Ensuring an adequate supply of clean water is an urgent global issue. In 1992, the United Nations (UN) designated March 22 of every year as World Water Day (WWD) to increase awareness of the importance of clean water conservation. Also, Population Action International (PAI) has reported a shortage of fresh water in developing and under-developed countries.[1] The demand for clean water will continue to increase due to industrialization and population growth. Thus, the development of technologies that can effectively purify contaminated water has been an emerging area of research.
Adsorption-based technologies have been used to remove a variety of toxic chemicals from contaminated water via batch or continuous flow processes. The carboxyl and amine groups of activated carbon[2] and polysaccharides such as alginate[3] and chitosan[4] are the most widely implemented adsorbents due to their ability to chelate toxic heavy metals.[3a] However, several limitations of existing adsorbents can be identified. First, the attachment of polysaccharides onto solid phases is essential, yet these adsorbents lack inherent adhesive properties to facilitate their immobilization onto substrates. Second, the generation of secondary pollutants during chemical processing of adsorbents is a serious environmental issue. In the case of activated carbon adsorbent, a strongly acidic solution—typically 10 – 50 % (v/v) HNO3—has been used.[5] Third, the variety of toxic chemicals that can be removed by existing adsorbents is limited- they often show excellent performance in the removal of heavy metals but perform poorly in the removal of toxic organic molecules, particularly in the case of polysaccharide adsorbents. Fourth, methods for regenerating adsorbents and isolating adsorbed toxic chemical complexes have not been adequately developed. Finally, the cost of carbon materials is rapidly increasing,[6] a particular concern for developing and resource-poor settings. Thus, novel approaches to overcome the aforementioned limitations, in whole or in part, may lead to improved and more cost-effective water detoxification processes.
Adaptations of chemical and physical principles found in the biological world can offer good alternative approaches to water detoxification. In this case, biological strategies that combine surface adhesion with the ability to isolate, bind and sequester heavy metals and other toxins are of great interest. The proteins comprising the byssal attachments of marine mussels share many of these qualities. The byssal adhesive pads are effective at attaching to substrates, and have been reported to be enriched in various metals (Fe, Mn, Zn, Cu, Ni, etc.).[7] As an indicator of the protein’s metal binding ability, the concentration of iron in dried byssus is approximately a million-fold higher than the typical concentration in seawater.[7] Furthermore, the strong metal binding property of mussel byssus has led to investigations of byssal tissue as a sensitive biomonitoring organ for heavy metals in the marine environment.[8]
Thus, we hypothesize that a mussel-inspired approach combining the substrate adhesion and metal binding affinity of catechol and amine functional groups would be useful to remove toxic metals from contaminated water. Furthermore, quinones formed by catechol oxidation may provide additional capability of removing selected toxic organic compounds, as they are known to react with amines and thiols.[9] In fact, we have demonstrated that this chemical reaction occurs at interfaces at the single-molecule level.[9c]
Polydopamine is a synthetic mimic of mussel adhesive proteins that deposits as a thin (monolayer to 50 nm or more) coating on virtually any material by spontaneous oxidation of dopamine in an alkaline aqueous solution (Scheme 1b).[10] Compared to other methods of coating substrates, polydopamine has the advantage of being inexpensive, adherent, and simple to deposit onto substrates without the need for surface pre-treatment. Polydopamine nano-layers form on virtually any material surface, including noble metals, oxides, semiconductors, ceramics, synthetic polymers, and graphene oxide, as well as on superhydrophobic surfaces.[11] The strength of catechol adhesion on TiO2 and gold surfaces was reported to be stronger than the well-known avidin-biotin interaction at a single-molecule level, explaining the robustness of the polydopamine coating[9c]. The binding force on glass substrates, however, has not been reported, but it has been observed that the coating remained stably under a vigorous mechanical stirring condition (~ 1000 rmp) Also, the catecholamines that do not participate in surface binding can perform a variety of chemical reactions, resulting in water detoxification.
Scheme 1.
