Abstract
Little is known about the prevalence and outcomes of patients with atrial fibrillation/flutter (AF) who receive a kidney transplant. We identified all patients who had >1 year of uninterrupted Medicare A+B coverage before receiving their first kidney transplant (1996–2009). The presence of pre-transplant AF was ascertained from diagnosis codes in Medicare physician claims. We studied the post-transplant outcomes of death, all-cause graft failure, death-censored graft failure, and stroke using multivariable Cox regression. Of 62,706 eligible first kidney transplant recipients studied, 3794 (6.4%) were diagnosed with AF prior to kidney transplant. Over a mean follow-up of 4.9 years, 40.6% of AF patients and 24.9% without AF died. All-cause and death-censored graft failure were 46.8% and 16.5%, respectively, in the AF group and 36.4% and 19.5%, respectively, in those without AF. Ischemic stroke occurred in 2.8% of patients with and 1.6% of patients without AF. In patients with AF, multivariable-adjusted hazard ratios (95% confidence intervals) for death, graft failure, death-censored graft failure, and ischemic stroke were 1.46 (1.38–1.54), 1.41 (1.34–1.48), 1.26 (1.15–1.37) and 1.36 (1.10–1.68), respectively. Pre-existing AF is associated with poor post-transplant outcomes. Special attention should be paid to AF in pre-transplant evaluation, counseling, and risk stratification of kidney transplant candidates.
Keywords: arrhythmia, end-stage renal disease, risk assessment, organ allocation
Introduction
Atrial fibrillation/flutter (AF) is a common diagnosis that will affect an estimated 15.6 million people in the US by 2050 and is associated with significantly increased risk of embolic stroke and mortality (1–3). AF is a particular problem the dialysis population where its prevalence has increased from 3.5% in 1992 to 10.7% in 2006 and remains associated with a doubling of one-year mortality (4). In dialysis patients, AF is associated with older age and an increased burden of cardiovascular disease. In addition, the benefits of anticoagulation in AF, which are well established in the general population, have not consistently been demonstrated in end stage renal disease (ESRD) (5–7).
Kidney transplantation is the best treatment for ESRD in terms of life expectancy and quality of life (8). However, the survival benefit of transplantation becomes evident only after an initial increase in short-term mortality from surgery. Detailed pre-transplant evaluation is key in identifying candidates that will benefit from renal transplantation with particular focus on the diagnosis and treatment of cardiovascular disease prior to wait listing and transplantation. While the incidence of new AF after kidney transplantation and associated outcomes have been studied, little is known about how patients with pre-existing AF will fare after receipt of their kidney transplant (9,10).
The goal of this study was 1) to establish the prevalence of previously diagnosed AF in U.S. ESRD patients undergoing renal transplantation and 2) to determine its association with post-transplant outcomes.
Materials and Methods
Study Population
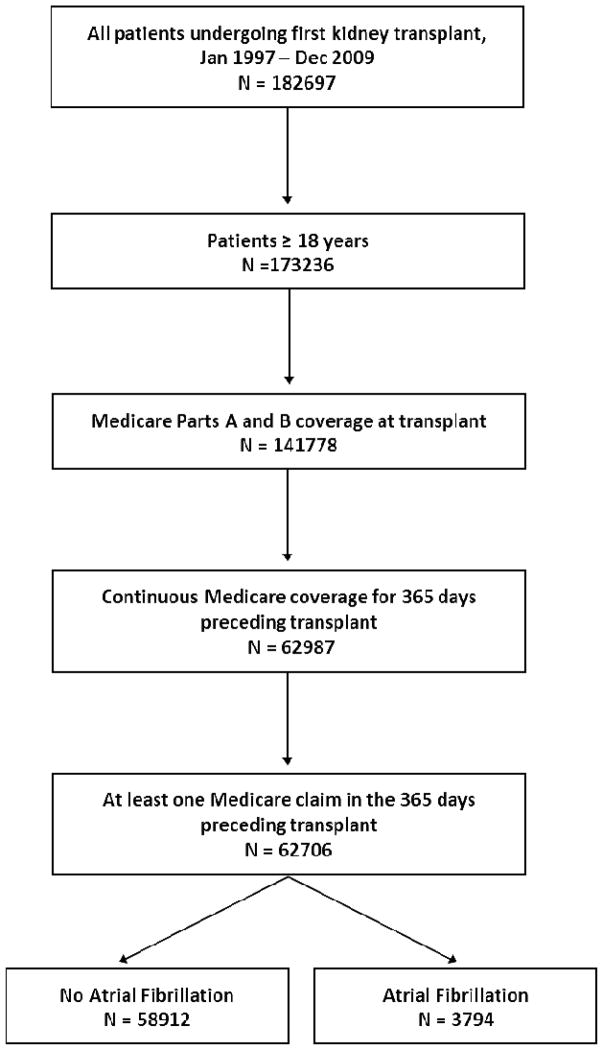
We identified all adult patients (≥18 years) in the US Renal Data System who received their first kidney transplant between January 1997 and December 2009. We restricted the cohort to all patients who had 1) uninterrupted Medicare Part A and B coverage (per payor history file) in the year prior to transplant and 2) in whom at least one valid claim was filed to Medicare during that period.
Variable of Interest
Whether patients had been diagnosed with AF prior to the day of their kidney transplant surgery (= index date) was the variable of interest, which was ascertained from all inpatient and outpatient physician billing claims during the 365 days prior to the index date. We considered patients to have been diagnosed with AF if any International Classification of Diseases, 9th revision, codes of 427.3, 427.31, or 427.32 were listed as diagnoses on 1 inpatient or 2 outpatient claims. Such an approach has been used to identify Medicare patients for the National Registry of Atrial Fibrillation (11) and other claims-based research studies of AF (12, 13). This approach has been shown to have a sensitivity of 94%, specificity of 99% and positive predictive value of 97% (14,15).
