant Pep, the murine homolog of Lyp (Supplemental Figure 1D). Pep was utilized in these experiments to limit potential confounding events due to interaction of the overexpressed protein with endogenous Lyp. Enforced expression of WT Pep did not alter peak Ca2+flux as compared with gated cherry-negative Ramos cells (SupplementalFigure 1D, upper left panel). In contrast, Ca2+ flux was significantly impaired in B cells expressing PEP-R619W as shown by comparison of the response in cherry+(red trace) vs. cherry -negative cells (blue trace; Supplemental Figure 1D, lower left). Control responses to ionomycin were equivalent in all populations (Supplemental Figure 1D, right panels). These data recapitulate the impaired Ca2+ signal previously observed in Lyp620W-expressing B cells (Rieck et al., 2007)and T cell lines (Vang et al., 2005); and support the idea that the increase in transitional B cells in subjects heterozygous for PTPN22 1858T is mechanistically linked to an altered BCR signaling threshold.
To determine whether transitional B cells that carry the PTPN22 variant allele may exhibit a selective advantage, we next evaluated pro-apoptotic signaling in this population. As previously described (Suryani et al., 2010), transitional B cells exhibited a significant increase in basal apoptosis compared with naïve mature B cells (Figure 1C and 1D, media). The relative frequency of active caspase-3 in transitional cells from 1858C/T individuals, however, was significantly less compared to C/C controls at 18 hours in mediaalone; and this population also exhibited a trend toward reduced caspase activity following anti-IgM stimulation (Figure 1D). The enhancement in survival in the absence of in vitro activation suggests that Lyp620W may impact basal signals mediated by the BCR and/or other receptors, contributing to expansion of this population.
To further assess this basal signaling difference, we next examined the expression of key pro-and anti -apoptotic BCL family proteins (Bim, BCL-2) in naïve mature and transitional B cells from 1858C/C and C/T healthy control individuals by intracellular flow cytometry. As previously reported (Suryani et al., 2010), significantly higher Bcl-2 protein levels were present in naïve vs. transitional B cells (p<0.0001; Figure 1E). However, when comparing expression in each subset by PTPN22 genotype, basal levels of BCL-2 were significantly higher in 1858C/T vs. C/C transitional B cells (p=0.003), whereas BCL-2 levels did not differ significantly between 1858C/T and C/C naïve B cells (Figure 1E, left). Basal expression of pro-apoptotic Bim protein was detected at similar levels in naïve and transitional B cells from both non-carriers and carriers of PTPN22 C/T (data not shown); consistent with a more favorable ratio of anti-to pro -apoptotic proteins in transitional B cells from 1858C/T subjects vs. 1858C/C subjects (p=0.002; Figure 1E, right).
Collectively, these data demonstrate increased survival of Lyp620W-expressing transitional and mature B cells, and implicate blunted BCR signaling as one mechanism by which the variant allele may contribute to inefficient negative selection. Importantly, the altered balance of BCL family proteins of Lyp620W-expressing transitional B cells is likely significant, as apoptosis (via decreased BCL2 expression and increased caspase 3 activity) is the major tolerogenic program in this cell population (King and Monroe, 2000; Norvell et al., 1995). Thus, diminished signaling mediated via Lyp620W may provide a mechanism by which self-reactive transitional B cells exhibit a selective advantage.
PTPN22 1858T B cells exhibit no evidence for altered receptor light-chain editing
The altered naïve B cell compartment and reduced BCR signaling observed in PTPN22 1858C/T subjects suggested that Lyp620W might impact central tolerance mechanisms operative within the bone marrow compartment; and bypass, or reduce, editing at light chain loci important for expression of an innocuous, non-self-reactive receptor (Nemazee, 2006). To evaluate this idea, we utilized a quantitative PCR-based assay for Recombining Sequence-Kappa Deleting Element (iRS-KDE), a marker for receptor editing (Panigrahi et al., 2008). The iRS-KDE assay measures the overall level of kappa light chain gene rearrangement in a Bcell population. The level of light chain gene rearrangement is correlated with receptor editing (Panigrahi et al., 2008). We found no significant difference in the frequency of iRS-KDE rearrangements per genome copy in highly purified, Igκ+ or Igλ+ immature/transitional and mature B cells isolated from healthy control 1858C/C vs. C/T subjects (Supplemental Figure 3). These data suggest that light chain receptor editing is largely intact in PTPN22 1858C/T subjects.