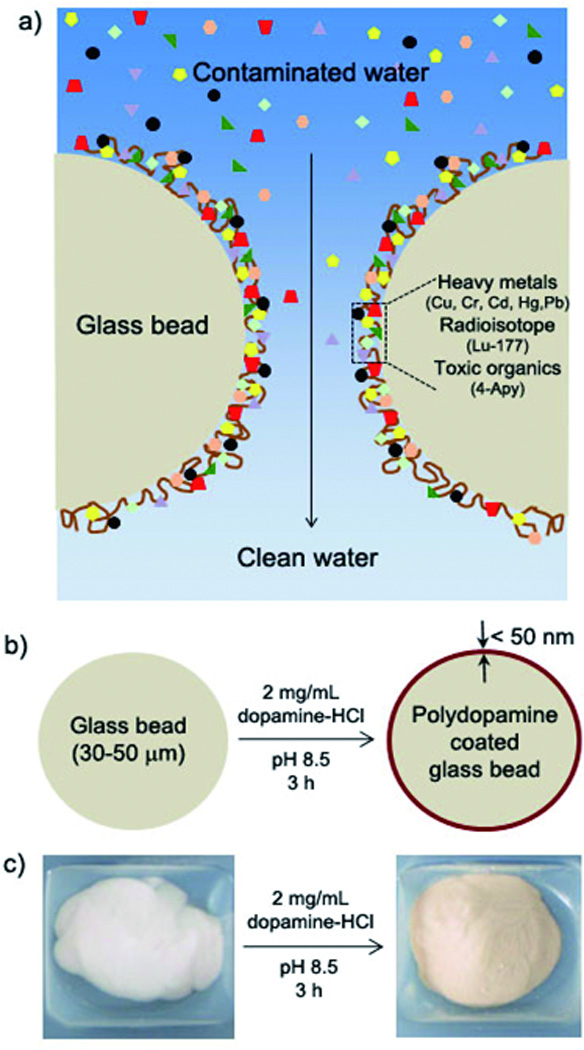
a) A schematic description of detoxification process by polydopamine. Water contaminated by heavy metals, radioisotopes, and organic compounds is passed through a packed column of polydopamine-coated beads. b) A schematic description of polydopamine coating process. c) Photographs of glass beads (left) and polydopamine-coated beads (right).
We chose glass beads in micro size as the material to be functionalized by polydopamine due to its large specific surface area. The amount of polydopamine on the glass beads was estimated by optical waveguide lightmode spectroscopy (OWLS). Under conditions identical to those used in preparation of the polydopamine coated glass beads, we were able to estimate that 360 µg polydopamine was deposited per gram of glass bead. (Figure S1)
In a typical experiment, an aqueous solution containing heavy metal ions was passed through the column (Scheme 1a) and the eluate was analyzed by inductively coupled plasma-optical emission spectroscopy (ICP-OES) to determine the remaining concentration of each metal species. Passage of 10ppm solutions of CrVI, HgII, PbII, CuII, and CdII through a column containing 0.1g of polydopamine glass beads reduced the metal ion concentration below the detection limit of ICP-OES (< 0.05 ppm). The metal binding capacity of the polydopamine beads was determined by continuing the filtration until the unbound ions were detected. The results show that the capacity is comparable to or better than a widely used activated carbon material for all of the toxic metals tested (CuII[12], CrVI[13], HgII[14], CdII[2b] and PbII[15]) as shown in Figure 1a. These results indicate that polydopamine is a promising adsorbent for removal of toxic heavy metal ions.
Figure 1.

a) The toxic metal binding capacity of polydopamine (black, n=3) compared to activated carbon (grey); b) XPS analysis (O1s) of polydopamine before (top) and after (bottom) Cu(II) metal binding; c) XPS analysis (N1s) of polydopamine before (top) and after (bottom) Cr(IV) metal binding.
