Abstract
As a neurodegenerative disorder, Alzheimer disease (AD) is the most common form of dementia found in the aging population. Immunotherapy with passive or active immunizations targeting amyloid beta (Aβ) build-up in the brain may provide a possible treatment option and may help prevent AD from progressing. A number of passive immunizations with anti-Aβ42 antibodies are in different phases of clinical trials. One active immunization approach, AN-1792, was stopped after the development of autoimmune encephalitis in 6% of patients and a second one, CAD106, in which a small Aβ epitope is used, is currently in safety and tolerability studies. Besides active immunizations with proteins or peptides, active immunizations using DNA which codes for the protein against which the immune response will be directed, so called genetic immunizations, provide additional safety as the immune response in DNA immunizations differs quantitatively and qualitatively from the response elicited by peptide immunizations. In this review, we summarize our data using DNA Aβ42 immunizations in mouse models and discuss the results together with the results presented by other groups working on a DNA vaccine as treatment option for AD.
Keywords: Alzheimer’s disease, amyloid-beta, immunotherapy, vaccination
The concept of immunotherapy as a treatment option for AD
Alzheimer disease (AD) is the most common form of age related dementia and it is estimated that worldwide nearly 36 million people have AD. Within the United States, AD is the 6th leading cause of death. Currently, no cure has been found for this disease and only symptomatic treatment options are available.
The pathologic features of extracellular amyloid plaques and intraneuronal neurofibrillary tangles are considered hallmarks for a definitive identification of this disease, which is only possible post-mortem. AD pathogenesis has been strongly associated with the accumulation and aggregation of amyloid beta (Aβ) peptides in the brain. It has been documented in a triple transgenic mouse model of AD that Aβ accumulation precedes the development of neurofibrillary tangles [1, 2]. Twenty years ago the amyloid cascade hypothesis was formulated, which postulated that Aβ deposition is the initial event in the multifactoral pathogenesis of AD [3–5]. There is significant evidence that Aβ peptides play a major role in the onset and progression of AD [6, 7]. Important findings showing that Aβ42 may not only serve as a marker, but may contribute to the development of AD came from a genetic study in Iceland. In this study, a single mutation (A673T) within the AMYLOID PRECURSOR PROTEIN (APP) gene resulted in a 40% reduction of the β-secretase cleavage product of APP: Aβ1–42; and carriers of this rare mutations were protected against developing AD [8].
Outcomes from a recently approved clinical trial to prevent AD will provide important information whether the amyloid hypothesis is valid in this regard. In this study, cognitively healthy patients who carry a Presenilin 1 genetic mutation and are highly likely to develop AD at around 45 years of age will be passively vaccinated with a humanized monoclonal antibody (Crenezumab). The antibody will binds to Aβ42 and likely interfere with formation of amyloid plaques and thus disease progression (New York Times, May 15, 2012, and [9]). This particular antibody is a non-inflammatory Th2 type antibody of the IgG4 isotype [10], very similar to the IgG1 antibody isotype we find after DNA Aβ1–42 immunizations in mice in our model.
Two other large clinical trials using passive immunization with monoclonal antibodies recognizing Aβ42, Bapineuzumab (Elan, Pfizer, Johnson&Johnson ) and Solanezumab (Eli Lilly and Company) have failed to achieve the projected results. Main outcomes from the Bapineuzumab study were slight differences for CSF Tau but not for CSF Aβ between treatment and placebo groups [11]. Solanezumab showed no clinical benefit of therapy at twelve weeks (12). However, a later report in the media indicated clinical benefit and mild effects in patients with early AD[New York Times, July 24, 2012]. At present, clinical trials using Bapineuzumab have ended (New York Times, July 24, 2012), whereas trials using Solanezumab are still ongoing. The concept in using anti-Aβ antibodies has also been the basis for another small clinical trial in which patients received the injection of intravenous immunoglobulins (IVIG). This study was based on the hypothesis that IVIG contains naturally occurring auto-antibodies (nAbs-Aβ) that specifically recognize and block the toxic effects of Aβ and showed positive results [13, 14].
Alzheimer therapy must begin before symptoms become apparent. In a recently published longitudinal study it was found that the Aβ42 concentrations in CSF decline 25 years before the onset of clinical symptoms indicating that Aβ deposition in brain has had begun; and using positron-emission tomography with fibrillar Aβ specific Pittsburgh compound B, Aβ deposition becomes visible as early as 15 years before the onset of symptoms [15]. To establish a better time frame as to how early treatment must be started for effective prevention of Alzheimer disease, three new trials will investigate this question. Participants in the DIAN (Dominantly Inherited Alzheimer Network) study, which is currently enrolling subjects, are carriers of genetic mutations predisposing them to develop AD at early age, as are the participants of the Alzheimer Prevention Initiative (API) members of an extended FAD family in Columbia. A third study, Treatment of Asymptomatic Alzheimer (A4), will focus on patients who are not carriers of gene mutations but whose brains already display signs of Aβ accumulation [16].
