Abstract
We show by high-resolution atomic force microscopy analysis that drebrin A (a major neuronal actin binding protein) induced F-actin structural and mechanical remodeling involves significant changes in helical twist and filament stiffness (+55% persistence length). These results provide evidence of a unique mechanical role of drebrin in the dendrites, contribute to current molecular-level understanding of the properties of the neuronal cytoskeleton, and reflect the role of biomechanics at the nanoscale, to modulate nanofilament-structure assemblies such as F-actin.
Keywords: F-actin remodeling, drebrin, AFM, neuron cytoskeleton, nanofilament mechanics
A fundamental aspect of bionanostructures, such as cytoskeletal F-actin filaments, is the ability to reorganize their spatial structure and nanoscale mechanics. Drebrin A is a major neuronal actin-binding protein (ABP), localized in dendritic spines1 that participates in synaptic signal transmission. The stability and dynamics of the spines are tightly linked to the actin cytoskeleton,2 which is regulated by ABPs.3 Reduced drebrin level, up to 80%, is a hallmark of Alzheimer's disease and Down syndrome.4 To date, remodeling of neuronal cytoskeleton by drebrin and the molecular mechanisms by which it modulates spine plasticity and dynamics in vivo is poorly understood.5 Measuring F-actin changes induced by ABPs, such as drebrin, requires high-resolution structures of F-actin and its complexes. X-ray diffraction, EM,6–8 and atomic force microscopy (AFM)9,10 have been used earlier for F-actin image analysis. AFM has never been applied to obtain molecular level structural details of F-actin complexes with ABPs at nanoscale resolution. Here, we show a high-resolution AFM study of single actin filaments decorated with drebrin. The observed remodeling of F-actin by drebrin involves changes of helical twist and filament stiffness (55% persistence length, Lp, increase). These results provide evidence of a unique mechanical role of drebrin in the dendrites and contribute to the current molecular level understanding of the properties of the neuronal cytoskeleton as well as the role of nanoscale mechanics in remodeling of nanofilament structures such as F-actin.
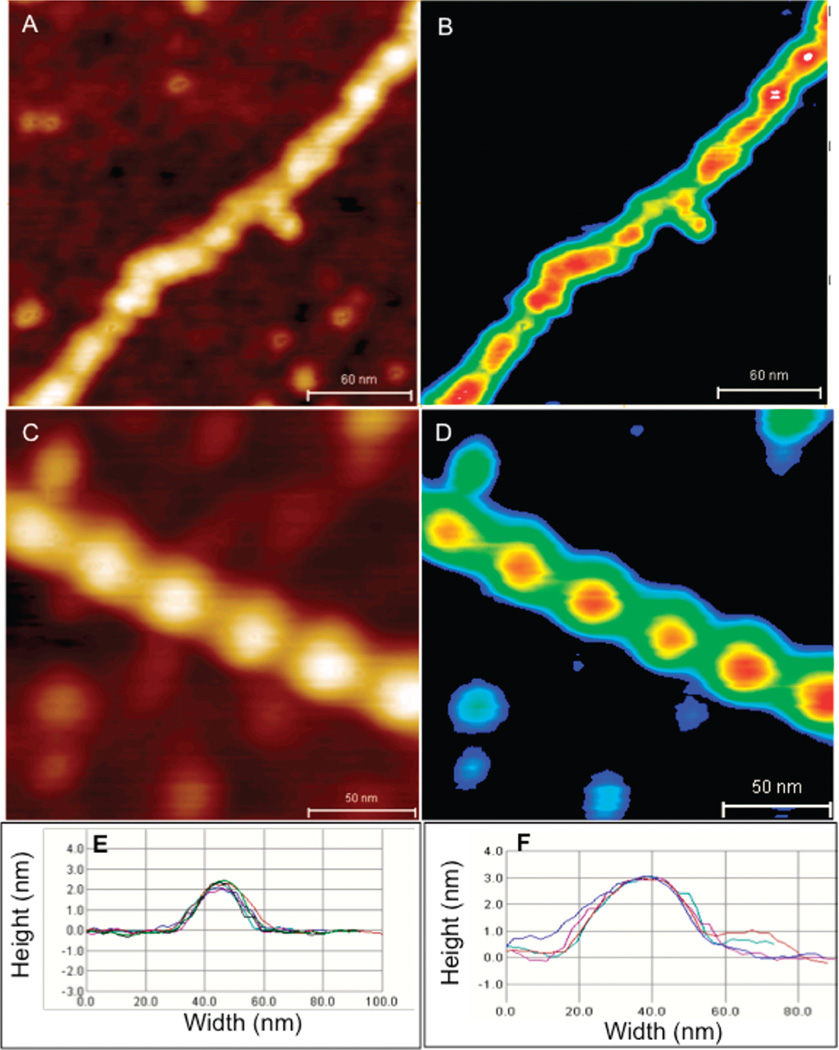
Figure 1 shows AFM images of actin filaments alone (A, C) and decorated with drebrin (B, D) at lower (A, B) and higher resolution (C, D) over mica substrate. Single isolated actin filaments were observed to have a mean width of ~20 nm and height ~2 nm of variable lengths. The helical pitch of single filaments was 36 ± 2 nm (n = 50 filaments), consistent with previous studies.9 The remarkably regular periodicity of drebrin bound actin structures, showing “pearl necklace” like morphology, was observed to extend along the entire length of the actin filament showing a mean width of ~40 nm and height of ~3 nm. Cosedimentation assays to examine binding of recombinant drebrin A to actin filaments estimated Kd as ~0.1 µM and the binding was saturated at 1:5 mol ratio of drebrin/actin (Supplementary Figure 2 in the Supporting Information) as per previous reports.11
Figure 1.
