Abstract
Visualization of living E. coli nucleoids, defined by HupA-mCherry, reveals a discrete, dynamic helical ellipsoid. Three basic features emerge. (i) Nucleoid density efficiently coalesces into longitudinal bundles, giving a stiff, low DNA density ellipsoid. (ii) This ellipsoid is radially confined within the cell cylinder. Radial confinement gives helical shape and drives and directs global nucleoid dynamics, including sister segregation. (iii) Longitudinal density waves flux back and forth along the nucleoid, with 5–10% of density shifting within 5s, enhancing internal nucleoid mobility. Furthermore, sisters separate end-to-end in sequential discontinuous pulses, each elongating the nucleoid by 5–15%. Pulses occur at 20min intervals, at defined cell cycle times. This progression is mediated by sequential installation and release of programmed tethers, implying cyclic accumulation and relief of intra-nucleoid mechanical stress. These effects could comprise a chromosome-based cell cycle engine. Overall, the presented results suggest a general conceptual framework for bacterial nucleoid morphogenesis and dynamics.
Introduction
Bacterial chromosomes are intriguing subjects for study. They are apparently “simpler” than their eukaryotic counterparts but nonetheless carry out all of the basic processes required for successful transmission of heredity, i.e. DNA replication, sister chromosome segregation in coordination with cell division. The present study investigates E. coli chromosomes from this perspective, focusing on the organization, organizational dynamics and the dynamics of sister chromosome segregation.
In eukaryotic organisms, sister segregation is usually discussed in terms of ropes and pulleys operating on compact, discrete objects: sister DNAs are organized initially into chromatin fibers and then into higher order coherent shapes, all the while kept together by specific cohesin molecules. Sisters then segregate into well-separated spaces by the combined effects of progressive cohesin release and pulling forces generated by the mitotic spindle. Bacterial chromosomes, in contrast, spatially segregate sister chromosomes to opposite ends of the cell in the apparent absence of such apparatus. We have been interested to understand more about how this process might occur, in part because underlying principles might turn out also to be relevant to eukaryotic chromosomes.
For bacterial sister segregation, two general issues are important. First, the process of placing sisters in distinct spaces cannot be conceptually separated from the physical nature and organization of the nucleoid. At one extreme, it has been proposed that the nucleoidal fiber can be treated as a randomly-oriented polymer, with sister fibers separated by the effects of entropic forces as they operate in the elongated space defined by the cylindrical cell periphery (Jun and Mulder, 2006). At the opposite extreme, sister nucleoid domains might comprise coherent, non-interacting entities that separate by mechanically pushing one another apart in space, with concomitant release of constraining inter-sister tethers (Bates and Kleckner, 2005; Joshi et al., 2011). Another model, where sister nucleoids are pumped outward in opposite directions from a “replication factory” (Lemon and Grossman, 2001; but see Bates, 2008), similarly necessitates an intrinsic tendency for non-intermingling of sister fibers. Yet other models invoke centromere-like sequences that move via molecular motors along railroad tracks or are passively attached to the cell periphery on either side of midcell, with segregation driven by incorporation of cell wall material at that site (Toro et al., 2008; Toro and Shapiro, 2010; Banigan et al., 2011; Norris, 1995). These latter models ignore the physical state of the nucleoid which, however, is likely highly relevant.
The second critical underlying issue for segregation of sisters is physical movement of nucleoid material which, in turn, requires energy. Where does this energy come from? Are thermal forces operating on a passive polymer fiber sufficient? Do molecular events place chromosomes in a high energy, mechanically-stressed conformation which then drives ensuing segregation? Are ATP-driven processes directly involved in segregation and, if so, at which stages, by what mechanism, and in what type of interplay with intrinsic physical features and effects?
To further address these questions, we developed and applied a new experimental system for analysis of E. coli chromosome dynamics, at high resolution in time and three-dimensional space.
Results
Experimental System
Previous studies of nucleoid organization and structure have been limited by technical constraints. Analysis of fixed cells or isolated nucleoids have been informative but cannot detect dynamic behaviors; also, the possibility of artifacts is always a concern. Analysis of living cells avoids fixation artifacts. However, light microscope imaging permits rapid image acquisition but provides very low spatial resolution while, oppositely, super-resolution methods give high spatial resolution but require data collection over time scales that preclude definition of rapid dynamic changes.
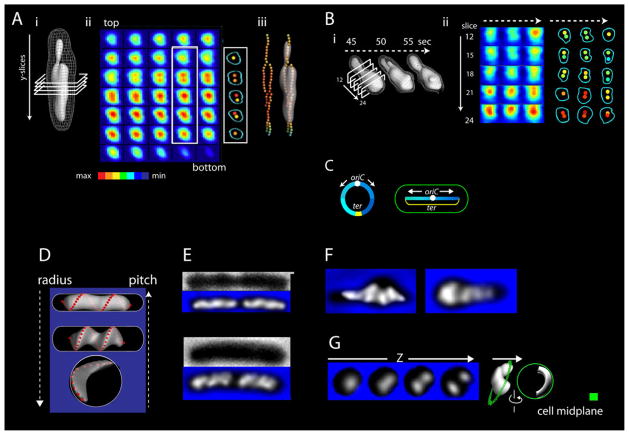
The current studies were carried out in living cells with a system that combines high spatial resolution and high temporal resolution (Figure 1; Experimental Procedures; Extended Experimental Procedures). Nucleoids were visualized using the general nucleoid-associated protein HU, fluorescently tagged via its HupA subunit (HupA-mCherry) and imaged by wide-field epi-fluorescence microscopy. Imaging provides spatial resolution of <260nm in the XY dimension and <470nm in the Z dimension. An entire nucleoid can be imaged in three-dimensions (3D) by a series of Z-stacks (Figure 1B) in as few as 2s. Successive 3D images of a single nucleoid can be taken as frequently as once every 5s. Concomitantly, the boundaries of the cell periphery are accurately defined for the mid-cell plane from phase contrast images (Figure 1A bottom).
Figure 1.
The G1 nucleoid is a discrete, but dynamic, helical ellipsoid. (A) Top: Normally growing E. coli cells were imaged in microfluidic chambers, held in place by gentle hugging in the Z-dimension, with fluid flowing around both sides. Bottom: The cell periphery, nucleoid and desired FROS foci (here marking oriC) were imaged by phase contrast and wide-field epifluorescence, in 3D, via collection of successive Z-stacks over < 2s. (B) Z-stack of HupA- mCherry images; nucleoid dimensions are 1.64μm by 0.48μm. (C) Iso-intensity PyMOL reconstruction of a G1 nucleoid, alone and with cell midplane outline (total signal (blue) and 50% and 20% of total signal (red and white)). The helical ellipsoid fills the cell radially but does not contact the cell at its new pole end. Radially-decreasing signal intensity suggests radially-decreasing density, subject to imaging resolution limits. (D) Iso-intensity reconstructions and longitudinal density centroid paths for three nucleoids; curvature handedness of curvature in red and green. Left-most nucleoid is that in (B). (E–H) Dynamic shape changes via longitudinal density waves in a single nucleoid imaged at 5s intervals. (E) 3D iso-intensity shape reconstructions. (F) Nucleoid intensities were summed by projection in the Z-dimension (left). Color map representations (right) reveal rapid longitudinal fluxes of density over distances comparable to nucleoid length. (G) Nucleoid intensities of cross-sectional slices along the length of the nucleoid (percentage of total intensity as a function of slice position), at the indicated time points (i, ii, iv). (iii) For pairs of time points, the difference in intensity at each position/slice is calculated and absolute values of these differences summed for all slices. (H) The nucleoid in (E–G) in relationship to its cell midplane at the beginning and end of the time series. Green shapes are the cell periphery in the midplane section of the corresponding phase contrast image; ivory shapes are suitably-oriented iso-intensity reconstructions. Flat bottom end to cell outline reflects close juxtaposition to its sister cell; the junction was approximated by a straight line. All images from 1× deconvolved data.
For maximum spatio-temporal resolution, cells were imaged while growing in microfluidic channels, within which they are immobilized by gentle “hugging” in the Z dimension (Figure 1A top; Figure S1A–D). Growth medium still flows continuously around the immobilized cells. These cells progress through the cell cycle with the same kinetics as occur in exponential phase growing under the same conditions in a standard liquid culture.