Besides passive vaccination, where patients receive injections of the antibody itself; active immunization, in which antibodies are produced by the individual upon contact with the antigen have great potential for preventive AD therapy. The benefits of immunizations targeting Aβ peptide and the production of Aβ antibodies respectively were first shown in animal models, in which mice transgenic for human APP were immunized with Aβ1–42 peptide [17, 18]. This mouse model resembles the human disease with the formation of amyloid plaques in cortex and hippocampus and immunization with Aβ1–42 peptide did indeed reduce senile plaque counts and improved cognitive behavior in the treated animals [18–20]. Based on these findings a clinical trial was started in which AD patients received Aβ1–42 peptide immunizations. However, this trial came to an abrupt end when 6% of participants developed meningoencephalitis, apparently due to an inflammatory Th1 auto immune response targeting the self antigen Aβ in brain [21, 22]. A clinical follow-up showed that the immunization with Aβ42 peptide led to a reduction in plaque load in patients who had been treated with Aβ42 peptide compared to the placebo control patients, thus providing proof for the possibility of amyloid removal by immunotherapy [23]. Furthermore, other positive functions of Aβ immunotherapy have been directly associated with anti-Aβ42 antibodies in additional studies. In the AD mouse model it was shown that removal of Aβ42 depositions in brain with antibody therapy preserves synaptic structures and improves neuron morphology, and these positive effects were attributed to the antibodies only [24, 25].
A new study using active Aβ immunization in Swedish AD patients has recently been published [26]. This vaccine, CAD106, consists of a B cell epitope peptide, Aβ1–6, which is coupled to a carrier protein displaying a second protein, 180 copies of a bacteriophage coat protein, to provide T cell help for the anticipated immune response. This study was performed to provide safety, tolerability, and antibody responses to this particular vaccine, and the outcome showed a positive antibody response in most patients and no signs for adverse autoimmune inflammation were noted. Data from this study are limited and longer lasting studies with more patients are needed but due to the lack of negative side effects this study will progress into a phase 2 study and is ongoing [26].
Mechanisms of how Aβ42 specific antibodies lower the amyloid burden in brain
The answer to this very important topic can be addressed only hypothetically as it has not been shown which mechanism is predominantly used, and there will likely be more than one which is used. In general, it is believed that there are three major pathways of antibody action to remove excess amyloid peptides from the brain: First, the antibody can bind directly to the Aβ peptides and thereby lead to dissociation of the amyloid fibrils and neutralization of neurotoxic Aβ oligomers. Second, the antibody can bind the amyloid plaque leading to Fc-receptor mediated phagocytosis by brain microglia; the Aβ specific antibody opsonizes the Aβ fibrils for removal via cellular immune mechanisms. Lastly, the antibody does not enter the brain but binds to Aβ in the plasma and is thus leading to a concentration gradient producing a net efflux of amyloid from the brain. This mechanism is called the peripheral sink mechanism. It is highly likely that all of these mechanisms play a role in the beneficial action of Aβ specific antibodies. A recent study showed sustained binding of Aβ42 specific antibodies to brain areas with high amyloid levels in an AD mouse model (27) and preliminary findings from our laboratory are also in support of the direct antibody binding to plaques in brain of Aβ42 immunized mice (unpublished).
The role of Aβ42 specific T cells in immunotherapy
Much less is known about the action of Aβ specific T cells in Alzheimer disease or their influence in immunotherapy. It has been shown that increased levels of Aβ42 specific T cells can be found in elderly patients with AD as well as in patients without any symptoms for AD and still these T cells were present without any previous immunization [28]. There is also strong evidence that the meningoencephalitis found in patients which had received the active Aβ42 peptide immunizations was caused by a Th1 inflammatory T cell response targeting the self antigen Aβ in brain [29]. It is clear that this auto-inflammatory T cell response has to be avoided in order to increase the safety for an active immunization protocol. Conversely, T cell help is needed to mount an effective antibody immune response. Without T cell help, there is no antibody isotype switching and no somatic hypermutation to create high affinity antibodies which are needed to optimize an effective Aβ clearance. Furthermore, regulatory T cells have an enormous effect on the down regulation and contraction of an expanding T cell response and these cells are also clearly needed. Thus far, Aβ42 specific T cells have not yet been studied in detail, and their contribution to the progression and/or regulatory effects on the prevention of an autoimmune inflammation directed to Aβ42 in brain are unknown. It is possible that polymorphisms in TCR V gene usage in AD patients might contribute to differences in the clearance and turnover processes of amyloid beta from brain, substantiating the clinical relevance of this topic.