Drebrin–F-actin complex. AFM images of unbound F-actin (A, C) and drebrin decorated F-actin (B, D) at lower (A, B) and higher resolution (C, D). Panels E and F represent periodicity and height profiles (obtained from arrows shown in panels A and B) along F-actin and drebrin bound F-actin, respectively.
The molecular volumes of “bare” and drebrin-decorated F-actin (Figure 2) were calculated from AFM images. Sedimentation equilibrium and sedimentation velocity runs confirmed the monomeric state of drebrin and yielded s020,w = 3.5 S. (Supplementary Figure 1 in the Supporting Information). Assuming a prolate ellipsoid model, drebrin is 28.4 nm long and 2.5 nm wide. The overall increase in the volume of the actin filament upon drebrin binding and the above drebrin shape considerations reveal that approximately three drebrin monomers bind to F-actin per helical pitch, consistent with the binding stoichiometry determined in solution experiments. The actin-drebrin images suggest their cooperative binding. The absence of such cooperativity would probably result in incomplete actin filament coverage by drebrin, leading to gaps between the characteristic beadlike structures (Figure 1).
Figure 2.
F-Actin volume analysis. AFM images (A, C), corresponding filament height contour maps (B, D) and cross-section profiles (E, F) of F-actin and drebrin bound F-actin filaments.
The height undulations along the actin filament backbone are shown in panels E and F of Figure 1 as cross-section profiles measured along the length of the filament. Fourier analysis of cross-section profiles obtained from several individual actin filaments (n = 20 per filament) revealed an increase in the peak periodicity profile of F-actin filaments (unbound 36 ± 2 nm) to 40.0 ± 0.8 nm for drebrin-bound F-actin.
To date, only few examples of ABPs changing the helical twist of F-actin have been reported.12–14 The most striking case is a multifunctional ABP, cofilin, which exerts its severing function by capturing and stabilizing an “overtwisted” actin conformation and, consequently, leading to filament severing.12 Interestingly, drebrin which contains N-terminal actin depolymerizing factor (ADF) homology domain, changes F-actin morphology in the opposite direction to that induced by ADF/cofilins. To confirm this conclusion and test the resolution and analysis of the AFM actin filament images, cofilin-bound actin filaments were used as controls (Supplementary Figure 3 in the Supporting Information). Compared to the drebrin-decorated F-actin, the cofilin-decorated F-actin appeared thinner (~30 nm) and lower in height (2.6 nm). The mass of filaments increased proportionately to that expected for a 16 kDa protein bound to actin. While drebrin binding increased the helical pitch of actin filaments, cofilin-decorated F-actin had a significantly reduced helical pitch (mean = 28.7 nm ± 2.1), which is in good agreement with earlier studies.12
Drebrin- and cofilin-induced changes in F-actin helical periodicity fall within the range of the angular component values in the random angular disorder model of F-actin.15 These changes are consistent with the scenario in which ABPs capture one of the internal modes of F-actin twist, which favors their binding, and stabilize this mode locally or over a long-range of filament length due to cooperative interactions.
