Summary
BACKGROUND
Lynch syndrome is caused by germline mutations in mismatch repair genes (MSH2, MLH1, MSH6 or PMS2), which lead to a high risk of predominantly colorectal and endometrial cancer. Recently, we found that also constitutional 3′ end deletions of EPCAM can cause Lynch syndrome through epigenetic silencing of MSH2 in EPCAM expressing tissues. This results in a tissue specific MSH2-deficiency, which may evoke a different cancer risk and spectrum. To optimize the care for EPCAM deletion carriers we studied their cancer risk and spectrum.
METHODS
Clinical data of 194 carriers from 41 EPCAM families were systematically collected and compared to those of 431 carriers from 91 families with mutations in MLH1, MSH2, or MSH6.
FINDINGS
EPCAM deletion carriers exhibited a 75% [95%CI 65–85%] cumulative risk of colorectal cancer before the age of 70 years, with a mean age at diagnosis of 43 years, which is comparable to that of carriers of a combined EPCAM-MSH2 deletion (69% [95%CI 47-91%], p=0·8609) or of a mutation in MSH2 (77% [95%CI 64-90%], p=0·5892) or MLH1 (79% [95%CI 68-90%], p=0·5492) and higher than that of MSH6 mutation carriers (50% [95%CI 38-62%], p<0·0001). In contrast, women with EPCAM deletions (n=87) exhibited a 12% [95%CI 0-27%] cumulative risk of endometrial cancer, which is significantly lower than in carriers of a combined EPCAM-MSH2 deletion (55% [95%CI 20-90%], p<0·0001) or of a mutation in MSH2 (51% [95%CI 33-69%], p=0·0006) or MSH6 (34% [95%CI 20-48%], p=0·0309) and lower than in MLH1 (33% [95%CI 15-51%] p=0·1193) mutation carriers. This risk seems to be restricted to large deletions that extend close to the MSH2 gene promoter. Overall, a relatively high incidence of duodenal (n=3) and pancreatic (n=4) cancers was observed.
INTERPRETATION
EPCAM deletion carriers do have a high risk of colorectal cancer. Only those with deletions extending close to the MSH2 promoter have an increased risk of endometrial cancer. These results underscore the impact of mosaic MSH2-deficiency on cancer risk and are indicative for a protocol revision for surveillance and preventive surgery in EPCAM deletion carriers.
Keywords: Lynch syndrome, cancer risk, TACSTD1, EPCAM, MSH2, genotype-phenotype correlation
INTRODUCTION
Lynch syndrome, or hereditary nonpolyposis colorectal cancer, is caused by pathogenic germline mutations in one of the DNA mismatch repair genes MLH1, MSH2, MSH6 and PMS2. Lynch syndrome is characterized by a high risk of early onset colorectal cancer and several extra-colonic malignancies, in particular endometrial cancer (1). Carriers of mutations in MLH1, MSH2, or MSH6 have a 30-80% risk of developing colorectal carcinoma by age the age of 70 years. Women with Lynch syndrome have an additional 27-71% risk for developing endometrial cancer at this age (2-4). In asymptomatic mutation carriers from Lynch syndrome families surveillance for colorectal cancer starting at an early stage is recommended in order to improve survival. Similarly, surveillance and prophylactic surgery for endometrial cancer are widely applied (4). As yet, it is unclear for which other extra-colonic malignancies surveillance would be beneficial, but based on the occurrence of Lynch syndrome-associated extra-colonic malignancies within a specific family, additional surveillance is often considered (2;5).
Recently, we identified germline deletions in the EPCAM gene, previously known as TACSTD1, as a novel cause of Lynch syndrome (6;7). These deletions disrupt the 3′ end of EPCAM, leading to transcriptional read-through into, and subsequent epigenetic silencing of, its neighbouring gene MSH2, thus causing Lynch syndrome (6). Since this silencing phenomenon is restricted to cells expressing EPCAM, subjects with EPCAM deletions show mosaic patterns of MSH2 inactivation which, compared to carriers of a mutation in MSH2, may lead to differences in tumour incidence and/or spectrum. The relatively high expression of EPCAM in colorectal cancer stem cells (8;9) explains why subjects with an EPCAM deletion have a significantly increased risk of colorectal cancer. Since very little is known about the expression of EPCAM in stem cells of extra-colonic malignancies, the risk of developing other Lynch syndrome-associated tumours in EPCAM deletion carriers is as yet unclear. Also, since EpCAM can modulate both cell adhesion and proliferation (10;11), the inactivation of EPCAM itself may affect tumour risk.
Multiple families with such deletions have been reported by others (7;12-15). Determination of the possibly specific tumour spectrum and age-specific cancer risk in families carrying EPCAM deletions is required to generate optimal recognition and surveillance strategies. Here, we employed deletion scanning in conjunction with clinical inventories to establish EPCAM deletion-associated cancer risks and compared these risks with those of Lynch syndrome patients carrying either a mutation in MLH1, MSH2, MSH6, or a deletion affecting both EPCAM and its neighbouring gene MSH2 (EPCAM-MSH2)..
