Abstract
Lipofuscin accumulation has been observed in a number of neurodegenerative diseases. We recently found that autofluorescent particles also occur in the aged human optic nerve. In this study we sought to determine the nature of these particles and their correlation with aging, age-related macular degeneration (AMD) and primary open angle glaucoma (POAG). Groups of eight optic nerves from patients diagnosed with primary open angle glaucoma, age-related macular degeneration, age-matched controls and four optic nerves derived from controls younger than 42 years were used for the study. All samples were fixed in paraformaldehyde and frozen frontal sections were prepared. Sections were analyzed with fluorescence microscopy, bright field microscopy, Sudan black staining and spectrofluorometry using a confocal laser scanning microscope. Sections were photographed and analyzed to establish the distribution, quantity, and size of the autofluorescent particles. Additionally, transmission electron microscopy was used to determine the ultrastructural location of the granules. On unstained sections under light microscopy granules are detectable as pale brown inclusions and are easily stained with oil-soluble dyes, such as Sudan black. Granules fluoresce when excited at all tested wavelengths but lose their fluorescence after staining with Sudan black. These particles are distributed throughout the axonal columns, but not in the septa, and appear to be located within the glia ensheathing optic nerve axons. The histologic properties of the granules seen in the optic nerve sections correspond to lipofuscin aggregates, a result of incomplete degradation of oxidized proteins. Our morphometric analyses indicate that overall the optic nerves from control, glaucoma, and AMD donors contain similar amounts of lipofuscin. However, optic nerves derived from donors with glaucoma contain lipofuscin particles that are larger than those observed in the age-matched control and AMD groups. Furthermore optic nerves from glaucoma donors display a smaller diameter than those from age-matched controls resulting in a higher concentration of lipofuscin in glaucomatous optic nerves.
Introduction
Lipofuscin is a pale yellow-brown lipopigment that is widely distributed throughout the animal kingdom and is a reliable morphologic marker of normal aging. Lipofuscin tends to accumulate throughout life in post-mitotic cells, such as neurons and glia, as these cell types appear to be unable degrade or exocytose this material. (Goyal, 1982; Idone et al., 2008) These deposits vary in their composition but are mainly composed from degraded proteins and a variety of lipid-like materials derived from the oxidation of polyunsaturated fatty acids. (Jolly et al., 2002) Lipofuscin is created when cellular waste is engulfed by autophagic vacuoles which later fuse with lysosomes in an attempt to degrade their constituents. Thus, lipofuscin particles are membrane bound and are located in the cytoplasm of cells.
Lipofuscin accumulates in multiple tissue types during aging. The age-related accumulation of lipofuscin in the retinal pigment epithelium (RPE) is striking, and this accumulation has been implicated as a major contributor in Mendelian forms of macular degeneration as well as AMD (Sparrow, 2010; Weingeist et al., 1982; Weng et al., 1999). In the optic nerve, the presence of lipofuscin has been previously noted (Dolman et al., 1980), but the extent and prevalence of lipofuscin deposition in this tissue has not been systematically evaluated. Advanced age is a very significant risk factor for the development of Primary Open Angle Glaucoma (POAG), a disease that affects the optic nerve (Coleman and Miglior, 2008). The events that lead to death of retinal ganglion cells and axonal loss in POAG are not completely understood (Kwon et al., 2009), but there is little doubt that the degradation of degenerating ganglion cell axons and their myelin sheaths requires the activity of lysosomal and proteosomal systems. For these reasons we set out to determine if lipofuscin accumulation in the optic nerve is correlated to the development of POAG or AMD.
The objective of this study is to establish the presence of lipofuscin in the optic nerve, and to determine the distribution, quantity, and size of the lipofuscin particles. These findings are compared between the optic nerves of healthy young eyes, those derived from donors with AMD or glaucoma, and healthy age-matched controls.
Materials and Methods
Human Donors
All experiments conformed to the Declaration of Helsinki. Human optic nerves were obtained in collaboration with the Iowa Lions Eye Bank (Iowa City, IA) and preserved within six hours postmortem. Following consent of the donors’ families medical records were obtained for all donors and reviewed for a diagnosis of primary open angle glaucoma or age related macular degeneration. In addition, young and age-matched control donors were selected who had received an eye exam within two years before death and had been found to be free of ocular disease.