Importantly, this analysis utilized a strain background and growth conditions where events of the chromosome cycle have previously been analyzed in detail by other methods (CM735; Bates and Kleckner, 2005; Bates et al., 2005; Joshi et al., 2011). Under these conditions, cells grow in a ~120min “linear” cell cycle. Cell division is followed by a ~10min “G1” period. DNA replication is then initiated. Replication lasts ~60min. An additional ~50min then elapses prior to the next cell division.
A final key aspect of this study is that oriC dynamics were analyzed by FROS, in parallel with whole nucleoid dynamics. Separation of sister oriC’s from one another is an easily-discerned event that occurs after initiation of replication, at a specific time in the cycle (below; Figure S1G). This event provides a fixed point of reference for defining the timing of events in any particular nucleoid and for temporal alignment of different, independent nucleoids.
Features of interest were confirmed in another strain background, with another fluorescent nucleoid-associated protein, and/or in cells imaged outside of channels and in the absence of flow, as detailed below.
The G1 nucleoid is a discrete, helical ellipsoid
3D imaging reveals that G1 nucleoids are well-defined ellipsoids that exhibit a variety of helix-like forms (e.g. Figure 1CD; Movie S1; hereafter referred to as “helical” for simplicity). This shape is clearly defined after one round of deconvolution, which eliminates out-of-focus information without introducing artificial sharpening or enhancement, but is also visible in raw images (Figure S1E).
The longitudinal paths of G1 nucleoids can be evaluated by slicing each 3D data set into a series of cross-sections perpendicular to the long axis of the nucleoid and computationally defining the density centroids of these slices. The paths of these centroids exhibit left- and/or right-handed helicity even along the length of a single nucleoid (Figure 1D). Thus: the important feature of nucleoid shape is the tendency for curvature, not any particular handedness.
HupA-mCherry fluorescence intensity reports nucleoid DNA density, not peculiarities of HupA binding or the disposition of free HU protein. The same basic nucleoid shape is observed in fixed cells where the nucleoid is illuminated by non-specific binding of SSB-GFP to single-stranded DNA regions created by the preparation procedure (Figure S1H) and in living cell nucleoids illuminated by FIS-GFP (Figure S1J). Also, helical shape is observed in cells growing outside of channels on agarose pads in the absence of flowing medium (Figure S1J, 2F, 3C–K) and in cells removed from synchronous liquid cultures and imaged within minutes after attachment to a glass slide (A.B).
Comparison of fluorescence nucleoid images and phase contrast mid-plane images (e.g. Figure 1H, ivory and green, respectively) further reveals that, at early G1 (left panel) the nucleoid is closely juxtaposed to the cell periphery in the radial dimension along its entire length but is well-separated from the old pole end of the cell, as previously inferred from fixed cell studies (Bates and Kleckner, 2005). Separation from the old pole end of the cell can be even more pronounced at later stages (below; Figure S2A, C). This same disposition has been observed in cells grown on agarose pads and imaged with FIS-GFP (Figure S2F). The same two features are also apparent in 3D STORM images of mEos2-labeled HU, which provide higher spatial resolution, but lower temporal resolution, than those of the current study (Wang et al., 2011). Thus: the nucleoid does not simply fill up the interior cell space; rather, it is a discrete, internally- delimited object.
The space at the end of the cell, while certainly containing proteins and small molecules, is nonetheless fully accessible to the nucleoid, rather than being solidly occluded by ribosomes or other non-visualized cellular components, because: (i) the nucleoid does extend to the ends of the cell at some stages (below); (ii) plasmid DNAs localize beyond the nucleoid at the ends of the cell (Kuhlman and Cox, 2012); and (iii) at some stages, individually-tagged chromosomal loci explore the space beyond the end of the nucleoid (J.F. and A.B. unpublished).
Since we can specifically define the limits of the nucleoid, we can further define additional features. First, all detectable HupA-mCherry intensity is involved in the shape (Extended Experimental Procedures). Second, nucleoid DNA density is very low: the atoms of the DNA duplex take up only ~2% of total cell volume while the nucleoid shape comprised of this DNA occupies ~75% of the total space (See supplemental experimental procedures).
The G1 nucleoid is highly dynamic due to oscillating longitudinal density waves
G1 nucleoid shape is highly dynamic. Significant global changes are apparent over intervals as short as 5s (e.g. Figure 1E; Movie S2). Furthermore, when total nucleoid density is summed in the Z-dimension (Figure 1F left), shape changes are seen to result from waves of density that flux longitudinally, up and down the shape, over distances comparable to the length of the nucleoid, with apparent periodicities of seconds-to-minute(s) (Figure 1F right). These “longitudinal density waves” are fluxing through the nucleoid in 3D to generate shape changes. Relatively modest changes in nucleoid density distribution are involved: between images taken 5s apart, ~5–10% of total intensity shifts position (Figure 1Gi–iii) with shifts of ~15% over longer intervals (Figure 1Giv).
Longitudinal density fluxes are a general, robust feature of nucleoid dynamics. Such fluxes have been observed in each of ~20 5s time series of G1 or G1/S transition nucleoids and each of ~100 time series of nucleoids at other cell cycle stages (below). 6 G1 nucleoids and 6 G1/S nucleoids, each imaged over periods of ~40s, exhibit average density shifts, per 5s interval, of 7.2% and 6.6% respectively. Furthermore, in G1 nucleoids of cells expressing both HupA-mCherry and FIS- GFP, both signals exhibit the same density waves (Figure S3A) which thus represent changes in underlying nucleoid density, not fluxes of HU or FIS protein alone irrespective of the nucleoid. Density waves are also seen in cells growing on agarose pads, without flowing medium and thus are not due to confinement in microfluidic channels nor to fluid flow past tethered or confined cells. Finally, density fluxes are not observed in fixed cells (Figure S3C–K) and thus are not artifacts of imaging or image processing.
These findings reveal the existence of a previously unrecognized source of chromosomal motion. This motion occurs prior to onset of DNA replication (and after completion of replication; below) and thus is replication-independent.
Previous studies show that G1 nucleoids also undergo coherent, longer time-scale, globally-directional motions. During and immediately following cell division, nucleoids are closely juxtaposed to the evolving new poles at midcell (Bates and Kleckner, 2005), with asymmetric shapes that are fatter at the new pole end (e.g. Figure 1H left). This configuration likely reflects molecular linkages between the pole and the terminus (ter) macrodomain (Espeli et al., 2012). As septum formation is completed, sisters move away from the emerging poles, without major internal reorganization, but with concomitant elongation (Bates and Kleckner, 2005; Figure 1H right). Time lapse further reveals that elongation concomitantly yields a more regular shape with a more even density distribution (e.g. Figure 1H compare left vs right). Longitudinal density fluxes presumably underlie these movements. However, these fluxes must in some way be directionally biased so as to give the observed effects (Discussion). Additionally, the G1 nucleoid retains its close contact with the cell periphery throughout dynamic shape and length changes (e.g. Figure 1H).
The G1 nucleoid has a substructure comprising dual longitudinal density bundles
When cross-sectional slices perpendicular to the length of the nucleoid (Figure 2Ai) are displayed in an intensity-coded array (Figure 2Aii), density substructure is revealed. Some slices exhibit a nearly symmetrical “bulls-eye” pattern; however, many exhibit two structures of high central intensity, one stacked above the other. The same features characterize fully individualized sister nucleoids immediately prior to cell division (pre-G1) (Figure S5B). Doubleness is seen in non-deconvolved images and thus is not a deconvolution artifact (Figure S1I).
Figure 2.
Nucleoid substructure comprises dual longitudinal density bundles. (A) Cross-sections of a G1 nucleoid (i) displayed in a color-coded array (ii). Dual longitudinal density bundles are revealed. Central densities occur along the nucleoid in continuous paths with a tendency for relational coiling (iii). (B) Bundle patterns change position, intensity and extent of duality in accord with changes in nucleoid shape as seen at 5s intervals. (C) Most of the E. coli G1 genome is arrayed linearly from one end of the cell to another, with the ~300kb terminus region stretched between the two nucleoid ends. Thus, G1 duality is not genomically specified. Images in (A,B) from 1x deconvolved data. (D–G) A change in cell radius results in a concomitant change in nucleoid helical radius and an inverse change in nucleoid helical pitch. (D) Predictions expected for a longitudinally stiff ellipsoid that is deformed into a helical shape by radial confinement. (E–G) Variations that match the predictions in (D) are observed in three cases. (E) Two different living cell types. (F) 100min growth in the presence of cell wall synthesis inhibitor mecillinam causes rounding up of cells at their (emerging) poles with changes in helical parameters in the expanded region. (G) 10min after spheroplasting by cell wall removal yields open low-pitch crescents. Images (E–G) are from 20x deconvolved data to emphasize shape.