DNA Aβ42 vaccination as a safe and effective alternative
Anti-amyloid immunotherapy for AD harbors the danger of an inflammatory autoimmune response targeting the self-antigen Aβ42 in brain, and this has happened in a clinical trial with Aβ42 peptide immunization (AN1792). To find a safer preventive therapy, many of us are studying the immune response following a DNA based immunization approach, in which the immunizing agent is DNA encoding Aβ1–42 [29–36]. DNA immunization via gene gun injections into the skin results in a strongly polarized immune response which greatly differs from a peptide generated immune response. Previously, it has been shown that Aβ42 DNA vaccination via gene gun generates a Th2 cellular immune response [ 30, 33, 36, 37]. We have also demonstrated that in-vitro T cell proliferation in response to Aβ1–42 peptide re-stimulation was absent in full-length DNA Aβ42 trimer immunized mice when compared to Aβ1–42 peptide immunized mice, thereby supporting the safety of this approach [38, 39].
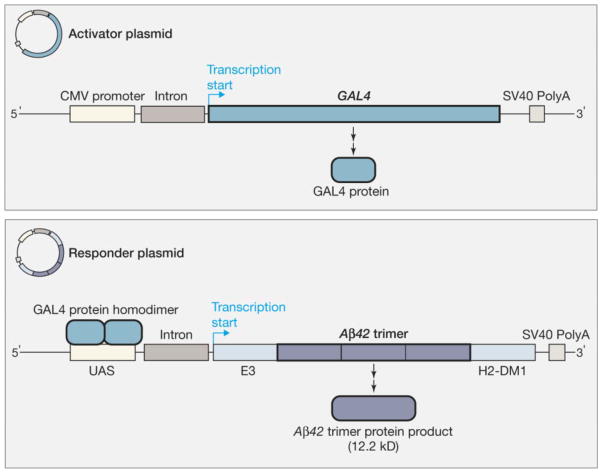
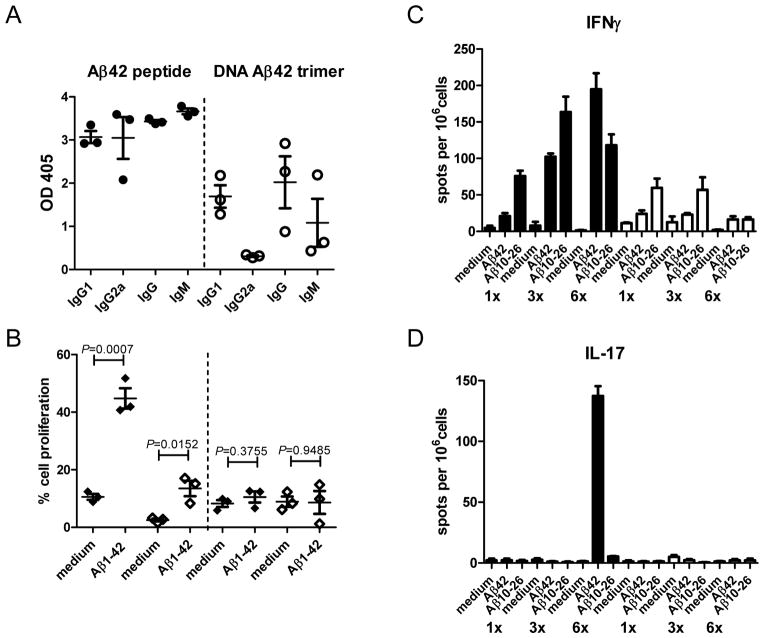
Our report on gene gun mediated DNA Aβ42 immunization with a constitutive promoter which induced a good antibody response against Aβ42 peptide in BALB/cJ mice [30] was the first to show that it is possible to use this methodology as an alternative to Aβ42 peptide immunization. In these studies, we have used one copy of the Aβ1–42 sequence in a plasmid vector in which the transcription and translation was driven by a CMV promoter. With the same plasmid system we further demonstrated that prophylactic DNA Aβ42 immunization in APPswe/PS1ΔE9 transgenic mice reduced the brain Aβ42 plaque load by 42% and that DNA immunization with this human Aβ42 sequence also lead to good antibody production in one monkey we have tested [31, 32]. The humoral response to DNA Aβ1–42 immunization was substantially improved when we started to use a binary Gal4/UAS system in combination with a novel Aβ1–42 trimer construct [33]. This binary system is comprised of a two plasmid system, which were injected into the skin via particle bombardment with the gene gun simultaneously. One plasmid codes for the DNA Aβ1–42 trimer (responder plasmid) and the other plasmid codes for the transcription factor Gal4 (activator plasmid), which drives the transcription of DNA Aβ1–42 trimer due to binding of Gal4 to an upstream UAS/Gal4 response element (Figure 1, from JAMA 2009 [38], with permission). Trimeric Aβ42 highly improved immunogenicity when compared to its monomeric forms [33]. Using this second generation DNA Aβ42 vaccine we compared the immune responses to DNA and Aβ1–42 peptide immunization side by side in a wild-type mouse model which clearly showed the characteristic features of genetic immunizations [38]. While we found a mixed Th1/Th2 (IgG1/IgG2a) antibody immune response in the Aβ42 peptide immunized mice with production of IFNγ and IL-17 indicative of a Th1 cellular immune reaction, the Aβ42 trimer DNA vaccination of wild-type mice resulted in sufficient antibody levels with a strongly polarized Th2 bias (IgG1 antibodies only) and no accompanying inflammatory T cell response (Figure 2, adapted from Cell. Mol. Neurobiol., Lambracht-Washington et al. 2011 [39]). Different from other Aβ42 DNA vaccine approaches in which only parts of the Aβ peptide were included in the respective plasmid sequences