Abstract
Objective
To examine whether spouses of patients with severe sepsis are at increased risk for depression independent of the spouse’s pre-sepsis history, whether this risk differs by sex, and is associated with a sepsis patient’s disability after hospitalization.
Design
Prospective longitudinal cohort study.
Setting
Population-based cohort of U.S. adults over 50 years old interviewed as part of the Health and Retirement Study (1993–2008).
Patients
929 patient-spouse dyads comprising 1,212 hospitalizations for severe sepsis.
Measurements and Main Results
Severe sepsis was identified using a validated algorithm in Medicare claims. Depression was assessed with a modified version of the Center for Epidemiologic Studies Depression Scale. All analyses were stratified by gender. The prevalence of substantial depressive symptoms in wives of patients with severe sepsis increased by 14 percentage points at the time of severe sepsis (from 20% at a median of 1.1 years pre-sepsis to 34% at a median of 1 year post-sepsis), an odds ratio (OR) of 3.74 (95% Confidence Interval [CI]: 2.20, 6.37), in multivariable regression. Husbands had an 8 percentage point increase in the prevalence of substantial depressive symptoms, which was not significant in multivariable regression (OR 1.90, 95%CI: 0.75, 4.71). The increase in depression was not explained by bereavement; women had greater odds of substantial depressive symptoms even when their spouse survived a severe sepsis hospitalization (OR 2.86, 95%CI 1.06, 7.73). Wives of sepsis survivors who were disabled were more likely to be depressed (OR 1.35 per ADL limitation of sepsis survivor, 95%CI: 1.12, 1.64); however, controlling for patient disability only slightly attenuated the association between sepsis and wives’ depression (OR 2.61, 95%CI: 0.93, 7.38).
Conclusions
Older women may be at greater risk for depression if their spouse is hospitalized for severe sepsis. Spouses of patients with severe sepsis may benefit from greater supports and depression screening, both when their loved one dies, but also when their loved one survives.
Keywords: sepsis, critical care, spouses, caregivers, depression, outcome assessment (health care)
INTRODUCTION
Hundreds of thousands of Americans are hospitalized for the treatment of severe sepsis annually, making it the most common non-cardiac cause of critical illness (1). Recently, it has been identified that patients who survive severe sepsis have diminished quality of life (2), and are at increased risk of new cognitive impairments as well as functional limitations (3). Furthermore, patients who survive severe sepsis have high rates of depression both before and after their sepsis-related hospitalization (4).
Although knowledge is increasing about the emotional well-being of survivors of severe sepsis, relatively little is known about the emotional toll severe sepsis takes on those patients’ loved ones. Patients with severe sepsis often require treatment in an intensive care unit (ICU) (1), which can be an extremely stressful experience for their family members (5). Prior studies have identified that some family members of critically ill patients can have high rates of depressive symptoms (5–9). Ascertaining the mental health of loved ones of critically ill patients is of particular importance since depression in family members could affect end-of-life care decisions in the ICU (5), and depression could impact a loved one’s ability to care for a critical illness survivor following their hospitalization (10). The latter point is particularly salient among survivors of severe sepsis, many of whom face levels of cognitive impairment and functional limitations that greatly increase the amount of caregiving they require (3, 11).
Although family members of severe sepsis patients may be at increased risk of depression, it is unknown to what extent this risk is due to the increased mortality of sepsis patients (1), the ongoing disability that can occur after sepsis (3), or other aspects of the experience of sepsis. Also, it is unknown if this risk of depression in family members of severe sepsis patients is independent of their own depression history prior to the patient’s sepsis hospitalization. Furthermore, although community-dwelling older women have higher rates of depression compared to men (12), it is unclear if wives of severe sepsis patients are at greater risk of depression than husbands.
The present study utilizes an ongoing longitudinal cohort of older Americans to examine whether husbands and wives of patients with severe sepsis are at risk for depression independent of their own prior history, if this risk is affected by whether a patient survives severe sepsis, and if this risk is associated with a sepsis patient’s disability following a sepsis hospitalization. This approach is novel and offers the distinct advantages of national scope and prospective assessment of depressive symptoms with a consistent instrument, avoiding the challenges of using proxy or retrospective assessment of baseline symptoms (13).
METHODS
Study Population
Our study cohort comes from the Health and Retirement Study (HRS), a nationally representative, longitudinal investigation of community-dwelling U.S. adults over the age of 50. The study began in 1992, and to date over 27,000 individuals have participated. Subjects (and their spouses if married) are re-interviewed every 2 years. The follow-up rate for the HRS has exceeded 90–95%, including proxies (14), and 16,772 participants have consented for linkage of their Medicare claims records with study data. The HRS protocol was approved by the University of Michigan Institutional Review Board. Study participants provided informed consent upon enrollment and again for linkage to Medicare claims.
The present study examines patient-spouse dyads with at least 1 HRS interview from 1993–2008 and for whom the patient had Medicare claims-based data for a subsequent hospitalization for severe sepsis from 1993–2008. All patients and/or their spouses were followed up through death or the 2008 HRS wave. Data were analyzed for up to 3 HRS interviews prior to severe sepsis and up to 4 interviews after severe sepsis.
Demographic and Clinical Characteristics
We obtained data on patient and spouse demographics (i.e., age, self-reported race and ethnicity, sex, education, employment status), alcohol use and smoking from the HRS interviews.
Baseline chronic medical conditions in spouses (Elixhauser comorbidity measures) (15), as well as clinical characteristics of the severe sepsis hospitalization, including an organ dysfunction score (the sum of the number of organ failures of cardiovascular, neurologic, hematologic, hepatic, renal, or respiratory origin) (1, 16) and length of stay for the severe sepsis hospitalization, were abstracted from the Medicare claims.