We used X-ray photoelectron spectroscopy (XPS) to detect the binding of metal ions to polydopamine. In polydopamine, two O1s photoelectron peaks were detected: one from the hydroxyl group of catechol (O-C; 532.6 eV) and the other from the quinone oxygen generated by catechol oxidation (O=C; 530.9 eV) (Figure 1b, upper).[16] Exposure of polydopamine to Cu resulted in an increase in the oxygen 1s core-level binding energies: 533.1 eV for Cu-O-C (0.5 eV increase) and 531.7 eV for Cu-O=C (0.8 eV increase). The binding-energy values of O1s have been reported to increase upon metal binding.[17] However, the lack of change in the binding energy of N1s upon Cu adsorption, as shown in Figure S2a, indicated that nitrogen did not participate in the binding of CuII. However, a similar analysis of XPS binding-energy changes of both O1s and N1s orbitals upon exposure of polydopamine to Cr(IV) revealed similar changes in the O1s binding energy (Figure S2b), as well as a 0.8 eV increase in N1s binding energy (401.4 eV to 402.2 eV) (Figure 1c). Apparently, the nitrogen atom of polydopamine is involved in binding of Cr(IV) but not Cu(II). These results suggest that the site of metal chelation in polydopamine may vary according to the nature of the adsorbed metal ion.
The removal of radioisotopes from water has recently become a critical issue. Common sources of radioisotopes are nuclear power plants and hospitals. As demonstrated during the recent weather-related destruction of nuclear power plants in Japan, radioisotopes can be released into the environment through radioactive water leakage from the reactor core and rain.[18] Also, several radioisotopes have important medical imaging and therapeutic applications and are widely used in hospitals. Tracking and disposal of these and other radioisotopes is important for preserving the safety of the environment, and there are significant concerns related to inadvertent release of these compounds. Thus, a radioisotope adsorbent device is desirable for these reasons. We tested whether polydopamine adsorbent can be used to remove a radioisotope from an aqueous liquid. We chose 177Lu as a model radioisotope because it has been widely used in radiotherapy and imaging.[19] At 10 µCi (1.66 ng of 177Lu), 29.3mg of polydopamine/glass beads removed nearly all (99.5% n = 3) 177Lu in a single-pass filtration (Figure S3). Water containing a higher level of 177Lu radioactivity (2000 µCi) was used to determine removal capacity of polydopamine/glass beads. Approximately 4% of the 177Lu was detected (n=3) (Figure 2a) after a single passage, from which we calculated the removal capacity to be approximately 181,000 mCi/g polydopamine (1920 µCi177Lu/10.6 µg polydopamine). Because the amount for a single dose of 177Lu for medical use is on the order of several tens of pCi,[20] the adsorption capacity of only 30mg of polydopamine/ glass beads would be more than sufficient in practice.
Figure 2.
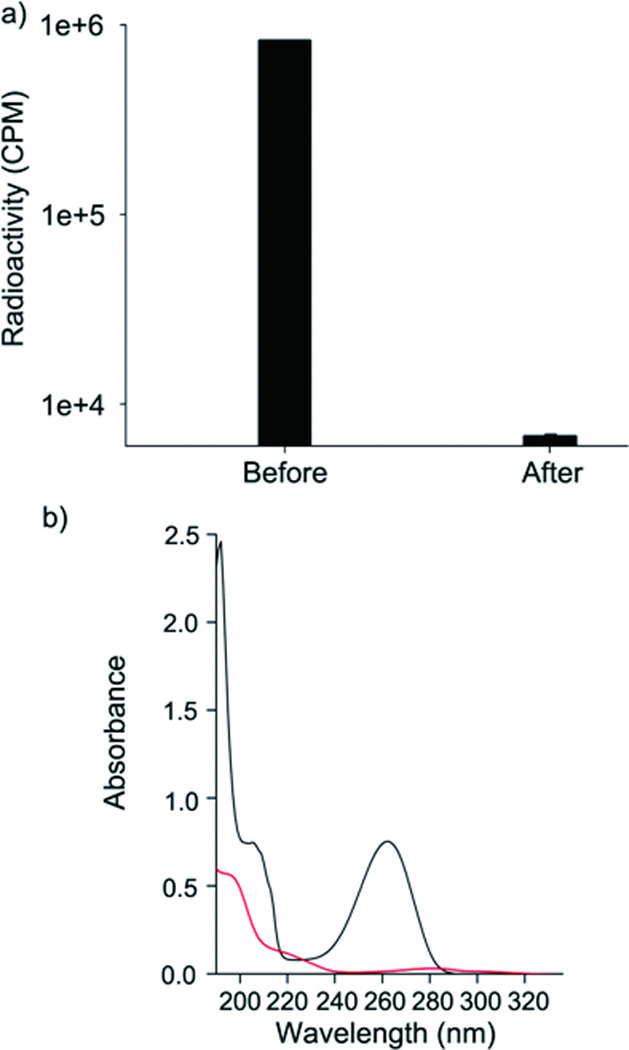
Removal of a radioisotope and a toxic organic molecule by polydopamine/glass beads. a) Scintillation counts of the 177Lu-containing water (2000 µCi) before and after passing through the polydopamine/glass bead column (~ 30 mg) (n=3). b) UV-Vis analysis of 4-Apy before (black) and after (red) filtration.