to avoid a possible harmful Th1 T cell response [35, 37, 40–42], the Aβ1–42 trimer we used is full-length and contains both, B- and T-cell epitopes. T cell help is needed at the early stages of the immune response to maintain and further the humoral immune response. From our findings, we speculate that T cells were reduced to levels below detection at the time of the cellular recall experiments, but T cells were clearly present in the DNA Aβ42 trimer immunized mice at earlier immunization time points as shown with the antibody isotype switch to IgG1 at two and three immunization time points [39]. It is possible that DNA Aβ42 immunization induces a regulatory T cell response which is the reason for the low level of Aβ42 specific T cell reactivity in our mouse models [43, manuscript in preparation].
Figure 1.
(with permission from JAMA, Lambracht-Washington et al., 2009, (38)) Constitutive expression of the GAL4 transcription factor is driven by a cytomegalovirus (CMV) promoter on the activator plasmid. The GAL4 protein binds as a homodimer to the responder plasmid at sites in the upstream activator sequence (UAS), as part of a minimal promoter. GAL4 binding drives transcription of the β-amyloid1–42 (Aβ42) trimer sequence that has been cloned into a DNA fragment between an adenovirus E3 (early region 3) leader sequence and an endosomal targeting sequence derived from the mouse major histocompatibility complex class II gene H2-DM. The endosomal targeting sequence is in directing the messenger RNA (mRNA) transcript to the endoplasmic reticulum for protein synthesis and secretion. The simian virus 40 (SV40) polyadenylation (PolyA) sequence on both the activation and responder plasmids stabilizes the respective mRNA transcripts.
Figure 2.
(adapted and with permission from Cell. Mol. Neurobiol., Lambracht-Washington et al., 2011 (39))
A) Anti-Aβ42 antibody production in response to Aβ42 peptide and DNA Aβ42 trimer immunization: Shown were OD readings for six immunization time points from three mice immunized with peptide (black circles) or DNA Aβ42 trimer (open circles). Antibody isotypes (IgG1, IgG2a, IgG, IgM) were indicated on the x axis. DNA Aβ42 immunization resulted in a strong polarized immune response with the production of IgG1 antibodies only. Aβ42 peptide immunized mice had a mixed immune response with similar levels of IgG1 and IgG2a antibodies. B) Proliferation of CD4 and CD8 T cells from six times immunized mice (Aβ42 peptide and DNA Aβ42 trimer) as assessed by CFSE dilution. Percentage of cell proliferation was analyzed in the life cell gate, followed by further gating on CD4 and CD8 positive cells and compared for the medium control and the Aβ42 peptide re-stimulated cell cultures. The left hand side of the graphs shows CD4 (black diamonds) and CD8 (open diamonds) T cell proliferation from Aβ42 peptide immunized mice, the right hand side of the graphs shows CD4 and CD8 T cell proliferation from DNA Aβ42 trimer immunized mice. Pooled splenocytes from three mice each were analyzed in triplicates for proliferative responses after in-vitro re-stimulation with Aβ1–42 and Aβ10–26 peptides and compared to medium controls. DNA Aβ42 immunized mice showed no Aβ42 specific CD4 or CD8 T cell proliferation after six immunization time points, whereas splenocytes from Aβ42 peptide immunized mice showed both, increased CD4 and CD8 T cell proliferation.