Definition of Severe Sepsis
We utilized a clinically validated and widely-used claims-based definition of severe sepsis (1, 17–20). The definition requires evidence of a concomitant infection and new-onset organ dysfunction during a single hospitalization, consistent with the international consensus conference definitions of severe sepsis (18). We focus on severe sepsis as a single syndrome, rather than the underlying infectious processes, in line with current thought that emphasizes the importance of the common host response in the pathogenesis, treatment and outcome of severe sepsis (21–24). For patients who had more than 1 distinct hospitalization for sepsis, each hospitalization was included, with appropriate adjustment of the standard errors as described below.
Depressive Symptoms
The HRS assessed depressive symptoms at each wave with an 8-item version of the Center for Epidemiologic Studies Depression Scale (CES-D) (25). Prior studies have reported that this modified version loses little of the structure and precision of the original scale (26, 27). Using a cutoff of 3 or more has been found to have a sensitivity of 71% and specificity of 79% for the diagnosis of major depression compared to structured diagnostic interview (28). We used a cutoff score of 4 or higher on the 8-item CES-D to define substantial depressive symptoms because this threshold was estimated to be comparable to the cutoff score of 16 or higher on the full CES-D by HRS investigators (29) and has been used in several prior studies (30–32).
Disability
To examine disability, respondents (or their proxies) were asked at each interview if they required assistance with any of 6 activities of daily living (ADLs): walking, dressing, bathing, eating, getting into or out of bed, and toileting.
Statistical Analysis
Since major depression is more prevalent among older women than men (12), and given an extensive sociological literature on gender differences in caregiving and bereavement (33), we stratified all analyses by gender.
Our unit of analysis for all analyses was the hospitalization. For unadjusted analyses, we grouped spouses of patients with severe sepsis hospitalizations by the number of HRS interviews that they completed after the hospitalization. For example, we compared all spouses at the most recent interview before the patient’s severe sepsis episode with spouses at their first interview after the patient’s hospitalization.
In order to test the hypothesis that severe sepsis is associated with an increased risk of substantial symptoms of depression among patients’ spouses using multivariable models, we used so-called “fixed effects” logistic regression models which use the longitudinal nature of the data to control for all stable characteristics of the spouses (3). Our data was organized at the interview level, one line per interview per severe sepsis hospitalization. Our independent variable was time from admission date for severe sepsis, measured to the day of each interview, as a continuous variable. We allowed the rate of developing substantial depressive symptoms per unit time to change from before sepsis to afterwards by parameterizing time as a linear spline with a knot at the day of admission for the severe sepsis hospitalization. We also included a dichotomous indicator variable that distinguished interviews prior to the severe sepsis hospitalization date from interviews after, grouping spouses by the number of interviews they had completed since the patient’s severe sepsis episode. We used a hospitalization-level fixed effect, sometimes called conditional models (34). This definition of “fixed effects” is different from the term “fixed effect” referring to regression seen commonly in the biostatistical literature (35). These results controlled for the spouse’s depressive symptom status before the patient’s severe sepsis episode. In other words, spouses served as their own controls. Only within-person variation over time was used to estimate the effect of severe sepsis on spouses. No parameters or limitations were set on the hospitalization-specific intercept terms, thus making these models very flexible. This analysis was implemented using clogit in STATA 11 (Stata Corporation, College Station, TX).
To examine the extent that levels of spousal post-sepsis depressive symptoms were affected by patient mortality, we performed an analysis where we stratified by whether or not the patient survived severe sepsis. Finally, to study whether the risk of substantial depressive symptoms in spouses of patients who survived severe sepsis was associated with the degree of patient disability after sepsis, we performed an analysis in which we adjusted for the number of ADL impairments that a severe sepsis survivor had.
We used two-sided significance tests for all analyses with statistical significance set at a p value of 0.05. Analyses were performed with appropriate components of the IBM SPSS Statistics 18 (SPSS Inc., Chicago, IL) and STATA 11 (Stata Corporation, College Station, TX) statistical software programs.
RESULTS
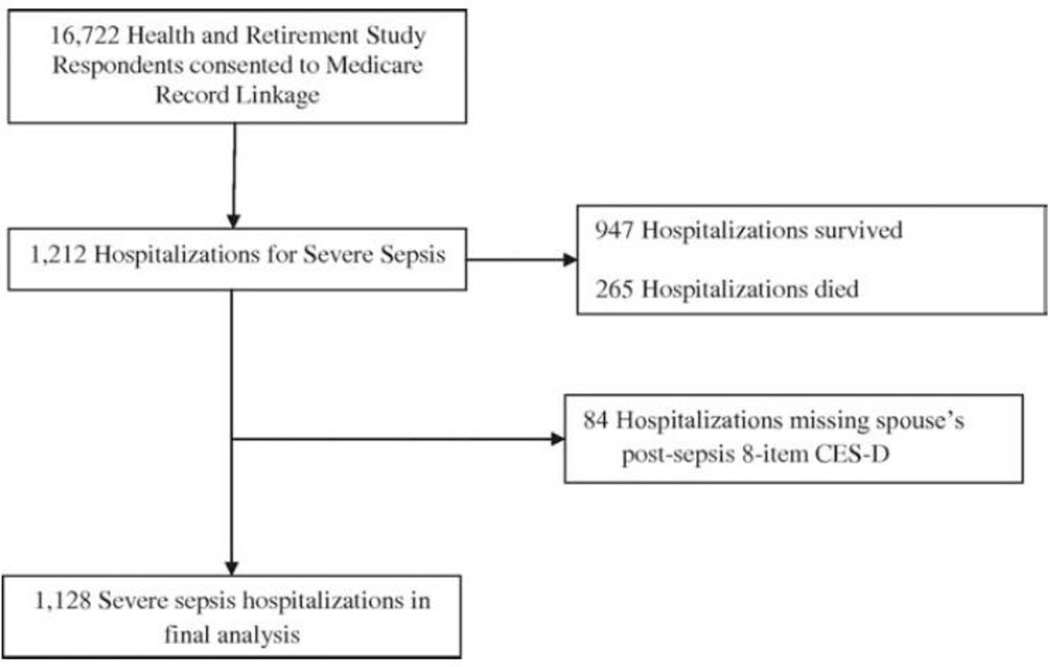
From 1993–2008, 929 HRS respondents had a spouse who was hospitalized for severe sepsis, comprising 1,212 hospitalizations for severe sepsis during that time period (Figure 1). Slightly more than one-fifth of the patients required their spouse to serve as a proxy respondent. Just over one-fifth of the severe sepsis hospitalizations ended in patient death (Table 1). Table 1 describes the baseline characteristics of patients and spouses as well as clinical characteristics of the severe sepsis-related hospitalization. Spouses were followed for up to 3 interviews before severe sepsis (mean of 5.1 years) and 4 interviews after severe sepsis (mean of 7 years). Of the hospitalizations, 865 (93%) spouses completed at least one follow-up depression assessment.