Patients Characteristics
We ascertained the following characteristics from the USRDS patient, treatment history and transplant files: recipient age (on transplant date), sex, race (white, black, other), cause of ESRD, body mass index (BMI) at time of transplant, dialysis duration and most recent dialysis modality, patient blood type, transplant type (living, standard deceased, expanded criteria deceased, donation after cardiac death), donor age and sex, HLA-match, panel reactive antibody, and cold ischemia time.
We additionally identified the following co-morbidities using the appropriate ICD-9 codes (see Technical Appendix, Supplemental Table S1): diabetes, cancer, coronary artery disease, cerebrovascular disease, alcohol misuse, peripheral vascular disease (PVD), hypertension, valve disease, heart failure, chronic pulmonary disease, and prior solid organ transplant (heart, lung, liver, pancreas). Co-morbidities were ascertained in the 365 day period prior to the date of transplant surgery and were established by at least 1 inpatient or 2 outpatient claims not on the same day. We also quantified health care utilization in the year prior to transplant by determining whether the patient was in a skilled nursing facility, the number of days spent in hospital, and the number of non-nephrology outpatient visits.
Outcomes
Our outcomes of interest were death from any cause, graft failure, death-censored graft failure, and ischemic stroke. Graft failure was identified from the USRDS patient files and defined as death, need for dialysis or re-transplant. Ischemic stroke was identified from billing claims and was defined by the presence of one inpatient claim with an ICD-9 primary diagnosis code of 433.x1, 434.x1, 436, or 437.1 or by stroke as cause of death. For the outcomes of death and graft failure, patients were censored at end of study, December 31, 2009. For ischemic stroke, patients were censored at end of study, loss of Medicare Parts A and B coverage and at 3 years post-transplant (when most patients under 65 years of age lose their Medicare coverage).
Statistical Analysis
Our cohort was divided according to the presence or absence of AF prior to transplant. Categorical variables were expressed as counts and percentages and continuous variables as medians with an interquartile range. Differences between the two groups were tested using the Chi-squared test for categorical and the Kolmogorov-Smirnov test for continuous variables. Unadjusted incidence rates, defined as the number of events over person-time observed, were calculated for each outcome.
We used multivariate Cox proportional hazards models, stratified by year of transplant, to calculate adjusted hazard ratios (HR) for each outcome. Hazard ratios were generated using three incrementally adjusted models: model 1, adjusted for demographic variables; model 2, additionally adjusted for BMI, comorbidities, healthcare utilization and dialysis history; and model 3, additionally adjusted for transplant related variables. The large sample size and number of outcomes allowed us to present comprehensive models without selecting from the available set specific variables based on significance thresholds or confounding criteria. We used log (− log) survival curves and plots of Loess smoothed scaled Schoenfeld residuals to assess the adequacy of the Cox proportional hazards models. We also analyzed death as a competing risk for both graft loss and ischemic stroke. To do this the same series of Cox proportional hazards models were fit with death as a censoring event. Detailed description of the analysis is available in the Technical Appendix.
We also analyzed death as a competing risk for both graft loss and ischemic stroke. To do this, the same series of Cox proportional hazards models were fit with death as a censoring event. A detailed description of the analytical approach is available in the Technical Appendix.
Missing Data
About 42% of patients had at least one variable missing, with the proportion of variables with data missing ranging from less than 1% (recipient race) to 20% (BMI; table 1). To handle the missing data we used standard multiple imputation (MI) techniques (using SAS proc mi) to obtain 9 imputed datasets. One dataset was used for model checking and to the remaining 8 we fitted the stratified Cox proportional hazard models. The results of the 8 fitted models were then combined using the rules described by Roderick and Little (16) (using SAS proc mianalyze). The imputation model included all variables. To help the imputation of BMI at transplant, we also included BMI at time of transplant wait-listing or, if missing, BMI from the Medical Evidence Report, and time between BMI reporting and transplantation. For technical details, see the Technical Appendix.
Table 1.
Characteristics of First Kidney Transplant Recipients, by Presence of Atrial Fibrillation
| All N = 62,706 |
No Atrial Fibrillation N=58,912 |
Atrial Fibrillation N=3,794 |
P-value1 | |