The dual density centroid(s) of cross-sectional slices run continuously along the length of the nucleoid (Figure 2Aiii). We thus describe the revealed sub-shapes as longitudinal density bundles. In accord with the fact that they underlie an ellipsoid shape, bundles are wider in the middle of the nucleoid than at the ends. In accord with the helical shape of the ellipsoid, dual bundle paths tend to be relationally twisted (Figure 2Aii, iii). And since the entire nucleoid is involved in the helical ellipsoid shape and its component density bundles, the nucleoid does not comprise a central scaffold surrounded by disordered material.
Further, in accord with dynamic nucleoid shape changes, bundle patterns change in intensity and position as density waves flux through the nucleoid (Figure 2B). These dynamics likely reflect local changes in inter-fiber proximity rather than global movement of a fixed proteinaceous core (Discussion).
Intriguingly, G1 nucleoids are single genomes; no sister is present. Moreover, while the E. coli chromosome is a single circular DNA molecule, ~95% of the genome is linearly organized along the cell length, with a thin elongated strand completing the circle (Wang et al., 2006; Wiggins et al, 2010; Figure 2C). This organization was defined in ABll57 which, we show, also exhibits dual longitudinal bundles at G1 (Figure S1F). Thus, dual bundles do not reflect the “left” and “right” sides of the circular chromosome but instead imply the existence of an intrinsic tendency for duality (or, more generally, “splitting”; below) due to some biochemical and/or physical feature of the system.
Nucleoid radius and nucleoid pitch are both defined, inversely with respect to each other, by the cell radius
The nucleoid is in close contact with the cell periphery. We further find that a change in the radius of the cell confers both a matching change in the helical radius of the nucleoid plus an inverse change in nucleoid helical pitch (Figure 2D) in three types of studies:
The standard strain for this study exhibits spontaneous variation between two cell types, longer/thinner and shorter/fatter, whose nucleoids respectively exhibit smaller radius with greater pitch and larger radius with smaller pitch (Figure 2E).
Inhibition of cell wall synthesis causes rounding-up of mid-cell regions where, locally, the nucleoid exhibits increased radius and decreased pitch (Figure 2F).
Enzymatic cell wall removal, carried out in the absence of DNA condensing agents converts the cell to a spherical shape and, concomitantly, the nucleoid becomes a very large radius, low-pitch ellipsoidal crescent, still juxtaposed against the edge of the cell (Figure 2G).
These observations imply that helicity of the nucleoid shape is not determined internally (e.g. by the longitudinal density bundles described above); instead, the helical aspect is determined by interaction of the basic ellipsoid form with the radial cell periphery. Further, since cells become spheroplasts by cell wall removal in only a few minutes, such interaction is required not only to establish helical dimensions but to maintain those dimensions.
Strikingly, the observed correlations are precisely those predicted if the nucleoid is a longitudinally stiff ellipsoid that has been forced into a helical path within a too-small cylinder (i.e. a cylinder whose radius is smaller than the persistence length of the ellipsoid). If the nucleoid could be forced into a radially confined helical shape as it evolves (below), the resulting state could then be maintained by outward-directed pushing forces along the nucleoid length.
The observed effects might be explained, alternatively, if the nucleoid were a soft ellipsoid that was linked to the inner cell periphery specifically along a peripheral helical path (red dots in Figure 2D). We do not favor this model because: (i) the nucleoid does not exhibit a tightly defined external helical peripheral path that would encourage such association; (ii) the nucleoid is of relatively low density around its entire outer surface, implying that molecular contacts between the nucleoid and cell periphery strong enough to change the shape of the nucleoid would likely rip the nucleoid apart; and (iii) contrary to early reports, there is no continuous peripheral helical cytoskeleton to which the edge of the nucleoid might attach (Swulius and Jensen, 2012).
All described features of G1 nucleoids are also present during and after DNA replication
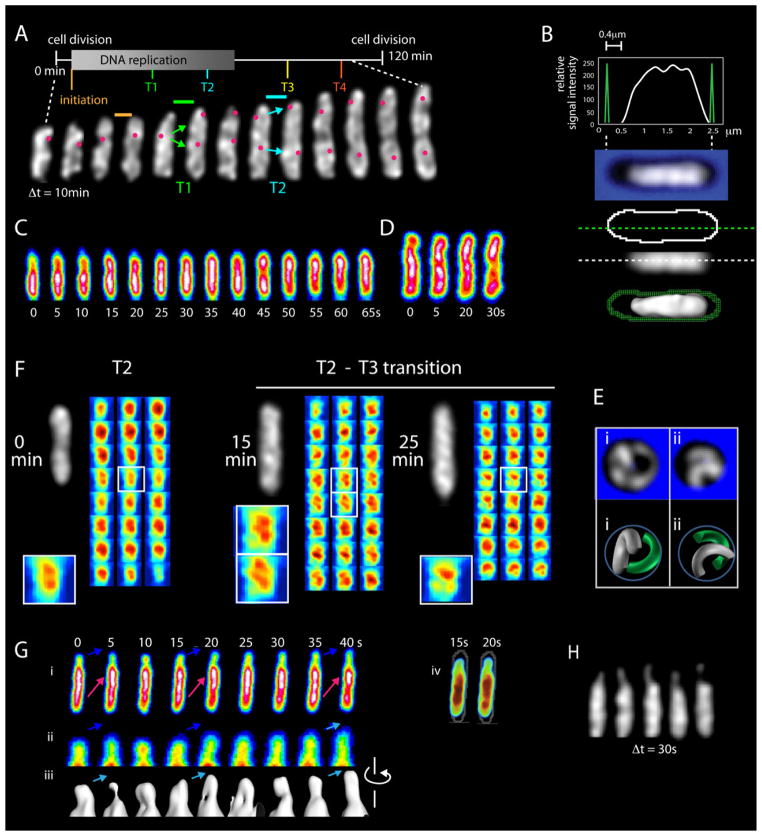
After G1, nucleoids exhibit helical shapes that evolve over time (Figure 3A). Under the conditions analyzed, the cell cycle is demarked by four transitions (Figure 3A; below). Among these, T1 and T2 are accompanied by diagnostic oriC dynamics (Figure S1G; below).
Figure 3.
Post-G1 nucleoids. (A–F) Basic features of G1 nucleoids also occur at later stages. (A) A single nucleoid imaged at 10min intervals reveals progressively evolving helix-like shapes with radius and pitch analogous to those at G1. Four transitions (T1–T4; below). Diagnostic origin movements at T1 and T2 are documented by concomitant imaging of oriC. Fully individualized sister nucleoids emerge only at the very end of the cell cycle (after completion of this imaging series). (B) Post-G1 nucleoids fill the cell radially but often, as in this case, do not come close to the old pole end of the cell (as in Figure 1C; also Figure S2). (C, D) Longitudinal density waves occur during DNA replication (C) and after completion of replication (D) (as in Figure 1F; quantification in Figure S3B). (E) Spheroplasting of a late-stage cell creates low pitch crescents (as in Figure 2G; Z-series in Figure S4). Maximal separation implies a tendency for non-intermingling. (F) Longitudinal density bundle patterns (as in Figure 2A, B) for the same nucleoid at successive times in the T2 to T3 period, showing duality (left), a multiple-bundled state (middle), and a peculiar mid-cell pattern (right). The latter two morphologies are not seen in G1 nucleoids. (G) Elongation of a post-G1 nucleoid seen by imaging at 5s intervals. Z-projections illustrate protrusion of nucleoid density into empty space at the old pole end of the cell (blue arrows in i, ii) with accompanying longitudinal density waves that move up and back through the shape in the same direction (i; red arrows) analogously to an incoming tide. Iso-intensity PyMOL thresholding of the same nucleoid (iii) reveals dual longitudinal density bundles in tightly juxtaposed or open states. Variations in thickness reveal incorporation of fluxing density into the shape. (iv) 3D PyMOL rendering of the same nucleoid illustrating protrusion of density into nucleoid-free space at the old cell pole. (H) Mid-plane images, taken at 30 sec intervals, of a nucleoid exhibiting a long thin protruding finger that is curving around the radial cell periphery with concomitant density fluxes. All images from 1x deconvolved data.