Light Microscopy
For light microscopy human optic nerves were fixed in 4% paraformaldehyde in phosphate buffered saline. A portion of the optic nerve, located approximately 3 to 5 mm posterior to the lamina cribrosa, was infiltrated with sucrose, embedded in OCT, and 7 μm thick frozen sections were collected in the frontal plane.
Sections were either stained with Sudan Black B (Fisher Biotech) or coverslipped unstained. Untreated sections were photographed under an inverted fluorescence microscope (Olympus IX81) using a 20 x objective lens at excitation wavelengths of 350 nm, 488 nm and 550 nm and bright field.
Spectrofluorometry
Using a confocal laser scanning microscope (Zeiss LSM 710) the fluorescence profile of the granules from unstained sections from each group was determined. The instrument’s software (Zen 2009) was used to measure the emission intensity at increasingly higher wavelengths ranging from 421 to 724nm on defined potions of the image containing lipofuscin particles. An excitation wavelength of 458 nm was used. A section containing autofluorescent granules in the retinal pigment epithelium (RPE) of an AMD positive donor was used to calibrate the confocal microscope settings of percentage of transmission and detector gain to avoid saturation. All other samples were measured with the same settings.
Transmission Electron Microscopy
Additional optic nerves were fixed in half strength Karnoksy fixative (Schneeberger-Keeley and Karnovsky, 1968) and embedded in Spur’s resin. Ultrathin sections were collected on formvar coated copper slotted grids using an ultramicrotome (EM UC7; Leica Microsystems) and photographed with a digital camera on a transmission electron microscope (JEM-1230, JEOL).
Morphometric Analyses
From each optic nerve sections were obtained as described above and two non-overlapping images each representing a microscopy field of 877x660 μm were randomly obtained at 200X magnification using a FITC filter set. Images were analyzed using ImageJ software (Abramoff et al., 2004) and the “Particle Analysis” plug – in. Initially, we selected a set of images from ten donors, representing a wide variety of lipofuscin granule density, and manually determined the number of particles. The same images were then converted to a binary format using several threshold settings. After each conversion the number of granules was determined computationally, findings were correlated with the numbers obtained manually, and the most accurate conversion threshold was determined by regression analysis (max r2=0.97). These settings were subsequently used on all images to determine the total number of granules, their individual size, and the total autofluorescent area. The overall area of each section was determined by outlining the digital image and calculating the enclosed space. Statistical comparison between groups of donors was carried out using one way ANOVA followed by an unsigned Tukey HSD post-hoc test.
Results
Donors
Eight optic nerves from patients clinically diagnosed with primary open angle glaucoma (POAG), eight with age-related macular degeneration (AMD), eight age-matched controls and four young controls were used for the study. The average age of the glaucoma group is 82.8 years, 83.0 years for the AMD group, 80.9 years for the age-matched controls, and 35.0 years for the young controls.
Histology
Initial examination of untreated human optic nerve transverse sections obtained from an area located 3–5 mm posterior to the lamina cribrosa revealed the presence of numerous autofluorescent particles ranging from 2 to 7 μm2 in size, which can be visualized at excitation wavelengths of 350 nm, 488 nm and 550 nm, consistent with the supposition that they are comprised of lipofuscin (Figure 1). Careful examination of sections with brightfield microscopy allows visual detection of these inclusions as pale brown granules. One additional characteristic of lipofuscin particles is that they readily react with oil-soluble dyes such as Sudan Black, resulting in a dark brown stain. Staining of lipofuscin with Sudan Black reduces its autofluorescence, thereby serving to additionally confirm the identity of the complex. Our findings demonstrate that the optic nerve granules are markedly sudanophilic in all donors (Fig. 2a). After staining with Sudan black autofluorescence is almost completely abolished when excitation wavelengths of 354, 488, or 594nm are used (Fig. 2b). These data are highly indicative that the observed optic nerve granules contain lipofuscin.