|---|---|---|---|---|
| Patient Demographics | ||||
| Patient age | 51 (40–61) | 50 (39–60) | 60 (52–67) | <0.001 |
| Female | 39.3 | 39.8 | 31.2 | <0.001 |
| Race2 | ||||
| White | 57.5 | 56.7 | 70.3 | <0.001 |
| Black | 34.8 | 35.5 | 23.9 | |
| Other | 7.7 | 7.8 | 5.7 | |
| BMI at transplant (kg/m2) | ||||
| < 20 | 2.0 | 2.0 | 1.6 | <0.001 |
| 20– 25 | 27.7 | 28.0 | 23.6 | |
| 26–30 | 26.7 | 26.6 | 29.1 | |
| >30 | 22.9 | 22.6 | 27.0 | |
| Missing | 20.7 | 20.8 | 18.8 | |
| Cause of ESRD | ||||
| Diabetes | 31.6 | 31.4 | 33.9 | <0.001 |
| Hypertension | 24.3 | 24.2 | 25.4 | |
| Glomerulonephritis | 25.0 | 25.2 | 21.1 | |
| Other | 18.2 | 18.1 | 18.6 | |
| Missing | 1.0 | 1.0 | 1.1 | |
| Dialysis Vintage (years) | ||||
| < 2.5 years | 26.2 | 26.1 | 26.7 | 0.01 |
| 2.5 – 5 years | 43.6 | 43.7 | 41.4 | |
| > 5 years | 30.3 | 30.1 | 31.9 | |
| Dialysis Modality | ||||
| Hemodialysis | 85.7 | 85.4 | 90.9 | <0.001 |
| Peritoneal Dialysis | 14.2 | 14.5 | 8.9 | |
| Missing | 0.2 | 0.2 | 0.2 | |
| Skilled Nursing Facility Utilization | 2.5 | 2.2 | 7.0 | <0.001 |
| Hospital days (N) | 4 (2–10) | 4 (2–9) | 10 (4–20) | <0.001 |
| Non-nephrology clinic visits (N) | 18 (9–30) | 17 (9–29) | 27 (16–42) | <0.001 |
| Comorbidities | ||||
| Diabetes | 42.3 | 39.3 | 49.0 | <0.001 |
| Cancer | 3.7 | 3.3 | 6.0 | <0.001 |
| Coronary artery disease | 11.8 | 9.2 | 24.0 | <0.001 |
| Cerebrovascular disease | 8.4 | 7.2 | 16.2 | <0.001 |
| Cerebral hemorrhage | 0.5 | 0.5 | 1.2 | <0.001 |
| Alcohol | 1.1 | 0.8 | 1.0 | 0.12 |
| Tobacco | 6.3 | 4.5 | 5.0 | 0.23 |
| PVD | 16.6 | 13.6 | 26.6 | <0.001 |
| Hypertension | 95.4 | 80.5 | 95.0 | <0.001 |
| Valve disease | 12.6 | 9.7 | 29.8 | <0.001 |
| Heart failure | 22.8 | 18.1 | 44.9 | <0.001 |
| Chronic pulmonary disease | 13.0 | 10.3 | 22.9 | <0.001 |
| Previous Solid Organ Transplant | ||||
| Heart transplant | 0.6 | 0.6 | 1.0 | <0.001 |
| Lung transplant | 0.1 | 0.1 | 0.4 | <0.001 |
| Liver transplant | 0.9 | 0.8 | 1.5 | <0.001 |
| Pancreas transplant | 0.4 | 0.4 | 0.2 | 0.03 |
| Patient blood type | ||||
| O | 48.5 | 48.6 | 45.7 | <0.001 |
| A | 33.0 | 32.8 | 37.1 | |
| B | 14.6 | 14.7 | 13.0 | |
| AB | 3.8 | 3.7 | 4.1 | |
| Missing | 0.1 | 0.1 | 0.1 | |
| Panel reactive antibody | ||||
| 0–10% | 65.2 | 65.2 | 67.5 | 0.001 |
| 11–80% | 18.6 | 18.6 | 18.3 | |
| >80% | 6.3 | 6.3 | 6.0 | |
| Missing | 9.9 | 10.0 | 8.2 | |
| Transplant Characteristics | ||||
| Donor age | 39 (24–51) | 39 (24–50) | 43 (28–53) | <0.001 |
| Missing | 7.2 | 7.2 | 6.1 | |
| Donor Gender | ||||
| Female | 43.5 | 43.4 | 44.7 | 0.03 |
| Missing | 0.2 | 0.2 | 0.1 | |
| Transplant type | ||||
| Living donor | 16.6 | 16.5 | 17.6 | <0.001 |
| Standard deceased donor | 62.5 | 62.7 | 58.2 | |
| Expanded criteria donor | 8.6 | 8.4 | 11.7 | |
| Donation after cardiac death | 5.3 | 5.2 | 6.6 | |
| Missing | 7.2 | 7.3 | 5.9 | |
| Number HLA mismatches | ||||
| 0 | 8.7 | 8.6 | 10.0 | <0.001 |
| 1–3 | 26.2 | 26.2 | 25.6 | |
| 4–6 | 59.0 | 58.9 | 60.2 | |
| Missing | 6.1 | 6.3 | 4.3 | |
| Cold ischemia time (hours) | ||||
| <10 | 20.2 | 20.1 | 21.0 | <0.001 |
| 10 – 23 | 41.0 | 41.0 | 40.7 | |
| > 23 | 21.9 | 21.8 | 23.5 | |
| Missing | 17.0 | 17.1 | 14.8 | |
All values are presented as percent or median (interquartile range).
p-values based on Chi-square tests for categorical variables and Kolmogorov-Smirnov test for continuous variables.
Fourteen patients had missing race all belonging to the No Atrial Fibrillation group.
All estimates are accompanied by their corresponding 95% confidence intervals (CI). Analyses were performed using SAS software, version 9.2 (SAS Institute, Inc., Cary, NC) and R (The R Project for Statistical Computing; Vienna, Austria). The Institutional Review Board of Stanford University approved the study.
Results
We identified 62,706 patients undergoing their first kidney transplant between 1997 and 2009 who met our study inclusion criteria (Figure 1). Of those, 3794 (6.4%) patients were diagnosed with AF prior to transplantation. Patients with atrial fibrillation (AF+) were older and had a higher burden of comorbidities than patients without atrial fibrillation (AF−). Donor age was also higher in patients with AF reflecting the higher number of expanded criteria kidneys performed in this group. Most comorbid conditions, especially cardiovascular ones, were much more prevalent among AF+ patients. Baseline characteristics for the cohort as a whole and stratified according to presence of AF at baseline are shown in Table 1.
Figure 1.
Cohort Flow Chart
During a mean follow-up period of 4.9 years 1539 (40.6%) of AF+ patients and 14,642 (24.9%) of AF− patients died. All-cause graft loss occurred in 1775 (46.8%) in AF+ and 21,466 (36.4%) AF− patients, while death-censored graft failure occurred in 624 (16.5%) in AF+ and 11,482 (19.5%) AF− patients. During a mean follow-up period of 2.2 years, 107 (2.8%) of AF+ patients and 921 (1.6%) of patients AF− patients had an ischemic stroke (all p<0.001; Table 2).
Table 2.