PATIENTS and METHODS
Study population and data collection
Families with EPCAM deletions
All 41 families with a 3′ end EPCAM deletion that were known at the department of Human Genetics of the Radboud University Nijmegen Medical Centre by November 2009, were eligible for this study. In all families the deletion was confirmed not to include the defined promoter region and open reading frame of the MSH2 gene (R. Kuiper et al, manuscript in preparation). The deletion in 14 of these 41 families has been reported before (6;7;12;14;16). Collection of the remaining families was based on the occurrence of as yet unexplained MSH2-deficient tumours in the Netherlands and Germany, and by analysis of germline DNA samples of subjects with unexplained MSH2-deficient tumours that were referred to the Radboud University Nijmegen Medical Centre. Only subjects tested positive for a deletion and obligate carriers were included in the current study. Genetic counsellors collected the following variables: gender, year of birth, year of death and year of tumour diagnosis, and clinicopathological and molecular data, including location of the tumour, microsatellite instability status, immunohistochemical status of mismatch repair proteins and methylation status of the MSH2 gene promoter.
At the Radboud University Nijmegen Medical Centre clinical data of deletion carriers were collected until February 1, 2010. In total the data of 16 families harbouring 105 carriers of a Dutch founder deletion (6), 2 families harbouring 42 carriers with an identical Swiss deletion (14) and 23 families harbouring 47 carriers with various different deletions from Germany (n=9), Hungary (n=5), USA (n=4), Hong Kong (n=2), Canada (n=1), United Kingdom (n=1) and, the Netherlands (n=1) were included. In total, information on 194 EPCAM deletion carriers representing 16 different deletions was collected. Ethics: The Committee on Research Involving Human Subjects Region Arnhem-Nijmegen approved the study (project approval: 2009/167).
Families with MLH1, MSH2 or MSH6 mutations
The collection of clinical data of a cohort of 95 Lynch syndrome families has been described before (5). From this cohort 4 families with an EPCAM deletion were excluded, as they were already incorporated as EPCAM deletion families, and 7 families with a deletion involving both EPCAM and the 5′ part of MSH2, reported as EPCAM-MSH2, were considered separately. Only data on subjects tested positive for a given mutation and obligate carriers were included in the analyses. This resulted in a set of 91 families with an EPCAM-MSH2 (n=7), MSH2 (n=32), MSH6 (n=26), or MLH1 (n=26) mutation representing 42, 143, 160, and 128 subjects, respectively.
Immunohistochemistry for EpCAM
Immunohistochemistry was performed on formalin fixed, paraffin embedded tissues with the antibody Ep-CAM Ab-1(Clone VU-ID9; Thermo Fisher) using standard procedures.
Statistical analysis
Differences in mean age of cancer occurrence between the five mutation groups were analyzed using the one-way ANOVA method. The follow-up time for each carrier was calculated as time lapse between date of birth and date of cancer diagnosis, date of last contact or date of death, whichever came first. Kaplan-Meier (KM) survival analyses were used to calculate the risk (plus 95% confidence interval) of cancer until specific ages. The age of 70 years was chosen as censoring age. The log-rank test was used for comparisons of risks. The SPSS version 16.0 software package was used for analyses.
Role of the funding source
The Sacha Swarttouw-Hijmans foundation, the Dutch Cancer Society, the Deutsche Krebshilfe (German Cancer Aid), the Hong Kong Cancer Fund, the Hungarian Research Grant OTKA and the Norwegian EEA Financial Mechanism (Hungarian National Institute of Oncology) and the National Cancer Institute supported this study financially, but had no access to the raw data and no involvement in study design, data collection, data interpretation, writing of the report or in the decision to submit the paper for publication. The corresponding author had full access to all of the data and the final responsibility to submit for publication.
RESULTS
Onset and risk of colorectal cancer
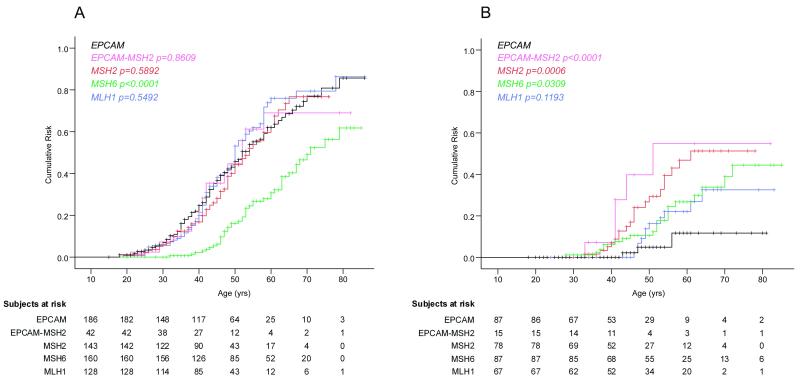
Clinical data were collected from 667 mutation carriers representing 132 independent Lynch syndrome families. Amongst these were 41 families encompassing 194 EPCAM deletion carriers (Table 1). During follow-up, 93 EPCAM deletion carriers were diagnosed with colorectal cancer at a mean age at first diagnosis of 43 ± 12 years (range 18-79 yrs), being 43 years for men and 42 years for women. This mean age was not significantly different from that of Lynch syndrome patients with an EPCAM-MSH2, MSH2, or MLH1 mutation, but it was significantly lower than that of MSH6 mutation carriers (p<0·0001). The cumulative risk of colorectal cancer among EPCAM deletion carriers until age 70 was 75% [95% CI 65-85], being 75% for men [95%CI 63-87] and 74% for women [95%CI 56-92], which was again similar to carriers of EPCAM-MSH2, MSH2, or MLH1 mutations, but higher than that of MSH6 mutation carriers (p<0·0001, Table 1, Figure 1A).
Table 1.