The mechanical properties of actin filaments may be correlated with the helical structure and flexibility. The Lp of actin filaments, determined from measurements of local angle distribution16(see supplementary methods in the Supporting Information), yield 7.05 µm for bare actin, 10.90 µm for drebrin-bound actin filaments (an increase of ~55% in Lp), and a dramatic decrease to 1.47 µm, for cofilin-decorated actin filaments. The Lp values obtained from AFM images are comparable to those previously reported for bare actin and the F-actin–cofilin complex, validating the approach used in this work.17–19
From indirect evidence, it has been tentatively proposed that drebrin as well as tropomyosin may increase the stiffness and alter the stability and elasticity of F-actin by binding along its sides.11 Our data show direct quantitative evidence that drebrin modulates F-actin by significantly increasing Lp and elastic modulus of actin filaments (Table 1). If drebrin and tropomyosin induce similar changes in F-actin, one may ask, why both proteins are needed in the cell. It is known that ABPs such as gelsolin and cofilin can cause dramatic changes in spine morphology due to their filament severing activity.3,20,21 It is possible that drebrin-induced stabilization of “undertwisted” (as opposed to cofilin-induced destabilization of “overtwisted”) F-actin conformation would result in a weakening of the cofilin binding to the filaments and thus prevent the cofilin-induced severing. In line with this prediction, our preliminary experiments indicated a drebrin-induced protection of F-actin against cofilin severing. However, it has also been proposed that drebrin, as opposed to tropomyosin, does not protect actin filaments from severing by gelsolin.11 Consequentially, it is tempting to speculate that drebrin and tropomyosin may address the different temporal or spatial needs for spine remodeling.
Table 1.
Comparison of ABP-Bound F-Actin Filaments
In summary, the structural differences between bare and drebrin-decorated F-actin, elucidated in this high-resolution AFM study, provide a model for how this actin binding protein changes the mechanical properties of actin filaments in neuronal cells and hence affect the remodeling and dynamics of the actin cytoskeleton in dendritic spines. The ability of AFM to quantitatively analyze remodeling of F-actin provides a deeper insight into the structural and mechanical modulation of F-actin complexes at nanoscale resolution.
Supplementary Material
ACKNOWLEDGMENT
Funding from USPHS and NIMS, Japan is acknowledged. We acknowledge the use of the Scanning Probe Microscope at the Nano and Pico Characterization Laboratory at the California NanoSystems Institute.
Footnotes
ASSOCIATED CONTENT
Supporting Information. Detailed experimental methods and analysis. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Lin YC, Koleske AJ. Mechanisms of synapse and dendrite maintenance and their disruption in psychiatric and neurodegenerative disorders. Annu. Rev. Neurosci. 2010;33:349–378. doi: 10.1146/annurev-neuro-060909-153204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hotulainen P, Hoogenraad CC. Actin in dendritic spines: connecting dynamics to function. J. Cell Biol. 2010;189(4):619–629. doi: 10.1083/jcb.201003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crystal G, Pontrello IME. Accelerators, Brakes, and Gears of Actin Dynamics in Dendritic Spines. Open Neurosci J. 2009;3:67–86. doi: 10.2174/1874082000903020067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harigaya Y, Shoji M, Shirao T, Hirai S. Disappearance of actin-binding protein, drebrin, from hippocampal synapses in Alzheimer's disease. J. Neurosci. Res. 1996;43(1):87–92. doi: 10.1002/jnr.490430111. [DOI] [PubMed] [Google Scholar]