Follow-up and Study Outcomes
| Outcome | No Atrial Fibrillation N=58,912 |
Atrial Fibrillation N=3,794 |
|---|---|---|
| Death | ||
| Number of events | 14,642 | 1,539 |
| Person-time at risk (years) | 292,976 | 13,403 |
| Incidence rate (events/100 person-years) | 4.998 | 11.482 |
| All cause graft loss | ||
| Number of events | 21,446 | 1,775 |
| Person-time at risk (years) | 254,538 | 12,052 |
| Incidence rate (events/100 person-years) | 8.43 | 14.73 |
| Death-censored graft loss | ||
| Number of events | 11,482 | 624 |
| Person-time at risk (years) | 254,538 | 12,052 |
| Incidence rate (events/100 person-years) | 4.51 | 5.18 |
| Death-censored Ischemic stroke1 | ||
| Number of events | 921 | 107 |
| Person-time at risk (years) | 131,126 | 7336 |
| Incidence rate (events/100 person-years) | 0.70 | 1.46 |
Ischemic stroke includes non-fatal events and death from any type of stroke
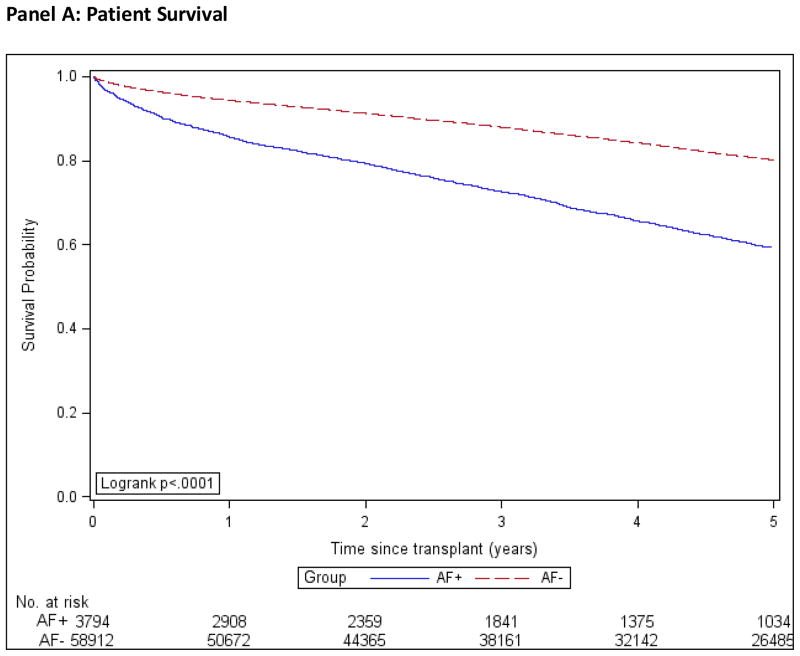
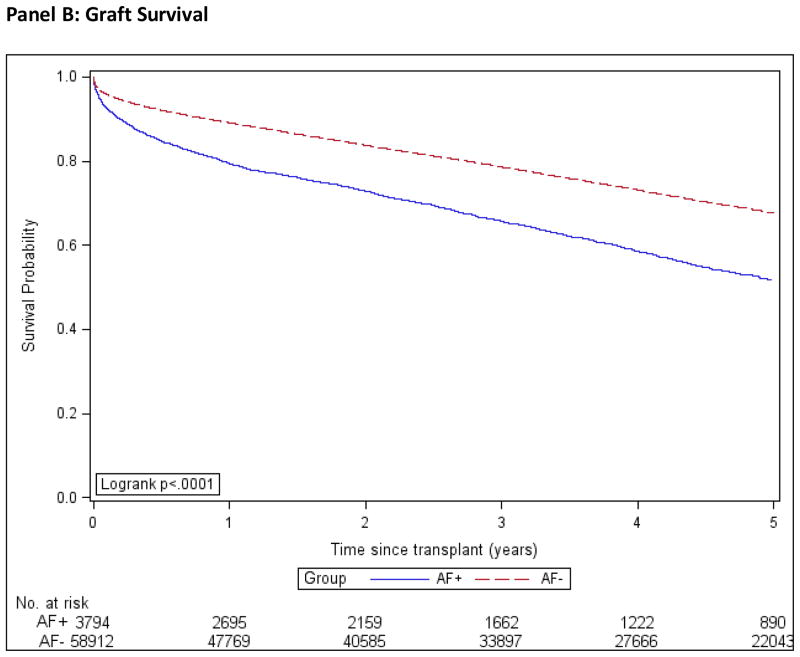
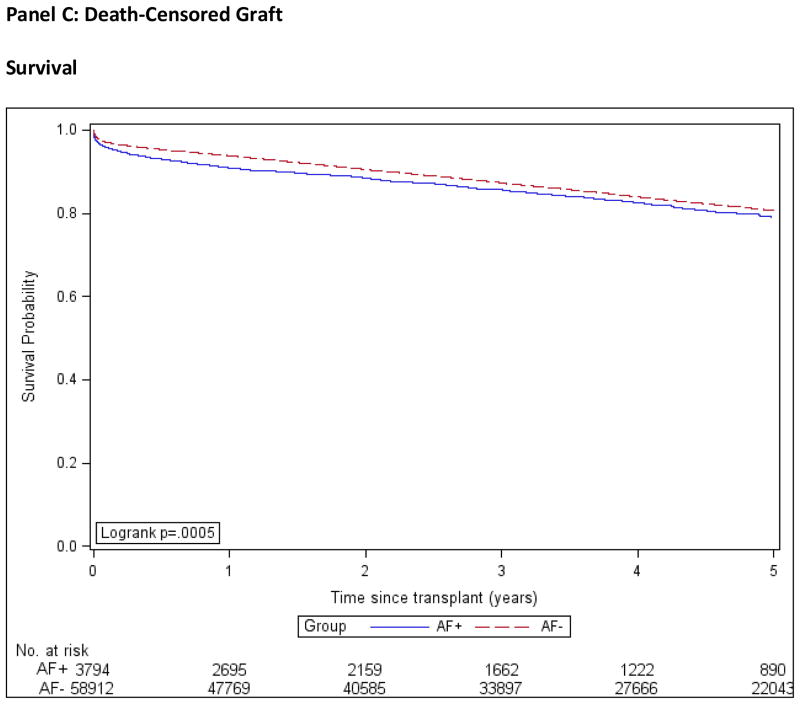
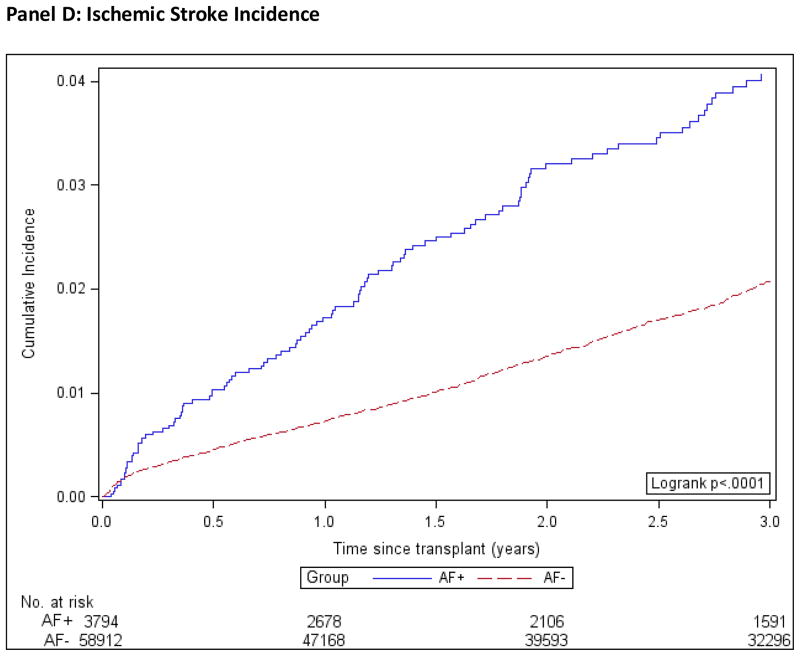
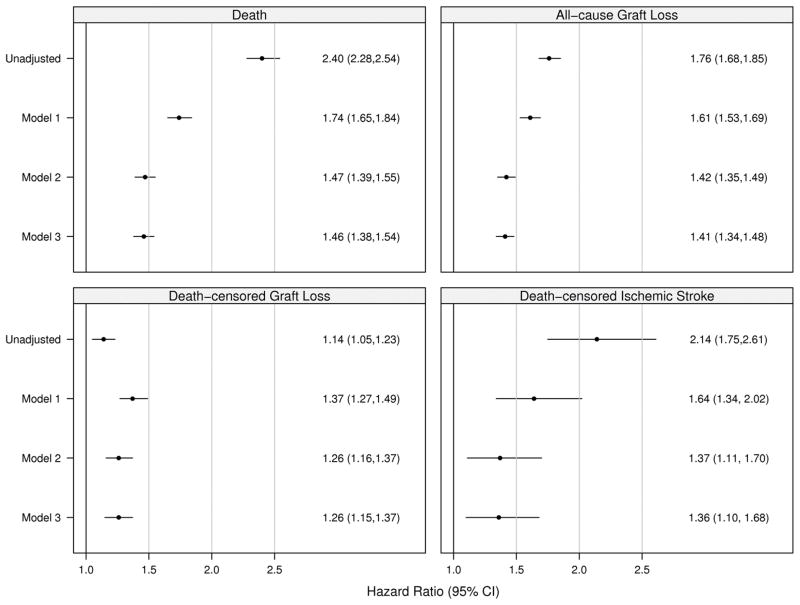
Patient, graft and death-censored graft survival were calculated using the Kaplan-Meier method and are shown in Figure 2. We used multivariable Cox regression to compare the outcomes of death, all-cause graft failure, death-censored graft failure, and stroke in the AF+ and AF− groups, while controlling for important confounding characteristics. We used three different models; model 1 was adjusted for demographic factors only, model 2 for demographics and co-morbidities and model 3 for demographics, co-morbidities and transplant covariates, Figure 3. The estimates of association for all co-variables are shown in the Technical Appendix, Supplemental Table S2.
Figure 2. Kaplan-Meier Product Limit Estimates of Study Outcomes.
AF+: Patients with atrial fibrillation.
AF−: Patients without atrial fibrillation.
Incidence computed by the Kaplan-Meier method.