The nucleoid elongates throughout the cell cycle, during and after DNA replication; moreover, fully morphologically-individualized sister nucleoids do not emerge until the very end of the cell cycle, long after completion of DNA replication (Figure 3A; below). Post-G1 shapes often comprise multiple helical turns and can be ellipsoidal, with elongated ends, or can have openly curved ends (Figure 3A).
Post-G1 nucleoids exhibit all features described above for G1 nucleoids, both during replication and during the lengthy ensuing post-replication pre-division period.
Nucleoid helical radius corresponds to cell radius, with radius and pitch both closely similar to those seen at G1 (Figure 3A).
The nucleoid is always in contact with the radial cell periphery, but is often far from the old pole end of the cell, particularly at the T1/T2 transition (Figure 3B; below; Figure S2).
Longitudinal density waves occur in all nucleoids, during and after replication, as documented by 5s time lapse analysis of ~100 nucleoids representing the entire cell cycle (e.g. Figure 3CD).
Cell radius defines both nucleoid radius and pitch, in inverse correlation (Figures 3E and S4). Cells at quite late stages rendered spherical by cell wall removal exhibit pairs of nucleoids, each with greatly increased curvature and greatly decreased pitch. Additionally, the two nucleoids are disposed so as to minimize overlap, suggesting that they are intrinsically discrete, non-intermingling objects (Figures 3E and S4; Discussion).
Nucleoids exhibit longitudinal density bundles that are often split. Bundle duality is common at all stages (e.g. Figure 3F left), including pre-G1 (Figure S5B). Moreover, in the latter stages of DNA replication, interesting morphologies occur that are not seen at other stages. In the example shown, a single nucleoid exhibits duality just after the T2 transition and then, during the run-up to T3, first exhibits three (or more) relatively equivalent bundles and then a unique mid-cell pattern with unequal-sized bundles (Figures 3F and S5E). These more complex patterns could reflect intermediate stages in the evolution of mother/sister/sister nucleoid states.
Time series at 5s intervals reveal elongation-biased density waves plus coalescence of new material into existing longitudinal density bundles
Longitudinal density waves underlie nucleoid elongation. In roughly half of all 5s time series, a significant increase in overall nucleoid length can be detected over the 1–2min visualization window, and all of these cases exhibit dynamic fluxing of density towards one or both ends of the nucleoid (e.g. Figure 3C). In favorable cases, e.g. when there is significant “space” at the end of the cell, specific morphological details can be discerned, as in Figure 3G. Here, a protrusion emerges from the new pole end of the nucleoid into previously unoccupied space, then retracts, then protrudes and then retracts, cyclically, with net forward extension, analogous to an incoming tide (blue arrows, Figure 3Gi, ii). Each protrusion is the leading edge of a longitudinal density wave that fluxes through the shape towards the growing end (red arrows, Figure 3Gi).
Furthermore, at a suitable iso-intensity threshold, dual longitudinal density bundles can be seen within the protruding material, fluctuating between tightly juxtaposed and open configurations as seen on a larger scale (above). Most importantly, these bundles evolve by coalescence of the fluxing material into already existing bundles: as a wave of density moves towards the growing end, it is efficiently incorporated into existing bundles to give smooth elongation of the shape.
The occasional nucleoid exhibits an extremely thin protrusion that curves progressively around the radial cell periphery with concomitant fluxes of density along its length (Figure 3H; Movie S6). These and other images (e.g. Figures 3G; S2E) suggest that the structure is capable of exploring space and, together with post-G1 nucleoids showing “space” at the end of the cell (e.g. Figures 3B; S2A; S2F), confirm that the helical shape does not arise by pushing of the nucleoid up against the end of the cell.
The post-G1 nucleoid elongates discontinuously via sequential pulses that occur at defined times in the cell cycle
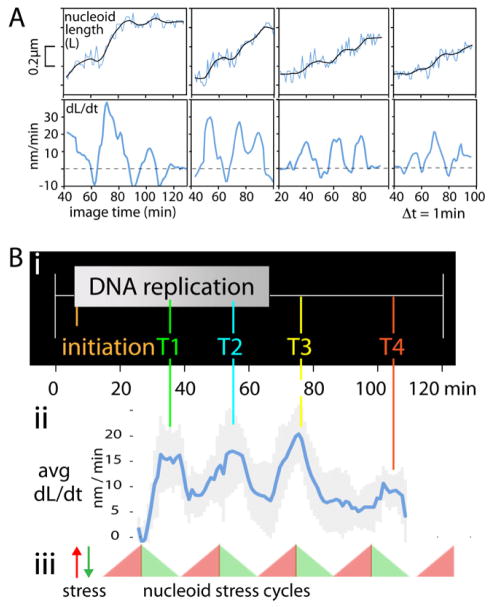
Time lapse analyses with 3D images of a single nucleoid are collected at 1min intervals reveals a striking, unanticipated behavior: the E. coli nucleoid elongates discontinuously over time. ~10min pulses of rapid length increase occur every ~20min, as seen in primary length curves and changes in the rate of nucleoid length increase (Figure 4A top and bottom). On average, in each pulse, nucleoid length increases ~100–200nm, or ~5–15% of nucleoid length. In contrast, end-to-end cell length increases monotonically over time throughout the cell cycle (Figure S6). Interestingly, a period of length increase is usually preceded by a short period of nucleoid shortening (Figure 4A top) and a corresponding negative rate of length increase (Figure 4A bottom), suggesting that elongation is immediately preceded by a global change of internal nucleoid state.
Figure 4.
The nucleoid elongates in 10min pulses at defined 20min intervals during the cell cycle. (A) Lengths of four individual nucleoids, imaged at successive 1min intervals, show pulses of increase, usually immediately preceded by a short period of nucleoid shortening, as seen in primary length curves (top) and rates of increase given by the slopes of those curves (bottom). In contrast, cell length increases monotonically throughout (Figure S6). (B) (i, ii) Averaging of rate increases for multiple data sets (N=14) reveals that pulses of length increase occur at 20min intervals, at specific times through the analyzed period of the cell cycle, in temporal correlation with the times of the T1–T4 transitions. (iii) Pulses could correspond to periodic accumulation and release of nucleoid stress.
When multiple data sets (N=14) are aligned with respect to cell cycle timing and nucleoid elongation rates averaged over time, it further emerges that pulses of nucleoid elongation occur at specific times in the cell cycle. Maximum elongation rates are similar in each period (15–20nm/min; Figure 4Bii). The ~100min period analyzed, which extends from early-mid DNA replication to ~40 min after the end of replication, exhibits four sequential length increase pulses. Since the last two pulses occur at the end of, and well after, DNA replication, respectively, these pulses are independent of concomitant replication.
We infer that, during each ~10 min period of nucleoid length increase, elongation is implemented by the sum of many short-time-scale longitudinal density waves. For example: the sequence of waves and length increases defined by imaging at 5s intervals in Figure 3G presumably represents ~40s out of a ~10min elongation period.
Tether-mediated sister separation transitions are temporally and morphologically correlated with nucleoid elongation pulses
A further striking finding is that the cell cycle times of the four pulses of nucleoid elongation match the times of four previously- described nucleoid transitions, each of which corresponds to a discrete increase in global and/or local sister separation and has either been shown, or is suspected, to involve release of one or more programmed tethers that would constrain separation (Figure 4Bi, ii; Bates et al., 2005; Joshi et al., 2011; below). Real time analysis confirms that temporal correspondence is accompanied by direct morphological correspondences.