C and D) ELISPOT analysis for the pro-inflammatory cytokines IFNγ and IL-17: Pooled splenocytes from three Aβ42 peptide immunized mice and three DNA Aβ42 trimer immunized mice (1, 3 and 6 immunization time points, 1 x, 3 x, and 6 x as indicated below the y axis) were analyzed in triplicates for cytokine secretion after in-vitro re-stimulation with Aβ1–42 and Aβ10–26 peptides and compared to medium controls. Black bars indicate Aβ42 peptide immunized mice; white bars indicate DNA Aβ42 trimer immunized mice. DNA Aβ42 immunized mice produced very little IFN and no IL-17 after in-vitro re-stimulation with Aβ peptides, while Aβ42 peptide immunized mice showed with the immunization time points increasing numbers of IFNγ producing cells and a very high number of IL-17 producing cells (indicative of a Th17 T cell immune response) after six immunizations.
The antibody production in response to DNA immunizations is much lower compared to antibody levels which can be obtained following peptide or protein immunizations with the respective antigen. To overcome this significantly lower antibody production, so called prime-boost regimens have been shown to be highly effective [34, 37, 44]. The antigen is applied via different routes and immunization sites: The first immunization initiates the immune response and subsequent heterologous immunizations (via different routes) lead to further expansion of antigen specific cells with a selection of cells with high antigen avidity to boost the specific responses [45]. For further studies on the effectiveness of the DNA Aβ1–42 trimer immunization protocol, we analyzed two different boost regimens: The peptide boost of a DNA primed immune response and the DNA boost of a peptide primed immune response. We believe this to be the first time that a DNA boost has shown such a strong effect on the immune response. While both appear to be effective to increase the antibody responses; we found about 350 μg of Aβ42 specific antibody per ml plasma in the DNA prime/peptide boost groups and 250 μg/ml of plasma in the peptide prime/DNA boost groups, marked differences were observed in regard to the antigen specific cellular immune response. Consistent with previous results we did not find Aβ42 specific T cell proliferation or the production of inflammatory cytokines in mice which have received the peptide prime/DNA boost regimen. The latter immunization regimen, the DNA boost, influenced strongly the cellular immune reaction. Thus, a benefit from this vaccination approach (peptide prime/DNA boost) may lay in the down regulation of a T cell response which could otherwise lead to the complication of an inflammatory auto immune response in any immunotherapy approach using a self antigen [46].
Plans to test the DNA Aβ42 Vaccine in Human Subjects
In 2009, the United States Patent and Trademark Office (USPTO) issued a patent for “Amyloid β Gene Vaccines” to the University of Texas Southwestern Medical Center at Dallas for our development of this vaccine and listed one of this paper’s authors (R. N. Rosenberg) as an inventor of the vaccine. The patent indicated that our vaccine was a new concept and provided new technology that could be developed into a clinically beneficial method of immunotherapy for Alzheimer disease. In 2011, the University licensed the vaccine’s technology to a Dallas company to raise funds to complete pre-clinical studies required by the Food and Drug Administration (FDA), as outlined to us by the FDA in 2010, and also to obtain an Investigational New Drug License from the FDA to conduct a Phase 1 clinical trial for safety, toxicity and tolerability.
The phase 1 clinical trial would test the vaccine for safety, toxicity and tolerability in asymptomatic 70 year old subjects who were accumulating amyloid as measured by non-invasive positron emission tomographic (PET) brain scans utilizing F-18 florbetapir [47]. Subsequently, a clinical benefit of the DNA Aβ42 vaccine would be tested in a phase 2 clinical trial in asymptomatic 70 year old subjects and the surrogate endpoint would be evidenced by a rate and amount of amyloid accumulation being reduced in treated versus control subjects using PET scanning. A phase 3 clinical trial would measure clinical benefit in asymptomatic 70 year old subjects by showing a slowing of deficits or improvement on neuropsychological test scores and also slowing or reduction in amyloid accumulation in brain using PET scanning with F-18 florbetapir. These are our current plans to test the vaccine for safety and efficacy in human subjects.
The DNA Aβ42 vaccine has the potential to safely delay the onset, slow the rate of progression or even possibly prevent AD. In contrast from Aβ42 peptide immunization, our recent results indicate the DNA Aβ42 vaccine produces high levels of non-inflammatory Th2 anti-Aβ42 antibodies, down regulates T cell proliferation after providing initial T cell help for IgG antibody production by B cells which is leading to the prevention of potential inflammatory T cells from transiting through brain. Furthermore, it shows no cytotoxic CD8 T cell proliferation and no production of the inflammatory cytokines IL-17 and INFγ. Our recent finding that Aβ42 peptide prime-DNA Aβ42 boost resulted in significant increases in anti-Aβ42 antibody and still down regulated later T cell proliferation offers a vaccination approach that may be even more effective than DNA Aβ42 vaccination alone [46].