Mean age at diagnosis and cumulative risk by age 70 of colorectal and endometrial cancer in Lynch mutation carriers
| EPCAM | EPCAM-MSH21 | MSH2 | MSH6 | MLH1 | |
|---|---|---|---|---|---|
| Families (n) | 41 | 7 | 32 | 26 | 26 |
| Mutation carriers (n) | 194 | 42 | 143 | 160 | 128 |
| Colorectal cancer: | |||||
| Carriers affected (n) | 93 | 18 | 60 | 45 | 68 |
| Mean age at diagnosis (yrs (range)) | 43 (18-79)2 | 41 (21-58) | 44 (19-65) | 54 (32-79) | 44 (22-78) |
| Cumulative risk (%[95%CI]) | 75 [65-85]3 | 69 [47-91] | 77 [64-90] | 50 [38-62] | 79 [68-90] |
| Excess risk (%)4 | 73 | 67 | 75 | 48 | 77 |
| Endometrial cancer: | |||||
| Female carriers (n) | 92 | 15 | 78 | 87 | 67 |
| Carriers affected (n) | 3 | 5 | 20 | 20 | 11 |
| Mean age at diagnosis (yrs (range)) | 49 (43-56) | 42 (33-51) | 47 (33-61) | 50 (28-72) | 52 (46-64) |
| Cumulative risk (%[95%CI]) | 12 [0-27]5 | 55 [20-90] | 51 [33-69] | 34 [20-48] | 33 [15-51] |
| Excess risk (%)5 | 11 | 54 | 50 | 33 | 32 |
| Ratio of females colorectal to endometrial cancer (n/n) |
12.3 | 0.8 | 1.6 | 1.1 | 2.9 |
Combined deletion of EPCAM and MSH2
Mean age at diagnosis of first colorectal cancer in EPCAM deletion carriers was based on the data of 91 affected carriers, because in two carriers the exact age at onset was not known
The cumulative risk of colorectal cancer was based on the data of 186 EPCAM deletion carriers
In the Netherlands, the cumulative risk at 70 years of age (for both sexes combined) of developing colorectal cancer is 2.5%. For endometrial cancer this risk is 1.6% (source Kiemeney et al (17) and Netherlands Cancer Registry at www.ikcnet.nl(18)). In the USA, these risks are 1.9% and 1.6%, respectively among caucasians (source www.seer.cancer.gov(19)).
The cumulative risk of endometrial cancer was based on the data of 87 EPCAM deletion carriers
Figure 1.
Cancer risk in EPCAM deletion carriers. Cumulative risk until the age of 70 of colorectal cancer (A) and endometrial cancer (B) in EPCAM (black lines), EPCAM-MSH2 (pink lines), MSH2 (red lines), MSH6 (green lines), and MLH1 (blue lines) mutation carriers. Indicated log-rank p-values are comparisons relative to EPCAM deletion carriers. The number of subjects in the table below the graphs indicate the number of mutation carriers, that are at risk for their first colorectal (A) or endometrial cancer (B) at the given age. Eight EPCAM deletion carriers were excluded from the Kaplan Meier curves because the exact age at colorectal cancer diagnosis (n=2) or at follow up (n=6) were unknown.
Onset and risk of endometrial cancer
Among the 92 women carrying an EPCAM deletion, 3 endometrial cancers were diagnosed (Table 1). Two of the endometrial cancers occurred in the family originally described by Chan et al (16): patient II-1 first developed colorectal cancer at age 30 and subsequently endometrial cancer at age 56; patient II-3 was diagnosed with endometrial cancer at age 43. Both were confirmed as MSH2-deficient. The other endometrial cancer was reported by family history as the only tumor in an obligate carrier at age 47. The age at diagnosis of these 3 endometrial cancers fell within the range observed for that of the other four mutation groups. However, the incidence of endometrial cancer among women with an EPCAM deletion was found to be >12-fold lower compared to colorectal cancer, which is in sharp contrast to the other mutation groups (Table 1). Overall, based on a Kaplan Meier analysis EPCAM deletion carriers had a 12% [95% CI 0–27%] cumulative risk of endometrial cancer by the age of 70 years (Table 1, Figure 1B), which was significantly lower than that of carriers of an EPCAM-MSH2 (p<0·0001), MSH2 (p=0·0006), or MSH6 (p=0·0309) mutation.
Endometrial cancer risk and EPCAM deletion size
We previously showed a direct correlation between EPCAM expression and MSH2 promoter methylation in EPCAM deletion carriers (9). The low incidence of endometrial cancer in this group of patients may, therefore, be related to lower expression levels of the EPCAM-MSH2 fusion transcript in the tumour-initiating endometrial cells. In mature endometrial carcinomas EpCAM appeared to be present, as detected by immunohistochemistry of 72 sporadic and 12 Lynch syndrome-related endometrial carcinomas (MSH2-EPCAM n=3; MSH2 n=2; MSH6 n=5; MLH1 n=2). In line with this, methylation of the MSH2 promoter was detected in the one endometrial carcinoma that was available for methylation testing.
Remarkably, we noticed that all 3 endometrial tumours occurred in subjects from families with an EPCAM deletion extending closely to the MSH2 promoter region (Figure 2A and Supplementary Table 1, <2.5 kb upstream of the MSH2 gene). Within these families there were only 13 confirmed female deletion carriers.These observations suggest that EPCAM deletions that extend close to MSH2 may more efficiently inactivate MSH2. In order to explore this suggestion, we divided the EPCAM deletion families into two subgroups (Figure 2A): subgroup 1 containing carriers with deletions located at least 10 kb upstream of the MSH2 gene (69 male and 62 female carriers), and subgroup 2 containing carriers with deletions extending to 5·5 kb upstream of the MSH2 gene (33 male and 30 female carriers). The risk of colorectal cancer until the age of 70 years was similar for both subgroups being 78% [95% CI: 67-90%] and 66% [95% CI: 46-85], respectively, and did not significantly differ from that in carriers of an EPCAM-MSH2 deletion or a MSH2 mutation (Figure 2B). The risk of endometrial cancer in subgroup 2 being 31% [95% CI 0-65%] seems still lower than that of carriers of an EPCAM-MSH2 deletion or a MSH2 mutation (Figure 2C), suggesting that either not all carriers in subgroup 2 have an increased endometrial cancer risk or that the risk per individual is lower. These findings suggest that an increased risk of endometrial cancer is dependent on the size and location of the EPCAM deletion.