Figure 1.
Health and Retirement Study cohort for analyses of depression in spouses of severe sepsis patients
Table 1.
Characteristics of patients and spouse dyads comprising severe sepsis hospitalizations
| Variables | n = 1,212 | |
|---|---|---|
| Panel A: Patient characteristics (baselinea and severe sepsis episode clinical factors) | ||
| Age (years) | 73.4 (8.3) | |
| Male | 790 (65.2%) | |
| Race | ||
| White | 996 (82.2%) | |
| Black | 194 (16.0%) | |
| Other | 22 (1.8%) | |
| Highest level of education | ||
| High school diploma or less | 1,050 (86.6%) | |
| Two or four year college degree | 97 (8.0%) | |
| Master’s or professional degree | 65 (5.4%) | |
| Employed | 137 (11.3%) | |
| Alcohol use (days/week) | 0.9 (2.0) | |
| Smoking Status | ||
| Never smoked | 350 (28.9%) | |
| Former smoker | 702 (57.9%) | |
| Current smoker | 160 (13.2%) | |
| ADL impairments | 1.6 (2.1) | |
| Severe sepsis episode clinical factors | ||
| Hospital length of stay (days) | 12.1 (12.8) | |
| Organ Dysfunction Score | 1.3 (0.6) | |
| Cardiovascular dysfunction | 304 (25.1%) | |
| Neurologic dysfunction | 104 (8.6%) | |
| Hematologic dysfunction | 224 (18.5%) | |
| Hepatic dysfunction | 13 (1.1%) | |
| Renal dysfunction | 494 (40.8%) | |
| Respiratory dysfunction | 370 (30.5%) | |
| Died during severe sepsis hospitalization | 265 (21.9%) | |
| More than one distinct sepsis hospitalization | 284 (23.4%) | |
| Panel B: Spouse baseline characteristicsa | ||
| Age (years) | 71.3 (9.5) | |
| Female | 791 (65.3%) | |
| Race | ||
| White | 1,000 (82.5%) | |
| Black | 189 (15.6%) | |
| Other | 23 (1.9%) | |
| Highest level of education | ||
| High school diploma or less | 1,045 (86.2%) | |
| Two or four year college degree | 122 (10.1%) | |
| Master’s or professional degree | 45 (3.7%) | |
| Employed | 242 (20.0%) | |
| Alcohol use (days/week) | 0.8 (1.8) | |
| Smoking Status | ||
| Never smoked | 548 (45.2%) | |
| Former smoker | 453 (37.4%) | |
| Current smoker | 211 (17.4%) | |
| Elixhauser chronic medical conditions15 | ||
| Congestive heart failure | 48 (4.0%) | |
| Diabetes mellitus | 84 (6.9%) | |
| Chronic pulmonary disease | 81 (6.7%) | |
| Peripheral vascular disease | 44 (3.6%) | |
| Neurologic disorders | 98 (8.1%) | |
| Cancer | 30 (2.5%) | |
| Collagen vascular disease | 36 (3.0%) | |
| ADL impairments | 0.6 (1.3) | |
All values are mean ± SD or n (%) unless otherwise indicated.
Abbreviations: ADL = Activities of Daily Living.
Patient and spouse baseline characteristics are from the last HRS interview before the severe sepsis hospitalization.
