Abstract
Background
Research has generally examined institutional review boards (IRBs) in isolation, but critical questions arise of how these entities fit into the larger institutional contexts in which they operate and what the implications may be.
Methods
Semi-structured interviews were conducted with leaders of IRBs from among the top 240 institutions receiving funding from the National Institutes of Health.
Results
Interviewees felt that institutions may affect IRBs through both broad, indirect features (e.g., size, type of research, and culture of the institution), and more direct, IRB-related factors (e.g., amount of leadership and resource support for the IRB). Interviewees thought that institutional support of IRBs ranged from financial to non-financial, direct and indirect, and that these institutional factors can mold amounts of IRB staff and education, audits, and education of principal investigators (PIs), and tensions IRBs had to address. Respondents felt that these factors can in turn potentially affect IRB reviews of protocols and interactions with principle investigators (PIs). Within the complex systems of an institution, IRBs felt that PIs' experiences and complaints about the IRB to institutional leaders may also shape how the institution related to the IRB.
Conclusions
These data are the first to show how IRBs perceive themselves as working within the contexts of dynamic local institutional relationships and systems that pose challenges and tensions that can potentially affect critical aspects of IRB functioning. The findings have implications for practice, future research, and policy.
Keywords: research ethics, organizational culture, dynamic systems theory, academic medical centers
Introduction
Research has examined several logistical aspects of institutional review boards (IRBs). Studies have probed IRB members' education and sociodemographics and the length of time that transpires before approval (De Vries et al. 2006; Greene and Geiger 2006; Larson et al. 2004) as well as discrepancies in IRBs' decisions in multi-site studies (Dziak et al. 2005; Greene and Geiger 2006; McWilliams et al. 2003).
Yet research on IRBs has tended to view these committees as isolated entities rather than as operating within larger institutional or social contexts. Research has demonstrated that other organizations operate in complex social systems, highlighting the importance of viewing institutions, in general, not as static, but as engaged in dynamic relationships (Emirbayer 1997). For instance, managers in an academic medical center involved in governmental environmental safety regulations (e.g., regarding radioactive and chemical wastes) work in highly dynamic and interactive systems (Silbey 2011). Many questions thus remain concerning the relationships of IRBs to the broader local institutional and social systems in which they work.
Recent proposals have been made to change IRBs (Emanuel and Menikoff 2011; US Department of Health and Human Services 2011), seeking to alter phenomena that occur in complex social and institutional milieu that can potentially shape whether and how any alterations to IRBs are implemented. Hence, the outcomes and effectiveness of attempts to change current regulations may depend in large part on these relationships, which need to be understood. These proposals are also being made at a time of wider financial constraints in health care and the rest of the economy, challenging the abilities of academic medical centers and their IRBs to respond to any new regulations.
Systems theory has explored how components of diverse types of social systems, including those involved in health care (Begun et al. 2003; Parsons 1951), interact and provide feedback in complex ways (von Bertalanffy 1950). Hospitals, medical schools, physicians, and the rest of universities can have intricate relationships with each other. But surprisingly, prior studies of IRBs have not systematically examined these issues – whether local institutions affect IRBs, and if so, when, in what ways, to what degree, and how often.
I recently conducted an in-depth semi-structured interview study of IRBs, focusing on views and approaches toward research integrity (RI), broadly defined, among chairs, directors, administrators, and members (Klitzman 2011a). Interviewees revealed how they understood RI issues in relation to how they viewed and approached conflicts of interests (COIs) (Klitzman 2011b); central IRBs (Klitzman 2011c); variations between IRBs (Klitzman 2011d); studies conducted in the developing world (Klitzman 2012); researchers (Klitzman 2011e); and so-called “community” members (Klitzman in press).
Though IRBs often argue that variations among them are due to differences in local community values, this may not often be the case (Klitzman 2011d). Rather, different IRBs at a single institution – all within the same community – may vary, and these inconsistencies often appear to arise due to other phenomena, including personalities and strong views held by a chair or particular members, and potentially social and institutional factors. Yet many critical questions remain concerning the nature and quality of these institutional factors – of what they consist, how they operate, and how they may interface and interact with IRBs. Since the study used qualitative research methods, it allowed exploration of these domains as they arose. The present paper explores respondents' perceptions of how the larger local institutions in which they worked affected and related to their IRBs and their views and decisions on that committee.
Methods
I conducted in-depth telephone interviews of two hours each with 46 chairs, directors, administrators, and members, as described elsewhere (Klitzman 2012; Klitzman 2011a; Klitzman 2011b; Klitzman 2011c; Klitzman 2011d; Klitzman 2011e; Klitzman in press). To identify the interviewees, I contacted leaders of 60 US IRBs (every fourth one in the list of the top 240 institutions by National Institutes of Health (NIH) funding). I interviewed a chair/director as well as an administrator from some institutions because, for example, the chair thought that the administrator might be better able to address particular areas. I also asked half of these leaders (every other one participating) to distribute an announcement about the study to members of their IRBs to recruit one member of each IRB.
The interviews focused on respondents' views of RI, IRB responses, and relevant factors, and shed light on many other, broader issues concerning IRBs. Appendix A provides portions of the semi-structured interview guide through which I sought to obtain a “thick description” (Geertz 1973) of these phenomena. These probes initiated the interviews and elicited data concerning IRBs' relationships with institutions.
I adapted elements from Grounded Theory (Strauss and Corbin 1990), specifically, using techniques of “constant comparison” in which data from different contexts are compared for similarities and differences to see if they suggest hypotheses. This technique of “constant comparison” generates new analytic categories and questions and checks them for reasonableness. During the ongoing process of in-depth interviewing, how participants resembled or differed from each other and what social, cultural, and medical contexts and factors contributed to differentiation were constantly considered.
Grounded theory also involves both deductive and inductive thinking, building inductively from the data to an understanding of themes and patterns within the data, and deductively, drawing on frameworks from prior research and theories. In conducting thematic content analyses, methods were also triangulated based on the published literature described above. I drafted the questionnaire, drawing on prior research and published literature. Interviews were transcribed and initially analyzed during the period in which the interviews were still being conducted, and influenced subsequent interviews.