Figure 2.
Cancer risk in EPCAM deletion carriers in relation to deletion breakpoint and size. (A) Schematic representation of the size of each EPCAM deletion (depicted by bars) and its position relative to the MSH2 CpG island (CGI) promoter. The deletions found in the endometrial cancer patients are depicted in black, the others are presented in grey. For each deletion the number of carriers as well as the number of patients with colorectal cancer (CRC) or endometrial cancer (EC) are indicated on the left. ST: subtotal; T: total. The cumulative colorectal (B) and endometrial (C) cancer risk of subgroup 1 (grey lines), subgroup 2 (black lines) in comparison to those of MSH2 (red lines) and MSH2-EPCAM (bleu lines) mutation carriers. Indicated log-rank p-values are comparisons relative to MSH2 mutation carriers. The number of subjects in the table below the graphs indicate the number of mutation carriers, that are at risk for their first colorectal (B) or endometrial cancer (C) at the given age. Eight EPCAM deletion carriers were excluded from the Kaplan Meier curves because the exact age at colorectal cancer diagnosis (n=2) or at follow up (n=6) were unknown.
Occurrence of other extra-colonic malignancies
Among EPCAM deletion carriers, 16 malignancies other than colorectal or endometrial cancer were detected (Table 2), of which 2 occurred in a single patient. Duodenal cancer was detected in 3 such carriers. Two of these cancers were available for analysis, and showed microsatellite instability (MSI-high), negative immunohistochemical staining for MSH2 and methylation of the MSH2 promoter, indicative of a role of the EPCAM deletion in the development of the DNA mismatch repair deficiency. Pancreatic cancer was reported in 4 EPCAM deletion carriers. Unfortunately, no tumour specimens were available for further analysis. No duodenal cancer and only one pancreatic cancer were detected among 473 carriers of an EPCAM-MSH2, MSH2, MLH1, or MSH6 mutation.
Table 2.
Extra-colonic and extra-endometrial cancer in EPCAM deletion carriers
| Tumor type | No of patients | MSI* status | Age at diagnosis |
|---|---|---|---|
| Duodenum | 3 | high/high/unknown | 52/54/unknown |
| Pancreas | 4 | Unknown | 46/51/65/unknown |
| Breast | 2 | Unknown | 57/59 |
| Urothelial carcinoma | 1 | Stable | 60 |
| Kidney | 1 | unknown | Unknown |
| Prostate | 1 | unknown | 71 |
| Basal cell carcinoma | 1 | unknown | 41 |
| Brain | 1 | unknown | Unknown |
| Gall bladder | 1 | unknown | 69 |
| Myelodysplastic syndrome | 1 | unknown | 79 |
MSI = microsatellite instability
DISCUSSION
To our knowledge, this is the first study describing the cancer profile and risk estimate in a large cohort of Lynch syndrome families exhibiting EPCAM deletions. We observed a high (75%) risk of colorectal cancer among the deletion carriers, which was similar to that of carriers with a mutation in the MSH2 gene or a deletion affecting both the EPCAM and MSH2 genes. In addition, a relatively high risk of duodenal and pancreatic cancer was observed. In contrast, the overall cumulative risk by the age of 70 years of endometrial cancer was only 12%, and appeared to be consistently low in carriers with EPCAM deletions located further upstream of the MSH2 gene, as all 3 endometrial cancers were found in women with the two EPCAM deletions that extend closest to the MSH2 gene. Together, these results indicate that carriers of EPCAM deletions in families with Lynch syndrome have a distinct cancer risk, and that this risk is dependent on the size and location of the deleted region.
In our study for all different types of mutations (EPCAM; EPCAM-MSH2; MSH2; MSH6 and MLH1) the index patients are included in the risk estimates. Because of ascertainment bias this will have led to an overestimation of the actual cancer risk for each of the mutations. Indeed in our cohort of Lynch syndrome families with an MSH2 mutation, the colorectal cancer risk appeared somewhat higher than reported by others, whereas the endometrial cancer risk for MSH2 mutation carriers and both the colorectal and endometrial cancer risks of MLH1 and MSH6 mutation carriers were in conformity with that reported by others (20-24)., Remarkably, we observed several duodenal and pancreatic cancers in EPCAM deletion carriers, while no duodenal and only one pancreatic cancer was observed in carriers with a mutation in one of the mismatch repair genes, which is in line with the very low incidence of duodenal and pancreatic cancer in families harbouring a mismatch repair gene mutation reported by others (23;25;26). It remains to be established whether the risk for these cancers is indeed higher in individuals with an EPCAM deletion as compared to individuals with a mismatch repair gene mutation. Comparison of a larger cohort of families with an EPCAM deletion, a combined EPCAM-MSH2 deletion or a mutation in MSH2 may unravel whether the inactivation of EPCAM is important for the apparently increased risk of these latter cancers.