Background
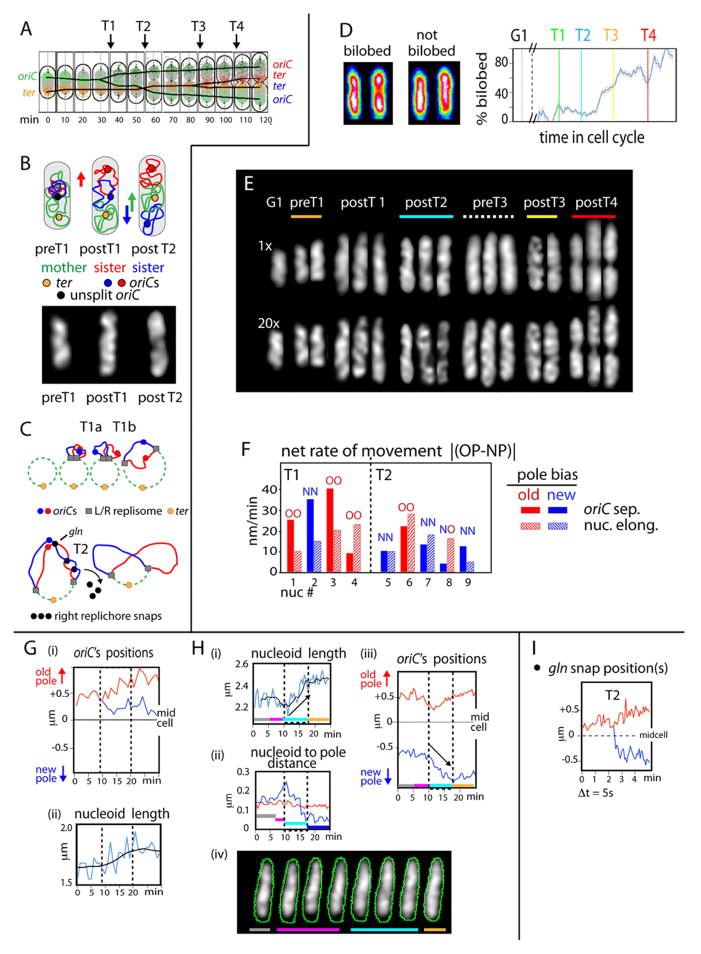
Transitions T1–T4 are each characterized by increased sister separateness for oriC (T1/T2) or the terminus region (ter) (T3/T4) (Figure 5A; Bates et al., 2005; Joshi et al., 2011). The best-studied transition, T2, is a global nucleoid reorganization that additionally includes: (i) a discrete increase in the separateness of sister loci throughout the genome; (ii) appearance of strongly bilobed nucleoid morphology, with one sister locus in each lobe; and (iii) movement of the terminus region inwards towards midcell (Bates et al., 2005; Joshi et al., 2011). A subtler global increase in sister separateness occurs at T1. Such data permit a specific model for sister/mother dynamics (Figure 5B left): DNA replication initiates at a position towards one end of the nucleoid (A.B. unpublished). At T1, one sister domain moves towards one cell pole, away from the mother nucleoid, leaving the other sister domain near midcell, proximal to the mother domain. At T2, the midcell sister domain moves to the other cell pole, switching places with the mother domain, which moves inward (along with ter). Sister oriC movements match this pattern (Figures 5A, 5B right). T3 is defined by splitting sister ter’s, which remain at the edge of one nucleoid lobe. At T4, separated sister ter’s move to the inner edges of sister nucleoids (Figure 5A).
Figure 5.
Nucleoid elongation pulses correspond to global increases in sister separation. (A) T1–T4 transitions as previously-described (Bates and Kleckner 2005; Joshi et al., 2011; text). (B) Top: model for spatial evolution of sister and mother regions via T1 and T2 transitions (Joshi et al., 2011). Bottom: matching whole nucleoid images occur at appropriate stages. (C) Programmed tethers that modulate T1 and T2 (Joshi et al., 2011; text). (D) Bilobed character was defined for >1000 nucleoids from known times throughout the cell cycle by analysis of longitudinal density distributions in Z-projections. Left: examples of bilobed and non-bilobed states. Right: Frequency of nucleoids showing bilobed character increases in discrete steps at the T1–T4 transitions. (E) Pronounced nucleoid morphologies characteristic of the indicated stages. Midplanes from 1x deconvolved and 20x deconvolved images (top and bottom). (F) Sister oriCs usually exhibit differential separation towards the old pole (filled red bars) and new pole (filled blue bars) at the T1 and T2 transitions, respectively. Y-axis = Δ = |(rate of increase towards old pole - rate of increase towards new pole)|. Bias is more pronounced at T1 vesus T2: Δ = 28nm/min (+/− 1.4) and 12nm/min (+/− 6.5) respectively. Concomitant nucleoid length increases (hatched bars) occur differentially in the same direction as oriC movement in 4/4 T1 nucleoids and 4/5 T2 nucleoids.
(G) A T1 nucleoid imaged at 1min intervals. oriC separates differentially towards the old pole (i) during a pulse of nucleoid length increase (ii) that also occurs preferentially towards the old pole end of the cell (not shown). (H) A T2 nucleoid, imaged at 30s intervals. (i) A pulse of length increase (turquoise) preceded by a period of nucleoid shortening (pink). (ii) Nucleoid length increases towards the new pole end of the cell (turquoise). (iii) Concomitant differential movement of the midcell sister oriC towards the new pole end at 50nm/min (turquoise). (I) Separation of sisters at the T2 “snap” locus gln, defined by 2D imaging at 5s intervals. Loci separate at approximately 380nm/min, significantly faster than oriC splitting at T1 or T2.
T1 involves sequential release of two programmed tethers that link, respectively, sister oriC’s and leftward and rightward replisomes (Figure 5C top). The two release steps occur ~5min apart (Bates and Kleckner, 2005; D. Bates, personal communication). T2 involves a unique set of inter-sister “snaps” in the right replichore where sisters remain cohered much longer than at intervening and flanking loci (Figure 5C bottom; Bates et al., 2005; Joshi et al., 2011). Moreover, even though snap loci are non-adjacent, they undergo sister splitting coordinately at T2. T3 involves loss of linkage between sister ters. T4 could involve release of any of several other known terminus-region tethers.
Step-wise emergence of bilobed nucleoid morphology at T1–T4
Bilobed nucleoid morphology evolves in discrete steps corresponding to the T1–T4 transitions; thus, all four transitions result in increased global separation of sister domains, as known for T2.
Bilobed character, defined in Z-projections, corresponds to a tendency for the longitudinal intensity distribution to exhibit two peaks separated by a valley (Figure 5D left). Analysis of >1000 nucleoids representing times throughout the cell cycle show that probability of bilobed character increases during the cell cycle in good correspondence to the four transitions: negligible before T1; low but significant from T1 to T2 onset; dramatically increased at/after T3; and further increased at T3 and again at T4 (Figure 5D right).
Additionally, nucleoid morphologies have been analyzed in hundreds of 3D time series, with images taken at intervals ranging from 5s to 10min. Despite dynamic variations, certain pronounced morphologies occur uniquely at certain specific periods and document a step-wise increase in bilobed character (Figure 5E). (i) Following T1, nucleoids can exhibit a terminal projection or “bud”, usually at the old pole end of the cell. (ii) Following T2, nucleoids can exhibit two lobes of similar size, less or more distinct, and linked by substantial intervening material. (iii) Following T3, the tendencies seen at T2 become more pronounced, with larger separation between the two lobes. (iv) Following T4, the tendencies seen after T2 and T3 continue, with sisters finally linked only by a thin thread. Notably, T1 and T2 morphologies match those predicted by the proposed model (Figure 5B bottom; left versus right).
Remarkably and unexpectedly, immediately prior to T3, the probability of bilobed character diminishes and essentially single continuous shapes can be seen (Figure 5D; also Figure 3F). An analogous effect may occur prior to T2. Apparently, during periods when sister separation is slowed or stalled, awaiting release of the next set of tethers, density fills in the region between already-separating lobes to give a single continuous shape. Then, upon tether release, duality re-emerges in more pronounced state than before. This same alternation of morphologies is also seen in snapshots of individual living cells from large synchronous populations imaged under different conditions.
Thus, nucleoid elongation during the T1–T4 period reflects progressive increases in the end-to-end separation of sister domains, which is alternately impeded by constraining tethers and then licensed by tether release.
At T1 and T2, sequentially separating sister oriC’s are carried in opposite directions on sequential pulses of nucleoid elongation
Individual nucleoids were examined for the relative rates at which sister origins move towards their respective poles during periods of nucleoid length increase (Figure 5F, closed bars). At T1, the origin moving towards the old pole of the cell usually moved faster than the origin moving towards the new pole (Figure 5F, closed red bars). At T2, oppositely, the origin moving towards the new pole of the cell moved faster than the origin moving towards the old pole (Figure 5F, closed blue bars). Moreover, the magnitude of the difference in rate of movement was less for T2 than for T1, in accord with the fact that the lagging origin had already previously carried out some of its poleward movement during T1 (Figure 5A).
Furthermore: in these same cells, nucleoid elongation occurs with the same sequential directional bias as oriC movement. The nucleoid usually elongates more rapidly towards the old pole at T1 and more rapidly towards the new pole at T2 (Figure 5F red and blue hatched bars; other examples in Figures 3G and 3H). Correspondingly, oriC and nucleoid directionality exhibit the same directional bias on a per-nucleoid basis, in 8/9 examined cases (Figure 5F, OO and NN). In essence, oriC’s are riding along with the differential elongation of the nucleoid, first in one direction and then in the other.