Figure 1.
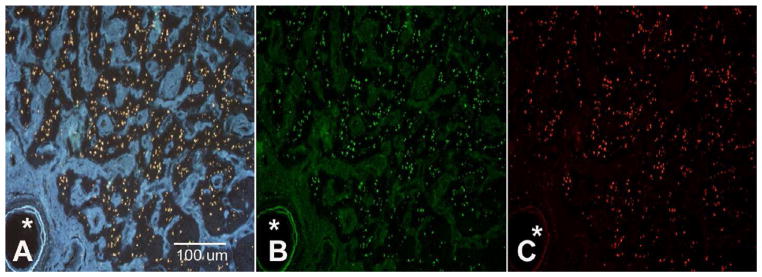
Autofluorescent particles observed in frontal of a human optic nerve derived from a donor with POAG. The section plane is located approximately 3–5 mm posterior to the lamina cribrosa. Particles are located almost exclusively within the remaining axonal bundles. Excitation wavelength: (A) 354 nm (B) 488 nm (C) 594 nm. Images taken at 100X magnification. Asterisk: Central artery
Figure 2.
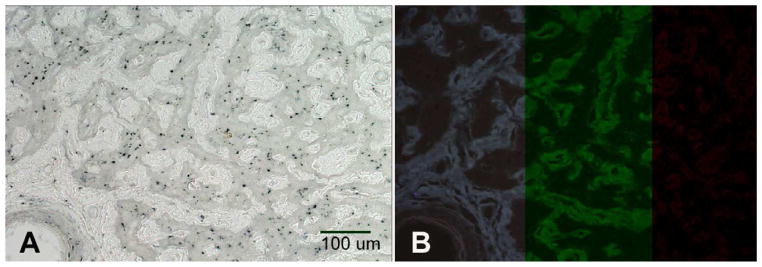
Photomicrograph of an optic nerve section stained with Sudan black B. This lipophilic dye stains all autofluorescent particles (A) and in the process abolishes their autofluorescent properties at 354, 488, and 594 nm (B). Depicted are images from the same donor as in figure 1. Magnification: 100 X.
Regardless of the detection method, the number of particles observed varies widely between donors. In those donors whose optic nerves contain lipofuscin it appears to be distributed throughout the entire cross section of the nerve. Particles are typically detected within the axonal columns and are largely absent from the septa or areas of glial hypertrophy.
Ultrastructural localization
The optic nerve axonal columns are comprised of myelin sheathed retinal ganglion cell axons as well as astroglia and oligodendrocytes, both of which maintain an intimate connection to the axons. In order to determine the ultrastructure and location of the granules we conducted transmission electron microscopy of the optic nerve of an 89 year old donor diagnosed with POAG. In this individual numerous dense, coarse electron dense granules were identified along with large lipid droplets, and small electron-dense granules (Figure 3). The observed granules vary in size and shape, but uniformly exhibit high electron density. These deposits resemble the density, shape and size of lipofuscin granules found elsewhere the central nervous system (Peters, 2007).
Figure 3.
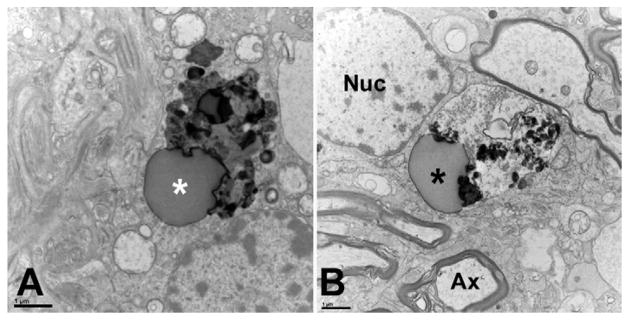
Transmission electron microscopy of lipofuscin granules in the optic nerve of an 89 year old donor with diagnosed primary open angle glaucoma. Multiple axons (Ax) with the characteristic banded myelin sheath and abundant mitochondria can be seen. Dense deposits (asterisks) are apparent in the cytoplasm of glial cells located within the axonal fascicles. Granules were not observed within the axons. The transverse section plane is located 3–5 mm posterior to the lamina cribrosa. Nuc: Nucleus. Scale bar: 1 μm
In the sections examined as part of this study the observed deposits appeared to occur exclusively within the cytoplasm of glial cells located within the axonal fascicles. Granules were rarely observed within the ganglion cell axons of the optic nerve.