After the full set of interviews was completed, a trained research assistant (RA) and I conducted subsequent analyses in two phases. First, we independently examined a subset of interviews, evaluating factors that affected IRB respondents' experiences and identifying categories of recurrent themes and issues, to which we subsequently gave codes. We read each interview, coding blocks of text systematically, to assign “core” codes or categories (e.g., descriptions of characteristics of institutions and interactions between institutions and IRBs). A topic name (or code) was inserted beside each excerpt of the interview to categorize the themes being discussed. Then we worked together to reconcile the independently developed coding schemes into a single scheme. Next we developed a coding manual, defining each code and examining areas of disagreement until reaching consensus. We discussed new themes that did not fit into the original coding framework and modified the manual when appropriate.
We independently performed content analysis of the data in the second phase of the analysis to identify the principal subcategories and ranges of variation within each of the major codes. We each identified sub-themes that were reconciled into a single set of “secondary” codes and an elaborated set of core codes. We assessed subcategories and other situational and social factors. Subcategories included specific types of interactions between IRB chairs and institutions, such as chairs asking for, and receiving or not receiving, more support, and feeling pressured by institutional leaders.
We then used the codes and sub-codes in analyzing all of the interviews. Where necessary, we used multiple codes. We examined similarities and differences between participants, categories that emerged, ranges of variation within categories, and variables that may have been involved. We probed areas of disagreement through closer analysis and checked regularly for consistency and accuracy by comparing earlier and later coded text. Core codes and secondary codes were systematically developed and well-documented.
The study was approved by the Columbia University Department of Psychiatry IRB, and all participants gave informed consent.
Results
This article reports on selected themes that are “incidental” to the study's primary focus on research integrity. The results are thus hypothesis-generating, providing ideas for further research.
IRB leaders from 34 of the 60 institutions contacted were interviewed (response rate = 55%). The institutions varied in location, size, and public/private status. In total, there were 46 respondents: 28 IRB chairs/co-chairs; 1 IRB director; 10 administrators; and 7 IRB members. Respondents varied in gender, ethnicity, and location. (See Table 1 [Klitzman 2011d].)
Table 1. Characteristics of the sample.
| Total | % (N=46) | |
|---|---|---|
| Type of IRB Staff | ||
| Chairs/Co-Chairs | 28 | 60.87% |
| Directors | 1 | 2.17% |
| Administrators | 10 | 21.74% |
| Members | 7 | 15.22% |
| Gender | ||
| Male | 27 | 58.70% |
| Female | 19 | 41.30% |
| Institutional Rank in NIH funding* | ||
| 1-50 | 13 | 28.26% |
| 51-100 | 13 | 28.26% |
| 101-150 | 7 | 15.22% |
| 151-200 | 1 | 2.17% |
| 201-250 | 12 | 26.09% |
| State vs. Private | ||
| State | 19 | 41.30% |
| Private | 27 | 58.70% |
| Region | ||
| Northeast | 21 | 45.65% |
| Midwest | 6 | 13.04% |
| West | 13 | 28.26% |
| South | 6 | 13.04% |
| Total # of Institutions Represented | 34 | |
Note. From Klitzman (2011c) and Klitzman (2011d).
From list of top 240 institutions by total amount of NIH funding
In brief, interviewees suggested that local institutions, each with their particular history, culture, finances, and leaders, could affect IRBs in various ways, both directly and indirectly, which can in turn affect principal investigators (PIs). Specifically, respondents felt that institutions could affect IRBs through both broad features (e.g., size, type of research, and culture of the institution), and more directly IRB-related factors (e.g., amount of economic and political support for the IRB). These individuals felt that institutional support of IRBs ranged from financial to non-financial, and direct and indirect, and that these institutional factors molded many characteristics of IRBs (e.g., amount of IRB staff and training, auditing, and education of PIs) and tensions IRBs had to address. Respondents suggested that these characteristics of IRBs could in turn affect IRB reviews of protocols and interactions with PIs. Within the complex systems of an institution, feedback loops may exist through which PIs' experiences (e.g., scandals) and complaints about the IRB to institutional leaders may also shape how the institution relates to the IRB. Table II presents a schematized view of many of these issues, suggesting how perceptions concerning a range of institutional and IRB characteristics and interactions may affect IRB decisions, which can in turn affect PIs as well as research participants.
Table 2. Issues concerning IRB-related characteristics of perceptions of relationships between IRBs and their institutions.
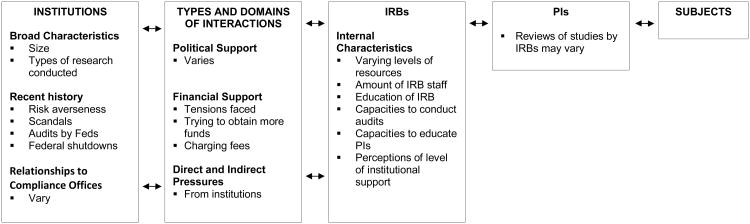
|
Broad Institutional Characteristics
Interviewees believed that an institution's size and culture could each impact the other as well as the IRB. Several chairs suggested that at smaller institutions “everybody knows everybody,” mitigating against problems with PIs. As one chair at a smaller institution said:
I've been here long enough that I know almost all the doctors and surgeons. Everybody knows everybody. That makes it harder for a researcher to be defiant or a cowboy. Everybody knows you. Researchers tend to be honest with us – what their problem is, or what they want from us. It's cultural, though. I've worked here so long, maybe I am just blind to our problems. I don't know what other institutions' cultures are like. IRB32
Responses indicated that trust and a closer community may increase social pressures to conform.
Interviewees felt that an institution's size can also potentially shape types of research, funding, and hence, possibly, ethical problems that may occur there. One chair, for instance, described his institution as “a small, sleepy” school, which consequently does not have big drug development programs, which can generate problems. Ironically, and perhaps revealingly, he wishes that his school did conduct such research:
We don't have big drug development programs, but wish we did. We don't have the capabilities of coming up with a molecule in a test tube, and then scaling it up, and putting it into humans for the first time, and then pushing for FDA approval. We just don't have the scientific enterprise capacity to do that – investigators with the laboratory back-up to develop gene therapy. Doing that has more opportunities for an ethical misstep. Jesse Gelsinger never could have happened here. That capability gets those researchers in trouble. They're doing great stuff, and move things forward – when these ethical glitches don't get in the way. So, that's the good news and the bad news about us: we are still a small sleepy place. We do some good science, but are not breaking new ground here. So, we don't have the stresses and strains that allow those lapses to happen. IRB14
The respondent felt that the opportunity for financial conflicts of interest might be less as well. “Nobody says, ‘I'm going to develop a new drug, and retire with huge stock options.'” (IRB14) Interviewees often felt that larger institutions may also be better resourced.