Although the cumulative risk at age 70 of endometrial cancer in EPCAM deletion carriers of 12% is still higher than the population risk of 1,6% (17), this 12% risk is much lower than that of MSH2 mutation carriers (51%) and the combined MSH2-EPCAM deletion carriers (55%). This most likely relates to the mosaic tissue-specific pattern of MSH2 inactivation in these carriers which is dependent on the tissue-specific level of EPCAM expression. As we previously reported, transcriptional read-through of EPCAM results in in cis epigenetic silencing of the MSH2 gene, whereas in tissues that lack EPCAM expression, MSH2 remains active (6). We, therefore, assume that the low incidence of endometrial cancer could be explained by an insufficient level of EPCAM expression in the endometrial cells during early stages of tumour development, resulting in a normal activity of MSH2 and, consequently, a normal risk for tumour development.
It is unlikely that the relatively low incidence of endometrial cancer in EPCAM deletion carriers can be attributed to a selection bias for colorectal cancer families. All EPCAM deletion carriers included in this study were derived from cohorts of patients with a clinical picture suggestive of Lynch syndrome, similar to the cohort from which the families with MSH2, MLH1, and MSH6 mutations were selected. It is also unlikely that this low incidence of endometrial cancer is affected by an unintended selection of the tumour type carried by the index patients, as 74% of the women included in this study were either derived from one large Dutch family (55% of women) or two large Swiss families (19% of women), in which relatives up to the 5th degree of the original index patient have been tested for the presence of a mutation. Although we cannot exclude that a modifying genetic factor acts in cis with either the Dutch or the Swiss founder deletion, this seems unlikely as, to the best of our knowledge, a lack of endometrial cancers in families with specific MSH2 mutations has not been reported before. Moreover, inactivation of the EPCAM gene is not a protective factor in itself, as the risk of endometrial cancer of individuals with a combined EPCAM-MSH2 deletion is similar to that of individuals with a single mutation in MSH2.
The only three early-onset endometrial cancers that we found occurred in women with a deletion that extends close to the MSH2 promoter region (27;28). There are several possible scenarios that may contribute to this phenomenon. Firstly, the efficiency of MSH2 inactivation could, for example be associated with the distance of the EPCAM and MSH2 promoters on the allele carrying the deletion. Larger EPCAM deletions extending close to the MSH2 gene will put the two promoters into closer proximity, thus enabling endometrial cells to drive MSH2 methylation, despite the weaker EPCAM promoter activity in these cells. Secondly, in subjects with deletions that extend close to the MSH2 gene the inactivation of MSH2 may be less dependent on high levels of EPCAM expression due to loss of a regulatory element. The presence of such an element in this region has thus far not been reported, but we did notice that the region overlaps with a punctuate site of enriched di- and tri-methylation of histone H3 on lysine 4 (H3K4Me2 and H3K4Me3) in HepG2 cells (29;30), which strongly correlate with active promoters or enhancers (31;32).
Whatever the mechanism may be, our data indicate that the risk for endometrial cancer in carriers of EPCAM deletions is dependent on the size and location of the deletion. The exact criteria of deletions that confer a low endometrial cancer risk remain to be defined by further assessments of endometrial cancer incidences in carriers of different EPCAM deletions and analyses of the EPCAM-MSH2 intergenic region for transcription-mediating capacity.
Surveillance programs for Lynch syndrome families are typically aimed at early detection of colorectal and endometrial tumours, sometimes supplemented with surveillance for other Lynch syndrome-associated malignancies that occur within the family (24;33). Recently, for example, surveillance for urinary tract cancer in MSH2 mutation carriers has been recommended (5). However where the predicted incidence of cancer is low, a targeted cancer prevention programme is less likely to offer clinical benefit, especially where evidence for its efficacy is limited. Therefore, our current findings suggest that surveillance and preventive surgery for endometrial cancer could reasonably be omitted for carriers of a smaller EPCAM deletion extending further away from the MSH2 promoter.
In conclusion, we report that EPCAM deletion carriers have a high risk of developing colorectal cancer that is comparable to that of MLH1 or MSH2 mismatch repair gene mutation carriers. The risk of endometrial cancer, however, is significantly lower. This low risk may be due to an insufficient level of EPCAM expression in endometrial cancer progenitor cells. Our data are indicative for an optimized protocol for the recognition and targeted prevention of cancer in EPCAM deletion carriers.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank the families and their counsellors that have so kindly contributed to this study. This work was financially supported by the Sacha Swarttouw-Hijmans foundation, the Dutch Cancer Society grant 2009-4335, the Deutsche Krebshilfe (German Cancer Aid), the Hong Kong Cancer Fund, the Hungarian Research Grant NKTH-OTKA K-80745, the Norwegian EEA Financial Mechanism Hu0115/NA/2008-3/ÖP-9. The City of Hope Hereditary Cancer Registry is supported in part by award number RC4CA153828 from the National Cancer Institute
FUNDING This study was supported by unrestricted financial grants of the Sacha Swarttouw-Hijmans foundation, the Dutch Cancer Society, the Deutsche Krebshilfe (German Cancer Aid), the Hong Kong Cancer Fund, the Hungarian Research Grant OTKA, the Norwegian EEA Financial Mechanism (Hungarian National Institute of Oncology) and the National Cancer Institute.
Footnotes
Conflicts of interest All authors state that there are no conflicts of interest.