Figure 3. Unadjusted and Adjusted Hazard Ratios for Study Outcomes.
Stratified Cox analysis with multiple imputation to handle missing data.
All models stratified by Year of transplant.
Model 1 is adjusted for age, sex, and race.
Model 2 is additionally adjusted for BMI at transplant, cause of ESRD, dialysis vintage, dialysis modality, skilled nursing facility utilization indicator, number of hospital days, number of non-nephrology clinic visits, previous solid organ transplant, and all comorbidities.
Model 3 is additionally adjusted for patient blood type, panel reactive antibody, donor age, donor sex, transplant type, number of HLA mismatches, and cold ischemia time.
Ischemic stroke includes events related to death from any kind of stroke.
In the AF+ group, estimated 1- and 5-year patient survival rates were 85.7% (CI, 84.5%–86.8%) and 59.3% (CI, 57.4% to 61.2%) respectively. In the AF− group, 1- and 5-year patient survival rates were 94.4% (CI, 94.2% to 94.6%) and 80.2% (CI, 79.8% to 80.5%) respectively. The hazard ratio for death in the AF+ group compared to AF− was 2.40 (CI, 2.28 to 2.54), which was attenuated to 1.46 (CI, 1.38 to 1.54) after multivariate adjustment in model 3.
In the AF+ group, estimated 1- and 5-year graft survival rates were 79.4% (CI, 78.1% to 80.7%) and 51.7% (CI, 49.7% to 53.6%) respectively and for the AF− group were 89.1% (CI, 88.9 to 89.4) and 67.6% (CI, 67.2% to 68.1%) respectively. The unadjusted hazard ratio for all-cause graft failure comparing the AF+ to AF− group was 1.76 (CI, 1.68 to 1.85), and 1.41 (CI, 1.34 to 1.48) after adjustment for all observed factors.
In the AF+ group, estimated 1- and 5-year death-censored graft survival rates were 90.9% (CI, 89.9% to 91.8%) and 79.0% (CI, 77.2% to 80.7%) respectively and in the AF− group were 93.8% (CI, 93.6% to 94%) and 80.6% (CI, 80.2% to 81%). The unadjusted hazard ratio for death-censored graft failure comparing the AF+ to AF− group was 1.14 (CI, 1.05 to 1.23), and 1.26 (CI, 1.15 to 1.37) after adjustment.