Respondents suggested that such a “small town” culture may in turn be self-perpetuating, attracting fewer “high proceed” players. Ecologically, within institutions, processes of mutual selection and implicit social feedback loops may occur:
People here know each other. It's much more of a small town, interpersonal ecology. You can't make enemies here. If you tick off the orthopedic surgeon today, if his daughter's on the field when your daughter's playing field hockey, and your daughter breaks her leg, he's going to be the first one there. So, we are much more interdependent in this small community, off the hospital grounds. That reverberates: we don't have the big institutional scale to attract high-power go-getters. We mostly tend to be here because we like the quality of life. If we wanted to make our jobs the absolutely number one thing in our lives, we'd probably go elsewhere. So, we don't have those strains because of both the interpersonal politics, and the drive that people don't have here. IRB14
Other cultural aspects of institutions can also mold perceptions concerning institutional contexts. Beliefs arose, for instance, that pediatric hospitals, might differ from other institutions due to the types of patients seen, diseases treated, and priorities of staff.
The culture here is due to the fact that we're pediatrics. The type of people that work with children just have a different attitude. They can explain things. There's a different ethics. It shows in a culture of an institution, too. Maybe that's why we don't have so many problems. IRB31
Aspects of an institution can also potentially affect issues of trust that can, in turn, potentially affect IRB reviews of protocols in several ways, and decrease certain tensions.
IRB-Related Characteristics of Institutions
Political support of IRBs
Interviewees felt that institutional leaders range in the degrees to which they understand or fully back the IRB. Chairs felt that institutional leaders needed to “buy into” and “be committed to the culture of compliance,” but varied widely in the extent to which they did so. Respondents thought that the standing and clout of IRBs varied, sometimes as a result of particular leaders or past historical events, which in turn shaped the institutions' relationships with IRBs.
One IRB chair felt well supported because a research ethicist was high up in the university administration,
Our university is very supportive of our IRB. One of the vice presidents is an ethicist, and brings status to our IRB and what we do. So the institution allots money. IRB39
Anecdotally, having an ethicist powerfully placed in an institution may not be the norm. Moreover, in this particular case, in which this institution had experienced difficulties with the Office for Human Research Protections (OHRP), it is unclear which came first – the ethicist high up in the university or the university valuing ethics (perhaps as a result of a scandal).
Yet having an ethicist or IRB leader in a powerful administrative position in the institution is not utterly unique and can provide many direct and indirect benefits to an IRB. An IRB director said that at his previous institution, the IRB had power because the associate dean was the committee's chair, buffering the group from PIs' criticisms:
Each institution has a certain expectation of what its IRBs are supposed to mean and do. At my previous institution, the IRB ruled the roost. Here, it's the exact opposite – it's an uphill battle. There, an associate dean for research who had an iron fist oversaw the IRB, and never really faced any resistance or noise from PIs. The primary difference relates to culture – the perception, related largely to what type of resources the dean gives the IRB. If the dean is a huge advocate and supporter, and gets involved as a buffer for IRB chairs, the IRB gets respect. IRB9
This IRB director highlights how institutional relationships occur against a backdrop of widespread criticism of IRBs. Institutional leaders can thus respond to PIs' criticism of IRBs by protecting and helping the IRB or trying to change it. As a response, leaders can, for instance, provide more resources.
A few years back, no members were paid. Now we compensate our members. They spend time, and put themselves in positions that may not be favorable with some of their colleagues. The support IRBs get from institutions highlights the differences in how they are viewed in different locations. It's a matter of perception and support, which pretty much determines the IRB's standing from institution to institution. IRB9
Because perception of an IRB can be important, some IRBs attempt to enhance these perceptions through “PR work” – sending out newsletters about their work and “helpful hints” to PIs, for example.
Still, these relationships may be mixed and ambivalent. Institutions may themselves be undergoing strains and changes that can affect an IRB. With mergers between hospitals and medical systems, an institution may include several varying entities (e.g., a medical school and various hospitals) that can have different cultures and priorities and be in conflict. Given the multifaceted complexities of such academic medical centers, often consisting of multiple institutional entities, an IRB may have to simultaneously engage with organizations at odds with each other:
The institution is and is not supportive of the IRB. We're about to have a potential blow up. Our hospital system is independent of the medical school. The IRB is now joint, so all the hospital research has to go through the university IRB. But there's a conflict. IRB3
This IRB then has to review research for both institutions, while often not knowing the PIs at the other institutions, which made this interviewee feel uncomfortable. Hospital PIs may be less receptive to input from an IRB with which they otherwise have had no historical, organizational, social, or personal relationship. Interviewees often felt more comfortable when they knew the PI in some way through past protocol reviews or other interactions, facilitating a sense of trust.
Institutions' Financial Support of IRBs: Getting More Resources
A major source of tension concerns the amount of resources institutions give their IRBs, which vary across institutions. Interviewees see these resources as critical, concrete manifestations of the relationship between an IRB and the institution. Chairs face difficulties concerning the extent of funds their IRBs received.
Almost all interviewees felt that they received insufficient support. As one administrator said, “there are never enough staff.” (IRB16) Chairs usually ask for more resources, but compete against other institutional priorities. At times, scandals at one's own or other institutions prompted institutional leaders to support IRBs more via increased resources.
At times, respondents felt the lack of resources, exacerbated by the recent economic downturn, were the major challenge their IRBs faced.
Losing a few million in research funds decreases staffing throughout the institution. There's a hiring freeze. That's the biggest challenge – the lack of resources to support the traditional expectation of growth. IRB9
Several interviewees thought that IRBs at smaller institutions might have more difficulty staying abreast of relevant regulations. One administrator was surprised at the naïveté of questions that she felt small IRBs asked at national meetings:
It's difficult to stay on top of IRB regulations. But at national meetings, it always amazes me that people from IRBs in smaller hospitals or communities will ask questions that to me are really obvious – about IRB composition, how many people they have to have, the difference between the majority vote and a quorum, the differences between IRB determinations. IRB13
Whether the “obvious” questions do indeed all come from individuals at smaller institutions is unclear, but this interviewee's perception that such attendees ask such questions is itself of note.
Trying to get more resources
While recognizing that universities have numerous other competing demands, priorities, and agendas, many chairs nonetheless try to strategize in various ways to obtain additional resources from institutional administrations. One chair had a new incoming dean and was planning to ask for more money:
We have a new dean starting in June, so we are taking advantage of that. We are hoping to negotiate to revert back to the good old days where we had discretionary spending, and we don't have one hand tied behind our backs when we decide to improve. We don't have to go and beg for resources – they're there for us. We do have responsibility for revenue collection. IRB9
IRB chairs recognize their institution's competing demands, but often try to argue that IRB support is crucial not only as a moral value in and of itself, but for pragmatic reasons (to avoid potential mishaps and/or scandals).