Alterations in the B cell compartment in T1D subjects phenocopy PTPN22 1858T controls
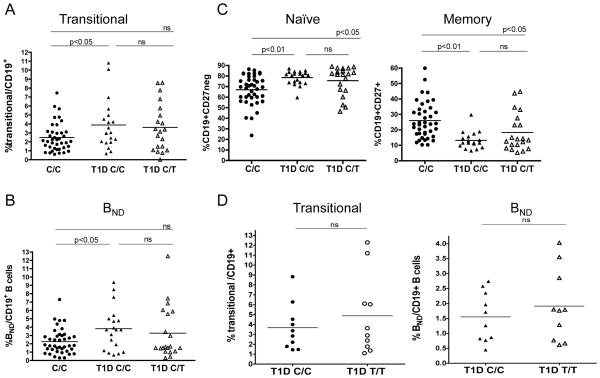
Previous work has implicated B cells in initiation of T1D and as precursors for relevant autoantibody-producing cells (Bouaziz et al., 2007; Miao et al., 2007; Silveira and Grey, 2006; Skyler, 2007; Xiu et al., 2008), However, little is known regarding defects in peripheral B cell tolerance that may favor development of T1D. Our observations with respect to the B cell compartment in control subjects who carry PTPN22 1858T led us to hypothesize that analogous alterations might be present in individuals with T1D. Thus, we examined the B cell profiles of T1D subjects and assessed the frequency of transitional, naïve, anergic and memory B cells. While we found no difference in total CD19+ B cell numbers between T1D subjects and healthy controls (data not shown and (Panigrahi et al., 2008)), the frequency of both CD19+CD10+CD27negCD24hi CD38hi transitional and CD19+CD27negIgD+ IgMneg BND B cells were significantly increased in T1D subjects compared to 1858C/C healthy controls (Figures 2A and 2B). These differences were most significant when comparing control PTPN22 1858C/C and T1D C/C subjects. In contrast, comparing paired T1D subjects by PTPN22 genotype, we found that heterozygosity of the 1858T risk allele is not associated with any further expansion of immature and BND anergic B cells (Figures 2A and 2B), implying that Lyp620Wis no t the sole factor leading to this phenotype in T1D. Similarly, the naïve and memory B cell profiles of T1D subjects paralleled 1858C/T healthy controls, as we observed a significant expansion in the naïve B cell subset and a reduction in the memory B cell pool in T1D subjects, again irrespective of PTPN22 genotype (Figure 2C). However, upon examination of a separate small cohort of age-matched T1D 1858C/C and T1D 1858T/T subjects, we found a modest trend toward increased transitional and BND B cells in hom ozygous carriers of 1858T, suggesting that Lyp620W contributes to this phenotype in T1D subjects who carry the variant (Figure 2D). We also measured serum BAFF levels in all subjects and found no correlation between BAFF levels and disease or PTPN22 1858 genotype that would explain the expansion of the transitional and BND B cells in either healthy controls or T1D subjects (Supplemental Figure 2B).
Figure 2. The composition of the B cell compartment in T1D subjects parallels healthy PTPN22 1858T controls.
Previously frozen PBMC from control 1858C/C subjects (ages 19–46 years), T1D 1858C/C subjects, and T1D 1858C/T subjects (ages 19–36 years) were stained and analyzed as in Figure 1. (A) Transitional B cells are increased in T1D individuals irrespective of genotype (n=40 control 1858C/C; n=20 T1D 1858C/C and n=20 T1D 1858C/T subjects). (B) Increased frequency of BND B cells in T1D individuals irrespective of genotype (n=40 control 1858C/C, n=19 T1D 1858C/C, and n=20 T1D 1858C/T subjects). (C) Significantly increased naïve CD19+CD27neg B cells and significantly reduced memory CD19+CD27+ B cells in heterozygous PTPN22 1858T subjects (left panel, naïve B cells: n=40 control 1858C/C, n=20 T1D 1858C/C, and n=20 T1D 1858C/T subjects; right panel, memory B cells: n=40 control 1858C/C, n=18 T1D 1858C/C, and n=20 T1D 1858C/T subjects). (D) B cells from T1D 1858T/T subjects display homeostatic alterations. Previously frozen PBMC from 10 age -and gender -matched T1D 1858C/C and T1D 1858T/T subjects (ages 27–57 years) were stained and analyzed as in Figure 1 for the relative frequencies of transitional and BND B cells. Bars represent the mean frequency relative to total CD19+ B cells.