Unlike widely used polysaccharide adsorbents, the polydopamine adsorbent was also able to remove toxic organic molecules. We chose 4-aminopyridine (4-Apy), an insecticide with broad toxic effects on mammals.[21] The optical property of 4-Apy exhibited a maximum adsorption at 260 nm (Figure 2b, black). When solutions containing 4-Apy (10 µg/mL) were passed through a polydopamine/glass bead column, UV analysis showed the nearly complete disappearance of UV absorption characteristic of 4-Apy (Figure 2b red). The removal capacity was found to be approximately 116 mg of 4-Apy per gram of polydopamine (Figure S4), determined by detecting unbound 4-Apy in the eluted solution by UV-vis spectrophotometry. Fourier-transformed-infrared (FT-IR) spectroscopy experiments using 4-Apy immobilized on the polydopamine adsorbent showed indications of the benzene ring (1531 cm−1 for C-C stretching of an aromatic ring (I); 1199 cm−1 for in-plane C-H bending (II); and 834 cm−1 for out-of-plane C-H bending (III)) and secondary amine (3100–3300 cm−1, IV) (Figure S5), confirming the attachment of 4-Apy.[22,23] The removal mechanism might be covalent binding of 4-Apy onto the polydopamine by Michael addition, or Schiff base formation.[24]
Appropriate methods for regeneration of adsorbent and safe disposal of adsorbent/toxic compound complexes are equally important considerations in the development of new remediation technologies for toxic compounds. Currently, entire adsorbate/adsorbent complexes, such as metal/alginate/substrates or adsorbate/activated carbon are collected for disposal. In contrast, the polydopamine adsorbent exhibits the potential advantage of being able to be regenerated through treatment of polydopamine glass beads with dilute acid or hydrogen peroxide (Figure 3a). The polydopamine layer remained intact after treatment in 10% acetic acid for 2h, as indicated by the persistence of the polydopamine-specific N1s photoelectron peak (398.1 eV) after the acid treatment (Figure 3b, blue). At the same time, the adsorbed toxic metals CuII (Figure 4b, black, Cu2p = 932.6 eV), PbII (red, Pb4f = 138.3 eV), and CdII (blue, Cd3d = 408.0 eV) were undetectable by XPS after treatment with acid, indicating their removal from the coating and regeneration of the polydopamine layer. We found that complete removal of the polydopamine layer by treatment with hydrogen peroxide was necessary for recovery of 4-Apy. The XPS results showed that the polydopamine N1s peak completely disappeared after 1 h of exposure to 30% H2O2. Also, the underlying glass bead surfaces were exposed by this treatment as indicated by the emergence of substrate-specific Si2p (100.1 eV) and Si2s (150.5 eV) peaks (Figure 3c). In this case, the polydopamine coating can be re-applied to the regenerated beads so that a subsequent water detoxification procedure can be performed. The polydopamine coating–removal cycle was repeatable, which we confirmed by measuring the N1s peak between steps of several coating and removal cycles (Figure 3d). Both regeneration methods offer the possibility for reuse of solid supports, which may translate into economic advantages over other technologies.
Figure 3.