- 5.Ivanov A, Esclapez M, Ferhat L. Role of drebrin A in dendritic spine plasticity and synaptic function: Implications in neurological disorders. Commun. Integr. Biol. 2009;2(3):268–270. doi: 10.4161/cib.2.3.8166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holmes KC, Popp D, Gebhard W, Kabsch W. Atomic model of the actin filament. Nature. 1990;347(6288):44–49. doi: 10.1038/347044a0. [DOI] [PubMed] [Google Scholar]
- 7.Egelman EH. A robust algorithm for the reconstruction of helical filaments using single-particle methods. Ultramicroscopy. 2000;85(4):225–234. doi: 10.1016/s0304-3991(00)00062-0. [DOI] [PubMed] [Google Scholar]
- 8.Steinmetz MO, Hoenger A, Tittmann P, Fuchs KH, Gross H, Aebi U. An atomic model of crystalline actin tubes: combining electron microscopy with X-ray crystallography. J. Mol. Biol. 1998;278(4):703–711. doi: 10.1006/jmbi.1998.1717. [DOI] [PubMed] [Google Scholar]
- 9.Shao Z, Shi D, Somlyo AV. Cryoatomic force microscopy of filamentous actin. Biophys J. 2000;78(2):950–958. doi: 10.1016/S0006-3495(00)76652-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lehto T, Miaczynska M, Zerial M, Muller DJ, Severin F. Observing the growth of individual actin filaments in cell extracts by time-lapse atomic force microscopy. FEBS Lett. 2003;551(1–3):25–28. doi: 10.1016/s0014-5793(03)00867-6. [DOI] [PubMed] [Google Scholar]
- 11.Ishikawa R, Hayashi K, Shirao T, Xue Y, Takagi T, Sasaki Y, Kohama K. Drebrin, a development-associated brain protein from rat embryo, causes the dissociation of tropomyosin from actin filaments. J. Biol. Chem. 1994;269(47):29928–29933. [PubMed] [Google Scholar]
- 12.McGough A, Pope B, Chiu W, Weeds A. Cofilin changes the twist of F-actin: implications for actin filament dynamics and cellular function. J. Cell Biol. 1997;138(4):771–781. doi: 10.1083/jcb.138.4.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmid MF, Sherman MB, Matsudaira P, Chiu W. Structure of the acrosomal bundle. Nature. 2004;431(7004):104–107. doi: 10.1038/nature02881. [DOI] [PubMed] [Google Scholar]
- 14.Tsaturyan AK, Koubassova N, Ferenczi MA, Narayanan T, Roessle M, Bershitsky SY. Strong binding of myosin heads stretches and twists the actin helix. Biophys J. 2005;88(3):1902–1910. doi: 10.1529/biophysj.104.050047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Egelman EH, Derosier DJ. Image-Analysis Shows That Variations in Actin Crossover Spacings Are Random, Not Compensatory. Biophys J. 1992;63(5):1299–1305. doi: 10.1016/S0006-3495(92)81716-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frontali C, Dore E, Ferrauto A, Gratton E, Bettini A, Pozzan MR, Valdevit E. An absolute method for the determination of the persistence length of native DNA from electron micrographs. Biopolymers. 1979;18(6):1353–1373. doi: 10.1002/bip.1979.360180604. [DOI] [PubMed] [Google Scholar]
- 17.Takebayashi T, Morita Y, Oosawa F. Electronmicroscopic investigation of the flexibility of F-actin. Biochim. Biophys. Acta. 1977;492(2):357–363. doi: 10.1016/0005-2795(77)90086-1. [DOI] [PubMed] [Google Scholar]
- 18.Isambert H, Venier P, Maggs AC, Fattoum A, Kassab R, Pantaloni D, Carlier MF. Flexibility of actin filaments derived from thermal fluctuations. Effect of bound nucleotide, phalloidin, and muscle regulatory proteins. J. Biol. Chem. 1995;270(19):11437–11444. doi: 10.1074/jbc.270.19.11437. [DOI] [PubMed] [Google Scholar]
- 19.McCullough BR, Blanchoin L, Martiel JL, De la Cruz EM. Cofilin increases the bending flexibility of actin filaments: implications for severing and cell mechanics. J. Mol. Biol. 2008;381(3):550–558. doi: 10.1016/j.jmb.2008.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wan-Hsin Lin DJW. Actin and Actin-Binding Proteins: Masters of Dendritic Spine Formation, Morphology, and Function. Open Neurosci J. 2009;3(13):54–66. doi: 10.2174/1874082000903020054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kojima N, Shirao T. Synaptic dysfunction and disruption of postsynaptic drebrin-actin complex: a study of neurological disorders accompanied by cognitive deficits. Neurosci. Res. 2007;58(1):1–5. doi: 10.1016/j.neures.2007.02.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.