Pre- and Post-Sepsis Depressive Symptoms among Spouses
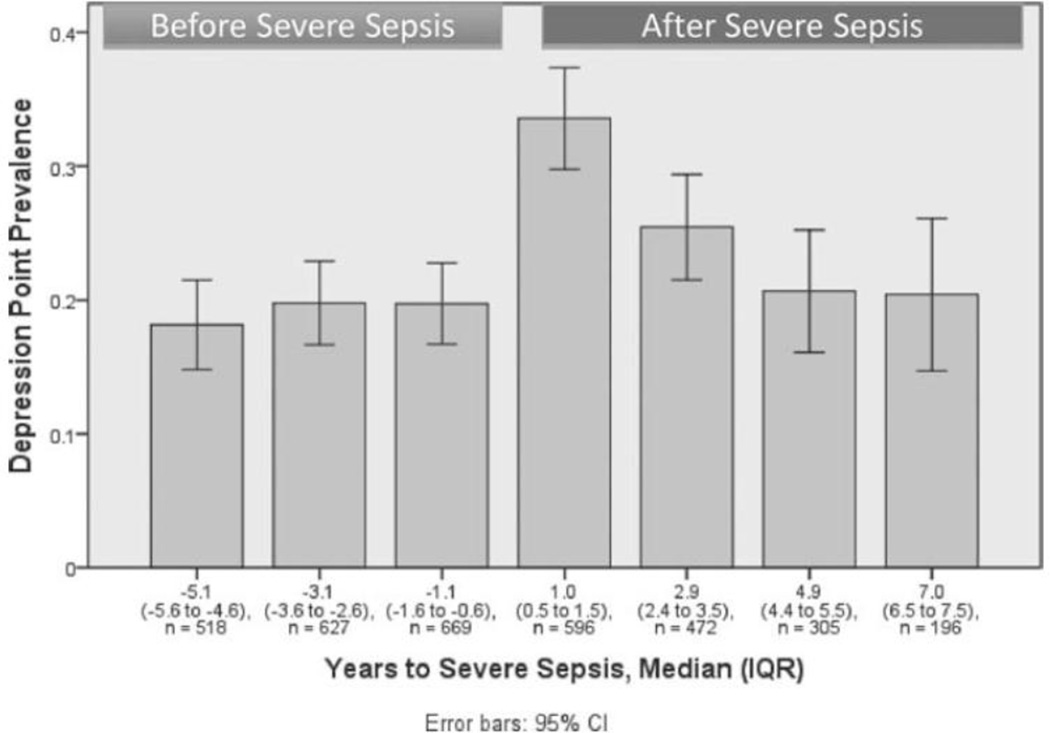
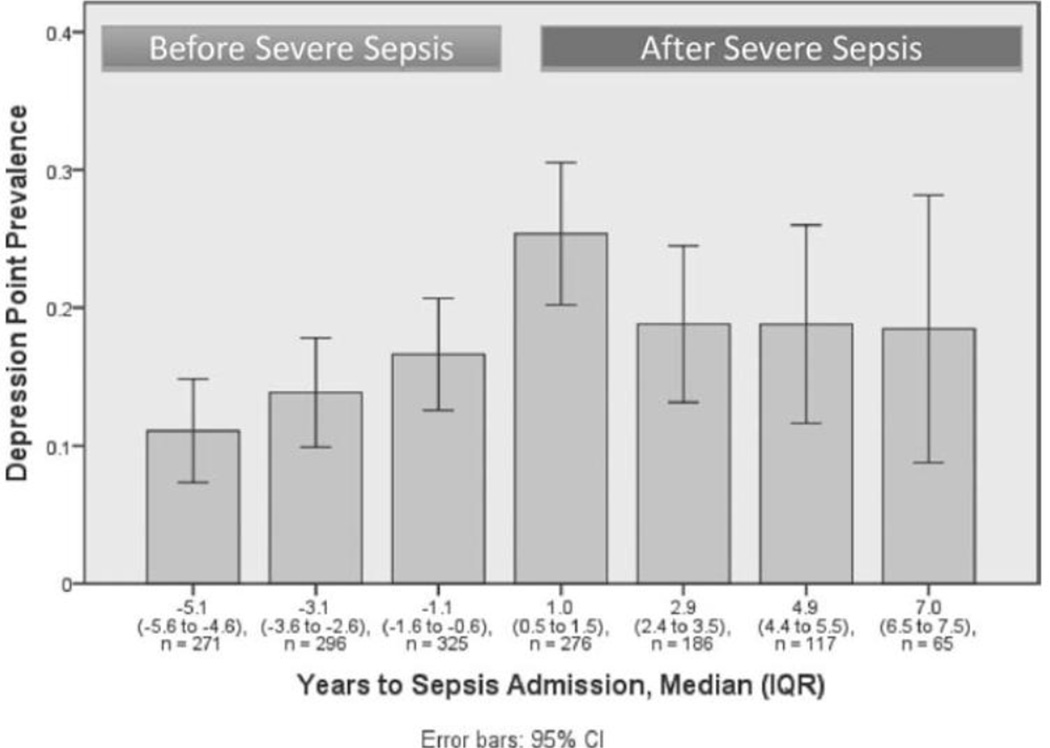
Figure 2 presents the prevalence of substantial depressive symptoms in wives who had a husband hospitalized for severe sepsis before and after sepsis. The prevalence of substantial depressive symptoms among wives increased from 20% (95%Confidence Interval [CI]: 17%, 23%) at the most recent interview before sepsis, a median of 1.1 years pre-sepsis, to 34% (95%CI: 30%, 37%) at the first interview after severe sepsis, a median of 1 year later (14 percentage point increase, p < 0.001 by χ2 test). Among husbands, the prevalence of substantial depressive symptoms increased from 17% (95%CI: 13%, 21%) at the most recent interview before severe sepsis to 25% (95%CI: 20%, 31%) at the first interview after sepsis (8 percentage point increase, p < 0.001 by χ2 test) (Figure 3).
Figure 2.
Prevalence of substantial depressive symptoms among wives of patients with severe sepsis
Figure 3.
Prevalence of substantial depressive symptoms among husbands of patients with severe sepsis
Effects of Severe Sepsis on Subsequent Substantial Depressive Symptoms among Spouses
In fixed effects logistic regression, which controls for all spousal characteristics that do not change over time, having a husband hospitalized for severe sepsis was associated with 3.74-times the odds (95%CI: 2.20, 6.37) of subsequent substantial depressive symptoms among wives (Table 2). However, having a wife hospitalized for severe sepsis was not significantly associated with subsequent substantial depressive symptoms among husbands, although there is a large confidence interval for the estimate (Odds Ratio [OR] 1.90, 95%CI: 0.75, 4.71) (Table 3). Women continued to have greater odds of substantial depressive symptoms even when their spouse survived a severe sepsis hospitalization (OR 2.86, 95%CI: 1.06, 7.73).
Table 2.