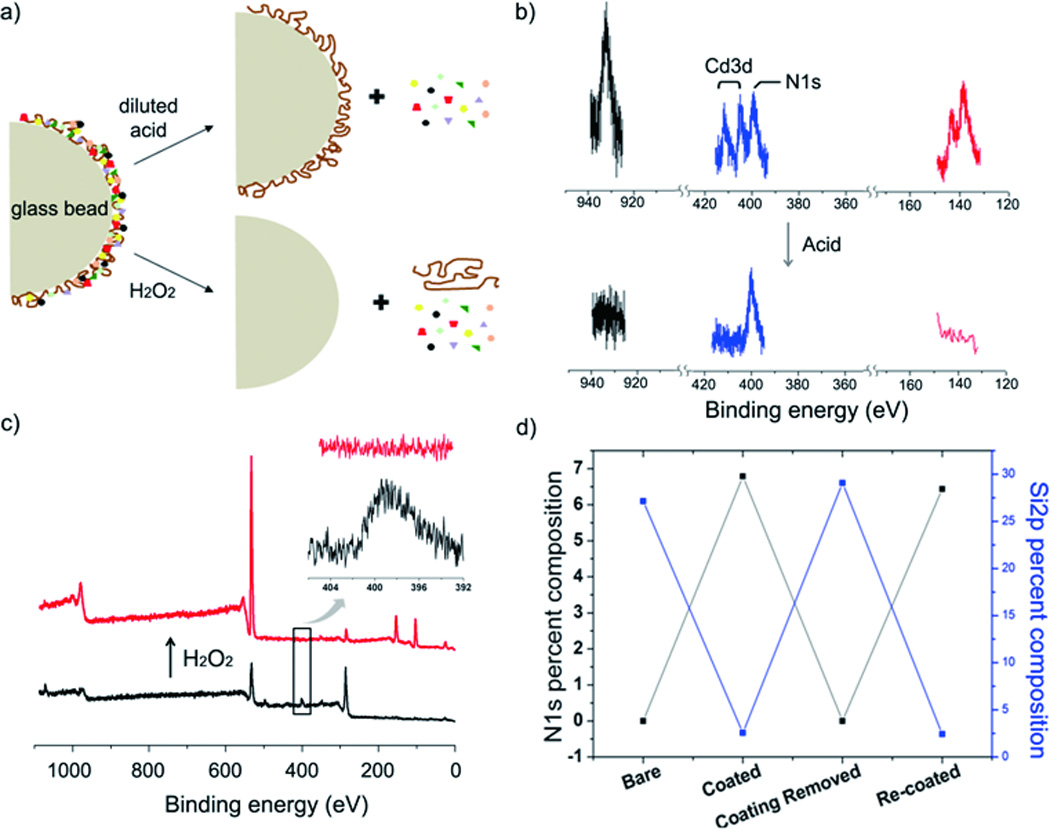
Regeneration of polydopamine adsorbent after exposure to toxic metal. a) Acid treatment (10% acetic acid, v/v) dissociates the bound toxic metals from the polydopamine/metal complexes, whereas treatment with hydrogen peroxide removes adsorbed metal as well as polydopamine. b) XPS analysis before (above) and after (below) acetic acid treatment showed removal of Cu2p (black, 932.6 eV), Cd3d (blue, 408.0 and 411.7 eV), and Pb4f (red, 138.3 eV), however the characteristic N1s peak of polydopamine was preserved (blue, 398.1 eV, after acid treatment) c) Hydrogen peroxide effectively removed the polydopamine coating from glass surface. The disappearance of N1s and re-appearance of Si2p and Si2s demonstrated the polydopamine layer removal. d) Deposition and removal of polydopamine can be repeated, as shown by a plot of N1s and Si2p percent composition as detected by XPS analysis.
In summary, we have described a novel, facile, and scalable method for batch or continuous flow removal of toxic heavy metals and organic compounds from water. The method exploits a simple and versatile approach to coating solid supports with an adherent film of mussel-adhesive-protein inspired polydopamine film that exhibits a high affinity for metal ions and certain organic compounds. The method is inexpensive, scalable, and the solid supports can be easily regenerated by treatment in dilute acid or hydrogen peroxide. To our knowledge this constitutes the first report of polydopamine related to environmental remediation, adding to a rapidly growing list of uses for polydopamine coatings that includes nanotechnology, bio-interfaces, medical devices, polymer science, and energy storage.[9c, 11a, 11c, 25]
Supplementary Material
Acknowledgements
The authors are grateful for financial support: Development of Biomedical Technology Program (2012-0006085, H.L.); Molecular-level Research Center (2012-0000909, H.L.); World Class University (R31-10071, H.L.); The Initiative for Sustainability and Energy at Northwestern (P.B.M.); and National Institutes of Health (R37 DE014193, P.B.M.)