I understand the institution has less money than they need for all the things everybody's asking them to do. But clearly, this is crucial. And it's the right thing to do. If things go badly, much is at stake for the institution. It's a time bomb – if you don't have the resources, you can almost predict it's eventually going to happen. That's sad. IRB40
To get more resources, several chairs advocate informing their institutions about lapses and scandals elsewhere.
I advise other IRB chairs about what I call the raindrop effect – they need to keep their institutional officials informed when things happen elsewhere – not telling these officials, “This means we need more resources,” but just sort of raindrop them throughout: “Geez, this went on at that institution. We're trying to figure out how to prevent this here.” IRB31
Respondents felt, however, that ultimately, institutions may be wary of additional expenditures, and appear more interested in protecting themselves than protecting subjects.
Most institutions don't want to spend a lot of money if they don't have to. They tend to do what they have to do – based on regulations or needs to get accredited and maintain accreditation. ccreditation seems to be one of the main drivers – not necessarily the ethics. IRB28
Fears of lawsuits can also impel changes and increased support, but have disadvantages as well as advantages.
Liability drives a lot of issues. Our institution has restructured to become more institutionalized, bureaucratized, and centralized, with unilateral management, removing people that want to question, and handle issues in more of a lawyer fashion. Before that, the administration support wasn't there to adequately review and support IRB functions. IRB28
Yet the institution's direct aim here may be to protect itself more than subjects. The former may lead to the second, but not necessarily as effectively or efficiently as possible.
Limited resources can restrict an IRB's ability to broaden its scope, moving beyond strictly legal or regulatory concerns. One chair wanted to make it an Ethics Office, more than a Compliance Office, and include more education. “Some of that happened; most of it didn't.” (IRB28)
Charging fees?
Increasingly, to obtain additional resources, many IRBs charge industry-sponsored studies fees, as do private, for-profit IRBs. This poses several challenges. Questions emerge of how much to charge and why and where the money does or should go (i.e., to the IRB or the institution). For evaluating protocols, some IRBs charge industry sponsors, for instance, $2,500 for initial reviews, and $500 for continuing reviews.
It used to be collected as a discretionary fund, used as IRBs chose. Now, it's been expropriated by senior officials and absorbed as part of the whole school revenue stream, which has affected our turnaround time. There is less of an incentive for IRBs to decrease their turnaround time if they know they are not going to reap the rewards. IRB9
This expropriation of funds may thus disincentivize IRBs from undertaking improvements.
In addition, these IRBs may then compete either intentionally or unintentionally with private IRBs. Interviewees frequently saw themselves as offering benefits – charging less than for-profit IRBs.
As more companies are going to central IRBs, which charge even more, they're realizing that universities are a good bargain – not faster, but at least not any more expensive. IRB3
This quote may not reflect actual relative costs, but reflects this IRB chair's beliefs.
Direct and Indirect Pressures from Institutions
A few IRB chairs felt direct or indirect pressures to approve research protocols. Occasionally, CEOs or other institutional officials may seek to have particular protocols approved, but this appeared very rare and seemed to have occurred more in the past. In the end, it seemed, institutions tended to demure, and IRBs prevailed.
I've gotten calls from the Dean and the hospital president, who say, “We need to approve this right away: we have 25 people lined up for this surgery, and it needs to be approved.” I say, “Well, the PI didn't fill out the forms,” or, “We can't approve it. I'm happy to work with them, but they need to go through the process. I'm not going to speak for the whole committee.” So, there has been pressure to circumvent things. Generally we resist, and say forget it. They eventually understand. It can take a bit of time. The hospital administrators “get it” the least, because they're just interested in getting the money, and don't understand the whole process and the issues. But they understand administrative process: it's no different from trying to cut a check. It's just new to them as a bureaucratic hurdle. They view it as a barrier or wrinkle to getting studies done. But it's just one of the necessary steps. IRB3
In recent years, some IRBs have also grown in prominence and importance, counterbalancing and diminishing these forces.
More than 10 or 12 years ago, the regime was different here, and there were attempts to force things down the throats of the IRB. But that's long gone history. The regulatory issues have gotten much stricter. Public awareness has increased. IRB4
Nevertheless, at times, some chairs still felt implicit pressure to help their institutions by encouraging research and researchers. Researchers' complaints about these committees can also prompt institutional leaders to side with PIs over IRBs in cases of conflict.
Chairs can respond to these pressures in one of several ways, including resisting rather than succumbing to such forces or in some cases resigning. One administrator said,
I'm trying to do what I think is right, which is going to upset some people. You only hear about the bad cases. Most of it runs quite fine. But chairs are going to still get pressured to get studies approved. Unfortunately, there are politics everywhere. In these situations, chairs may cave in, and change what they do, or get frustrated, or nervous, or say, “I've had enough. I can't work in this!” IRB23
Institutional leaders may also become dissatisfied with, and decide to replace, an IRB chair – in part in response to complaints from researchers. “He had a number of conflicts with the researchers, who started saying, ‘Can't we get a new chair? Isn't there a term limit?’” (IRB35) This chair then stepped down.
IRBs may face questions, too, of whether to allow representatives from the institution to sit in on or participate in IRB meetings, which may allow the institution to influence the IRB in various ways. One chair allowed an institutional official to be involved. Yet these decisions can be controversial, and IRB members and chairs can disagree.
The old-timers on the board say the IRB is a faculty committee, and that the administration shouldn't have any input – that the administration's always the dark side. They were upset that I'm asking an administrator to look at informed consent form. I didn't see the harm in getting input. I make sure the institution's protected. IRB29
Other Factors Involved
Role of scandals at institutions
Interviewees thought that variations between IRBs can stem in part from the fact that some institutions have had “scandals,” and been cited or investigated by federal authorities, increasing sensitivity, attention, and resources devoted to research ethics. Indeed, most IRBs that felt relatively well supported had in fact had major scandals in recent years.
As a result of a federal shutdown of research, one institution more than doubled the number of IRBs and staff and now had regulatory and consent form specialists, a dean for clinical research, and IRB retreats.