It is our view that the DNA Aβ42 vaccine represents an immunotherapeutic approach that needs to be tested following passive anti-Aβ42 vaccination, assuming that passive immunotherapy which is currently tested in several clinical trials can be shown to be effective and safe in delaying the progression or preventing AD from developing by at least 5 years. Active DNA Aβ42 vaccination compared to passive antibody infusion is less expensive and easier to administer to large populations at risk for AD, which will be identified by serum bio-markers, including PET brain scanning.
We are entering a new phase of treating AD by identifying at risk persons for AD utilizing bio-markers and the surrogate endpoint of amyloid accumulation with amyloid scanning. Identifying persons at risk for AD years before the onset of symptoms offers the best opportunity to prevent AD from occurring and active vaccination with the DNA Aβ42 vaccine may be an important method to provide clinical benefit.
Acknowledgments
This study was funded by grants from NIH/NIA Alzheimer’s Disease Center (P30AG12300-17), Rudman Partnership; and McCune Foundation.
References
- 1.Götz J, Chen F, van Dorpe J, Nitsch RM. Formation of neurofibrillary tangles in P301L tau transgenic mice induced by Abeta 42 fibrils. Science. 2001;293:1491–1495. doi: 10.1126/science.1062097. [DOI] [PubMed] [Google Scholar]
- 2.Oddo S, Caccamo A, Kitazawa M, Tseng BP, LaFerla FM. Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer’s disease. Neurobiol Aging. 2003;24:1063–1070. doi: 10.1016/j.neurobiolaging.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 3.Hardy J. New insights into the genetics of Alzheimer’s disease. Ann Med. 1996;28:255–258. doi: 10.3109/07853899609033127. [DOI] [PubMed] [Google Scholar]
- 4.Selkoe DJ. Amyloid beta-protein and the genetics of Alzheimer’s disease. J Biol Chem. 1996;271:18295–18298. doi: 10.1074/jbc.271.31.18295. [DOI] [PubMed] [Google Scholar]
- 5.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 6.Bertram L, Tanzi RE. The current status of Alzheimer’s disease genetics: what do we tell the patients? Pharmacol Res. 2004;50:385–396. doi: 10.1016/j.phrs.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg RN. Translational research on the way to effective therapy for Alzheimer’s disease. Arch Gen Psychiatry. 2005;62:1186–1192. doi: 10.1001/archpsyc.62.11.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jonsson T, Atwal JK, Steinberg S, Snaedal J, Jonsson PV, Bjornsson S, et al. A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature. 2012;488:96–99. doi: 10.1038/nature11283. [DOI] [PubMed] [Google Scholar]
- 9.Wadman M. US government sets out Alzheimer’s plan. Nature. 2012;485:426–427. doi: 10.1038/485426a. [DOI] [PubMed] [Google Scholar]
- 10.Adolfsson O, Pihlgren M, Toni N, Varisco Y, Buccarello AL, Antoniello K, et al. An Effector-reduced anti-β-amyloid (Aβ) antibody with unique Aβ binding properties promotes neuroprotection and glial engulfment of Aβ. J Neurosci. 2012;32:9677–9689. doi: 10.1523/JNEUROSCI.4742-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blennow K, Zetterberg H, Rinne JO, Salloway S, Wei J, Black R, et al. Effect of immunotherapy with bapineuzumab on cerebrospinal fluid biomarker levels in patients with mild to moderate Alzheimer disease. Arch Neurol. 2012;69:1002–1010. doi: 10.1001/archneurol.2012.90. [DOI] [PubMed] [Google Scholar]
- 12.Farlow M, Arnold SE, van Dyck CH, Aisen PS, Snider BJ, Porsteinsson AP, et al. Safety and biomarker effects of solanezumab in patients with Alzheimer’s disease. Alzheimers Dement. 2012;8:261–271. doi: 10.1016/j.jalz.2011.09.224. [DOI] [PubMed] [Google Scholar]
- 13.Relkin NR, Szabo P, Adamiak B, Burgut T, Monthe C, Lent RW, et al. 18-Month study of intravenous immunoglobulin for treatment of mild Alzheimer disease. Neurobiol Aging. 2009;30:1728–1736. doi: 10.1016/j.neurobiolaging.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 14.Relkin N, Bettger L, Tsakanikas D, Ravdin L. Three-year follow-up on the IVIg for Alzheimer’s phase II study. Alzheimer’s Dement. 2012;8(Suppl):P589–P3-381. [Google Scholar]
- 15.Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012;367:795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller G. News focus: Stopping Alzheimer’s before it starts. Science. 2012;337:790–792. doi: 10.1126/science.337.6096.790. [DOI] [PubMed] [Google Scholar]