Author contributions and statement M.J.L.L. and N.H. designed the study. C.W.O., M.J.E.K. and R.S.v.d.P. set up the database, collected and analyzed the clinical data. R.P.K. and M.J.L.L. characterized the different deletions. P.O.C, P.H., N.R, H.K.S., V.S., E. H.-F., M.M., M.K., R.C.N., R.H.S., I.K, F.B.L.H., E.M.L, J.J.P.G., C.M.A., E.J.W.R., F.J.H., C.M.J.T., B.P.M.v.N., M.E.v.G., E.B.G.G., D.M.E., D.J.B., S.S., E.M.S., J.O.C., M.R.P., T.G., L.V., J.P., E.O., T.L.C., S.Y.L., were responsible for clinical and/or molecular data acquisition; E.V. performed bio-informatic analyses of the intergenic region. J.H.v.K., I.D.N, M.G. and R.B. collected and analyzed pathological materials. L.A.L.M.K. and M.J.E.K. were responsible for the statistical analyses, M.J.L.L. and N.H. supervised the work. C.W.O. M.J.E.K., R.P.K, A.G.v.K., N.H. and M.J.L.L. wrote the manuscript, with assistance and final approval from all coauthors. As corresponding author, M.J.L.L., states that she had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Contributor Information
Marlies JE Kempers, Department of Human Genetics, Radboud University Nijmegen Medical Centre, Nijmegen, The Netherlands.
Roland P Kuiper, Department of Human Genetics, Radboud University Nijmegen Medical Centre, Nijmegen, The Netherlands.
Charlotte W Ockeloen, Department of Human Genetics, Radboud University Nijmegen Medical Centre, Nijmegen, The Netherlands.
Pierre O Chappuis, Oncogenetics and Cancer Prevention Unit, Division of Oncology, Department of Internal Medicine, Division of Genetic Medicine, Department of Genetic Medicine and Laboratory, University Hospitals, Geneva, Switzerland.
Pierre Hutter, Unit of Genetics, Institut Central des Hôpitaux Valaisans, Sion, Switzerland.
Nils Rahner, Institute of Human Genetics, University of Bonn, Germany.
Prof Hans K Schackert, Department of Surgical Research, Technische Universität Dresden, Germany.
Verena Steinke, Institute of Human Genetics, University of Bonn, Germany.
Elke Holinski-Feder, University Hospital of the Ludwig-Maximilians-University, Campus Innenstadt, Center of Medical Genetics, Munich, Germany.
Monika Morak, University Hospital of the Ludwig-Maximilians-University, Campus Innenstadt, Center of Medical Genetics, Munich, Germany.
Matthias Kloor, Institute of Pathology, Department of Applied Tumor Biology, University Hospital Heidelberg, Heidelberg, Germany.
Reinhard Büttner, Institute of Pathology, University of Bonn, Germany.
Eugene TP Verwiel, Department of Human Genetics, Radboud University Nijmegen Medical Centre, Nijmegen, The Netherlands.
J. Han van Krieken, Department of Pathology, Radboud University Nijmegen Medical Centre, Nijmegen, The Netherlands.
Iris D Nagtegaal, Department of Pathology, Radboud University Nijmegen Medical Centre, Nijmegen, The Netherlands.
Monique Goossens, Department of Pathology, Radboud University Nijmegen Medical Centre, Nijmegen, The Netherlands.
Rachel S. van der Post, Department of Pathology, Radboud University Nijmegen Medical Centre, Nijmegen, The Netherlands.
Renée C Niessen, Department of Genetics, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands.
Rolf H Sijmons, Department of Genetics, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands.
Irma Kluijt, Family Cancer Clinic, The Netherlands Cancer Institute, Amsterdam, The Netherlands.
Frans BL Hogervorst, Family Cancer Clinic, The Netherlands Cancer Institute, Amsterdam, The Netherlands.
Edward M Leter, Department of Clinical Genetics, VU University Medical Center, Amsterdam, The Netherlands.
Johan JP Gille, Department of Clinical Genetics, VU University Medical Center, Amsterdam, The Netherlands.
Cora M Aalfs, Department of Clinical Genetics, Academic Medical Centre, University of Amsterdam, The Netherlands.
Egbert JW Redeker, Department of Clinical Genetics, Academic Medical Centre, University of Amsterdam, The Netherlands.
Frederik J Hes, Department of Clinical Genetics, Leiden University Medical Centre, The Netherlands.
Carli MJ Tops, Department of Clinical Genetics, Leiden University Medical Centre, The Netherlands.
Bernadette PM van Nesselrooij, Department of Medical Genetics, Utrecht, The Netherlands.
Marielle E van Gijn, Department of Medical Genetics, Utrecht, The Netherlands.
Encarna B Gómez García, Department of Genetics and Cell Biology, University Medical Centre, and Research Institute for Growth and Development (GROW), Maastricht, The Netherlands.
prof Diana M Eccles, Wessex Clinical Genetics Service, Southampton University Hospitals NHS Trust. Southampton, United Kingdom.
David J Bunyan, Wessex Regional Genetics Laboratory, Salisbury District Hospital, Salisbury, Wiltshire, United Kingdom.
Sapna Syngal, Dana Farber Cancer Institute, Boston Massachusetts.
Elena M Stoffel, Dana Farber Cancer Institute, Boston Massachusetts.
Julie O Culver, Division of Clinical Cancer Genetics, City of Hope Comprehensive Cancer Center, Duarte, California, USA.
Melanie R Palomares, Division of Clinical Cancer Genetics, City of Hope Comprehensive Cancer Center, Duarte, California, USA.
Tracy Graham, Odette Cancer Centre, Sunnybrook Health Sciences Centre, Toronto, Canada.