We went from two committees to five, and regular members are now paid 10% salary support. Before, nobody was. Now, there's a huge IRB central office with consent form specialists, a regulatory person who's a lawyer, and administrative support. A whole leadership structure was put in place, that didn't exist: a dean for clinical research, retreats once or twice a year. The institution takes it extremely seriously now – making resources available to do this the right way. IRB40
Yet unfortunately it took the shutdown to produce these beneficial changes. Almost all respondents felt that their IRBs were underfunded. Crises alone may generate additional funds.
Getting shut down had an absolutely seismic effect on this institution – all for the positive, I think. After our tragedy, everything got upgraded. Unless there's a crisis, it's very hard to get resources. Trying to get money and support for ethics has not been easy. Before the tragedy, I asked the former dean for money, and was told, “You're doing great work. Keep it up.” No money. Then there was a disaster, and suddenly the coffers opened. Unfortunately, that's the way institutions work. It takes exceptionally strong, smart leadership to identify these things ahead of time. IRB40
Interviewees felt that such scandals can help IRB chairs in negotiating for support. Federal audits can take years, and make both IRBs and PIs anxious, but in the end may compel institutions to fund IRBs more.
I came in the middle of a big federal investigation, and the school was desperate for help in getting out of it. It was pretty bad, but a blessing for me. It gave me leverage to get resources, hire staff, and make changes. IRB18
Institutional leaders vary in whether and to what degree they pay attention to federal shutdowns at other, similar institutions. Crises at one's own institution generally have far more impact.
In addition to increasing resources, such crises can galvanize institutions and IRBs to change in other ways as well. In response to complaints and problems, many IRBs have recently worked on improving or reorganizing themselves to varying degrees – impelled from within or without. For instance, as a result of an audit, one vice chair reported:
With restructuring, we decreased our turnaround time for reviews from six months to six weeks. Before, the IRB “wasn't functioning” – we would just sit on studies. IRB39
Institutional leaders cannot directly dictate IRB decisions, but can replace IRB chairs. New IRB leaders can in turn potentially improve IRB processes, making these more efficient and helping researchers. As one vice chair said about a new chair who had replaced an older, less effective one:
The new chair is very linear and structural: “Here's what the process needs to look like – the steps we need to take.” It is much more conducive to researchers. IRB39
Yet not all IRBs underwent such changes as a result of scandals or audits.
With fewer federal shutdowns of research in the past few years, less money may now go to IRBs and compliance given other competing needs.
It's not that deans don't respect IRBs, but the government is taking money away from NIH to fund the Iraq War. In the absence of resources, the expectation is that the senior administration would come to bat for the IRB. But I haven't seen that. They look at us as a regular department, like the clinical trials or technology transfer office. Money speaks loudly here, and is more important than compliance these days. There is very little money going around. Support is going more towards offices that bring in revenue: the grants office. The IRB is simply compliance. These days, we don't have much noncompliance, because everyone pretty much knows what they should and shouldn't do. Everyone involved in the IRB business knows what needs to be in place to avoid a shutdown. As a result, there are fewer shutdowns, and attention goes to the money-making, revenue-generating groups. That's the problem. IRB9
In part, shutdowns occurred in a different era. For example, well-publicized shutdowns occurred, at Duke in 1999, Virginia Commonwealth University in 2000, and Johns Hopkins in 2001.
Yet IRBs that do not get more resources cannot expand and are instead beholden to the institution's “good graces.” Interviewees often felt that institutions aim implicitly to determine how little they can give the IRB while avoiding federal audits and/or shutdowns.
These days, IRB review fees that we collect from industry-sponsored research – our discretionary funds – have been taken away from many IRBs. Schools have absorbed any revenue that our invoicing brings in. We rely more on direct support from the school, instead of generating revenue from these fees. So, making the judgment call as to whether or not we have the funds to hire another two people largely depends on the good graces of the school, instead of how much revenue we generate, and whether we can afford it. But we need expansion. That's a problem. IRB9
The need for expansion can generate tensions. These pressures may also potentially fuel conflicts of interests (COIs), as IRBs may feel they need to obtain support from their institutions in order to function optimally, and may thus lean toward supporting their institutions and colleagues (in seeking to obtain research grants) more than protecting subjects as much as possible.
Moreover, interviewees felt that even at institutions where scandals have occurred, IRBs may initially receive more resources, but over time this added support slips back down to some degree.
Initially, there was a lot of support: you need this much in staff, this much in office supplies. Before, things weren't quite as forthcoming. Investigators protested about all the training requirements, all these things that they're putting us through. You got to do it. Now, it's not in the forefront. We're certainly, at our institution, light years ahead of what we were in the late 90s. But to a degree, we may have lost some of that edge that we had right after the government shutdown. IRB6
These phenomena can create pressures, even if only indirect and unconscious.
The IRB is supposed to be very autonomous and free from all those pressures. But subliminally, they are still there. Investigators are unhappy about review turnaround times, and complain up the chain, and then the institution says: “Hey you guys have to do better at review times, or this and that.” IRB6
Risk Averseness
Institutions may vary in the degree to which they are risk averse, related in part to other institutional events, past and present. For instance, an institution or close institutional affiliate may have recently had a large debt or investigation, or have gone bankrupt. Yet risks are inevitable in clinical care and research. Since the bankruptcy of an affiliated institution, one medical center has become far more cautious, and the IRB asked the medical center to get an insurance policy. But this committee has not yet received a response to this request.
Since the bankruptcy of an affiliate hospital, our institution has been immensely risk averse. An IRB does its best to protect human subjects, but risks are intrinsic to research. The university ought to live with that, and get an insurance policy. Our “in case of injury” clause says the university will take no responsibility. In the old days, it used to say that in case of injury, they'll cover it. They had an insurance policy. That's appropriate. We've appealed to the administration to change that, and have never even gotten a formal answer. IRB7
Insurance raises other intricate issues that institutions, even if not especially “risk averse,” may need to consider. But this respondent's perceptions regarding these issues are valuable even if he does not examine all of the complexities potentially involved.
Risk and financial adhesiveness may affect multiple aspects of IRB processes and reviews, even informed consent forms. Institutions may request, for instance, that all informed consent forms state that the institution will not provide any care to research participants if they are injured during a study. The IRB may object, but then face conflict.