Associations between having a husband hospitalized with severe sepsis and subsequent substantial depressive symptoms among wivesa
| Odds Ratio (95% Confidence Interval) | |||
|---|---|---|---|
| Among all wives | Among wives with surviving husbands (stratified by patient survival) |
Among wives with surviving husbands adjusted for husband’s post-sepsis disability |
|
| Before sepsis (per additional year) | 1.07 (0.87–1.32) | 1.11 (0.73–1.68) | 0.98 (0.62–1.55) |
| Effect of sepsis | 3.74 (2.20–6.37)‡ | 2.86 (1.06–7.73)* | 2.61 (0.93–7.38) |
| After sepsis (per additional year) | 0.73 (0.60–0.90)† | 0.84 (0.45–1.15) | 0.82 (0.43–1.59) |
| Sepsis survivor ADL impairment | 1.35 (1.12–1.64)† | ||
Abbreviations: ADL = Activities of Daily Living
Results of fixed-effects logistic regression with hospitalization-level fixed effects stratified by gender, controlling for all time-invariant characteristics of the spouse.
P < 0.05
P < 0.01
P < 0.001
Table 3.
Associations between having a wife hospitalized with severe sepsis and subsequent substantial depressive symptoms among husbandsa
| Odds Ratio (95% Confidence Interval) | |||
|---|---|---|---|
| Among all husbands | Among husbands with surviving wives (stratified by patient survival) |
Among husbands with surviving wives adjusted for wife’s post-sepsis disability |
|
| Before sepsis (per additional year) | 1.36 (0.92–2.01) | 1.87 (0.86–4.04) | 1.65 (0.76–3.62) |
| Effect of sepsis | 1.90 (0.75–4.81) | 0.72 (0.12–4.25) | 0.63 (0.10–4.01) |
| After sepsis (per additional year) | 0.83 (0.64–1.08) | 1.12 (0.42–2.94) | 1.17 (0.43–3.15) |
| Sepsis survivor ADL impairment | 1.19 (0.90–1.57) | ||
Abbreviations: ADL = Activities of Daily Living
Results of fixed-effects logistic regression with hospitalization-level fixed effects stratified by gender, controlling for all time-invariant characteristics of the spouse.
Wives of severe sepsis survivors who were disabled were more likely to have substantial depressive symptoms (OR 1.35 per ADL limitation of sepsis survivor, 95%CI: 1.12, 1.64). However, controlling for patient disability appeared to somewhat attenuate the association between husband sepsis and spousal substantial depressive symptoms (OR 2.61, 95%CI: 0.93, 7.38). For husbands, there was no evidence of increased depressive symptoms if their wife survived her sepsis episode (Table 3). For husbands, there was also no statistically significant association of substantial depressive symptoms with greater post-sepsis disability in their wives.
DISCUSSION
In this nationally representative longitudinal investigation of older adults, we found that having a husband hospitalized for severe sepsis is associated with a near quadrupling of the odds of substantial symptoms of depression among wives, and a near tripling of the odds of substantial depressive symptoms if their husband survived sepsis, suggesting that our results are not explained by bereavement. Furthermore, each additional impairment of ADLs that a severe sepsis survivor had was associated with a 35% increase in the odds of substantial depressive symptoms in their wife, an association that could be reflective of the increased burdens of informal caregiving for a disabled older adult. To our knowledge, this study is the first investigation of depressive symptoms in spouses of older patients with severe sepsis that uses a standardized measure of depressive symptoms administered to spouses prospectively and prior to the patient’s illness, and thereby to show clear evidence of a dramatic increase in rates of depressive symptoms among caregivers of severe sepsis.
While our data for wives are clear, we must acknowledge that the data for husbands are less definitive. There was a significant unadjusted association between having a wife hospitalized for severe sepsis and an increased prevalence of subsequent substantial depressive symptoms. However, this association was not significant in multivariate models, nor was the association between increasing ADL impairments in sepsis-surviving wives and substantial depressive symptoms in husbands. These findings could be due to response bias because of older men possibly being less willing to report depressive symptoms (36), as well as to insufficient statistical power given the wide confidence intervals surrounding the adjusted odds ratios for depressive symptoms in husbands. A clinically meaningful effect of sepsis on husbands is not completely ruled out by these results.
These findings have important implications for the growing literature on the central importance of spouses to the care of critically ill patients. Spouses have been well recognized to play a crucial but challenging role as surrogate decision-makers for critically ill patients (5, 37, 38). Similar to prior studies of depression in community-dwelling older adults using questionnaire measures (39, 40), spouses of older patients hospitalized for severe sepsis in our study had relatively high rates (20% in wives and 17% in husbands) of substantial depressive symptoms during the year prior to the sepsis episode. Depression in spouses of critically ill sepsis patients could affect patient care decisions due to the spouse overestimating the risks and underestimating the benefits of a suggested course of treatment and/or impairing their decision-making abilities (5, 41). This may explain some of the remarkable challenges in information processing noted in decision-making by surrogates (42). Even as a spouse’s pre-sepsis depression may have implications for ICU decision-making, there is evidence that the processes of ICU decision-making and care may impact spousal mental health after the critical illness experience (9). For example, one important randomized controlled trial showed that relatively modest changes to enhance communication during family meetings in French ICUs could reduce short-term levels of anxiety and depression among spouses of dying critically ill patients (43). Additional study is warranted to see if these or other ICU-based interventions could prevent longer-term depressive symptoms in spouses of older adults who are hospitalized with severe sepsis.
