Abstract
Background and Aims
Graft survival in HCV (hepatitis C virus) infected recipients is worse than those transplanted for other liver diseases. We studied whether several donor cardiovascular risk factors (including advanced age, smoking, hypertension, and diabetes mellitus) contribute to worse outcomes for HCV positive and HCV negative liver transplant recipients.
Methods
We obtained data from the United Network for Organ Sharing on all adult liver transplants performed in the United States between January 1, 1998 and December 31, 2003. In total, 27,033 transplant cases were evaluated. Independent predictors of graft survival were determined using Cox proportional hazards regression analysis after controlling for factors previously found to be associated with differences in transplant outcomes.
Results
Donor diabetes was a strong independent risk factor for graft failure [hazard ratio (HR) = 1.20, p = 0.006] only in HCV positive recipients. Neither donor smoking status nor hypertension predicted graft loss in either cohort. Consistent with previous studies, advanced donor age, donation after cardiac death, height, and African American donor all predicted graft loss amongst both cohorts.
Conclusion
Accounting for donor diabetes in relation to recipient HCV status in the selection of liver recipients may result in improved graft survival.
Keywords: Liver transplantation, Hepatitis C, Liver donor, Diabetes mellitus type II
Introduction
End stage liver disease due to HCV-induced cirrhosis is the leading indication for liver transplantation in the United States and Western Europe [1]. Unfortunately, long-term graft and patient survival in HCV-infected recipients is lower than that in recipients transplanted for other underlying liver diseases [2]. Several mechanisms likely contribute to this observation, including disease recurrence and subsequent rapid fibrosis progression. HCV recurs post transplantation in 95 % of patients. HCV-induced hepatitis occurs in 90 % of patients, and HCV-induced cirrhosis occurs in 30 % of patients by five to seven years after transplantation [2]. Long term graft survival at five years was 10 % lower for patients with HCV infection as compared to recipients without HCV infection after orthotopic liver transplantation [3].
Since HCV-infected transplant recipients may be particularly vulnerable to graft failure, our study aims to elucidate some of the donor factors that can adversely affect graft survival in HCV positive recipients. In particular, given the high prevalence of cardiovascular disease in the United States and Western Europe, we retrospectively investigated whether donor cardiovascular risk factors such as hypertension, diabetes, older donor age and smoking contribute to worse outcomes for patients who underwent liver transplantation for HCV cirrhosis compared to those transplanted for other indications. Insufficient donor data exists to evaluate the contribution of hyperlipidemia.
Methods
Records of all adult liver transplants performed in the United States between January 1, 1998 and December 31, 2003 were obtained from United Network for Organ Sharing (UNOS) Standard Transplant Analysis and Research files. This time period was chosen to capture the post-MELD era, yet allow for adequate follow-up duration (10 years) after transplant. These files contain one record per transplant event, along with data from the most recent follow-up visit for each patient. Data are collected by each transplant center and transmitted to UNOS at the time of registration, time of transplant, 6 months after transplant, and annually thereafter.
The following exclusion criteria were applied: those with unknown hepatitis C status, multi-organ transplant, living donors, and patients with other viral hepatitis co-infection. HIV co-infected patients were not excluded due to a very small number of such patients. The hepatitis C cohort was defined as those patients with documented detection of an antibody to HCV (anti-HCV) and a diagnosis of either type C cirrhosis or chronic or acute hepatitis C. The HCV-negative cohort consisted of patients with documented negative anti-HCV and a diagnosis other than type C cirrhosis, or alcoholic cirrhosis with hepatitis C.
The primary outcome measure was recipient death or the need for re-transplantation. The dataset did not make it possible to ascertain exact cause of recipient death or need for re-transplantation. Overall survival was computed with the Kaplan–Meier estimator. Comparisons of survival between groups were performed with the log-rank test. Cox proportional hazards regression modeling was used to determine independent predictors of graft survival following transplantation. To build the model, we first performed univariate analysis for donor variables shown to be significant in previous studies to impact graft survival [2], including the seven donor factors of Feng et al’s Donor Risk Index [4]. The donor variables were: donor age, height, African American ethnicity, gender, cold ischemia time, warm ischemia time, Cr, INR, bilirubin, albumin. BMI donor data was not available from the database. We also included four cardiovascular risk factors available in the database: donor age, hypertension, diabetes, and smoking. Hyperlipidemia is a known cardiovascular risk factor that could not be included due to lack of sufficient data. Hypertension and diabetes were determined by a “yes/no” question posed by the transplant center.