The “in case of injury” clause changed from one that would cover the cost of care for injury, to one that would not – unless the sponsor covers it, in which case there are alternative clauses. In any research that is internally sponsored, or not fully sponsored, we say there is no coverage in case of injury. That's inappropriate. But the university is set on its policy, and not going to change. So, we face questions of disallowing a study, or using the present language. We have not yet refused to approve a study unless it was changed. IRB7
The federal regulations specify that consent forms should state whether treatment or compensation will be provided in case of injury, and if so, of what it will consist. However, the regulations do not specify the answer to these questions – that is, whether participants will receive treatment or compensation in these cases, and if so, what sort of compensation. IRBs, PIs, and their institutions may disagree in confronting these decisions.
Financial pressures may impede research in other ways as well. For example, PIs often know the areas in which IRBs or institutions are risk averse and may choose not to do certain studies as a result. As the previous interviewee stated:
Investigators in cardiology and oncology knew, if a study looked high risk, they didn't want to do it. (IRB7)
Relationships to other Institutional Offices
Institutions differ in how they configure the IRB in relation to compliance and other regulatory offices, which may then affect IRB functioning. In particular, interactions and boundaries between Compliance Offices and IRBs can vary widely – from close and collaborating to distant, with minimal, if any, communication. These two organizational entities may differ but have overlapping and potentially synergistic roles shaped by complex institutional histories and cultures that can facilitate or impede interactions. This may influence the culture and institutional support for research ethics within an institution.
One IRB chair did not know, for instance, if a PI, whose study the IRB had suspended because he had kept identifying information on samples when he should not have, was still conducting any research. The IRB had reported the problem to institutional officials, but not received any follow-up.
It isn't clear to me: maybe the PI's still doing research. I don't know if he's doing nonhuman research, or working on those samples properly. IRB7
Most chairs felt that their IRB was already over-worked and under-resourced, and hence neither could, nor should, do more. But uncertainties lingered. As the interviewee above continued, “There should be a way to find out what this faculty member was doing. But this is in the Compliance Office's domain.” (IRB7)
To assist researchers more fully, many institutions establish Quality Assurance/Quality Improvement (QA/QI) offices, yet the relationships between these entities and IRBs can also vary and evolve. The two offices may share members, and vary in what, how, and when they communicate.
Separating IRB and QA offices can have potential advantages and disadvantages, though these may not always be clear. A QA division can investigate studies for the IRB. One respondent, both a QA committee member and an IRB chair, said:
If the IRB has a concern about a study, they will ask the QA/QI committee to go in and take a look. But we are independent from the IRB. I don't know why, or whether that's good or bad. Sometimes I think communication would be better if we were a part of the IRB. But the independence means that when researchers ask us to come, our review is very confidential, unless we find something that's reportable to the IRB. IRB11
Internal IRB Characteristics
Effects of more resources
Additional resources can expand an IRB's capabilities, allowing it to have, for example, “consent form specialists,” full-time staff assigned to perform random audits, more in-depth orientations for new members, and community member specialists. Chairs also thought that added resources would allow more frequent and better reviews, audits, and monitoring of studies. At small institutions, an IRB may meet only nine times a year, lengthening delays for PIs.
Wide differences exist in whether IRBs provide any support and training to new members and education for researchers, and if so, the type and extent of this support. Yet many IRB members felt that not all members were equally knowledgeable about relevant issues and that many could benefit from additional training. When IRB conferences occur in other cities, institutions' financial constraints prevent most members from traveling there to attend. Often, only the chair can go, and sometimes even he or she cannot do so, given the costs.
Rarely, relatively well-resourced IRBs may have staff dedicated exclusively to student research, which appears to be helpful.
We have a student research web page, and a student research liaison at the IRB, for students and faculty who do certain kinds of studies. IRB5
Implications
These social and institutional relationships can affect IRBs in many ways. IRB staff can feel demoralized due to limited resources and perceptions that institutions are unsupportive.
Additional resources haven't come in to make up for the additional workload. That demoralizes people who want to work. Chairs are demoralized, because they see the same pattern going forward. IRB9
Overwork can further lower IRB staff morale.
We're all overworked. We're one to one-and-a-half positions less than we should be, so some of the staff take on a larger chunk of the workload. It doesn't help their morale. IRB9
Discussion
These data, the first to probe IRB personnel's perceptions of how they operate within their institutions, highlight how these committees see themselves as interacting with, and being affected by, social and institutional dynamics, relationships, and systems, both directly and indirectly. These views are important as they may potentially serve to justify variations between IRBs at different institutions in ways that may impede multi-site studies and frustrate researchers. Because these attitudes about institutions play critical roles, they need to be appreciated and understood as much as possible.
The data also underscore how IRBs' chairs and members may be affected by COIs, wanting to assist institutions and colleagues (e.g., in trying to get grants) more than protecting study participants as much as possible (Klitzman 2011b). IRB chairs and members may feel implicitly or explicitly, subtly or directly, pressured by their institutions, which produces and exacerbates conflicts. IRBs often try to fight these forces but doing so can be hard, especially since institutions determine how much support IRBs receive. These findings suggest how such forces generate circumstances that may foster COIs and the degree to which they are successfully resisted by IRBs (which depends in part on other factors). Importantly, while IRBs may be seen as making objective, unbiased decisions, these data highlight how these committees participate in complex systems and dynamics that can have various effects. IRBs are situated in dense systems of institutional relationships that can potentially influence the committees' resources, structure, functioning, and possibly decisions. Institutional officials, IRBs, and policymakers should thus be more aware of these phenomena.
Clearly, additional future research is needed to explore more fully when, in what ways, and to what extent perceptions of these institutional factors may affect IRB decisions. IRBs have been shown in the past to be underfunded (Woodward 1999), and the amounts of resources that they receive have been shown to vary (Speckman et al. 2007; Wagner et al. 2003) both between high and low volume IRBs, as well as within these two categories. The present data suggest that these funding differences can result in part from complex and dynamic institutional relationships that are subject to negotiation and fluctuation over time.
Of note, the interviewees' perceptions are important and relevant to institutional culture, but may not be wholly objective accurate statements of the “reality.” For instance, an IRB may see itself as differing from others because its institution is small, specializes in pediatrics, conducts only social-behavioral research, or is a top medical center in NIH funding. Yet these beliefs in institutional differences may be used in attempts to justify unnecessary inter-IRB variations. These committees may differ due to other factors (e.g., whether the chair and/or many members are themselves researchers).
The perception that IRBs are underfunded may result in part from IRB inefficiencies. Some IRBs that complain about underfunding may potentially be able to function with fewer staff. As mentioned above, studies indicate that IRB budgets vary widely without clear consensus of how much support is sufficient (Speckman et al. 2007; Wagner et al. 2003; Woodward 1999).