Moving beyond the acute care setting, there has been increasing recognition that the caregivers of survivors of critical illness may face substantial burdens (44, 45). In this context, our study shows that severe sepsis, in particular, may place a real and substantial burden on spouses, and extends beyond past work by providing evidence of several key potential mechanisms linking severe sepsis and subsequent psychiatric morbidity. We demonstrate for the first time that bereavement after severe sepsis is potentially associated with real and substantial depressive symptoms, as would be expected based on the ubiquitous and substantial bereavement symptoms after other causes of death (12, 46–48). However, more novelly, we document the burden of a substantial increase in depressive symptoms among the spouses of severe sepsis survivors, and its relationship to disability among those survivors.
Disability after severe sepsis is very common. In a related cohort of older Americans, over 59% of severe sepsis survivors had worse cognitive or physical function after their sepsis hospitalization (3). Our data demonstrate that each additional limitation in an activity of daily living for a survivor of severe sepsis is associated with a clinically and statistically significant increase in the odds of substantial depressive symptoms for his wife, consistent with prior studies that have found that wives are more likely to be the primary caregiver for their disabled husbands than vice versa (33), and that female caregivers are at increased risk of depression compared to male caregivers across a wide-range of populations due to the physical and emotional burdens of caregiving (49–52). The present study adds important urgency to ongoing clinical and research efforts to reduce disability in older adults surviving serious medical illnesses (53, 54) and to provide appropriate multidisciplinary resources to facilitate rehabilitation. Beyond the direct benefits to the patients and to society of having less disability in sepsis survivors, such efforts might also reduce the burden of depression on surviving spouses – particularly important in light of work demonstrating the adverse health outcomes of older caregivers with emotional distress (55), and the negative health effects of depression on older adults (56). To the extent that depressed spouses are less able to support patients in ongoing rehabilitation, mental health interventions for spouses might be hypothesized to be an efficacious way to improve the outcomes of patients themselves.
Our findings also argue for strong consideration among clinicians of providing ongoing support and potential mental health screening for spouses as part of a comprehensive program of patient and family-centered post-ICU care. Further, providers must learn to assess the burden that post-discharge follow-up plans may place on the patient’s spouse, and consider their feasibility and vulnerability to reduced spousal participation due to depression. Also, non-spousal family members and support networks could be brought in to help facilitate the patient’s post-ICU recovery. Prior work has identified that adult children of older adults with chronic illnesses could be an important resource for providing additional support (57), and future investigations in the context of critical illnesses could elucidate the importance of adult children and other support networks in the post-illness recovery period.
Our study does have several limitations. Since we studied older Americans, the associations with severe sepsis and spousal depressive symptoms may be different in younger spouses. Furthermore, due to the methodology of our cohort, we cannot comment on the potential effects of severe sepsis on the emotional well-being of non-spousal caregivers. Also, since we assessed depressive symptoms with a questionnaire and not a diagnostic interview, a diagnosis of major depression could not be made. The 8-item CES-D has been used in many relevant populations (4, 30–32), but it has not been specifically validated for use in spouses of patients with severe sepsis. Moreover, we used a claims-based definition of severe sepsis, which although not the same as prospective clinical assessment, has been validated and widely used (1, 17–20). In addition, we did not have a measure of illness severity related to the severe sepsis hospitalization, so we could not investigate the potential role of severity of sepsis as a predictor or effect-modifier of spousal depression. Finally, the possibility of residual confounding remains as in any observational study.
In conclusion, using a nationally representative sample of older adults, we found that wives of patients hospitalized with severe sepsis may be at significantly greater risk for substantial depressive symptoms following their spouse’s sepsis episode, and that this association is present for the majority of wives whose husband survives his hospitalization. We also identified that wives of older men who survive severe sepsis with lasting disabilities have increased odds of substantial depressive symptoms. Future research focusing on improving communication between providers and spouses of severe sepsis patients— along with interventions that increase identification and treatment facilitation of depression in spouses of patients with severe sepsis— could improve the health and quality of life for this growing population of older Americans.
Acknowledgments
This work was supported by grants KL2 RR025015-05, K08 HL091249, R01 AG030155, and U01 AG09740 from the National Institutes of Health. The Health and Retirement Study is performed at the Institute for Social Research, University of Michigan. We appreciate the expert programming of Tish Shapiro and Mohammed Kabeto, both at the University of Michigan.
Footnotes
Publisher's Disclaimer: Disclaimer: The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the US government.