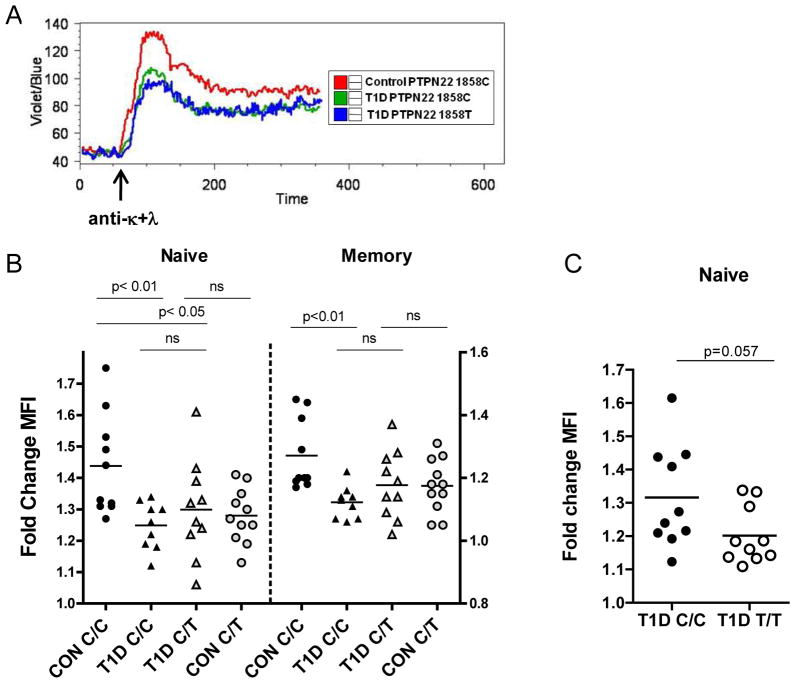
Based on our observation that blunted BCR signaling is correlated with expansion of the transitional and BND B cell subsets in control 1858C/T subjects, we investigated proximal BCR signaling in T1D subjects using both Ca2+ flux and intracellular staining. We found impaired peak Ca2+ mobilization in naïve B cells from T1D subjects following BCR crosslinking with anti-κ+λ (Figure 3A). These data correlated with a significant decrease in P-PLCγ2 levels in both naive and memory subsets from T1D PTPN22 1858C/C individuals, and in the naïve population of T1D PTPN22 1858C/T B cells (Figure 3B). The impairment in activation of T1D B cells mirrored that seen in control PTPN22 1858C/T subjects with the corresponding stimulation. Moreover, our analysis of a separate cohort of age-matched T1D 1858C/C and T1D 1858T/T subjects revealed further blunting of BCR signaling in naive B cells from T1D 1858T/T individuals (Figure 3C). These combined data reveal a striking similarity between T1D and the homeostatic alterations in the B cell compartment in control PTPN22 1858C/T subjects. Most notably, they suggest that blunted proximal BCR signaling and an altered transitional/anergic B cell compartment represent a common feature of T1D subjects, irrespective of the presence of Lyp620W. Importantly, Lyp620W can further alter the BCR signaling threshold and potentially impact the development of autoreactive B cells. Thus, in T1D subjects, both Lyp620W-dependent and -independent alterations in BCR signaling may enable autoreactive B cells to escape one or more key tolerance checkpoints; leading, ultimately, to an expanded pool of transitional, anergic and naïve B cells.
Figure 3. B cells from T1D subjects display BCR signaling defects that parallel PTPN221858T healthy controls.
(A). Impaired BCR -mediated Ca2+ flux in B cells from T1D individuals. Freshly isolated PBMC from healthy control and T1D subjects with the indicated 1858 genotype were loaded with indo-1 dye and stained with anti-CD19 and anti-CD27antibodies. Shown is a representative kinetics profile (of five separate experiments) depicting the mean indo-1 ratio (violet/blue) as a function of time in gated CD19+CD27neg naïve B cells before and after stimulation with 20 μg/ml of anti-κ+λF(ab′)2. (B) Total B cells from healthy control 1858C/C and C/T subjects (range 21–61 years), T1D 1858C/C and T1D 1858C/T subjects (range 20–55 years) were purified from fresh PBMC, rested for 1h, then stimulated with 20 μg/ml soluble anti-κ+λF(ab′)2 for 5 min a nd P-PLCγ2 was quantified by intracellular flow cytometry in gated naïve (CD20+CD27neg) and memory (CD20+CD27+) subsets (P-PLCγ2 assay, n=10 control C/C, n=11 control C/T; n=9 T1D C/C, n=10 T1D C/T). Bars show mean fold change in mean fluorescence intensity (MFI). (C) B cells from T1D 1858T/T subjects display signaling defects. Total B cells from T1D 1858C/C and T/T subjects shown in Figure 2D were purified from previously frozen PBMC, rested overnight, then stimulated as in (B); P-PLCγ2 was quantified by intracellular flow cytometry in gated naïve CD20+CD27neg B cells.