Spectroscopy
Additional confirmation that the detected autofluorescent particles in the optic nerve are lipofuscin was obtained by comparison of the emission spectrum with those observed in the lipofuscin accumulation in the RPE. Portions of sections containing lipofuscin were analyzed under a confocal microscope measuring emission at wavelengths between 421 and 724nm at 9 nm intervals. The emission intensity was recorded at each wavelength and then plotted to obtain a fluorescence profile for each of the study groups. The resulting data indicate a broad fluorescence spectrum with a peak around 570 nm as well as two marked peaks at 502 and 475 nm. Our data further demonstrate that the observed spectral properties of RPE lipofuscin are very similar to those of the autofluorescent particles in the optic nerve, providing additional evidence that they are lipofuscin (Figure 4). Additionally, the spectra obtained from lipofuscin particles of optic nerves derived from donors with POAG, AMD, or healthy individuals do not differ from one another, suggesting that the chemical composition of optic nerve lipofuscin is not affected by disease status.
Figure 4.
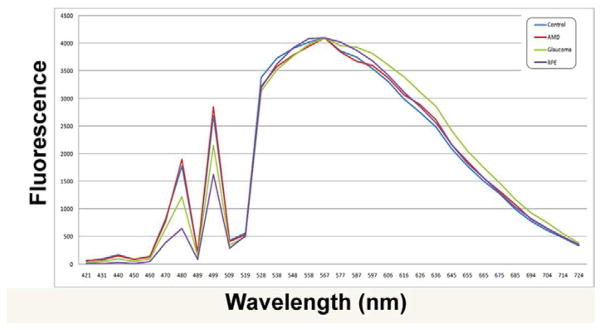
Excitation spectra of lipofuscin particles the optic nerve of donors with glaucoma, AMD, or age-matched healthy controls. In addition, one spectrum derived from lipofuscin within the RPE is displayed.
Association with Age and Disease
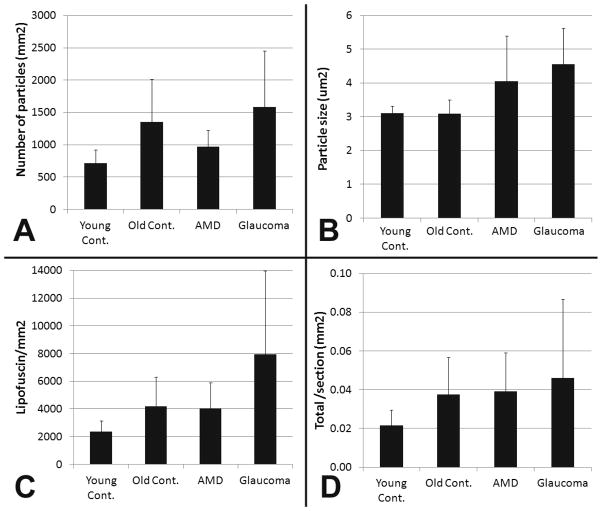
We initially observed that the extent to which lipofuscin particles are discernible varies between individual donor optic nerves. In order to determine if the density and size of the lipofuscin granules or the concentration and amount of lipofuscin is correlated to the presence of ocular disease, we compared these parameters in optic nerves derived from human eye donors with either AMD (N=8), POAG (N=8), or age-matched control donors with healthy eyes (N=8). Data from older healthy controls were also compared to those derived from optic nerves derived young controls (N=4). As expected, our data clearly demonstrate that the accumulation of lipofuscin granules in the optic nerve is age related. In optic nerves from young donors granules are more sparse than in older control donors (716 vs. 1,352 granules/mm2, p=0.02 by t-test), but those granules that do occur in younger donors are almost identical in size to those present in healthy older individuals (3.11 vs 3.09 μm2, p=0.9 by t-test) (Figure 5a and b).