We included in our Cox regression analysis those variables shown to be significant predictors (p < 0.05) of outcome in our univariate analysis. For the regression analyses, observations that contained a missing value for any predictor were excluded.
Results
Of the 27,033 adult liver transplants listed in the UNOS database between January 1998 and December 2003, 20,702 met inclusion criteria. Of these, 9587 (46 %) were HCV positive and 11,115 (54 %) were HCV negative. Baseline demographic and transplant characteristics are largely similar, as shown in Table 1. Overall, the HCV positive cohort had a lower MELD score at the time of transplantation, but slightly older donors (39.1 vs 38.9). As expected, the HCV positive cohort had a higher proportion of men. The median follow-up for the HCV positive group was 4.4 years, and 4.9 years for the HCV negative group.
Table 1.
Baseline characteristics
| Variablea | HCV positive | HCV negative | p value |
|---|---|---|---|
| N | 9,587 | 11,115 | – |
| Percent male | 73.2 | 58.0 | < 0.0001 |
| Percent African American | 7.6 | 8.0 | 0.31 |
| Age, years | 50.8 | 50.8 | 0.31 |
| Donor age, years | 39.1 | 38.9 | 0.30 |
| Cold ischemia time, hours | 8.1 | 8.0 | 0.31 |
| Warm ischemia time, min | 44 | 43 | 0.0023 |
| BMI, kg/m2 | 28.4 | 27.6 | < 0.0001 |
| Serum creatinine, mg/dl | 1.26 | 1.36 | < 0.0001 |
| INR | 1.81 | 1.99 | < 0.0001 |
| Serum bilirubin, mg/dl | 5.82 | 8.38 | < 0.0001 |
| Serum albumin, mg/dl | 2.80 | 2.90 | < 0.0001 |
| Median follow-up, years | 4.35 | 4.94 | < 0.0001 |
Mean values for all variables other than follow-up, which is a median value
Figure 1 shows the Kaplan–Meier survival curves for HCV positive versus HCV negative transplant recipients. Consistent with previous studies, survival was lower for the HCV positive cohort than the HCV negative cohort. This difference first became apparent 9 months after transplantation and continued throughout the study period.
Fig. 1.
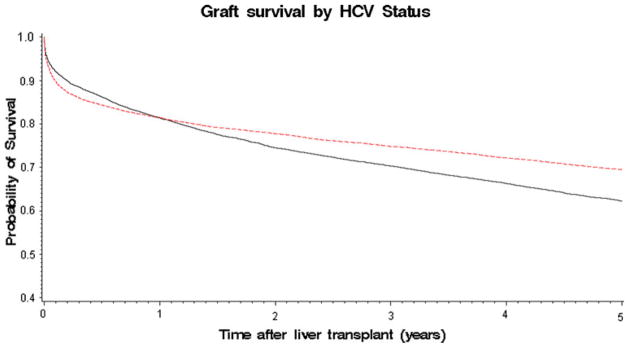
Kaplan Meier survival curves for hepatitis C virus (HCV) positive (solid line) versus HCV negative (dotted line) transplant recipients. Consistent with previous studies, survival was lower for the HCV positive cohort than the HCV negative cohort
In the univariate analysis (not shown), the presence of donor diabetes appeared to impart a survival disadvantage among both the HCV positive group and the HCV negative group (HR = 1.42, p < 0.0001 for HCV positive; HR = 1.27, p = 0.0006 for HCV negative). All variables shown to be significant predictors (p < 0.05) of outcome in our univariate analysis were then included in the Cox multivariate regression model except non-cardiac cause of donor death due to missing data.
Cox proportional hazards regression modeling showed that having a graft from a donor with diabetes was a strong independent risk factor for graft failure (Table 2, Fig. 2) with hazard ratio (HR) = 1.20, p = 0.006 in the HCV positive transplant recipients. However, this association was not seen in the HCV negative cohort (Table 3). In terms of other cardiovascular risk factors, there was no deleterious effect of either donor smoking or hypertension seen for either cohort (Tables 2 and 3).
Table 2.
Donor risk factors for graft failure in hepatitis C virus (HCV) positive cohort
| Parameter | Parameter estimate | Hazard ratio | p value |
|---|---|---|---|
| Donor diabetes | 0.181 | 1.20 | 0.0056 |
| Donor age (1 year increment) | 0.014 | 1.02 | < 0.0001 |
| Donor smoker | −0.041 | 0.96 | 0.2230 |
| Donor hypertension | −0.044 | 0.96 | 0.2881 |
| Split liver transplant | 0.321 | 1.38 | 0.5781 |
| Donation after cardiac death | 0.349 | 1.42 | 0.0089 |
| Donor height (1 cm increment) | −0.009 | 0.99 | < 0.0001 |
| African American donor | 0.006 | 1.006 | 0.0139 |
Fig. 2.