REFERENCES
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Winters BD, Eberlein M, Leung J, et al. Long-term mortality and quality of life in sepsis: a systematic review. Crit Care Med. 2010;38:1276–1283. doi: 10.1097/CCM.0b013e3181d8cc1d. [DOI] [PubMed] [Google Scholar]
- 3.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davydow DS, Hough CL, Langa KM, Iwashyna TJ. Symptoms of depression in survivors of severe sepsis. Paper presented at Academy of Psychosomatic Medicine. 2011 Nov 19; [Google Scholar]
- 5.Pochard F, Azoulay E, Chevret S, et al. Symptoms of anxiety and depression in family members of intensive care unit patients: Ethical hypothesis regarding decision-making capacity. Crit Care Med. 2001;29:1893–1897. doi: 10.1097/00003246-200110000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Azoulay E, Pochard F, Kentish-Barnes N, et al. Risk of post-traumatic stress symptoms in family members of intensive care unit patients. Am J Respir Crit Care Med. 2005;171:987–994. doi: 10.1164/rccm.200409-1295OC. [DOI] [PubMed] [Google Scholar]
- 7.Anderson WG, Arnold RM, Angus DC, Bryce CL. Posttraumatic stress and complicated grief in family members of patients in the intensive care unit. J Gen Intern Med. 2008;23:1871–1876. doi: 10.1007/s11606-008-0770-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McAdam JL, Dracup KA, White DB, Fontaine DK, Puntillo KA. Symptom experiences of family members of intensive care unit patients at high risk for dying. Crit Care Med. 2010;38:1078–1085. doi: 10.1097/CCM.0b013e3181cf6d94. [DOI] [PubMed] [Google Scholar]
- 9.Gries CJ, Engelberg RA, Kross EK, et al. Predictors of posttraumatic stress and depression in family members after patient death in the ICU. Chest. 2010;137:280–287. doi: 10.1378/chest.09-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cameron ID, Aggar C, Robinson AL, Kurrle SE. Assessing and helping carers of older people. BMJ. 2011;343:d5202. doi: 10.1136/bmj.d5202. [DOI] [PubMed] [Google Scholar]
- 11.Langa KM, Chernew ME, Kabeto MU, et al. National estimates of the quantity and cost of informal caregiving for the elderly with dementia. J Gen Intern Med. 2001;16:770–778. doi: 10.1111/j.1525-1497.2001.10123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cole MG, Dendukuri N. Risk factors for depression among elderly community subjects: a systematic review and meta-analysis. Am J Psychiatry. 2003;160:1147–1156. doi: 10.1176/appi.ajp.160.6.1147. [DOI] [PubMed] [Google Scholar]
- 13.Gifford JM, Husain N, Dinglas VD, Colantuoni E, Needham DM. Baseline quality of life before intensive care: a comparison of patient versus proxy responses. Crit Care Med. 2010;38:855–860. doi: 10.1097/CCM.0b013e3181cd10c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Health and Retirement Study: Sample sizes and response rates. [Accessed March 6, 2011]; http://hrsonlineisrumichedu/sitedocs/sampleresponsepdf. [Google Scholar]
- 15.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9 CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 17.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979–2000. N Engl J Med. 2003;34:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 18.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 19.Weycker D, Akrhas KS, Edelsberg J, Angus DC, Oster G. Long-term mortality and medical care charges in patients with severe sepsis. Crit Care Med. 2003;31:2316–2323. doi: 10.1097/01.CCM.0000085178.80226.0B. [DOI] [PubMed] [Google Scholar]
- 20.Lee H, Doig CJ, Ghali WA, et al. Detailed cost analysis of care for survivors of severe sepsis. Crit Care Med. 2004;32:981–985. doi: 10.1097/01.ccm.0000120053.98734.2c. [DOI] [PubMed] [Google Scholar]
- 21.Angus DC. Management of sepsis: a 47-year-old woman with an indwelling intravenous catheter and sepsis. JAMA. 2011;305:1469–1477. doi: 10.1001/jama.2011.438. [DOI] [PubMed] [Google Scholar]
- 22.Annane D, Bellissant E, Cacvaillon J-M. Septic Shock. Lancet. 2005;365:63–78. doi: 10.1016/S0140-6736(04)17667-8. [DOI] [PubMed] [Google Scholar]
- 23.Dellinger RP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 24.Zahar J-R, Timsit J-F, Garrouste-Orgeas M, et al. Outcomes in severe sepsis and patients with septic shock: pathogen species and infection sites are not associated with mortality. Crit Care Med. 2011;39:1886–1895. doi: 10.1097/CCM.0b013e31821b827c. [DOI] [PubMed] [Google Scholar]
- 25.Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 26.Soldo BJ, Hurd MD, Rodgers WL, Wallace RB. Asset and health dynamics of the oldest old: an overview of the AHEAD study. J Gerontol B Psychol Soc Sci. 1997;52:1–20. doi: 10.1093/geronb/52b.special_issue.1. [DOI] [PubMed] [Google Scholar]
- 27.Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J. Two shorter forms of the CESD (Center for Epidemiological Studies Depression) depression symptoms index. J Aging Health. 1993;5:179–183. doi: 10.1177/089826439300500202. [DOI] [PubMed] [Google Scholar]
- 28.Turvey CL, Wallace RB, Herzog R. A revised CES-D measure of depressive symptoms and a DSM-based measure of major depressive episodes in the elderly. Int Psychogeriatr. 1999;11:139–148. doi: 10.1017/s1041610299005694. [DOI] [PubMed] [Google Scholar]
- 29.Steffick DE. Documentation of affective functioning measures in the Health and Retirement Study. Ann Arbor, Michigan: Survey Research Center. 2000 [Google Scholar]
- 30.Langa KM, Valenstein MA, Fendrick AM, Kabeto MA, Vijan S. Extent and cost of informal caregiving for older Americans with symptoms of depression. Am J Psychiatry. 2004;161:857–863. doi: 10.1176/appi.ajp.161.5.857. [DOI] [PubMed] [Google Scholar]
- 31.Zivin K, Llewellyn DJ, Lang IA, et al. Depression among older adults in the United States and England. Am J Geriatr Psychiatry. 2010;18:1036–1044. doi: 10.1097/JGP.0b013e3181dba6d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mezuk B, Bohnert ASB, Ratliff S, et al. Job strain, depressive symptoms, and drinking behavior among older adults: results from the Health and Retirement Study. J Gerontol B Psychol Sci Soc Sci. 2011;66:426–434. doi: 10.1093/geronb/gbr021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katz SJ, Kabeto M, Langa KM. Gender disparities in the receipt of homecare for elderly people with disability in the United States. JAMA. 2000;284:3022–3027. doi: 10.1001/jama.284.23.3022. [DOI] [PubMed] [Google Scholar]