Figure 5.
Morphometric analysis of lipofuscin particles in human optic nerves. (A) Average number of particles detected per mm2 (B) Average size of lipofuscin particles (μm2), (C) Concentration of lipofuscin (average area (μm2) occupied by lipofuscin per mm2 ), (D) Total amount of lipofuscin per optic nerve section in mm2 (Number of particles x size x optic nerve area). Error bars: standard deviation
Comparison of data among the three groups of older donors indicated that the density of lipofuscin granules in the optic nerve was similar between age-matched control and glaucoma donors (1,352 vs. 1,583 granules/mm2) (Figure 5a). The particle density was lowest in optic nerves derived from donors with AMD (969 granules/mm2), but not significantly below that observed in healthy controls.
We also determined the average size of the observed lipofuscin particles in each of the four groups (Figure 5 b). The average particle size in older controls (Average size= 3.1 μm2) is smaller than in AMD (4.0 μm2) or in POAG (4.6 μm2). ANOVA indicated that these differences are statistically significant (p=0.003) and post-hoc analysis revealed that the strongest difference exists between the control and glaucoma groups (p<0.01).
The higher density and larger size of lipofuscin particles in POAG optic nerves results in a higher concentration of lipofuscin than observed in other older donors. A significantly higher fraction of the POAG optic nerve is occupied by lipofuscin (7,938 μm2/mm2) than either those derived from age-matched control (4,197 μm2/mm2) or AMD donors (4,043 μm2/mm2) (Figure 5 c). Statistical analysis indicates that these differences are significant (p=0.013 by ANOVA) and that POAG optic nerves contain a higher concentration of lipofuscin than those derived from either healthy donors or those with AMD (p<0.05 for each on post-hoc test).
However, glaucomatous optic nerves frequently posses a smaller crossectional area than those of healthy eyes, presumably due to the loss of ganglion cell axons, which might concentrate lipofuscin particles into a smaller space. In our samples glaucomatous optic nerves (6.63 +/− 2.1 mm2) were indeed smaller than those of age matched controls (8.75 +/− 0.8 mm2,) although this difference was not statistically significant (p=0.12). When these measurements are used to calculate the total amount of lipofuscin visible in each transverse section, the results indicate that crossectional area of glaucomatous optic nerves contain 0.046 mm2 lipofuscin compared to 0.039 mm2 in AMD and 0.038 mm2 in healthy control optic nerves (Figure 5d). This difference is not significant (p=0.56), in part due to the notable heterogeneity exhibited by the POAG group with respect to the number and size of detected particles.
Discussion
In the present study we demonstrate the presence of lipofuscin in the aged human optic nerve and evaluate whether the presence of age-related macular degeneration or POAG influences the accumulation of this lipopigment. To our knowledge this is the first study to analyze lipofuscin composition, distribution, and frequency in the human optic nerve.
Our data demonstrate that, as expected, there is a strong correlation between the amount of lipofuscin in the optic nerve and donor age. Furthermore, we demonstrate that optic nerve lipofuscin accumulation in donors with AMD does not significantly differ from that observed in age-matched healthy control donors. The pathophysiology of AMD does not typically involve optic nerve damage, but a heightened accumulation of potentially photocytotoxic lipofuscin in the RPE of patients with AMD has been repeatedly documented and is appears to be correlated to the development of the disease (Davies et al., 2001; Sparrow, 2010). Although the amount of RPE lipofuscin was not determined in the donors used in this study, our findings support the notion that independent pathophysiologic processes lead to the accumulation of lipofuscin in the RPE and the optic nerve. The lipofuscin accumulation in the RPE of older humans appears to be at least partially derived from retinol and photoreceptor phospholipids (Finnemann et al., 2002). In contrast, neuronal lipofuscin is generally believed to be derived from incomplete degradation of mitochondrial components or other oxidized cellular components (Sulzer et al., 2008). Yet in our study of the spectral properties of RPE lipofuscin was comparable to that found in optic nerve of all examined samples. The obtained spectrum with broad emission around a peak at 580 nm is largely consistent with RPE lipofuscin data presented by other investigators. (Boulton et al., 1990; Marmorstein et al., 2002; Warburton et al., 2007) Our data also indicated the presence of two peaks at shorter wavelengths that were not previously reported. Marmorstein et al., using a very similar approach albeit at a slightly different excitation wavelength, reported very little emission below 500 nm. In contrast, other investigators, using isolated preparations of RPE lipofuscin, obtained spectra featuring gradually increasing fluorescence beginning at approximately 450 nm. (Boulton et al., 1990; Warburton et al., 2007) Whether the peaks, or the valleys, observed in our study might be artifactual is difficult to resolve. However, our findings indicate that, while the source of RPE and optic nerve lipofuscin may be distinct, their emission spectra are comparable, suggesting that the chemical composition of the material is similar.