Lea Velsher, Odette Cancer Centre, Sunnybrook Health Sciences Centre, Toronto, Canada.
Janos Papp, Department of Molecular Genetics, National Institute of Oncology, Budapest, Hungary.
Prof Edith Oláh, Department of Molecular Genetics, National Institute of Oncology, Budapest, Hungary.
Tsun L Chan, Hereditary Gastrointestinal Cancer Genetic Diagnosis Laboratory, Department of Pathology, The University of Hong Kong, Queen Mary Hospital, Pokfulam, Hong Kong.
Suet Y Leung, Hereditary Gastrointestinal Cancer Genetic Diagnosis Laboratory, Department of Pathology, The University of Hong Kong, Queen Mary Hospital, Pokfulam, Hong Kong.
Prof Ad Geurts van Kessel, Department of Human Genetics, Radboud University Nijmegen Medical Centre, Nijmegen, The Netherlands.
Lambertus ALM Kiemeney, Department of Epidemiology, Biostatistics and HTA, Radboud University Nijmegen Medical Centre, Nijmegen, The Netherlands.
Prof Nicoline Hoogerbrugge, Department of Human Genetics, Radboud University Nijmegen Medical Centre, Nijmegen, The Netherlands.
Marjolijn JL Ligtenberg, Department of Human Genetics, Radboud University Nijmegen Medical Centre, Nijmegen, The Netherlands; Department of Pathology, Radboud University Nijmegen Medical Centre, Nijmegen, The Netherlands.
Reference List
- 1.Lynch HT, Lynch PM, Lanspa SJ, Snyder CL, Lynch JF, Boland CR. Review of the Lynch syndrome: history, molecular genetics, screening, differential diagnosis, and medicolegal ramifications. Clin Genet. 2009;76:1–18. doi: 10.1111/j.1399-0004.2009.01230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koornstra JJ, Mourits MJ, Sijmons RH, Leliveld AM, Hollema H, Kleibeuker JH. Management of extracolonic tumours in patients with Lynch syndrome. Lancet Oncol. 2009;10:400–8. doi: 10.1016/S1470-2045(09)70041-5. [DOI] [PubMed] [Google Scholar]
- 3.Hendriks YM, Wagner A, Morreau H, Menko F, Stormorken A, Quehenberger F, et al. Cancer risk in hereditary nonpolyposis colorectal cancer due to MSH6 mutations: impact on counseling and surveillance. Gastroenterology. 2004;127:17–25. doi: 10.1053/j.gastro.2004.03.068. [DOI] [PubMed] [Google Scholar]
- 4.Vasen HF, Moslein G, Alonso A, Bernstein I, Bertario L, Blanco I, et al. Guidelines for the clinical management of Lynch syndrome (hereditary non-polyposis cancer) J Med Genet. 2007;44:353–62. doi: 10.1136/jmg.2007.048991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Post RS, Kiemeney LA, Ligtenberg MJ, Witjes JA, Hulsbergen-van de Kaa CA, Bodmer D, et al. Risk of urothelial bladder cancer in Lynch syndrome is increased, in particular among MSH2 mutation carriers. J.Med.Genet. 2010;47(7):464–470. doi: 10.1136/jmg.2010.076992. Ref Type: Journal (Full) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ligtenberg MJ, Kuiper RP, Chan TL, Goossens M, Hebeda KM, Voorendt M, et al. Heritable somatic methylation and inactivation of MSH2 in families with Lynch syndrome due to deletion of the 3′ exons of TACSTD1. Nat Genet. 2009;41:112–7. doi: 10.1038/ng.283. [DOI] [PubMed] [Google Scholar]
- 7.Kovacs ME, Papp J, Szentirmay Z, Otto S, Olah E. Deletions removing the last exon of TACSTD1 constitute a distinct class of mutations predisposing to Lynch syndrome. Hum Mutat. 2009;30:197–203. doi: 10.1002/humu.20942. [DOI] [PubMed] [Google Scholar]
- 8.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–68. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 9.Todaro M, Francipane MG, Medema JP, Stassi G. Colon cancer stem cells: promise of targeted therapy. Gastroenterology. 2010;138:2151–62. doi: 10.1053/j.gastro.2009.12.063. [DOI] [PubMed] [Google Scholar]
- 10.Munz M, Kieu C, Mack B, Schmitt B, Zeidler R, Gires O. The carcinoma-associated antigen EpCAM upregulates c-myc and induces cell proliferation. Oncogene. 2004;23:5748–58. doi: 10.1038/sj.onc.1207610. [DOI] [PubMed] [Google Scholar]
- 11.Litvinov SV, Balzar M, Winter MJ, Bakker HA, Briaire-de B I, Prins F, et al. Epithelial cell adhesion molecule (Ep-CAM) modulates cell-cell interactions mediated by classic cadherins. J Cell Biol. 1997;139:1337–48. doi: 10.1083/jcb.139.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niessen RC, Hofstra RM, Westers H, Ligtenberg MJ, Kooi K, Jager PO, et al. Germline hypermethylation of MLH1 and EPCAM deletions are a frequent cause of Lynch syndrome. Genes Chromosomes Cancer. 2009;48:737–44. doi: 10.1002/gcc.20678. [DOI] [PubMed] [Google Scholar]