- 17.Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, Blackwell C, et al. Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. Nature. 1995;373:523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- 18.Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, et al. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 19.Janus C, Pearson J, McLaurin J, Mathews PM, Jiang Y, Schmidt SD, et al. A beta peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer’s disease. Nature. 2000;408:979–982. doi: 10.1038/35050110. [DOI] [PubMed] [Google Scholar]
- 20.Morgan D, Diamond DM, Gottschall PE, Ugen KE, Dickey C, Hardy J, et al. A beta peptide vaccination prevents memory loss in an animal model of Alzheimer’s disease. Nature. 2000;408:982–985. doi: 10.1038/35050116. [DOI] [PubMed] [Google Scholar]
- 21.Fox NC, Black RS, Gilman S, Rossor MN, Griffith SG, Jenkins L, et al. Effects of Abeta immunization (AN1792) on MRI measures of cerebral volume in Alzheimer disease. Neurology. 2005;64:1563–1572. doi: 10.1212/01.WNL.0000159743.08996.99. [DOI] [PubMed] [Google Scholar]
- 22.Gilman S, Koller M, Black RS, Jenkins L, Griffith SG, Fox NC, et al. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005;64:1553–1562. doi: 10.1212/01.WNL.0000159740.16984.3C. [DOI] [PubMed] [Google Scholar]
- 23.Holmes C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, Bayer A, et al. Long-term effects of Abeta42 immunisation in Alzheimer’s disease: follow-up of a randomised, placebo-controlled phase I trial. Lancet. 2008;372:216–223. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- 24.Buttini M, Masliah E, Barbour R, Grajeda H, Motter R, Johnson-Wood K, et al. Beta-amyloid immunotherapy prevents synaptic degeneration in a mouse model of Alzheimer’s disease. J Neurosci. 2005;5(25):9096–9101. doi: 10.1523/JNEUROSCI.1697-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Serrano-Pozo A, William CM, Ferrer I, Uro-Coste E, Delisle MB, Maurage CA, et al. Beneficial effect of human anti-amyloid-beta active immunization on neurite morphology and tau pathology. Brain. 2010;133:1312–1327. doi: 10.1093/brain/awq056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winblad B, Andreasen N, Minthon L, Floesser A, Imbert G, Dumortier T, et al. Safety, tolerability, and antibody response of active Aβ immunotherapy with CAD106 in patients with Alzheimer’s disease: randomised, double-blind, placebo-controlled, first-inhuman study. Lancet Neurol. 2012;11:597–604. doi: 10.1016/S1474-4422(12)70140-0. [DOI] [PubMed] [Google Scholar]
- 27.Bard F, Fox M, Friedrich S, Seubert P, Schenk D, Kinney GG, Yednock T. Sustained levels of antibodies against Aβ in amyloid-rich regions of the CNS following intravenous dosing in human APP transgenic mice. Exp Neurol. 2012;238(1):38–43. doi: 10.1016/j.expneurol.2012.07.022. [DOI] [PubMed] [Google Scholar]
- 28.Monsonego A, Zota V, Karni A, Krieger JI, Bar-Or A, Bitan G, et al. Increased T cell reactivity to amyloid beta protein in older humans and patients with Alzheimer disease. J Clin Invest. 2003;112:415–422. doi: 10.1172/JCI18104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monsonego A, Imitola J, Petrovic S, Zota V, Nemirovsky A, Baron R, et al. Abeta-induced meningoencephalitis is IFN-gamma-dependent and is associated with T cell-dependent clearance of Abeta in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA. 2006;103:5048–5053. doi: 10.1073/pnas.0506209103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qu B, Rosenberg RN, Li L, Boyer PJ, Johnston SA. Gene vaccination to bias the immune response to amyloid-beta peptide as therapy for Alzheimer disease. Arch Neurol. 2004;61:1859–1864. doi: 10.1001/archneur.61.12.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qu B, Boyer PJ, Johnston SA, Hynan LS, Rosenberg RN. Aβ42 gene vaccination reduces brain amyloid plaque burden in transgenic mice. J Neurol Sci. 2006;244:151–158. doi: 10.1016/j.jns.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qu B-X, Xiang Q, Li L, Johnston SA, Hynan LS, Rosenberg RN. Aβ42 gene vaccine prevents Aβ42 deposition in brain of double transgenic mice. J Neurol Sci. 2007;260:204–213. doi: 10.1016/j.jns.