In all donors lipofuscin deposition in the optic nerve is largely restricted to the axonal columns and is rarely observed in the glial septae. This is also true in POAG optic nerves, even though the area occupied by axons decreases as optic nerve degeneration progresses. Our transmission electron microscopy data indicates that the majority of the lipofuscin particles are located in the neuroglial cells residing within axon bundles, rather than axons themselves. It remains a matter of speculation whether these particles result from incomplete degradation of material phagocytosed by the glia from adjacent axons. Alternatively, lipofuscin aggregates may originate from abnormal protein degradation of the glia’s own components. In either case, the vicinity of axons appears to promote lipofuscin formation, which is much less prevalent in glial septae and areas of glial hypertrophy.
Our data further indicate that optic nerves derived from donors with POAG contain larger lipofuscin aggregates than those found in either healthy age-matched controls or donors with AMD. POAG optic nerves contain similar, or slightly elevated, numbers of lipofuscin particles than either of these two groups, resulting in higher concentration of lipofuscin. This may be, at least in part, due to the fact that the diameter of glaucomatous optic nerves is frequently reduced, presumably due to the loss of axons. (Jonas et al., 1995) This loss of optic nerve volume would result in increased lipofuscin concentration, but not total accumulation. This was indeed the case in the optic nerves used in this study; our analyses indicate that the total amount of lipofuscin in the glaucomatous optic nerve is not statistically above that observed in other older donors.
We observed pronounced variability within the POAG group with respect to the lipofuscin density, particle size, and total lipofuscin area. Statistical analyses designed to correlate these parameters with the severity of glaucoma, defined by either cup-to-disk ratio or degree of visual field loss, did not reveal a strict correlation (data not shown). Oxidative stress and mitochondrial dysfunction clearly contribute to the formation of lipofuscin aggregates. (Jarrett et al., 2008; Terman et al., 2010) The theory that reduced optic nerve head blood flow, either as a result of decreased perfusion pressure or due to structural changes in the optic nerve head, contributes to the pathophysiology of glaucoma has been debated for many years. Clinical evidence in support of this notion has been presented (recently reviewed in Mozaffarieh et al., 2008) as has been immunohistochemical and biochemical data demonstrating the presence of reactive oxygen species (ROS) and hypoxia-induced protein synthesis in both human donor tissue and animal models of the disease (Ferreira et al., 2010; Tezel and Wax, 2004; Tezel et al., 2007). In addition, some morphological evidence of mitochondrial damage in the ONH, at least in rodent models, has been presented (Ju et al., 2008). The fact that the largest lipofuscin particles and density was observed in POAG optic nerves may indicate that higher a level of oxidative stress persists in the some, but not all, glaucomatous optic nerves.
The functional consequences of lipofuscin deposition in the optic nerve are currently unclear. Biologically lipofuscin is not inert and appears to inhibit several cellular functions, such as lysosomal and proteosomal degradation. The elevated density of lipofuscin particles in glaucomatous optic nerves may be a consequence of optic nerve shrinkage, but it is likely that it further induces a pro-inflammatory phenotype in microglia and astrocytes (Nakanishi and Wu, 2009). Consequently the higher concentration of lipofuscin could potentially exacerbate optic nerve damage in glaucoma and as such further study is warranted.
Highlights.