Correspondingly, despite considerable diversity, some nucleoids exhibit the prototypical “population average” pattern for T1 or T2 movements. Figure 5G shows a T1 nucleoid: following splitting of sister origins, one oriC moves steadily to the old pole whereas its sister, after an initial separation, shows little net movement. Figure 5H shows a T2 nucleoid: a cycle of nucleoid shortening is followed by nucleoid lengthening, both occurring specifically at the new pole end of the cell (Figure 5H i, ii, iv) and accompanied by differential movement of the new pole-proximal oriC towards its pole (Figure 5Hiii).
These patterns, in toto, directly link increased separation of sister oriC’s, and their underlying sister domains, to pulses of nucleoid elongation and confirm the evolution of sister/mother relationships at T1 and T2 via sequential movement of sister domains in opposite directions (Figure 5C).
Tethers are under tension prior to splitting
Real time analysis of tether release at one T2 snap locus, gln (Figure 5C) shows that splitting of sister gln loci is accompanied by very rapid movement of one gln locus (400 +/− 131nm/min; N=3), specifically toward the new pole end of the nucleoid; in contrast, the other gln locus changes little in net position (e.g. Figure 5I). This behavior strongly suggests that intact inter-sister tethers are under tension due to the ongoing, but constrained, tendency for movement of one sister domain towards the new pole end of the cell (Figure 5B) and that, upon release of the tether, the corresponding snap locus undergoes rapid elastic retraction into its already separating domain.
Discussion
The presented results define E. coli nucleoid organization, shape, and short- and long- time scale dynamics in living cells. Four basic features emerge: longitudinal density bundling, radial confinement, longitudinal density waves, and modulation of sister segregation by programmed inter-sister tethers. The central role of radial confinement, plus the effects of tethers, suggest that the system can be viewed in mechanical terms, with physical features and effects playing critical governing roles. Taken together, the observed results provide a coherent conceptual framework for understanding and further analysis of nucleoid morphology, morphogenesis and dynamics, including segregation of sister chromosomes.
Nucleoid Shape and State
The nucleoid is revealed to be a discrete, internally-organized object. Internal organization comprises longitudinal density bundles that intrinsically tend to be dual (or, more generally, split), irrespective of genomic connectivity. Since bundle patterns are dynamic, involved associative forces are relatively weak. Physical effects (e.g. protein-mediated molecular crowding (Cunha et al., 2001; Adams and Fraden, 1998) and/or weak molecular linking interactions (reviewed in Reyes-Lamothe et al., 2008) are possibilities; negatively supercoiled plectonemes are likely involved (Figure 6A).
Figure 6.
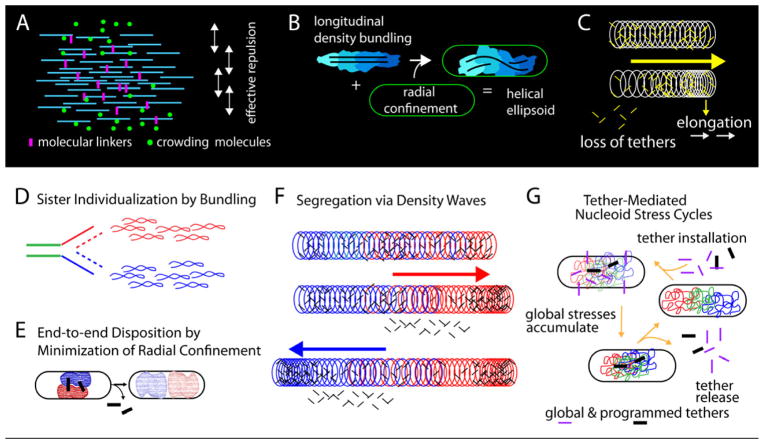
E. coli nucleoid shape, organization and dynamics. (A) Longitudinal density bundles could comprise sub-bundles (e.g. supercoiled plectonemes). Association via weak forces, to permit ready adjustment, could reflect molecular crowding and/or weak- binding linker proteins. Associative effects of bundling could be opposed by an effective repulsion to give low DNA density. (B) Longitudinal density bundles create ellipsoid shape that is helically deformed by interaction with the cell periphery (radial confinement). (C) Longitudinal density waves tend to promote release of tethers at the lagging end. Given directionality, they can underlie elongation at the leading end. (D–F) Sister segregation without a spindle. ( D ) Genomically-biased longitudinal bundling promotes individualization of sisters into distinct units. (E) Minimization of radial confinement stress promotes placement of sister units in an end-to-end disposition versus other relationships. (F) The back and forth motion of density waves facilitate sister segregation by “greasing” the system, removing constraining linkages to increase mobility. (G) Global tether-mediated nucleoid stress cycles (text).
Importantly, virtually all nucleoid material is contained within the shape, implying a strong tendency for coalescence. On the other hand, the density of DNA atoms is very low, comprising <2% of total nucleoid volume. Thus, coalescence into bundles is likely opposed by an effective repulsion. Together these features create a stiff, springy shape. Repulsion could reflect physical effects (excluded volume (Wiggins et al., 2010) or charge), and/or biochemical effects (e.g. a diffusion ratchet; Vecchiarelli et al., 2010).
The nucleoidal ellipsoid has a helix-like shape. This shape results from deformation of the ellipsoid by radial confinement (Figure 6B). This feature implies that the longitudinal persistence length of the ellipsoid is greater than the radius of the confining cell cylinder. Correspondingly, progressive evolution of helical nucleoid(s) during the cell cycle can reflect extension of existing helical path(s) by coalescence of material into stiff longitudinal bundles.
These results confirm and extend previous evidence for internal organization (Odijk, 1998; Cunha et al., 2001), nucleoid stiffness (Wiggins et al., 2010) and/or helix-like shape (Yazdi et al., 2012). They exclude models in which the nucleoid is a randomly-oriented polymer whose shape is defined by the cell cylinder (Jun and Mulder, 2006) or where helical shape arises by pushing of a linear object up against the cell pole(s) (e.g. Wiggins et al, 2010; Jun and Mulder, 2006; Chaudhuri and Mulder, 2012).
Replication-independent Longitudinal Density Waves Mediate Chromosome Mobility
Waves of nucleoid density flux longitudinally, back and forth, along the helical path of the shape, over distances comparable to the length of the nucleoid. Our preliminary qualitative impression is that the waves of density are likely to be oscillatory, with longer time-scale periodicities of the order of a minute. Since fluxes result in only small net changes in density distribution, we infer that they involve subtle changes in fiber proximities (Figure 6C) rather than global coherent nucleoid movement.
Longitudinal density waves are independent of ongoing DNA replication. They may involve an active ATP-driven biochemical process because they are highly dynamic; in contrast, thermal adjustments, which involve low energies, tend to be slow. Nucleoid dynamics are known to be strongly ATP -modulated (Weber et al., 2012).
The nature of density waves suggests that their general role is to promote mobility within the nucleoid (Figure 6C). As a wave passes, increased nucleoid density at the leading end will be accompanied by decreased nucleoid density at the lagging end. This, in turn, will tend to reduce constraining inter-fiber linkages (e.g. by disfavoring rebinding of dissociating unstable linker molecules). This effect will destabilize unwanted linkages that impede all types of nucleoid dynamics. The need to reduce non-specific meshwork linkages is notable, not often emphasized, concern. Many chromosomal molecules nonspecifically link pairs of DNA segments (Reyes-Lamothe et al., 2008). Linkages along a chromosome fiber could play positive roles (e.g as organizational loops); however, linkages between unrelated segments will tend to create an immobile gel incompatible with both global and local dynamics. The need for elimination of spontaneous meshwork linkages, and the ability of density waves to accomplish this task, could be important general features of chromosome biology.
Global Dynamics and Segregation of Sisters Without A Spindle
Taken together the above described nucleoid features, plus programmed and non-specific intra-nucleoid tethers, can explain global nucleoid dynamics, including sister segregation.
(1) Individualization (Figure 6D)
Longitudinal density bundling will promote individualization of sister chromosomes into their respective discrete units. As sisters emerge from the replication fork, bundling of adjacent positions along the same sister will be intrinsically favored over bundling of segments on different sisters. Thus, at this stage (contrary to G1), bundling will be genomically biased, but will be further sharpened by the intrinsic tendency for bundle duality. Complex bundle patterns at intermediate stages would thus reflect the simultaneous presence of bundles corresponding to the mother and two sister domains.