Footnotes
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/cplu.200xxxxxx.
References
- 1.Outlaw TG, Englman R. Population Action International report. Sustaining Water,Easing Scarcity: A Second Update. 1997 [Google Scholar]
- 2.a) Gray NF. Water Technology. 2nd ed. chapter 20. London: Elsevier; 2005. [Google Scholar]; b) Ramos RL, Rangel Mendez JR, Mendoza Barron J, Fuentes Rubio L, Guerrero Coronado RM. Water Sci. Technol. 1997;35:205–211. [Google Scholar]
- 3.a) Davis TA, Volesky B, Mucci A. Water Res. 2003;37:4311–4330. doi: 10.1016/S0043-1354(03)00293-8. [DOI] [PubMed] [Google Scholar]; b) Hamdy AA. Curr.Microbiol. 2000;41:232–238. doi: 10.1007/s002840010126. [DOI] [PubMed] [Google Scholar]
- 4.Huang CP, hung YCC, Liou MR. J.Hazard.Mater. 1996;45:265–277. [Google Scholar]
- 5.Leyva-Ramos R, Diaz-Flores PE, Aragon-Pina A, Mendoza-Barron J, Guerrero-Coronado RM. Sep.Sci.Tech. 2005;40:2079–2094. [Google Scholar]
- 6.Babel S, Kurniawan TA. J.Hazard. Mater. 2003;97:219–243. doi: 10.1016/s0304-3894(02)00263-7. [DOI] [PubMed] [Google Scholar]
- 7.a) Fowler SW. Mar. Environ. Res. 1990;29:1–64. [Google Scholar]; b) Szefer P, Frelek K, Szefer K, Lee CB, Kim BS, Warzocha J, Zdrojewska I, Ciesielski T. Environ. Pollut. 2002;120:423–444. doi: 10.1016/s0269-7491(02)00111-2. [DOI] [PubMed] [Google Scholar]
- 8.Yap CK, Ismail A, Tan SG. Environ. Intl. 2003;29:521–528. doi: 10.1016/S0160-4120(03)00008-4. [DOI] [PubMed] [Google Scholar]
- 9.a) Mason HS, Peterson EW. Biochim. Biophys. Acta. 1965;3:134–146. doi: 10.1016/0304-4165(65)90479-4. [DOI] [PubMed] [Google Scholar]; b) Bindoli A, Rigobello MP, Deeble DJ. Free radicals Biol. 1992;13:391–405. doi: 10.1016/0891-5849(92)90182-g. [DOI] [PubMed] [Google Scholar]; c) Lee H, Scherer NF, Messersmith PB. Proc. Natl. Acad. Sci. U. S. A. 2006;103:12999–13003. doi: 10.1073/pnas.0605552103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.a) Lee H, Dellatore SM, Miller WM, Messersmith PB. Science. 2007;318:426–430. doi: 10.1126/science.1147241. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Kang SM, Hwang NS, Yeom J, Park SY, Messersmith PB, Choi IS, Langer R, Anderson DG, Lee H. Adv. Func. Mater. 2012 doi: 10.1002/adfm.201200177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.a) Kang SM, You I, Cho WK, Shon HK, Lee TG, Choi IS, Karp JM, Lee H. Angew. Chem. Int. Ed. 2010;49:9401–9404. doi: 10.1002/anie.201004693. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Kang SM, Park SY, Kim D, Rouff RS, Lee H. Adv. Funct. Mater. 2010;21:108–112. [Google Scholar]; c) Ham HO, Liu Z, Lee H, Messersmith PB. Angew. Chem. 2011;123:758–762. doi: 10.1002/anie.201005001. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Ye W, Wang D, Zhang H, Zhou F, Liu W. Electrochim. Acta. 2010;55:2004–2009. [Google Scholar]; e) Ou J, Wang J, Liu S, Zhou J, Yang S. J. Phys. Chem. C. 2009;113:20429–20434. [Google Scholar]; f) Liao Y, Cao B, Wang WC, Zhang L, Wu D, Jin R. Appl. Surf. Sci. 2009;255:8207–8212. [Google Scholar]