We examine the distribution of lipofuscin in the human optic nerve in healthy eye donors as well as those with PAOG and AMD
Lipofuscin formation is common in aged optic nerves
Lipofuscin deposition occurs within the fascicles of the optic nerve
Lipofuscin particles are larger and more densely spaced in optic nerves derived from eye donors with a history of glaucoma
Acknowledgments
These studies were supported by NIH grants EY019485, EY022044, (MHK) and EY017451 (RFM). The authors appreciate the help of Dr. Kai Wang (University of Iowa Department of Biostatistics) for his help with the analysis of the data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature cited
- Abramoff M, Magelhaes P, Ram S. Image Processing with ImageJ. Biophotonics International. 2004;11:36–42. [Google Scholar]
- Boulton M, Docchio F, Dayhaw-Barker P, Ramponi R, Cubeddu R. Age-related changes in the morphology, absorption and fluorescence of melanosomes and lipofuscin granules of the retinal pigment epithelium. Vision Res. 1990;30:1291–303. doi: 10.1016/0042-6989(90)90003-4. [DOI] [PubMed] [Google Scholar]
- Coleman AL, Miglior S. Risk factors for glaucoma onset and progression. Surv Ophthalmol. 2008;53(Suppl1):S3–10. doi: 10.1016/j.survophthal.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Davies S, Elliott MH, Floor E, Truscott TG, Zareba M, Sarna T, Shamsi FA, Boulton ME. Photocytotoxicity of lipofuscin in human retinal pigment epithelial cells. Free Radic Biol Med. 2001;31:256–65. doi: 10.1016/s0891-5849(01)00582-2. [DOI] [PubMed] [Google Scholar]
- Dolman CL, McCormick AQ, Drance SM. Aging of the optic nerve. Arch Ophthalmol. 1980;98:2053–8. doi: 10.1001/archopht.1980.01020040905024. [DOI] [PubMed] [Google Scholar]
- Ferreira SM, Lerner SF, Brunzini R, Reides CG, Evelson PA, Llesuy SF. Time course changes of oxidative stress markers in a rat experimental glaucoma model. Invest Ophthalmol Vis Sci. 2010;51:4635–40. doi: 10.1167/iovs.09-5044. [DOI] [PubMed] [Google Scholar]
- Finnemann SC, Leung LW, Rodriguez-Boulan E. The lipofuscin component A2E selectively inhibits phagolysosomal degradation of photoreceptor phospholipid by the retinal pigment epithelium. Proc Natl Acad Sci U S A. 2002;99:3842–7. doi: 10.1073/pnas.052025899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal VK. Lipofuscin pigment accumulation in human brain during aging. Exp Gerontol. 1982;17:481–7. doi: 10.1016/s0531-5565(82)80010-7. [DOI] [PubMed] [Google Scholar]
- Idone V, Tam C, Goss JW, Toomre D, Pypaert M, Andrews NW. Repair of injured plasma membrane by rapid Ca2+-dependent endocytosis. J Cell Biol. 2008;180:905–14. doi: 10.1083/jcb.200708010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett SG, Lin H, Godley BF, Boulton ME. Mitochondrial DNA damage and its potential role in retinal degeneration. Prog Retin Eye Res. 2008;27:596–607. doi: 10.1016/j.preteyeres.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Jolly RD, Palmer DN, Dalefield RR. The analytical approach to the nature of lipofuscin (age pigment) Arch Gerontol Geriatr. 2002;34:205–17. doi: 10.1016/s0167-4943(01)00219-9. [DOI] [PubMed] [Google Scholar]
- Jonas JB, Schmidt AM, Muller-Bergh JA, Naumann GO. Optic nerve fiber count and diameter of the retrobulbar optic nerve in normal and glaucomatous eyes. Graefes Arch Clin Exp Ophthalmol. 1995;233:421–4. doi: 10.1007/BF00180945. [DOI] [PubMed] [Google Scholar]