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qu BX, Lambracht-Washington D, Fu M, Eagar TN, Stüve O, Rosenberg RN. Analysis of three plasmid systems for use in DNA A beta 42 immunization as therapy for Alzheimer’s disease. Vaccine. 2010;28:5280–5287. doi: 10.1016/j.vaccine.2010.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim HD, Jin JJ, Maxwell JA, Fukuchi K. Enhancing Th2 immune responses against amyloid protein by a DNA prime-adenovirus boost regimen for Alzheimer’s disease. Immunol Lett. 2007;15:30–38. doi: 10.1016/j.imlet.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Movsesyan N, Ghochikyan A, Mkrtichyan M, Petrushina I, Davtyan H, Olkhanud PB, et al. Reducing AD-like pathology in 3xTg-AD mouse model by DNA epitope vaccine - a novel immunotherapeutic strategy. PLoS ONE. 2008;3:e2124. doi: 10.1371/journal.pone.0002124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DaSilva KA, Brown ME, McLaurin J. Reduced oligomeric and vascular amyloid-beta following immunization of TgCRND8 mice with an Alzheimer’s DNA vaccine. Vaccine. 2009;27:1365–1376. doi: 10.1016/j.vaccine.2008.12.044. [DOI] [PubMed] [Google Scholar]
- 37.Davtyan H, Mkrtichyan M, Movsesyan N, Petrushina I, Mamikonyan G, Cribbs DH, et al. DNA prime-protein boost increased the titer, avidity and persistence of anti-Abeta antibodies in wild-type mice. Gene Ther. 2010;17:261–271. doi: 10.1038/gt.2009.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lambracht-Washington D, Qu BX, Fu M, Eagar TN, Stüve O, Rosenberg RN. DNA beta-amyloid (1–42) trimer immunization for Alzheimer disease in a wild-type mouse model. JAMA. 2009;302:1796–1802. doi: 10.1001/jama.2009.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lambracht-Washington D, Qu BX, Fu M, Anderson LD, Jr, Stüve O, Eagar TN, et al. DNA Immunization against Amyloid beta 42 has high potential as safe therapy for Alzheimer’s Disease as it diminishes antigen specific Th1 and Th17 cell proliferation. Cell Mol Neurobiol. 2011;31:867–874. doi: 10.1007/s10571-011-9680-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lemere CA, Maier M, Peng Y, Jiang L, Seabrook TJ. Novel Abeta immunogens: is shorter better? Curr Alzheimer Res. 2007;4:427–436. doi: 10.2174/156720507781788800. [DOI] [PubMed] [Google Scholar]
- 41.Maier M, Seabrook TJ, Lazo ND, Jiang L, Das P, Janus C, et al. Short amyloid-beta (Abeta) immunogens reduce cerebral Abeta load and learning deficits in an Alzheimer’s disease mouse model in the absence of an Abeta-specific cellular immune response. J Neurosci. 2006;26:4717–4728. doi: 10.1523/JNEUROSCI.0381-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zou J, Yao Z, Zhang G, Wang H, Xu J, Yew DT, et al. Vaccination of Alzheimer’s model mice with adenovirus vector containing quadrivalent foldable Aβ1–15 reduces Aβ burden and behavioral impairment without Aβ-specific T cell response. J Neurol Sci. 2008;272:87–98. doi: 10.1016/j.jns.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 43.Lambracht-Washington D, Qu BX, Fu M, Stüve O, Eagar TN, Rosenberg RN. A regulatory immune response after DNA vaccination against amyloid beta 42. Alzheimers Dement. 2012;8(Suppl):P201–P1-272. [Google Scholar]
- 44.Subramanian S, Divya Shree AN. Enhanced Th2 immunity after DNA prime-protein boost immunization with amyloid beta (1–42) plus CpG oligodeoxynucleotides in aged rats. Neurosci Lett. 2008;436:219–222. doi: 10.1016/j.neulet.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 45.Woodland DL. Jump-starting the immune system: prime-boosting comes of age. Trends Immunol. 2004;25:98–104. doi: 10.1016/j.it.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 46.Lambracht-Washington D, Qu BX, Fu M, Anderson LD, Jr, Stüve O, Eagar TN, et al. A peptide prime-DNA boost immunization protocol provides significant benefits as a new generation Aβ42 DNA vaccine for Alzheimer disease. J Neuroimmunol. 2012 doi: 10.1016/j.jneuroim.2012.09.008. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fleisher AS, Chen K, Liu X, Roontiva A, Thiyyagura P, Ayutyanont N, et al. Using positron emission tomography and florbetapir F18 to image cortical amyloid in patients with mild cognitive impairment or dementia due to Alzheimer disease. Arch Neurol. 2011;68:1404–1411. doi: 10.1001/archneurol.2011.150. [DOI] [PubMed] [Google Scholar]