Our data support a model wherein a range of newly identified (Concannon et al., 2009; Gregersen and Olsson, 2009; Kochi et al., 2009; Borowiec et al., 2009), and as yet uncharacterized, genetic factors similarly impact B cell tolerance checkpoints in the progression to T1D, leading ultimately to a common phenotypic outcome. In humans, immature B cells expressing auto-and/or polyreactive antibodies comprise a high frequency (55–75%) of the bone marrow repertoire (Yurasov et al., 2005). Based upon murine models, central tolerance is achieved predominantly via receptor editing with clonal deletion serving as a default mechanism when editing fails to silence self-reactive cells (Halverson et al., 2004; Nemazee, 2006). Notably, in our analyses of receptor editing, we did not observe a significant difference in the relative level of RS rearrangement in either immature or mature B cells purified from healthy 1858C/C vs. 1858C/T individuals. These data suggest either that central tolerance is largely intact in 1858C/T individuals, or alternatively, that while sufficient to drive an equivalent proportion of editing, blunted signaling may shift the overall target repertoire permitting escape of a larger number of low-affinity autoreactive cells. Additional selective events operating within the transitional B cell compartment, including clonal deletion and anergy, further restrict the repertoire of naïve human B cells (Chung et al., 2003; Su et al., 2004); and the altered BCR-driven apoptotic programand increase in anergic B cells observed in individuals with the PTPN22 variant implicates alterations in these processes. Interestingly, in contrast to our finding with healthy individuals, decreased receptor editing (RS levels) has been reported in both κ+ and λ+B cells isolated from patients with SLE or T1D (Panigrahi et al., 2008), where the level of RS rearrangement does not correlate with PTPN22 status. Thus, as suggested by our data, the events leading to altered tolerance in T1D are likely more diverse than in healthy individuals who carry the PTPN22 variant allele. Finally, consistent with our data implicating defective peripheral tolerance, recent studies using receptor profiling clearly demonstrate a significant increase in autoreactive B cells within the peripheral B cell compartment of healthy individuals who carry PTPN22 1858T; and a similar increase in autoreactive B cell frequencies in T1D patients irrespective of PTPN22 genotype (E. Meffre, submitted).
Our novel finding that an expanded population of transitional and naïve BND B cells is present in the periphery of healthy carriers of PTPN22 1858T, combined with the alterations in BCR signaling and survival associated with this variant, provide new insight into the mechanisms by which Lyp620W may compromise peripheral B cell tolerance. Importantly, our data also define similar, previously uncharacterized alterations in B cell homeostasis and function in T1D subjects. The identification of developmental checkpoints that are impacted by Lyp620W may have predictive value for predisposition to autoimmune diseases associated with this variant, as well as the response to B cell-directed therapies.
Materials and Methods
Subjects
Peripheral blood and frozen PBMC samples for this study were obtained from control and T1D participants in the Benaroya Research Institute Immune Mediated Disease Registry and JDRF Center for Translational Research. The control population was selected based on a lack of personal or family history of diabetes, autoimmunity, or asthma. Research protocols were approved by the Benaroya Research Institute Institutional Review Board. All experiments were performed in a blinded manner without prior knowledge of disease state, and each analysis consisted of carriers and non-carriers of PTPN22 1858T.
FACS analysis and cell sorting
The following antibodies were used for B cell immunophenotyping: PE Cy7-anti-CD19, APC Cy7-CD27, APC-CD10 (Biolegend); FITC-IgM (Southern Biotech); PE-CD24, PerCP Cy5.5-CD38, PE Cy5-CD21, Biotin-IgD (BD Biosciences); Alexa 700-Strepavidin (Invitrogen). Flow cytometry was performed using FACSAria, or LSR-II flow cytometers (BD Biosciences) and analysis was performed with FlowJo software (Tree Star, Inc., Ashland, OR, USA). Cell sorting of naïve mature and transitional B cells for survival and RS rearrangement assays was performed by labeling freshly isolated PBMC with PE-Cy7-CD19, APC-CD27, PE-CD24, and PerCP Cy5.5-CD38 (BD Biosciences); or PE-Cy7-CD19 (Biolegend), APC-CD27 (eBiosciences), PE -CD24, PerCP Cy5.5-CD38, FITC-Anti Kappa, Biotin–Anti Lambda (BD Bioscience), and Alexa 700-Streptavidin (Invitrogen), respectively. Sorting was performed using FACSVantage or FACSAria sorters with FACSDiva software (BD Biosciences).