Institutional IRBs frequently feel that they do an equal or better job than commercial IRBs, but no objective comparative evidence has been published. In part, no consensus exists concerning objective measures of “IRB quality” (e.g., whether “efficiency” should be part of such an assessment).
IRBs' perceptions of their institutional relationships and constraints may also shape how they implement new regulations. A major aim of the 2011 Advance Notice of Proposed Rule-Making (ANPRM) for revisions to the Common Rule is to alter IRBs, in part to reduce inter-IRB variations by establishing more central IRBs (US Department of Health and Human Services 2011). Yet the present data elucidate phenomena that could potentially fuel IRB variations and the implementation of any such regulatory changes and are thus important to explore. Policymakers, IRBs, and PIs should hence be aware of these issues in order to respond to these regulatory changes as best as possible.
Complex feedback loops may exist. Specifically, PIs complain about IRBs to institutions, which may in turn try to pressure and/or alter IRBs (e.g., by replacing the chair). Over time, PI complaints can also prompt federal agencies to alter regulations governing IRBs. Indeed, the ANPRM in part reflects such complaints from researchers (Emanuel and Menikoff 2011; US Department of Health and Human Services 2011). The death of a subject can also lead to attention from the media and the federal government in a way that can force a major investigation and shutdown of an IRB or research at an institution.
Questions emerge of how these complex systems can operate more effectively and efficiently. Presumably, events other than “scandals” should motivate heightened institutional support of IRBs. Unfortunately, perhaps human nature is such that only crises, not appeals to ethical standards by themselves, will impel such improvements. In addition, IRBs appear to often minimize or dismiss feedback that they receive from PIs (e.g., that IRBs are interpreting the regulations in ways that, PIs feel, are overly stringent, impeding research). Yet, arguably, IRBs might benefit from being more responsive to feedback that they receive from PIs.
While systems theory explores how some systems are homeostatic, due to feedback, the present data highlight how IRBs are not fully homeostatic, and the system is not fully self-regulatory. Though PIs provide feedback to IRBs and institutions, it may not always affect these committees, and other, external factors may be involved (e.g., federal agencies and new regulatory guidance).
The feeling that the amount of funding an IRB receives may vary over time based on publicized mishaps or “scandals” would benefit from more thorough and quantitative examination. This belief, if supported by further studies, would raise several concerns (e.g., that levels of such funding may at times thus be reactive rather than proactive). The belief that resources may increase after such a scandal, but subsequently decrease over time, if supported in further research, poses concerns, since scandals may then again occur. The findings also underscore, the importance of questions of how much support IRBs realistically do and should receive (i.e., how institutions should apportion resources between IRBs vs. other competing needs).
These findings are important, too, for improving relationships of IRBs with PIs and institutions, underscoring pressures that IRBs face, of which, anecdotally, PIs often do not appear aware. Many IRBs perceive pressures and constrictions due to the institutions in which they work that may in turn affect how these committees review research protocols. Enhancing explicit awareness and understanding of these institutional contexts may help ameliorate tensions between PIs and IRBs and wariness of PIs toward IRBs.
These interviews highlight key questions concerning IRBs charging fees for protocol reviews, namely who decides (and on what basis) when to charge fees and how much to charge as well as who does or should get the money. Charging fees may create COIs that affect IRB decision-making. Respondents felt that IRBs differ from most clinical departments within a medical center, in terms of not generating revenue and therefore receiving fewer resources.
Given proposed changes in IRB regulations, these data are particularly critical at the present time. How effectively, and in what ways any such changes are implemented, and how IRB processes and decisions will shift, may depend in part on the complex systems in which individual IRBs operate (e.g., levels of resources that an IRB receives, concerns of the larger institution, such as risk averseness or desire for industry funding) and IRB perceptions of these needs and priorities. These phenomena thus need further attention and study, looking at how IRBs, individually and as a group, adapt to changing regulatory, financial, and scientific environments.
Future studies can explore more fully, with larger samples, how IRBs view these tensions, and how much financial support they receive, given the amount of research that each institution conducts. Whether and to what degree more resources enhance the quality of reviews requires further investigation as well. For example, whether the size of an IRB's budget in relation to the number of protocols it reviews is statistically associated with the length of turnaround times, the number of violations of research ethics that occur, or levels of PI frustrations and tensions with IRBs. Yet several of these variables are difficult to assess and may be unknown by IRBs. For instance, IRBs may not fully detect the number of violations that occur since PIs may not in fact do what they say they will do, and may not report when they deviate from an approved protocol. Future research can also investigate whether large and small institutions differ in their policies and procedures for handling financial COIs (e.g., covering stock ownership).
Investigating the nature and quality of relationships that IRBs have with their institutions, PIs, and others is useful as these may affect the amount of financial and political support IRBs receive, the nature and quality of protocol reviews, and the type and quantity of violations of research integrity.
Limitations
This study has several other potential limitations. The response rate was 55%, although prior studies have shown that it is typical among surveys of physicians. Asch et al. (1997) found that of 187 published papers, the mean response rate among physicians was 54%. Baruch's (1999) analysis of published papers found an average response rate of 55.6%. Of medical school faculty on IRBs, 75.5% are physicians (Campbell et al. 2003).
This paper also reports on themes that arose in a study focused on issues concerning research integrity, broadly defined, rather than the local ecologies of IRBs. Hence, these data are hypotheses-generating, shedding light on critical areas that can be explored in future studies. I did not interview the institutional leaders (e.g., research directors, deans, department chairs) and other players potentially involved in the institutional dynamics in which IRBs operate to see how IRBs are viewed by, and interact with these other stakeholders. These interviews also probed experiences and perspectives at a single point in time.
Finally, this study did not include direct observation of IRB decision-making, which could potentially help corroborate interviewees' reports about these meetings. Future research may be difficult in this area. Anecdotally, many IRBs have declined efforts to be studied directly or indirectly, for example, requiring that all IRB members sign informed consent documents for the IRB to be observed or even for the chair to be interviewed.
Conclusion
The results of the present study suggest that IRBs often perceive institutional contexts and relationships as posing critical challenges and tensions that must be negotiated and confronted. As a result, key aspects of IRB functioning and decisions may be affected. Efforts to improve the effectiveness and efficiency of IRBs should be informed by a more detailed understanding of these local ecologies.
Acknowledgments
The author would like to thank Patricia Contino for her assistance with this manuscript.
Funding/Support: The National Institutes of Health (R01-NG04214) and the National Library of Medicine (5-G13-LM009996-02) funded this work.