(2) Minimization of Radial Confinement (Figure 6E)
Radial confinement is a non-equilibrium high energy (mechanically stressed) condition. Given two individualizing sister units, minimization of radial confinement stress will drive and direct these units into an end-to-end configuration, versus a longitudinally overlapping state, because radial confinement stress will be much reduced in the former state relative to the latter. Radial confinement stress (and thus segregation force) will increase due to synthesis of new material during DNA replication, but likely is also modulated in other ways (below). Minimization of radial confinement stress would thus be the functional bacterial analogue of eukaryotic mitotic spindle forces, promoting spatial separation as required for regular cell division.
More generally, minimization of radial confinement can provide a driving force and a directionality for all global nucleoid dynamics. For example: the two observed G1 changes - elongation and development of a more symmetrical shape - are those required to reduce radial confinement stress.
(3) Longitudinal density waves (Figure 6F)
Longitudinal density waves will facilitate sister segregation by “greasing” the process, increasing nucleoid mobility to permit implementation of confinement-driven changes in sister disposition. Density waves will similarly facilitate global G1 adjustments.
This three-component model for sister segregation accommodates our previous suggestion that sisters separate by inter-sister pushing (Bates and Kleckner, 2005). It differs qualitatively from other models (Introduction) because: (i) individualization of sisters is a specific feature, not simply a secondary consequence of spatial separation of chromosome fibers; (ii) end-to-end disposition arises from pushing forces, and involves evolution of coherent domains, rather than arising from pulling forces exerted on centromere-like loci; and/or (iii) no intra-cellular scaffolds or peripheral cytoskeletal “railroad tracks” are involved.
Generality
The basic features and processes described above for the E. coli nucleoid could apply to other bacteria as well.
With respect to nucleoid shape and state: B. subtilis nucleoids exhibit an analogous progression of helical shapes (Berlatzsky et al., 2009) and a linear E. coli-like genome organization (D. Rudner, personal communication). Also, the Caulobacter G1 nucleoid is a ~1.5 turn helical ellipsoid dual longitudinal density bundles which, due to polar tethering of ori and ter, is biased by genomic connectivity to give left and right replichores (Umbarger et al., 2011). Interestinglly, helical shape provides a continuous lower-density complementary space through which larger objects can freely move, which could accommodate rapid movement of one sister oriC from its original pole to the opposite end of the cell.
Three-component sister segregation could also occur in all rod-shaped bacteria. This idea is at odds with current views of Caulobacter segregation, often envisioned to involve polar oriC localization and replication-linked compaction towards the two cell poles. However, it is possible to envision collaboration between molecularly-promoted oriC localization and the types of physical effects described here, in both (all) organisms.
This model for segregation can also explain why bacteria occur only in three basic shapes - rods, spirals and spheres. Well-individualized chromosomes in a confined space will always position themselves into their lowest energy state, which will be the one that minimizes overlap. For a rod or a spiral, that state is end-to- end disposition. For a sphere of small enough radius, a pair of fat ellipsoidal nucleoids will be in a symmetrical disposition, also suitable for segregation.
Moreover, the same scenario could explain both sister segregation and cell division in evolving life: individualization will confer sister separateness; minimization of overlap in a confining proto-cell/membrane will place the sisters in a regular relationship; and the need for separateness under confinement would create mechanical weak points at the inter-nucleoid boundaries, giving a primordial mechanism that provokes cell division at the appropriate positions.
Cyclic release of programmed and non-specific tethers
End-to-end segregation of sister chromosomes evolves in discrete steps as mediated by programmed tethers. T1 and T2 probably promote regular sister nucleoid disposition: basic ori-centric orientation is set up for one sister at (T1), thus simplifying events at the major separation transition (T2). T2 concomitantly brings the terminus region into juxtaposition for capture at midcell, where T3 and T4 mediate ensuing ter-related events.
If sister segregation is driven by minimization of radial confinement stress, and since programmed tethers are under mechanical tension at the time of their release, tether-mediated sister separation cycles are most simply explained by cyclic accumulation and release of radial confinement stress (Figure 4Biii). However, several factors point to the existence of more general global nucleoid stress cycles, with sister segregation superimposed as one outcome. (i) T3 and T4 occur after completion of DNA replication, implying that ongoing replication-generated increase in radial confinement stress is not required. (ii) At T4, sister nucleoids are already well separated, so the source of nucleoid stress might not be sister overlap. (iii) Each transition is preceded by a tendency for nucleoid shortening, implying global changes in state throughout the nucleoid. (iv) Cycles may occur at all times: nucleoid release from midcell and ensuing relaxation at G1 could comprise a pre-replicative cycle; and preliminary results hint at an additional post-T4 cycle.
We propose that pulses of nucleoid length increase reflect the accumulation and release of intra-nucleoid stress mediated globally by non-specific tethers (Figure 6E). Basic chromosome metabolism would provoke chromosome fiber changes whose realization is constrained by the tether meshwork, despite the counteracting effects of longitudinal density waves. The result would be accumulation of an unfavorable (stressed) state along the fiber and, concomitantly, via increased radial confinement. When stress reaches a critical level it will provoke catastrophic release of meshwork tethers (and, concomitantly, programmed tethers) thus licensing a more relaxed state. By this scenario, a fundamental role of the observed pulses would be to periodically “cleanse” the genome of unwanted linkages, in addition to promoting specific programmed transitions. An obvious candidate for altered stress would be supercoiling, whose dynamically and regionally-modulated level is, in turn, determined primarily by transcription (Rovinskiy et al., 2012)).
The observed cycles essentially comprise a primordial cell cycle. Interestingly, eukaryotic organisms also undergo cyclic expansion and compaction of chromatin (Kleckner et al., 2004). It is not excluded that the two types of cycles correspond and that the chromatin stress cycle predates, and now works in parallel linkage with, the cell cycle engine.
Synthesis
The presented results provide a coherent general conceptual framework for understanding bacterial nucleoid morphogenesis and dynamics, including, but not limited to sister segregation. The central feature of this framework, which distinguishes it from previous considerations, is that the nucleoid is considered as a complex and evolving, but coherent object whose intrinsic mechanical features play critical governing roles. Underlying basic effects can thus be described mechanical terms, rather than in the language of molecular biology, biochemistry or DNA topology.
Experimental Procedures
Microbiology
Except as noted (Extended Experimental Procedures), studies used CM735-derived strain NK9386 (Bates et al., 2005) expressing hupA::mCherry (Marceau et al., 2011), plasmid-borne pBAD-TetR-mVenus (Wang et al., 2005) and an asnA::tetO (Lau et al., 2003) or gln::tetO (Joshi et al., 2011) array. Growth conditions were as described (Bates and Kleckner 2005; Joshi et al., 2011; Bates et al., 2005).
Microfluidics
Cells were imaged in a microfabricated Polydimethylsiloxane (PDMS) device (Whitesides 2006; Figures 1A top and S1A). Cells are injected at a high flow rate, which causes growth channels to expand slightly, after which the flow rate is reduced, gently trapping cells between the PDMS upper surface and the glass coverslip bottom surface (Figure 1A). Thereafter, fresh medium flowed through the channels at 0.7 ml/hour with temperature maintained at 30°C (Extended Experimental Procedures).
3D Microscopy
Z-section slice separation ranged from 45–200nm with 100–300ms exposure time per slice, and typically covered a range of 1.2μm. 3D data sets were deconvolved using the blind deconvolution algorithm of AutoQuant (Media Cybernetics, Inc.) with the Point Spread Function appropriate to our microscope (Extended Experimental Procedures) at each emission wavelength.
Further processing was done using ImageJ software (National Institutes of Health), MatLab, and Pymol (http://www.pymolwiki.org/index.php/Tiff2ccp4; D. Jeruzalmi and J. Vertrees). All analyses (3D rendering of nucleoid data sets, definition and analysis of cell boundaries from midplane phase contrast images, transformation of Z-stacks into a series of (XZ) slices along the length of the nucleoid in the Y dimension, calculation of density centroid paths, Z-projections, pseudo-color mapping, quantification of longitudinal density fluxes, determination of bi-lobed nucleoid character, localization of FROS foci) as well as detailed protocols for dynamics analyses are described in Extended Experimental Procedures.