B cell purification, activation, and survival
Total B cells were enriched from PBMC by negative selection using the Human B cell isolation kit II(Miltenyi). Purified total B cells were rested in RPMI 1640 medium supplemented with 1% human serum (MP Biomedicals, Inc.) then stimulated with F(ab′)2 fragment goat anti -human Kappa, 20 μg/mL, or F(ab′)2goat anti -human kappa plus F(ab′)2anti -human lambda (anti-κ+λ), 20 μg/mL (Southern Biotech)) for 5 minutes. Immediately thereafter, cells were fixed with BD Fix Buffer I, permeabilized with BD Perm Buffer III (BD Biosciences), and surface stained with anti-CD27-APC and CD20-Alexa-488 in combination with PE-conjugated anti-PLCγ2 (Y759) (BD Biosciences) for intracellular phosphoprotein staining according to the manufacturer’s instructions. To quantify naïve B cell apoptosis, sorted naïve CD19+CD27negCD38intCD24int B cells and transitional CD19+CD27neg CD38hiCD24hi B cells were immediately plated in complete RPMI media (10% FCS with L-glutamine, penicillin-streptomycin) in the presence or absence of 20 μg/ml F(ab′)2 anti-IgM. Cells were fixed with BD Cytofix and permeabilized as above after 0, 12, 18, and 24 hours in culture, then stained with anti-Cleaved Caspase-3-Alexa-488 (Asp 175, Cell Signaling Technology) as per the manufacturer’s instructions. Flow cytometry was performed using a BD FACSCalibur flow cytometer. To detect BCL-2 and Bim proteins, PBMC were first surface stained with anti-CD27-APC, -CD38-PerCP Cy5.5, and –CD24-FITC mAbs (for subsequent BCL-2 or isotype control antibody staining), or anti-CD27-APC, -CD38-PerCP Cy5.5, and -CD24-PE (for subsequent Bim or isotype control antibody staining), in combination with the fixable dead cell discrimination dye LIVE/DEAD-violet (Invitrogen) for 30 minutes at room temperature. Cells were then fixed and permeabilized with BD CytoFix-Cytoperm reagent as per the manufacturer’s protocol (BD Biosciences). Intracellular staining was performed simultaneously with anti-CD20-PE-Cy7 (BD Biosciences) using the anti-human BCL-2-PE kit (BDBiosciences), anti -Bim, or anti-rabbit IgG-Alexa -488 isotype control antibodies (Cell Signaling Technology), in 1X Permwash buffer (BD Biosciences) for 30 minutes at 4°C followed by incubation of Bim-stained samples with secondary Alexa-488 F(ab′)2 fragment of goat anti-mouse IgG (Invitrogen) in 1X Permwash reagent. Flow cytometry was performed using a LSR II flow cytometer. BCL-2 expression in gated naïve and transitional B cells is expressed as the BCL-2 MFI divided by the MFI of the corresponding isotype control antibody for each sample. The ratio of BCL-2/Bim expression is expressed as (BCL-2 MFI/isotype control MFI) divided by (Bim MFI/isotype control MFI) for each sample. All figures generated using the above methods consist of data combined from more than one experiment, where separate donor groups of mixed genotype were processed and analyzed sequentially in the same manner.
Inhibitor Studies
The Lyp specific inhibitor I-C11 was synthesized as described previously (Yu et al., 2007). Total B cells purified from previously frozen PBMC were pre-treated with 10 μM I-C11 or 0.2% DMSO vehicle and stimulated and previously described (Arechiga et al., 2009).
Lentiviral transduction
WT-PEP or R619W PEP was cloned into the lentiviral vector pRRL-SFFV-IRES-mCherry, where mCherry serves as a marker for the flow cytometry-based identification of lentiviral transduced cells. Lentiviral transductions were performed as previously described (Kerns et al., 2010). Ramos cells were infected with lentivirus in RPMI media (10% FCS, 4μM l-glutamine, 50 μM 2-ME, 10 mM HEPES and antibiotics) in the presence of 8 μg/ml polybrene. Two days post-transduction, cells were harvested and mCherry expression was analyzed on a LSRII flow cytometer (BD Biosciences) and protein expression levels of WT or R619W PEP were analyzed by Western blot as previously described (Dai et al., 2007). The following antibodies were used: Anti-PEP (provided by Dr. Andrew Chan); anti-HA (6E2, Cell Signaling); Anti-mouse β-actin (A-2066, Sigma) was used to verify equal protein loading.