For post-transplant ischemic stroke, 1- and 3- year incidence estimates, computed using the 1-KM method, were 1.72% (CI, 1.33% to 2.24%) and 4.07% (CI, 3.36% to 4.92%), respectively, in the AF+ group and 0.72% (CI, 0.65% to 0.80%) and 2.07% (CI, 1.92% to 2.19%), respectively, in the AF− group (Figure 2, Panel D). The ischemic stroke incidence curves crossed immediately (within 30 days) post-transplant violating the proportional hazards assumption, therefore as a sensitivity analysis, we re-fitted the models excluding stroke and death occurring within the first 30 days post-transplant and found a clear separation of the curves. Moreover, hazard ratio estimates from this conditional model did not differ substantially from those utilizing the entire data. The multivariable adjusted HR for stroke was 1.36 (CI, 1.10–1.68) for AF+ patients compared with those without the arrhythmia.
As a sensitivity analysis, we compared results using multiple imputation with a complete case analysis. Complete-case analysis is a commonly used alternative to handling missing data in which only subjects without missing data are utilized for analysis. While complete-case analysis is a simple approach, it does make greater assumptions about missing data than multiple imputation and is generally considered an inferior method (17,18). With the exception of ischemic stroke, the results were very similar for all outcomes. For post-transplant ischemic stroke there is a loss of efficiency due to the loss of 42% of the sample in complete case analysis and the HR are biased towards the null and not significant.
We also analyzed death as a competing risk for both graft loss and ischemic stroke. The same series of models were fitted with death as a censoring event. For both outcomes, the adjusted hazard ratios remained similar and statistically significant. Results from all sensitivity analysis as well as competing risk analysis are available in Supplemental Tables S3–S6 and Supplemental Figure S1 in the Technical Appendix.
Discussion
In our large population of US kidney transplant recipients, pre-existing AF was strongly associated with adverse post-transplant outcomes. To our knowledge, this is the first study to focus on patients with known AF prior to transplant surgery. In the hemodialysis population, AF is associated with increased mortality (4). However, ESRD patients who undergo kidney transplantation are highly selected individuals that have passed through rigorous pre-transplant screening and have in many cases, survived long deceased donor waiting times (19). Nonetheless, we found that post-transplant death, graft failure and ischemic stroke were all significantly more likely in patients with pre-existing AF.
We found patients with AF to be older and have a higher burden of cardiovascular and non-cardiovascular comorbidities. Several of these, including diabetes, heart failure, coronary artery disease and COPD, are independently associated with poor transplant outcomes (20,21). Patients with AF also received more kidneys from expanded criteria donors, a reflection of the older age and higher burden of diabetes in the group, which have a shorter median half-life than standard deceased donor kidneys (22). However, the presence of AF remained strongly associated with poor outcomes even after adjustment for demographics, co-morbidities and transplant variables.
Although AF could simply be a marker of patient frailty or advanced cardiovascular disease not otherwise captured in the dataset, there are several plausible explanations for a causal relationship between AF and poor post-transplant outcomes. It is well-established that AF causes stroke, and CKD has been shown to increase the risk of stroke in AF (7). Furthermore, CKD is a strong predictor of mortality after stroke (23,24). In our study, patients with baseline AF had a 37% higher risk of post-transplant stroke than patients without AF, which is consistent with these prior observations. The association between AF and increased risk of graft failure is consistent with findings in patients without chronic kidney disease, in whom AF was associated with the new development of chronic kidney disease (first decline of eGFR to <60 mL/min/1.73m2 and decline of >10 mL/min/1.73m2 or development of new proteinuria)(25). This association may be explained by a higher risk of recurrent microembolism into the (transplant) kidney in patients with AF.
In addition, the therapies used to treat AF may themselves be harmful. The use of anticoagulation in renal transplant recipients with atrial fibrillation has not been well studied and it is not clear whether their AF management is in line with current guidelines (26). Post-transplant patients may be particularly predisposed to proarrhythmia from antiarrhythmic and rate-control drugs, particularly for drugs that are excreted by the kidney. The risk of proarrhythmia may be heightened from irreversible ventricular hypertrophy and myocardial fibrosis that develops in ESRD pre-transplant (27,28). AF is also associated with increased systemic inflammation (29,30), which along with decreased cardiac output (31), may potentiate decline in renal graft function.
Our study further emphasizes the association of AF with poor outcomes in ESRD patients. The findings complement previous studies that demonstrate increased mortality associated with the arrhythmia in the hemodialysis population and increased mortality and graft loss in patients who develop the arrhythmia post-transplant as previously described by Lentine and colleagues. (4,10). However, prospective studies are necessary to determine whether interventions such as rate or rhythm control will improve outcome.
Our findings also impact the pre-transplant evaluation process. The evaluation is designed to both select patients that will gain a survival advantage from transplant and to maximize the benefit to society of a very scarce resource. While there are relatively few absolute medical contraindications to kidney transplantation, the identification of markers for high-risk candidates is vital to guide the frequency of pre-transplant follow-up, pre-transplant diagnostic testing and decision-making in borderline candidates (19).
Our study has a number of strengths; it was designed to capture medical claims data in the year preceding transplantation in a large population of US ESRD patients. The use of Medicare Parts A and B claims data allows us to ascertain detailed medical and health care utilization data and link it to transplant outcomes through the US Renal Data System. Limitations of the study include the use of diagnosis codes, which likely underestimate the prevalence of AF in the ESRD population because AF may be overlooked in patients with numerous other co-morbidities. In addition, diagnostic coding for AF does not distinguish among persistent, paroxysmal or transient atrial fibrillation, although all forms of atrial fibrillation are associated with increased stroke risk (32,33). As all observational studies, we cannot rule out the presence of residual confounding by unobserved or inaccurately measured characteristics. By limiting our study group to patients with 1 year of Medicare Part A and B coverage we are selecting patients of relatively longer dialysis vintage, which is itself associated with poor transplant outcome (34). In addition, we do not have data on medication use, and specifically anti-coagulation and anti-platelet medications that impact the outcome of atrial fibrillation in the general population (5).