Supplementary Material
The E. coli nucleoid exhibits longitudinal density bundling and radial confinement
Longitudinal density waves flux through the E. coli nucleoid over seconds-to-minutes
A mechanical model explains segregation of bacterial chromosomes without a spindle
Sisters segregate in pulses via cyclic accumulation/relief of mechanical stress
Acknowledgments
We thank G. Guidotti, K. Mizuuchi, E. Garner and Kleckner laboratory members for helpful comments; D. Jeruzalmi and J. Vertrees for adaptation of PyMOL to nucleoid images; and R. Johnson for a FIS-GFP plasmid. Research was supported by NIH grant GMS-RO1-025326 to N.K. and Swiss National Science Foundation grant PBELP3-135860 to G.W. We acknowledge the Center for Computer Integrated Systems for Microscopy and Manipulation at UNC Chapel Hill, funded by NIH grant NIBEB-5-P41-EB002025, and the Center for Nanoscale Systems (CNS) at Harvard University, a member of the National Nanotechnology Infrastructure Network.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams M, Fraden S. Phase behavior of mixtures of rods (tobacco mosaic virus) and spheres (polyethylene oxide, bovine serum albumin) Biophys J. 1998;74:669–677. doi: 10.1016/S0006-3495(98)77826-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banigan EJ, Gelbart MA, Gitai Z, Wingreen NS, Liu AJ. Filament depolymerization can explain chromosome pulling during bacterial mitosis. PLoS Comput Biol. 2011;7:e1002145. doi: 10.1371/journal.pcbi.1002145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D. The bacterial replisome: back on track? Mol Microbiol. 2008;69:1341–1348. doi: 10.1111/j.1365-2958.2008.06378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Kleckner N. Chromosome and replisome dynamics in E. coli: Loss of sister cohesion triggers global chromosome movement and mediates chromosome segregation. Cell. 2005;121:1–13. doi: 10.1016/j.cell.2005.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Epstein J, Boye E, Fahrner K, Berg H, Kleckner N. The Escherichia coli baby cell column: a novel cell synchronization method provides new insight into the bacterial cell cycle. Mol Microbiol. 2005;57:380–391. doi: 10.1111/j.1365-2958.2005.04693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlatzky IA, Rouvinski A, Ben-Yehuda S. Spatial organization of a replicating bacterial chromosome. Proc Natl Acad Sci USA. 2008;105:14136–14140. doi: 10.1073/pnas.0804982105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri D, Mulder B. Spontaneous Helicity of a Polymer with Side Loops Confined to a Cylinder. Phys Rev Lett. 2012;108:268305. doi: 10.1103/PhysRevLett.108.268305. [DOI] [PubMed] [Google Scholar]
- Cunha S, Woldringh CL, Odijk T. Polymer-mediated compaction and internal dynamics of isolated Escherichia coli nucleoids. J Struct Biol. 2001;136:53–66. doi: 10.1006/jsbi.2001.4420. [DOI] [PubMed] [Google Scholar]
- Espeli O, Borne R, Dupaigne P, Thiel A, Gigant E, Mercier R, Boccard F. A MatP-divisome interaction coordinates chromosome segregation with cell division in E. coli. EMBO J. 2012;31:3198–3211. doi: 10.1038/emboj.2012.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi MC, Bourniquel A, Fisher J, Ho BT, Magnan D, Kleckner N, Bates D. Escherichia coli sister chromosome separation includes an abrupt global transition with concomitant release of late-splitting intersister snaps. Proc Natl Acad Sci USA. 2011;108:2765–2770. doi: 10.1073/pnas.1019593108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun S, Mulder B. Entropy-driven spatial organization of highly confined polymers: lessons for the bacterial chromosome. Proc Natl Acad Sci USA. 2006;103:12388–12393. doi: 10.1073/pnas.0605305103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N, Zickler D, Jones GH, Dekker J, Padmore R, Henle J, Hutchinson J. A mechanical basis for chromosome function. Proc Natl Acad Sci USA. 2004;107:12502–12597. doi: 10.1073/pnas.0402724101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman T, Cox E. Gene location and DNA density determine transcription factor distributions in Escherichia coli. Mol Sys Bio. 2012;8:610. doi: 10.1038/msb.2012.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau F, Filipe SR, Søballe B, Økstad OA, Barre FX, Sherratt DJ. Spatial and temporal organization of replicating Escherichia coli chromosomes. Mol Microbiol. 2003;49:731–743. doi: 10.1046/j.1365-2958.2003.03640.x. [DOI] [PubMed] [Google Scholar]
- Lemon KP, Grossman AD. The extrusion-capture model for chromosome partitioning in bacteria. Genes Dev. 2001;15:2031–2041. doi: 10.1101/gad.913301. [DOI] [PubMed] [Google Scholar]
- Marceau AH, Bahng S, Massoni SC, George NP, Sandler SJ, Marians KJ, Keck JL. Structure of the SSB–DNA polymerase III interface and its role in DNA replication. EMBO J. 2011;30:4236–4247. doi: 10.1038/emboj.2011.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris V. Hypothesis: chromosome separation in Escherichia coli involves autocatalytic gene expression, transertion and membrane-domain formation. Mol Microbiol. 1995;16:1051–1057. doi: 10.1111/j.1365-2958.1995.tb02330.x. [DOI] [PubMed] [Google Scholar]
- Odijk T. Osmotic compaction of supercoiled DNA into a bacterial nucleoid. Biophys Chem. 1998;73:23–29. doi: 10.1016/s0301-4622(98)00115-x. [DOI] [PubMed] [Google Scholar]
- Reyes-Lamothe R, Wang X, Sherratt DJ. Escherichia coli and its chromosome. Trends Microbiol. 2008;16:238–245. doi: 10.1016/j.tim.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Rovinskiy N, Agbleke AA, Chesnokova O, Pang Z, Higgins NP. Rates of Gyrase Supercoiling and Transcription Elongation Control Supercoil Density in a Bacterial Chromosome. PLoS Genet. 2012;8:e1002845. doi: 10.1371/journal.pgen.1002845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swulius MT, Jensen GJ. The helical MreB cytoskeleton in E. coli MC1000/pLE7 is an artifact of the N-terminal YFP tag. J Bacteriol. 2012;194:6382–6386. doi: 10.1128/JB.00505-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro E, Hong SH, McAdams HH, Shapiro L. Caulobacter requires a dedicated mechanism to initiate chromosome segregation. Proc Natl Acad Sci USA. 2008;105:15435–15440. doi: 10.1073/pnas.0807448105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro E, Shapiro L. Bacterial chromosome organization and segregation. Cold Spring Harb Perspect Biol. 2010;2:a000349. doi: 10.1101/cshperspect.a000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbarger MA, et al. The three-dimensional architecture of a bacterial genome and its alteration by genetic perturbation. Mol Cell. 2011;44:252–264. doi: 10.1016/j.molcel.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecchiarelli AG, Han YW, Tan X, Mizuuchi M, Ghirlando R, Biertümpfel C, Funnell BE, Mizuuchi K. ATP control of dynamic P1 ParA-DNA interactions: a key role for the nucleoid in plasmid partition. Mol Microbiol. 2010;78:78–91. doi: 10.1111/j.1365-2958.2010.07314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Li GW, Chen C, Xie XS, Zhuang X. Chromosome organization by a nucleoid-associated protein in live bacteria. Science. 2011;333:1445–1449. doi: 10.1126/science.1204697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Liu X, Possoz C, Sherratt DJ. The two Escherichia coli chromosome arms locate to separate cell halves. Genes Dev. 2006;20:1727–1731. doi: 10.1101/gad.388406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Possoz C, Sherratt DJ. Dancing around the divisome: asymmetric chromosome segregation in Escherichia coli. Genes Dev. 2005;19:2367–2377. doi: 10.1101/gad.345305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber SC, Spakowitz AJ, Theriot JA. Nonthermal ATP-dependent fluctuations contribute to the in vivo motion of chromosomal loci. Proc Natl Acad Sci USA. 2012;109:7338–7343. doi: 10.1073/pnas.1119505109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitesides GM. The origins and the future of microfluidics. Nature. 2006;442:368– 373. doi: 10.1038/nature05058. [DOI] [PubMed] [Google Scholar]
- Wiggins PA, Cheveralls KC, Martin JS, Lintner R, Kondev J. Strong intranucleoid interactions organize the Escherichia coli chromosome into a nucleoid filament. Proc Natl Acad Sci USA. 2010;16:4991–4995. doi: 10.1073/pnas.0912062107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdi NH, Guet CC, Johnson RC, Marko JF. Variation of the folding and dynamics of the Escherichia coli chromosome with growth conditions. Mol Microbiol. 2012;86:1318–1333. doi: 10.1111/mmi.12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.