- Ju WK, Kim KY, Lindsey JD, Angert M, Duong-Polk KX, Scott RT, Kim JJ, Kukhmazov I, Ellisman MH, Perkins GA, Weinreb RN. Intraocular Pressure Elevation Induces Mitochondrial Fission and Triggers OPA1 Release in Glaucomatous Optic Nerve. Invest Ophthalmol Vis Sci. 2008;49:4903–11. doi: 10.1167/iovs.07-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon YH, Fingert JH, Kuehn MH, Alward WL. Primary open-angle glaucoma. N Engl J Med. 2009;360:1113–24. doi: 10.1056/NEJMra0804630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmorstein AD, Marmorstein LY, Sakaguchi H, Hollyfield JG. Spectral profiling of autofluorescence associated with lipofuscin, Bruch’s Membrane, and sub-RPE deposits in normal and AMD eyes. Invest Ophthalmol Vis Sci. 2002;43:2435–41. [PubMed] [Google Scholar]
- Mozaffarieh M, Grieshaber MC, Flammer J. Oxygen and blood flow: players in the pathogenesis of glaucoma. Mol Vis. 2008;14:224–33. [PMC free article] [PubMed] [Google Scholar]
- Nakanishi H, Wu Z. Microglia-aging: roles of microglial lysosome- and mitochondria-derived reactive oxygen species in brain aging. Behav Brain Res. 2009;201:1–7. doi: 10.1016/j.bbr.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Peters A. The Effects of Normal Aging on Nerve Fibers and Neuroglia in the Central Nervous System. In: Riddle D, editor. Brain Aging: Models, Methods, and Mechanisms. CRC Press; Boca Raton: 2007. [PubMed] [Google Scholar]
- Schneeberger-Keeley EE, Karnovsky MJ. The ultrastructural basis of alveolar-capillary membrane permeability to peroxidase used as a tracer. J Cell Biol. 1968;37:781–93. doi: 10.1083/jcb.37.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow JR. Bisretinoids of RPE lipofuscin: trigger for complement activation in age-related macular degeneration. Adv Exp Med Biol. 2010;703:63–74. doi: 10.1007/978-1-4419-5635-4_5. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Mosharov E, Talloczy Z, Zucca FA, Simon JD, Zecca L. Neuronal pigmented autophagic vacuoles: lipofuscin, neuromelanin, and ceroid as macroautophagic responses during aging and disease. J Neurochem. 2008;106:24–36. doi: 10.1111/j.1471-4159.2008.05385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terman A, Kurz T, Navratil M, Arriaga EA, Brunk UT. Mitochondrial turnover and aging of long-lived postmitotic cells: the mitochondrial-lysosomal axis theory of aging. Antioxid Redox Signal. 2010;12:503–35. doi: 10.1089/ars.2009.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezel G, Wax MB. Hypoxia-inducible factor 1alpha in the glaucomatous retina and optic nerve head. Arch Ophthalmol. 2004;122:1348–56. doi: 10.1001/archopht.122.9.1348. [DOI] [PubMed] [Google Scholar]
- Tezel G, Yang X, Luo C, Peng Y, Sun SL, Sun D. Mechanisms of immune system activation in glaucoma: oxidative stress-stimulated antigen presentation by the retina and optic nerve head glia. Invest Ophthalmol Vis Sci. 2007;48:705–14. doi: 10.1167/iovs.06-0810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton S, Davis WE, Southwick K, Xin H, Woolley AT, Burton GF, Thulin CD. Proteomic and phototoxic characterization of melanolipofuscin: correlation to disease and model for its origin. Mol Vis. 2007;13:318–29. [PMC free article] [PubMed] [Google Scholar]
- Weingeist TA, Kobrin JL, Watzke RC. Histopathology of Best’s macular dystrophy. Arch Ophthalmol. 1982;100:1108–14. doi: 10.1001/archopht.1982.01030040086016. [DOI] [PubMed] [Google Scholar]
- Weng J, Mata NL, Azarian SM, Tzekov RT, Birch DG, Travis GH. Insights into the function of Rim protein in photoreceptors and etiology of Stargardt’s disease from the phenotype in abcr knockout mice. Cell. 1999;98:13–23. doi: 10.1016/S0092-8674(00)80602-9. [DOI] [PubMed] [Google Scholar]