- 34.Allison PD. Fixed Effects Regression Models. Thousand Oaks: Sage; 2009. [Google Scholar]
- 35.Farewell VT. Fixed Effects. In: Armitage P, editor. Encyclopedia of Biostatistics. Vol. 2. New York: John Wiley; 1998. p. 1533. [Google Scholar]
- 36.Eaton WW, Neufeld K, Chen LS, Cai G. A comparison of self-report and clinical diagnostic interviews for depression: diagnostic interview schedule and schedules for clinical assessment in neuropsychiatry in the Baltimore epidemiologic catchment area follow-up. Arch Gen Psychiatry. 2000;57:217–222. doi: 10.1001/archpsyc.57.3.217. [DOI] [PubMed] [Google Scholar]
- 37.Boyd EA, Lo B, Evans LR, et al. It’s not just what the doctor tells me:“ factors that influence surrogate-decision makers’ perceptions of prognosis. Crit Care Med. 2010;38:1270–1275. doi: 10.1097/CCM.0b013e3181d8a217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Apatira L, Boyd EA, Malvar G, et al. Hope, truth, and preparing for death: perspectives of surrogate decision makers. Ann Intern Med. 2008;149:861–868. doi: 10.7326/0003-4819-149-12-200812160-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Han B. The impact gender, and race on the relationship between depression and self-rated health in community-dwelling older adults: a longitudinal study. Home Health Care Serv Q. 2001;20:27–43. doi: 10.1300/J027v20n03_02. [DOI] [PubMed] [Google Scholar]
- 40.Solhaug HI, Romuld EB, Romild U, Stordal E. Increased prevalence of depression in cohorts of the elderly: an 11-year follow-up in the general population - the HUNT study. Int Psychogeriatr. 2012;24:151–158. doi: 10.1017/S1041610211001141. [DOI] [PubMed] [Google Scholar]
- 41.Blank K, Robison J, Doherty E, et al. Life-sustaining treatment and assisted death choices in depressed older patients. J Am Geriatr Soc. 2001;49:153–161. doi: 10.1046/j.1532-5415.2001.49036.x. [DOI] [PubMed] [Google Scholar]
- 42.White DB. Rethinking interventions to improve surrogate decision making in intensive care units. Am J Crit Care. 2011;20:252–257. doi: 10.4037/ajcc2011106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lautrette A, Darmon M, Megarbane B, et al. A communication strategy and brochure for relatives of patients dying in the ICU. N Engl J Med. 2007;356:469–478. doi: 10.1056/NEJMoa063446. [DOI] [PubMed] [Google Scholar]
- 44.Cameron JI, Herridge MS, Tansey CM, McAndrews MP, Cheung AM. Well-being in informal caregivers of survivors of acute respiratory distress syndrome. Crit Care Med. 2006;34:81–86. doi: 10.1097/01.ccm.0000190428.71765.31. [DOI] [PubMed] [Google Scholar]
- 45.Cox CE, Docherty SL, Brandon DH, et al. Surviving critical illness: acute respiratory distress syndrome as experienced by patients and their caregivers. Crit Care Med. 2009;37:2702–2708. doi: 10.1097/CCM.0b013e3181b6f64a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kersting A, Brähler E, Glaesmer H, Wagner B. Prevalence of complicated grief in a representative population-based sample. J Affect Disord. 2011;131:339–343. doi: 10.1016/j.jad.2010.11.032. [DOI] [PubMed] [Google Scholar]
- 47.Zivin K, Christakis NA. The emotional toll of spousal morbidity and mortality. Am J Geriatr Psychiatry. 2007;15:772–779. doi: 10.1097/JGP.0b013e318050c9ae. [DOI] [PubMed] [Google Scholar]
- 48.Taylor DH, Jr, Kuchibhatla M, Ostbye T, Plassman BL, Clipp EC. The effect of spousal caregiving and bereavement on depressive symptoms. Aging Ment Health. 2008;12:100–107. doi: 10.1080/13607860801936631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holtzman S, Abbey SE, Singer LG, Ross HJ, Stewart DE. Both patient and caregiver gender impact depressive symptoms among caregivers of organ transplant recipients. J Health Psychol. 2011;16:843–856. doi: 10.1177/1359105310393542. [DOI] [PubMed] [Google Scholar]
- 50.Sugiura K, Ito M, Kutsumi M, Mikami H. Gender differences in spousal caregiving in Japan. J Gerontol B Psychol Sci Soc Sci. 2009;64:147–156. doi: 10.1093/geronb/gbn005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pinquart M, Sörensen S. Gender differences in caregiver stressors, social resources, and health: an updated meta-analysis. J Gerontol B Psychol Sci Soc Sci. 2006;61:P33–P45. doi: 10.1093/geronb/61.1.p33. [DOI] [PubMed] [Google Scholar]
- 52.Covinsky KE, Newcomer R, Fox P, et al. Patient and caregiver characteristics associated with depression in caregivers of patients with dementia. J Gen Intern Med. 2003;18:1006–1014. doi: 10.1111/j.1525-1497.2003.30103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients. Lancet. 2009;373:1874–1882. doi: 10.1016/S0140-6736(09)60658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Needham DM, Korupolu R, Zanni JM, et al. Early physical medicine and rehabilitation for patients with acute respiratory failure: a quality improvement project. Arch Phys Med Rehabil. 2010;91:536–542. doi: 10.1016/j.apmr.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 55.Schulz R, Beach SR. Caregiving as a risk factor for mortality: the Caregiver Health Effects Study. JAMA. 1999;282:2215–2219. doi: 10.1001/jama.282.23.2215. [DOI] [PubMed] [Google Scholar]
- 56.Katon WJ. Clinical and health services relationships between major depression, depressive symptoms, and general medical illness. Biol Psychiatry. 2003;54:216–226. doi: 10.1016/s0006-3223(03)00273-7. [DOI] [PubMed] [Google Scholar]
- 57.Piette JD, Rosland AM, Silveira M, Kabeto M, Langa KM. The case for involving adult children outside of the household in the self-management support of older adults with chronic illnesses. Chronic Illn. 2010;6:34–45. doi: 10.1177/1742395309347804. [DOI] [PMC free article] [PubMed] [Google Scholar]