Calcium Flux assays
Lentiviral-transfected Ramos cells were incubated with AM ester Indo-1 (Invitrogen) at room temperature for 30 minutes. The indo-1 fluorescence ratio was acquired as a function of time on a LSRII flow cytometer. For each experiment, collection of a 1-minute baseline measurement was followed by stimulation with anti-IgM F(ab′)2 (Jackson Laboratories) or 500 ng/ml ionomycin as indicated. Freshly isolated PBMC were incubated with Indo-1 AM for 30 minutes at 37°C, then surface-stained with anti-CD19 and -CD27 to detect naïve B and memory B cells. Collection of a 1-minute baseline was followed by stimulation with F(ab′)2 fragment anti-κ+λ, or 500 ng/ml ionomycin. The indo-1 fluorescence ratio was acquired as a function of time, and kinetics curves were generated using FlowJo software.
Quantitative assay for RS rearrangement
Genomic DNA was isolated from sorted transitional (CD19+CD27negCD10+CD24hiCD38hi) and mature (CD19+CD27negCD24intCD38int) B cells separated by light chain isotype using the Gentra Puregene Tissue Kit (Qiagen). Quantitative PCR (60 cycles at 95°C for 10 s, 60°C for 30 s, and 72°C for 1 s) was performed on 15–50 ng template DNA in a 20-μl reaction mix containing 1x LightCycler 480 Probes Master Mix (Roche), 0.5 U LightCycler Uracil-DNA Glycosylase (Roche), 0.5 μM forward primer, 0.5 μM reverse primer, and 0.2 μM hydrolysis probe using aLightCycler 480 real-time PCR system (Roche). iRS-KDE rearrangement frequencies were determined by absolute quantification using a standard curve generated from serial dilution of a cloned iRS-KDE rearrangement resuspended in 100ng human fibroblast genomic DNA. Genome copy number was calculated from β-actin quantification using a standard curve generated from serially diluted human fibroblast genomic DNA. All reactions were performed in duplicate and samples with inconsistent replicates or β-actin cycle numbers >35 were excluded.
BAFF Immunoassay
Sera from control and T1D subjects were harvested and stored at −80°C. BAFF serum levels were measured using an enzyme-linked immunosorbent assay (ELISA) sandwich method with the Quantikine® Human BAFF/BLyS/TNFSF13B Immunoassay (R&D Systems, # DBLYS0) following the manufacturer’s instructions. Samples were analyzed in duplicates based on the use of a monoclonal anti-BAFF Ab and corresponding irrelevant IgG as negative control. The absorbance values in the isotype control wells were subtracted from the corresponding anti-BAFF capture wells.
Statistical Analysis
Differences between healthy control PTPN22 1858T-carrier and non-carrier populations were analyzed for statistical significance with a two-tailed Student’s t test using GraphPad Prism version4.02 for Windows. Paired t tests were used to analyze vehicle-vs. IC11 -treated populations in Supplemental Figure 1. Group comparisons between healthy control and T1D populations were performed using a one-way ANOVA (Dunnett’s multiple comparison’s test) and Student’s t tests. P-values <0.05 were considered significant.
Supplementary Material
Acknowledgments
The authors thank K. Arumuganathan for assistance with cell sorting. We thank Dr. Gerald Nepom for helpful discussions, Dr. Z.Y. Zhang for generously providing the Lyp inhibitor, the Benaroya Research Institute Translational Research Program and Diabetes Clinical Research Unit for subject recruitment, and Diana Sorus foradministrative assistance. This work was supported by the JDRF-Center for Translational Research; ELP and AP were supported for this work by the Alliance for Lupus Research. ELP also acknowledges support from NIH R56-AI-090842 and the Pennsylvania Department of Health.
Abbreviations
- BCR
B cell receptor
- Ca 2+
calcium
- T1D
type I diabetes
Footnotes
The authors declare that no conflicts of interest exist.