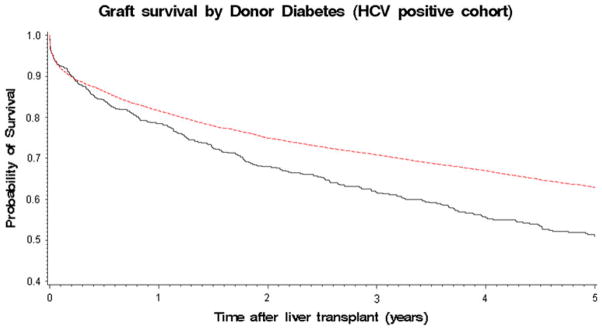
Solid line donor with diabetes. Dotted line donor without diabetes
Table 3.
Donor risk factors for graft failure in hepatitis C virus (HCV) negative cohort
| Parameter | Parameter estimate | Hazard ratio | p value |
|---|---|---|---|
| Donor diabetes | 0.0873 | 1.09 | 0.2153 |
| Donor age (1 year increment) | 0.0072 | 1.01 | < 0.0001 |
| Donor smoker | −0.0169 | 0.98 | 0.6259 |
| Donor hypertension | 0.0710 | 1.07 | 0.0971 |
| Split liver transplant | 0.8686 | 2.39 | 0.0092 |
| Donation after cardiac death | 0.5291 | 1.70 | < 0.0001 |
| Donor height (1 cm increment) | −0.0045 | 0.99 | 0.0002 |
| African American donor | 0.2433 | 1.28 | < 0.0001 |
Consistent with the donor risk index of Feng et al., donation after cardiac death was found to be a significant predictor of poor outcome in both HCV positive and negative cohorts (Tables 2 and 3). Also consistent with previous studies, the donor variables of older age, less height and African American ethnicity were independent risk factors for graft failure in both cohorts (Tables 2 and 3). Split liver transplants appear to be a strong independent predictor of poor outcome only among HCV negative recipients (Table 3).
Discussion
Our study reveals a novel finding that HCV positive transplant recipients have worse graft survival if their donor has diabetes. Feng et al’s donor risk index is one of the most widely known studies that identifies donor risk factors for poor orthotropic liver transplant outcomes. Feng’s study identified seven donor characteristics that are significantly and independently associated with increased failure of deceased donor liver transplants: Donor age over 40 years [and particularly over 60 years], donation after cardiac death (DCD), African-American race, less height, split/partial grafts, cerebrovascular accident and “other” causes of brain death were significantly associated with graft failure [4]. Our results concur in showing that advanced donor age, donation after cardiac death, less height, and African American ethnicity are significantly and independently associated with worse graft survival in both HCV positive and negative cohorts. In our study, split/partial grafts were strongly associated with graft failure in the HCV negative cohort only. Our study differs from Feng’s study in that the donor risk index did not distinguish between HCV positive and negative recipients. This lack of distinction in recipient HCV status may have masked the detrimental effect of donor diabetes on HCV positive liver transplant recipients. This may explain why diabetes in the donor was not identified as a significant risk factor previously in the donor risk index of Feng et al.
Few studies have addressed the impact of donor diabetes on liver transplant outcomes and even fewer on recipients with HCV. Many studies have associated Diabetes Mellitus Type II (DMII) with hepatic steatosis, a benign form of non-alcoholic fatty liver disease (NAFLD) [5]. Growing evidence suggests that NAFLD patients with diabetes are more likely to progress to non-alcoholic steatohepatitis (NASH) than NAFLD patients without diabetes [6]. NASH is a damaging form of NAFLD that leads to fibrosis and cirrhosis, resulting in poor graft function [7]. The worse outcomes of patients with DMII and NAFLD may be explained by chronic inflammation, oxidative stress [8, 9], and the up-regulation of hepatotoxic cytokines [7]. Studies have shown that grafts with moderate hepatic steatosis (>30 %) accelerate the progression of HCV disease and should not be used for HCV patients with high MELD scores [10], and another study found that moderate steatosis in combination with prolonged ischemic time resulted in worse transplant outcomes in HCV recipients [2, 11]. The association of worse graft outcomes in HCV positive recipients with diabetic donor livers seen in our study may be attributable to pre-existing graft steatosis and fibrosis induced by diabetes in the donor, which is further exacerbated by post-transplant HCV recurrence with subsequent fibrosis.