Appendix A. Sample questions from semi-structured interview*
Do you think IRBs differ in their views or approaches toward research integrity (RI), and if so, when, how, and why? Do you think IRBs apply standards regarding RI differently, and if so, when, how, and why?
What factors do you think affect how IRBs make decisions about RI and other areas? Do institutional factors affect these issues, and if so, how, when, why, and to what degree? What has happened?
What do you think makes an IRB work well or not in monitoring and responding to RI? Do institutional factors play a role, and if so, what, how, when, and to what degree?
Does your IRB encounter tensions with PIs about RI or other related issues? If so, how, when, and why?
What kinds of conflicts, if any, has your IRB faced with your institution? Why? What happened?
Should other regulations or guidelines concerning IRB reviews of RI or other areas be developed, and if so, what?
What do you think could be done to improve interactions with PIs concerning RI and related issues?
Do you have any other thoughts about these issues?
Note: Additional follow-up questions were asked, as appropriate, with each participant.
References
- Asch DA, Jedrziewski MK, Christakis NA. Response rates to mail surveys published in medical journals. Journal of Clinical Epidemiology. 1997;50(10):1129–1136. doi: 10.1016/s0895-4356(97)00126-1. [DOI] [PubMed] [Google Scholar]
- Baruch Y. Response rate in academic studies—A comparative analysis. Human Relations. 1999;52(4):421–438. [Google Scholar]
- Begun JW, Zimmerman B, Dooley K. Health care organizations as complex adaptive systems. In: Mick SM, Wyttenbach M, editors. Advances in health care organization theory. San Francisco: Jossey-Bass; 2003. pp. 253–288. [Google Scholar]
- Campbell EG, Weissman JS, Clarridge B, Yucel R, Causino N, Blumenthal D. Characteristics of medical school faculty members serving on Institutional Review Boards: Results of a national survey. Academic Medicine. 2003;78(8):831–836. doi: 10.1097/00001888-200308000-00019. [DOI] [PubMed] [Google Scholar]
- De Vries R, Anderson MS, Martinson BC. Normal misbehavior: Scientists talk about the ethics of research. The Journal of Empirical Research on Human Research Ethics. 2006;1(1):43–50. doi: 10.1525/jer.2006.1.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziak K, Anderson R, Sevick MA, Weisman CS, Levine DW, Scholle SH. Variations among institutional review boards in a multisite health services research study. Health Services Research. 2005;40(1):279–290. doi: 10.1111/j.1475-6773.2005.00353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuel EJ, Menikoff J. Reforming the regulations governing research with human subjects. The New England Journal of Medicine. 2011 doi: 10.1056/NEJMsb1106942. [DOI] [PubMed] [Google Scholar]
- Emirbayer M. Manifesto for a relational sociology. American Journal of Sociology. 1997;103(2):281–317. [Google Scholar]
- Geertz C. Interpretation of cultures: Selected essays. New York: Basic Books; 1973. [Google Scholar]
- Greene SM, Geiger AM. A review finds that multicenter studies face substantial challenges but strategies exist to achieve Institutional Review Board approval. Journal of Clinical Epidemiology. 2006;59:784–790. doi: 10.1016/j.jclinepi.2005.11.018. [DOI] [PubMed] [Google Scholar]
- Klitzman R. US IRBs confronting research in the developing world. Developing World Bioethics. 2012;12(2):63–73. doi: 10.1111/j.1471-8847.2012.00324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klitzman R. Views and experiences of IRBs concerning research integrity. Journal of Law, Medicine & Ethics. 2011a;39(3):513–528. doi: 10.1111/j.1748-720X.2011.00618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klitzman R. “Members of the same club”: Challenges and decisions faced by US IRBs in identifying and managing conflicts of interest. PLoS ONE. 2011b;6(7):e22796. doi: 10.1371/journal.pone.0022796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klitzman R. How local IRBs view central IRBs in the US. BMC Medical Ethics. 2011c;12(13) doi: 10.1186/1472-6939-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klitzman R. The myth of community differences as the cause of discrepancies between IRBs. The American Journal of Bioethics Primary Research. 2011d;2(2):24–33. doi: 10.1080/21507716.2011.601284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klitzman R. The Ethics police? IRBs' views concerning their power. PLoS ONE. 2011e;6(12):e28773. doi: 10.1371/journal.pone.0028773. Epub 2011 Dec 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klitzman R. “Community” IRB members: Who are they, what do they do, and do they represent anyone? Academic Medicine. doi: 10.1097/ACM.0b013e3182578b54. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson E, Bratts T, Zwanziger J, Stone P. A survey of IRB process in 68 U.S. hospitals. Journal of Nursing Scholarship. 2004;36(3):260–264. doi: 10.1111/j.1547-5069.2004.04047.x. [DOI] [PubMed] [Google Scholar]
- McWilliams R, Hoover-Fong JJ, Hamosh A, Beck S, Beaty T, Cutting G. Problematic variation in local institutional review of a multicenter genetic epidemiology study. Journal of the American Medical Association. 2003;290(3):360–366. doi: 10.1001/jama.290.3.360. [DOI] [PubMed] [Google Scholar]
- Parsons T. The Social System. New York: The Free Press; 1951. [Google Scholar]
- Silbey SS. The sociological citizen: Pragmatic and relational regulation in law and organizations. Regulation & Governance. 2011;5(1):1–13. [Google Scholar]
- Speckman JL, Byrne MM, Gerson J, Getz K, Wangsmo G, Muse CT, Sugarman J. Determining the costs of institutional review boards. IRB: Ethics & Human Research. 2007;29(2):7–13. [PubMed] [Google Scholar]
- US Department of Health and Human Services. Human subjects research protections: enhancing protections for research subjects and reducing burden, delay, and ambiguity for investigators. 2011 http://www.ofr.gov/OFRUpload/OFRData/2011-18792_PI.pdf.
- Von Bertalanffy L. An outline of general system theory. The British Journal for the Philosophy of Science. 1950;1(2):134–165. [Google Scholar]
- Wagner TH, Bhandari A, Chadwick GL, Nelson DK. The cost of operating institutional review boards (IRBs) Academic Medicine. 2003;78(6):638–644. doi: 10.1097/00001888-200306000-00019. [DOI] [PubMed] [Google Scholar]
- Woodward B. Challenges to human subject protections in US medical research. Journal of the American Medical Association. 1999;282(20):1947–1952. doi: 10.1001/jama.282.20.1947. [DOI] [PubMed] [Google Scholar]