References
- Arechiga A, Habib T, He Y, Zhang X, Zhang Z-Y, Funk A, Buckner JH. The PTPN22 allelic variant associated with autoimmunity impairs B cell signaling. J Immunol. 2009;182:3343–3347. doi: 10.4049/jimmunol.0713370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berard M, Mondiere P, Casamayor-Palleja M, Hennino A, Bella C, Defrance T. Mitochondria connects the antigen receptor to effector caspases during B cell receptor-induced apoptosis in normal human B cells. J Immunol. 1999;163:4655–4662. [PubMed] [Google Scholar]
- Borowiec M, Liew CW, Thompson R, Boonyasrisawat W, Hu J, Mlynarski WM, El Kattabi I, Kim SH, Marselli L, Rich SS, Krolewski AS, Bonner-Weir S, Sharma A, Sale M, Mychaleckyj JC, Kulkarni RN, Doria A. Mutations at the BLK locus linked to maturity onset diabetes of the young and beta-cell dysfunction. Proc Natl Acad Sci U S A. 2009;106:14460–14465. doi: 10.1073/pnas.0906474106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouaziz JD, Yanaba K, Venturi GM, Wang Y, Tisch RM, Poe JC, Tedder TF. Therapeutic B cell depletion impairs adaptive and autoreactive CD4+ T cell activation in mice. Proc Natl Acad Sci U S A. 2007;104:20878–20883. doi: 10.1073/pnas.0709205105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JB, Silverman M, Monroe JG. Transitional B cells: step by step towards immune competence. Trends Immunol. 2003;24:343–349. doi: 10.1016/s1471-4906(03)00119-4. [DOI] [PubMed] [Google Scholar]
- Concannon P, Rich SS, Nepom GT. Genetics of type 1A diabetes. N Engl J Med. 2009;360:1646–1654. doi: 10.1056/NEJMra0808284. [DOI] [PubMed] [Google Scholar]
- Dai X, Chen Y, Di L, Podd A, Li G, Bunting KD, Hennighausen L, Wen R, Wang D. Stat5 is essential for early B cell development but not for B cell maturation and function. J Immunol. 2007;179:1068–1079. doi: 10.4049/jimmunol.179.2.1068. [DOI] [PubMed] [Google Scholar]
- Dorner T, Jacobi AM, Lee J, Lipsky PE. Abnormalities of B cell subsets in patients with systemic lupus erythematosus. J Immunol Methods. 2010 doi: 10.1016/j.jim.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Duty JA, Szodoray P, Zheng NY, Koelsch KA, Zhang Q, Swiatkowski M, Mathias M, Garman L, Helms C, Nakken B, Smith K, Farris AD, Wilson PC. Functional anergy in a subpopulation of naive B cells from healthy humans that express autoreactive immunoglobulin receptors. J Exp Med. 2009;206:139–151. doi: 10.1084/jem.20080611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregersen PK, Olsson LM. Recent advances in the genetics of autoimmune disease. Annu Rev Immunol. 2009;27:363–391. doi: 10.1146/annurev.immunol.021908.132653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halverson R, Torres RM, Pelanda R. Receptor editing is the main mechanism of B cell tolerance toward membrane antigens. Nat Immunol. 2004;5:645–650. doi: 10.1038/ni1076. [DOI] [PubMed] [Google Scholar]
- Jacobi AM, Zhang J, Mackay M, Aranow C, Diamond B. Phenotypic characterization of autoreactive B cells--checkpoints of B cell tolerance in patients with systemic lupus erythematosus. PLoS ONE. 2009;4:e5776. doi: 10.1371/journal.pone.0005776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns HM, Ryu BY, Stirling BV, Sather BD, Astrakhan A, Humblet-Baron S, Liggitt D, Rawlings DJ. B cell-specific lentiviral gene therapy leads to sustained B-cell functional recovery in a murine model of X-linked agammaglobulinemia. Blood. 2010;115:2146–2155. doi: 10.1182/blood-2009-09-241869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King LB, Monroe JG. Immunobiology of the immature B cell: plasticity in the B-cell antigen receptor-induced response fine tunes negative selection. Immunol Rev. 2000;176:86–104. doi: 10.1034/j.1600-065x.2000.00609.x. [DOI] [PubMed] [Google Scholar]
- Kochi Y, Myouzen K, Yamada R, Suzuki A, Kurosaki T, Nakamura Y, Yamamoto K. FCRL3, an autoimmune susceptibility gene, has inhibitory potential on B-cell receptor-mediated signaling. J Immunol. 2009;183:5502–5510. doi: 10.4049/jimmunol.0901982. [DOI] [PubMed] [Google Scholar]
- Miao D, Yu L, Eisenbarth GS. Role of autoantibodies in type 1 diabetes. Front Biosci. 2007;12:1889–1898. doi: 10.2741/2195. [DOI] [PubMed] [Google Scholar]