There were several limitations to our study. Non-cardiac cause of donor death, which was a significant predictor of graft failure in the donor risk index of Feng et al. was not included in our model due to lack of data (missing for 98 % of our transplants). Insufficient donor graft data existed to assess for steatosis or fibrosis since donor biopsies were not routinely performed. The primary outcome measure was recipient death or the need for re-transplantation, but the dataset did not make it possible to ascertain exact cause of recipient death, which may not have been liver-related. There were no data available regarding patients with HCV antibody who lack HCV RNA. The definition of diabetes in donors was determined by the transplant center and thus may have lacked uniformity. Duration of diabetes and treatment information was not available, although it is unlikely that there would be bias in these regards in liver allocation between HCV positive and negative recipients.
Conclusions
In conclusion, this study suggests that donor diabetes negatively impacts graft survival in HCV positive recipients. Since HCV-infected transplant recipients may be particularly vulnerable to graft failure, accounting for donor diabetes concurrent with HCV status in the selection of liver recipients may result in improved graft survival.
Acknowledgments
This work was also supported by the Health Resources and Services Administration (contract 234-2005-370011C). There was no pharmaceutical and industry support. The funding source was Stanford University Gastroenterology Division.
Footnotes
Conflict of interest None.
Contributor Information
Ying Wu, Email: yingny@gmail.com, Division of Gastroenterology, Massachusetts General Hospital, 55 Fruit St, Blake 4, Boston, MA 02114, USA.
Aijaz Ahmed, Division of Gastroenterology and Hepatology, Stanford, University, Stanford, CA, USA.
Ahmad Kamal, Division of Gastroenterology and Hepatology, Stanford, University, Stanford, CA, USADivision of Gastroenterology, Santa Clara Valley Medical, Center, San Jose, CA, USA.
References
- 1.Verna EC, Brown RS., Jr Hepatitis C virus and liver transplantation. Clin Liver Dis. 2006;10(4):919. doi: 10.1016/j.cld.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 2.Berenguer M. Risk of extended criteria donors in hepatitis C virus positive recipients. Liver Transpl. 2008;14:S45–S50. doi: 10.1002/lt.21617. [DOI] [PubMed] [Google Scholar]
- 3.Forman LM, Lewis JD, Berlin JA, et al. The association between hepatitis C infection and survival after orthotopic liver transplantation. Gastroenterology. 2002;122:889. doi: 10.1053/gast.2002.32418. [DOI] [PubMed] [Google Scholar]
- 4.Feng S, Goodrich NP, Bragg-Gresham JL, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6:783–790. doi: 10.1111/j.1600-6143.2006.01242.x. [DOI] [PubMed] [Google Scholar]
- 5.Kotronen A, Juurinen L, Tiikkainen M, Vehkavaara S, Yki–järvinen H. Increased liver fat, impaired insulin clearance, and hepatic and adipose tissue insulin resistance in Type 2 diabetes. Gastroenterology. 2008;135:122–130. doi: 10.1053/j.gastro.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 6.Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999;306:1356–1362. doi: 10.1002/hep.510300604. [DOI] [PubMed] [Google Scholar]
- 7.Mulhall BP, Ong JP, Younossi ZM. Non-alcoholic fatty liver disease: an overview. J Gastroenterol Hepatol. 2002;17:1136–1143. doi: 10.1046/j.1440-1746.2002.02881.x. [DOI] [PubMed] [Google Scholar]
- 8.Evan JL, Goldfine ID, Maddux BA, Grudsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev. 2002;23:599–622. doi: 10.1210/er.2001-0039. [DOI] [PubMed] [Google Scholar]
- 9.Prolisso G, D’Amore A, Volpe C, et al. Evidence for a relationship between oxidative stress and insulin action in noninsulin dependent [type II] diabetic patients. Metabolism. 1994;43:1426–1429. doi: 10.1016/0026-0495(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 10.Nocito A, El-Bardy AM, Clavien PA. When is steatosis too much for transplantation? J Hepatol. 2006;45:494–499. doi: 10.1016/j.jhep.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 11.Briceno J, Ciria R, Pleguezuelo M, et al. Contribution of marginal donors to liver transplantation for hepatitis C virus infection. Transpl Proc. 2007;39:2297–2299. doi: 10.1016/j.transproceed.2007.07.069. [DOI] [PubMed] [Google Scholar]