In fact our study serves to highlight the great number of unanswered questions about atrial fibrillation management in renal transplant recipients. The role of anticoagulation, of questionable benefit in the dialysis population [35], has not been studied at all in those ESRD patients that go on to receive a transplant. Practical questions about the optimal peri-operative management of atrial fibrillation and the timing of anticoagulation initiation post-transplant also arise. In addition evidence for the benefit of cardio-protective drugs such ACE inhibitors or angiotensin receptor blockers, which may ameliorate left ventricular hypertrophy and myocardial fibrosis, both risk factors for AF, is conflicting in kidney transplant recipients [36,37].
In conclusion, we have shown that pre-transplant atrial fibrillation is present in approximately 6% of patients undergoing renal transplantation and is associated with significantly higher post-transplant mortality, graft loss and stroke. Special attention should be paid to patients with AF being evaluated or on the wait-list for kidney transplant.
Supplementary Material
Acknowledgments
This work has been supported by grants 1R21DK077336 and 1R01DK095024 from the National Institute of Diabetes, Digestive, and Kidney Diseases (NIDDK) to Dr. Winkelmayer. The manuscript was reviewed and approved for publication by an officer of the NIDDK. Dr. Turakhia is supported by a Veterans Health Services Research and Development Career Development Award (CDA09027-1). Dr. Lenihan received support from the Dr. Richard Steevens’ Scholarship from the Irish Health Service Executive.
Abbreviations
- AF
atrial fibrillation
- ESRD
end stage renal disease
- CI
confidence interval
- HR
hazard ratio
- ICD9
International Classification of Diseases, 9th revision
- USRDS
US Renal Data System
- PVD
peripheral vascular disease
- MI
multiple imputation
- eGFR
estimated glomerular filtration rate
Footnotes
Mandatory Disclaimers:
Data reported herein were supplied by the United States Renal Data System (USRDS). Interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as official policy or interpretation of the US government. The content and opinions expressed are solely the responsibility of the authors and do not necessarily represent the views or policies of the Department of Veterans Affairs.
References
- 1.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114(2):119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 2.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22(8):983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98(10):946–952. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 4.Winkelmayer WC, Patrick AR, Liu J, Brookhart MA, Setoguchi S. The increasing prevalence of atrial fibrillation among hemodialysis patients. J Am Soc Nephrol. 2011;22(2):349–357. doi: 10.1681/ASN.2010050459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146(12):857–867. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 6.Winkelmayer WC, Liu J, Setoguchi S, Choudhry NK. Effectiveness and safety of warfarin initiation in older hemodialysis patients with incident atrial fibrillation. Clin J Am Soc Nephrol. 2011;6(11):2662–2668. doi: 10.2215/CJN.04550511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olesen JB, Lip GY, Kamper AL, Hommel K, Køber L, Lane DA, et al. Stroke and bleeding in atrial fibrillation with chronic kidney disease. N Engl J Med. 2012 Aug 16;367(7):625–35. doi: 10.1056/NEJMoa1105594. [DOI] [PubMed] [Google Scholar]
- 8.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341(23):1725–1730. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 9.Abbott KC, Reynolds JC, Taylor AJ, Agodoa LY. Hospitalized atrial fibrillation after renal transplantation in the United States. Am J Transplant. 2003 Apr;3(4):471–6. doi: 10.1034/j.1600-6143.2003.00071.x. [DOI] [PubMed] [Google Scholar]
- 10.Lentine KL, Schnitzler MA, Abbott KC, Li L, Xiao H, Burroughs TE, et al. Incidence, predictors, and associated outcomes of atrial fibrillation after kidney transplantation\ Clin J Am Soc Nephrol. 2006 Mar;1(2):288–96. doi: 10.2215/CJN.00920805. [DOI] [PubMed] [Google Scholar]
- 11.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285(22):2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 12.Lakshminarayan K, Solid CA, Collins AJ, Anderson DC, Herzog CA. Atrial fibrillation and stroke in the general medicare population: a 10-year perspective (1992 to 2002) Stroke. 2006;37(8):1969–1974. doi: 10.1161/01.STR.0000230607.07928.17. [DOI] [PubMed] [Google Scholar]
- 13.Dublin S, French B, Glazer NL, Wiggins KL, Lumley T, Psaty BM, et al. Risk of new-onset atrial fibrillation in relation to body mass index. Arch Intern Med. 2006;166(21):2322–2328. doi: 10.1001/archinte.166.21.2322. [DOI] [PubMed] [Google Scholar]
- 14.Frost L, Vestergaard P. Caffeine and risk of atrial fibrillation or flutter: the Danish Diet, Cancer, and Health Study. Am J Clin Nutr. 2005;81(3):578–582. doi: 10.1093/ajcn/81.3.578. [DOI] [PubMed] [Google Scholar]
- 15.Kokotailo RA, Hill MD. Coding of stroke and stroke risk factors using international classification of diseases, revisions 9 and 10. Stroke. 2005;36(8):1776–1781. doi: 10.1161/01.STR.0000174293.17959.a1. [DOI] [PubMed] [Google Scholar]
- 16.Little Roderick JA, Rubin Donald B. Statistical Analysis with Missing Data. 2. Hoboken, NJ: John Wiley & Sons, Inc; 2002. [Google Scholar]