- 12.Shim JW, Park SJ, Ryu SK. Carbon. 2001;39:1635–1642. [Google Scholar]
- 13.Hamadi NK, Chen XD, Farid MM, Lu MGQ. Chem. Eng. J. 2001;84:95–105. [Google Scholar]
- 14.Babi BM, Milonji SK, Plovina MJ, Upi S, Kaludjerovi BV. Carbon. 2002;40:1109–1115. [Google Scholar]
- 15.Reed BE, Arunachalam SJ. Environ. Eng. 1994;120:416–436. [Google Scholar]
- 16.a) Fu R, Yoshizawa N, Dresselhaus MS, Dresselhaus G, Satcher JH, Baumann TF. Langmuir. 2002;18:10100–10104. [Google Scholar]; b) Cuesta A, Martinez-Alonso A, Tascon JMD, Bradley RH. Carbon. 1997;35:967–976. [Google Scholar]
- 17.a) Tait SL, Wang Y, Costantini G, Lin N, Baraldi A, Esch F, Petaccia L, Lizzit S, Kern K. J. Am. Chem. Soc. 2008;130:2108–2113. doi: 10.1021/ja0778186. [DOI] [PubMed] [Google Scholar]; b) Shen C, Hill IG, Kahn A, Schwartz J. J. Am. Chem. Soc. 2000;122:5391–5392. [Google Scholar]
- 18.a) Nicoll Alexander. IISS-Strategic Comments: The fallout from Fukushima. 2011;17 [Google Scholar]; b) Greenfield SM. J.Atmos.Sci. 1957;14:115–125. [Google Scholar]
- 19.a) Liu S. Adv. Drug Delivery Rev. 2008;60:1347–1370. doi: 10.1016/j.addr.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Ehrhardt GJ, Ketring AR, Ayers LM. Appl. Radiat. Isot. 1998;49:295–297. doi: 10.1016/s0969-8043(97)00038-9. [DOI] [PubMed] [Google Scholar]
- 20.Meredith Ruby F, Partridge Edward E, Alvarez Ronald D, Khazaeli MB, Plott G, Russell Charles D, Wheeler RH, Liu T, Grizzle WE, Schlom J, LoBuglio AF. J. Nucl. Med. 1996;37:1491–1496. [PubMed] [Google Scholar]
- 21.Schafer Edward W, R J, Brunton Ronald B, Cunningham Donald J. Toxicol. Appl. Pharm. 1973;26:532–538. doi: 10.1016/0041-008x(73)90291-3. [DOI] [PubMed] [Google Scholar]
- 22.a) Lambert JB, Shurvell HF, Lightner DA, Cooks RG. Organic structural spectroscopy. New Jersey: Prentice Hall, Inc.; 1998. pp. 175–196. [Google Scholar]; b) Heacock RA, Marion L. Can. J. Chem. 1956;24:1782–1795. [Google Scholar]
- 23.a) Akyuz S, Akyuz T, Davies JED. J. Struct. Chem. 1999;40:796–801. [Google Scholar]; b) Buyukmurat Y, Akyuz S. J. Mol. Struct. 2003;651–653:533–539. [Google Scholar]
- 24.a) Cavalieri EL, Li K-M, Saeed NBM, Devanesan P, Higginbotham S, Zhao J, Gross ML, Rogan EG. Carcinogenesis. 2002;23:1071–1077. doi: 10.1093/carcin/23.6.1071. [DOI] [PubMed] [Google Scholar]; b) Burzio LA, Waite JH. Biochemistry. 2000;39:11147–11153. doi: 10.1021/bi0002434. [DOI] [PubMed] [Google Scholar]
- 25.a) Ku SH, Park CB. Biomaterials. 2010;31:943. doi: 10.1016/j.biomaterials.2010.08.071. [DOI] [PubMed] [Google Scholar]; b) Ryou MH, Lee YM, Park JK, Choi JW. Adv.Mater. 2011;23:3066–3070. doi: 10.1002/adma.201100303. [DOI] [PubMed] [Google Scholar]; c) Hong S, Kim KY, Hwang JW, Park SY, Lee KD, Lee DY, Lee H. Nanomedicine. 2011;6:793–801. doi: 10.2217/nnm.11.76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