- Nemazee D. Receptor editing in lymphocyte development and central tolerance. Nat Rev Immunol. 2006;6:728–740. doi: 10.1038/nri1939. [DOI] [PubMed] [Google Scholar]
- Niiro H, Clark EA. Regulation of B-cell fate by antigen-receptor signals. Nat Rev Immunol. 2002;2:945–956. doi: 10.1038/nri955. [DOI] [PubMed] [Google Scholar]
- Norvell A, Mandik L, Monroe JG. Engagement of the antigen -receptor on immature murine B lymphocytes results in death by apoptosis. J Immunol. 1995;154:4404–4413. [PubMed] [Google Scholar]
- Panigrahi AK, Goodman NG, Eisenberg RA, Rickels MR, Naji A, Luning Prak ET. RS rearrangement frequency as a marker of receptor editing in lupus and type 1 diabetes. J Exp Med. 2008;205:2985–2994. doi: 10.1084/jem.20082053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieck M, Arechiga A, Onengut-Gumuscu S, Greenbaum C, Concannon P, Buckner JH. Genetic Variation in PTPN22 Corresponds to Altered Function of T and B Lymphocytes. J Immunol. 2007;179:4704–4710. doi: 10.4049/jimmunol.179.7.4704. [DOI] [PubMed] [Google Scholar]
- Samuels J, Ng YS, Coupillaud C, Paget D, Meffre E. Impaired early B cell tolerance in patients with rheumatoid arthritis. J Exp Med. 2005;201:1659–1667. doi: 10.1084/jem.20042321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira PA, Grey ST. B cells in the spotlight: innocent bystanders or major players in the pathogenesis of type 1 diabetes. Trends Endocrinol Metab. 2006;17:128–135. doi: 10.1016/j.tem.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Sims GP, Ettinger R, Shirota Y, Yarboro CH, Illei GG, Lipsky PE. Identification and characterization of circulating human transitional B cells. Blood. 2005;105:4390–4398. doi: 10.1182/blood-2004-11-4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skyler JS. Prediction and prevention of type 1 diabetes: progress, problems, and prospects. Clin Pharmacol Ther. 2007;81:768–771. doi: 10.1038/sj.clpt.6100179. [DOI] [PubMed] [Google Scholar]
- Stanford SM, Mustelin TM, Bottini N. Lymphoid tyrosine phosphatase and autoimmunity: human genetics rediscovers tyrosine phosphatases. Semin Immunopathol. 2010;32:127–136. doi: 10.1007/s00281-010-0201-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su TT, Guo B, Wei B, Braun J, Rawlings DJ. Signaling in transitional type 2 B cells is critical for peripheral B-cell development. Immunol Rev. 2004;197:161–178. doi: 10.1111/j.0105-2896.2004.0102.x. [DOI] [PubMed] [Google Scholar]
- Suryani S, Fulcher DA, Santner-Nanan B, Nanan R, Wong M, Shaw PJ, Gibson J, Williams A, Tangye SG. Differential expression of CD21 identifies developmentally and functionally distinct subsets of human transitional B cells. Blood. 2010;115:519–529. doi: 10.1182/blood-2009-07-234799. [DOI] [PubMed] [Google Scholar]
- Tussiwand R, Bosco N, Ceredig R, Rolink AG. Tolerance checkpoints in B-cell development: Johnny B good. Eur J Immunol. 2009;39:2317–2324. doi: 10.1002/eji.200939633. [DOI] [PubMed] [Google Scholar]
- Vang T, Congia M, Macis MD, Musumeci L, Orru V, Zavattari P, Nika K, Tautz L, Tasken K, Cucca F, Mustelin T, Bottini N. Autoimmune-associated lymphoid tyrosine phosphatase is a gain-of-function variant. Nat Genet. 2005;37:1317–1319. doi: 10.1038/ng1673. [DOI] [PubMed] [Google Scholar]
- vonBoehmer H, Melchers F. Checkpoints in lymphocyte development and autoimmune disease. Nat Immunol. 2010;11:14–20. doi: 10.1038/ni.1794. [DOI] [PubMed] [Google Scholar]
- Xiu Y, Wong CP, Bouaziz JD, Hamaguchi Y, Wang Y, Pop SM, Tisch RM, Tedder TF. B lymphocyte depletion by CD20 monoclonal antibody preventsdiabetes in nonobese diabetic mice despite isotype-specific differences in Fc gamma R effector functions. J Immunol. 2008;180:2863–2875. doi: 10.4049/jimmunol.180.5.2863. [DOI] [PubMed] [Google Scholar]
- Yu X, Sun JP, He Y, Guo X, Liu S, Zhou B, Hudmon A, Zhang ZY. Structure, inhibitor, and regulatory mechanism of Lyp, a lymphoid-specific tyrosine phosphatase implicated in autoimmune diseases. Proc Natl Acad Sci U S A. 2007;104:19767–19772. doi: 10.1073/pnas.0706233104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurasov S, Wardemann H, Hammersen J, Tsuiji M, Meffre E, Pascual V, Nussenzweig MC. Defective B cell tolerance checkpoints in systemic lupus erythematosus. J Exp Med. 2005;201:703–711. doi: 10.1084/jem.20042251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.