- 17.Little RJ, D’Agostino R, Cohen ML, Dickersin K, Emerson SS, Farrar JT, et al. The prevention and treatment of missing data in clinical trials. N Engl J Med. 2012 Oct 4;367(14):1355–60. doi: 10.1056/NEJMsr1203730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ware JH, Harrington D, Hunter DJ, D’Agostino RB. Missing Data. N Engl J Med. 2012 Oct 4;367( 14):1353–54. [Google Scholar]
- 19.Scandling JD. Kidney transplant candidate evaluation. Semin Dial. 2005;18(6):487–494. doi: 10.1111/j.1525-139X.2005.00094.x. [DOI] [PubMed] [Google Scholar]
- 20.van Walraven C, Austin PC, Knoll G. Predicting potential survival benefit of renal transplantation in patients with chronic kidney disease. CMAJ. 2010;182(7):666–672. doi: 10.1503/cmaj.091661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rabbat CG, Thorpe KE, Russell JD, Churchill DN. Comparison of mortality risk for dialysis patients and cadaveric first renal transplant recipients in Ontario, Canada. J Am Soc Nephrol. 2000;11(5):917–922. doi: 10.1681/ASN.V115917. [DOI] [PubMed] [Google Scholar]
- 22.Pascual J, Zamora J, Pirsch JD. A systematic review of kidney transplantation from expanded criteria donors. Am J Kidney Dis. 2008 Sep;52(3):553–86. doi: 10.1053/j.ajkd.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 23.Yahalom G, Schwartz R, Schwammenthal Y, Merzeliak O, Toashi M, Orion D, et al. Chronic kidney disease and clinical outcome in patients with acute stroke. Stroke. 2009 Apr;40(4):1296–303. doi: 10.1161/STROKEAHA.108.520882. [DOI] [PubMed] [Google Scholar]
- 24.Tsagalis G, Akrivos T, Alevizaki M, Manios E, Stamatellopoulos K, Laggouranis, et al. Renal dysfunction in acute stroke: an independent predictor of long-term all combined vascular events and overall mortality. Nephrol Dial Transplant. 2009 Jan;24(1):194–200. doi: 10.1093/ndt/gfn471. [DOI] [PubMed] [Google Scholar]
- 25.Watanabe H, Watanabe T, Sasaki S, Nagai K, Roden DM, Aizawa Y. Close bidirectional relationship between chronic kidney disease and atrial fibrillation: the Niigata preventive medicine study. Am Heart J. 2009 Oct;158(4):629–36. doi: 10.1016/j.ahj.2009.06.031. [DOI] [PubMed] [Google Scholar]
- 26.Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2011;123(10):e269–367. doi: 10.1161/CIR.0b013e318214876d. [DOI] [PubMed] [Google Scholar]
- 27.Losi MA, Memoli B, Contaldi C, Barbati G, Del Prete M, Betocchi S, et al. Myocardial fibrosis and diastolic dysfunction in patients on chronic haemodialysis. Nephrol Dial Transplant. 2010 Jun;25(6):1950–4. doi: 10.1093/ndt/gfp747. [DOI] [PubMed] [Google Scholar]
- 28.Mark PB, Johnston N, Groenning BA, Foster JE, Blyth KG, Martin TN, et al. Redefinition of uremic cardiomyopathy by contrast-enhanced cardiac magnetic resonance imaging. Kidney Int. 2006 May;69(10):1839–45. doi: 10.1038/sj.ki.5000249. [DOI] [PubMed] [Google Scholar]
- 29.Chung MK, Martin DO, Sprecher D, Wazni O, Kanderian A, Carnes CA, et al. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001 Dec 11;104(24):2886–91. doi: 10.1161/hc4901.101760. [DOI] [PubMed] [Google Scholar]
- 30.Marcus GM, Smith LM, Ordovas K, Scheinman MM, Kim AM, Badhwar N, et al. Intracardiac and extracardiac markers of inflammation during atrial fibrillation. Heart Rhythm. 2010;7(2):149–54. doi: 10.1016/j.hrthm.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naito M, David D, Michelson EL, Schaffenburg M, Dreifus LS. The hemodynamic consequences of cardiac arrhythmias: evaluation of the relative roles of abnormal atrioventricular sequencing, irregularity of ventricular rhythm and atrial fibrillation in a canine model. Am Heart J. 1983;106(2):284–291. doi: 10.1016/0002-8703(83)90194-1. [DOI] [PubMed] [Google Scholar]
- 32.Healey JS, Connolly SJ, Gold MR, Israel CW, Van Gelder IC, Capucci A, et al. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012;366(2):120–129. doi: 10.1056/NEJMoa1105575. [DOI] [PubMed] [Google Scholar]
- 33.Friberg L, Hammar N, Pettersson H, Rosenqvist M. Increased mortality in paroxysmal atrial fibrillation: report from the Stockholm Cohort-Study of Atrial Fibrillation (SCAF) Eur Heart J. 2007;28(19):2346–2353. doi: 10.1093/eurheartj/ehm308. [DOI] [PubMed] [Google Scholar]
- 34.Meier-Kriesche HU, Kaplan B. Waiting time on dialysis as the strongest modifiable risk factor for renal transplant outcomes: a paired donor kidney analysis. Transplantation. 2002;74(10):1377–1381. doi: 10.1097/00007890-200211270-00005. [DOI] [PubMed] [Google Scholar]
- 35.Shen JI, Turakhia MP, Winkelmayer WC. Anticoagulation for atrial fibrillation in patients on dialysis: are the benefits worth the risks? Curr Opin Nephrol Hypertens. 2012 Nov;21(6):600–6. doi: 10.1097/MNH.0b013e32835856fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Opelz G, Zeier M, Laux G, Morath C, Döhler B. No improvement of patient or graft survival in transplant recipients treated with angiotensin-converting enzyme inhibitors or angiotensin II type 1 receptor blockers: a collaborative transplant study report. J Am Soc Nephrol. 2006 Nov;17(11):3257–62. doi: 10.1681/ASN.2006050543. [DOI] [PubMed] [Google Scholar]
- 37.Heinze G, Collins S, Benedict MA, Nguyen LL, Kramar R, Winkelmayer WC, Haas M, Kainz A, Oberbauer R. The association between angiotensin converting enzyme inhibitor or angiotensin receptor blocker use during postischemic acute transplant failure and renal allograft survival. Transplantation. 2006 Dec 15;82(11):1441–8. doi: 10.1097/01.tp.0000244587.74768.f7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.