- 13.Nagasaka T, Rhees J, Kloor M, Gebert J, Naomoto Y, Boland CR, et al. Somatic hypermethylation of MSH2 is a frequent event in Lynch Syndrome colorectal cancers. Cancer Res. 2010;70:3098–108. doi: 10.1158/0008-5472.CAN-09-3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van der Klift H, Wijnen J, Wagner A, Verkuilen P, Tops C, Otway R, et al. Molecular characterization of the spectrum of genomic deletions in the mismatch repair genes MSH2, MLH1, MSH6, and PMS2 responsible for hereditary nonpolyposis colorectal cancer (HNPCC) Genes Chromosomes Cancer. 2005;44:123–38. doi: 10.1002/gcc.20219. [DOI] [PubMed] [Google Scholar]
- 15.Guarinos C, Castillejo A, Barbera VM, Perez-Carbonell L, Sanchez-Heras AB, Segura A, et al. EPCAM Germ Line Deletions as Causes of Lynch Syndrome in Spanish Patients. J Mol Diagn. 2010 doi: 10.2353/jmoldx.2010.100039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan TL, Yuen ST, Kong CK, Chan YW, Chan AS, Ng WF, et al. Heritable germline epimutation of MSH2 in a family with hereditary nonpolyposis colorectal cancer. Nat Genet. 2006;38:1178–83. doi: 10.1038/ng1866. [DOI] [PubMed] [Google Scholar]
- 17.Kiemeney LA, Lemmers FA, Verhoeven RH, Aben KK, Honing C, de NJ, et al. The risk of cancer in the Netherlands. Ned Tijdschr Geneeskd. 2008;152:2233–41. [PubMed] [Google Scholar]
- 18.Association of Comprehensive Cancer Centres (ACCC) The Netherlands; www.ikcnet.nl. [Google Scholar]
- 19.The Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute (NCI) USA: www.seer.cancer.gov. [PubMed] [Google Scholar]
- 20.Stoffel E, Mukherjee B, Raymond VM, Tayob N, Kastrinos F, Sparr J, et al. Calculation of risk of colorectal and endometrial cancer among patients with Lynch syndrome. Gastroenterology. 2009;137:1621–7. doi: 10.1053/j.gastro.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vasen HF, Wijnen JT, Menko FH, Kleibeuker JH, Taal BG, Griffioen G, et al. Cancer risk in families with hereditary nonpolyposis colorectal cancer diagnosed by mutation analysis. Gastroenterology. 1996;110:1020–7. doi: 10.1053/gast.1996.v110.pm8612988. [DOI] [PubMed] [Google Scholar]
- 22.Ramsoekh D, Wagner A, van Leerdam ME, Dooijes D, Tops CM, Steyerberg EW, et al. Cancer risk in MLH1, MSH2 and MSH6 mutation carriers; different risk profiles may influence clinical management. Hered Cancer Clin Pract. 2009;7:17. doi: 10.1186/1897-4287-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barrow E, Robinson L, Alduaij W, Shenton A, Clancy T, Lalloo F, et al. Cumulative lifetime incidence of extracolonic cancers in Lynch syndrome: a report of 121 families with proven mutations. Clin Genet. 2009;75:141–9. doi: 10.1111/j.1399-0004.2008.01125.x. [DOI] [PubMed] [Google Scholar]
- 24.Baglietto L, Lindor NM, Dowty JG, White DM, Wagner A, Gomez Garcia EB, et al. Risks of Lynch syndrome cancers for MSH6 mutation carriers. J Natl Cancer Inst. 2010;102:193–201. doi: 10.1093/jnci/djp473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kastrinos F, Mukherjee B, Tayob N, Wang F, Sparr J, Raymond VM, et al. Risk of pancreatic cancer in families with Lynch syndrome. JAMA. 2009;302:1790–5. doi: 10.1001/jama.2009.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koornstra JJ, Kleibeuker JH, Vasen HF. Small-bowel cancer in Lynch syndrome: is it time for surveillance? Lancet Oncol. 2008;9:901–5. doi: 10.1016/S1470-2045(08)70232-8. [DOI] [PubMed] [Google Scholar]
- 27.Scherer SJ, Seib T, Seitz G, Dooley S, Welter C. Isolation and characterization of the human mismatch repair gene hMSH2 promoter region. Hum Genet. 1996;97:114–6. doi: 10.1007/BF00218844. [DOI] [PubMed] [Google Scholar]
- 28.Iwahashi Y, Ito E, Yanagisawa Y, Akiyama Y, Yuasa Y, Onodera T, et al. Promoter analysis of the human mismatch repair gene hMSH2. Gene. 1998;213:141–7. doi: 10.1016/s0378-1119(98)00187-5. [DOI] [PubMed] [Google Scholar]
- 29.Public chromatin-state map by Broad/MGH ENCODE group http://genome.ucsc.edu/
- 30.Mendenhall EM, Bernstein BE. Chromatin state maps: new technologies, new insights. Curr Opin Genet Dev. 2008;18:109–15. doi: 10.1016/j.gde.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bernstein BE, Kamal M, Lindblad-Toh K, Bekiranov S, Bailey DK, Huebert DJ, et al. Genomic maps and comparative analysis of histone modifications in human and mouse. Cell. 2005;120:169–81. doi: 10.1016/j.cell.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 32.Eissenberg JC, Shilatifard A. Histone H3 lysine 4 (H3K4) methylation in development and differentiation. Dev Biol. 2010;339:240–9. doi: 10.1016/j.ydbio.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watson P, Vasen HF, Mecklin JP, Bernstein I, Aarnio M, Jarvinen HJ, et al. The risk of extra-colonic, extra-endometrial cancer in the Lynch syndrome. Int J Cancer. 2008;123:444–9. doi: 10.1002/ijc.23508. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.