Preface
Lymphocyte function is regulated by a network of ion channels and transporters in the plasma membrane of T and B cells. They modulate the cytoplasmic concentrations of diverse cations such as calcium, magnesium and zinc, which function as second messengers to regulate critical lymphocyte effector functions including cytokine production, differentiation and cytotoxicity. The repertoire of ion conducting proteins includes calcium release-activated calcium (CRAC) channels, P2X receptors, transient receptor potential (TRP) channels, potassium channels as well as magnesium and zinc transporters. This review discusses the roles of several ions channels and transporters in lymphocyte function and immunity.
Ion channels and transporters function as gateways for charged ions that cannot freely diffuse across lipid membrane barriers. They regulate the intracellular concentration of a variety of ions such as calcium (Ca2+), magnesium (Mg2+) or zinc (Zn2+). The movement of these cations across the plasma membrane depends on electrical gradients that are maintained in turn by potassium (K+), sodium (Na+) and chloride (Cl−) channels. In the past couple of years, fundamental progress has been made towards identifying the molecules that control the function of CRAC channels (the predominant antigen receptor-activated calcium channels in lymphocytes) and channels that mediate magnesium and zinc influx in T cells. We discuss the mechanisms regulating the function of these ion channels in lymphocytes and review their roles in immunity and their emerging potential for therapeutic immunomodulation.
Several other ion channels, pumps and organelles are also required for the regulation of ion homeostasis in lymphocytes. For example, transient increases in the intracellular Ca2+ concentration are mediated by the release of Ca2+ from endoplasmic reticulum (ER) stores via Ca2+ permeable inositol 1,4,5 triphosphate (InsP3) receptor and ryanodine receptor (RyR) channels. Conversely, Ca2+ is cleared from the cytoplasm by uptake into mitochondria and the ER via sarco/endoplasmic reticulum Ca2+ ATPases (SERCA) and Ca2+ export through plasma membrane Ca2+ ATPases (PMCA). Due to space limitations, these intracellular ion channels and transporters are not discussed here.
Store-operated Ca2+ channels
Ca2+ is a well-established second messenger in lymphocytes regulating proliferation, gene expression, motility and other functions. Similar to other mammalian cell types, the intracellular Ca2+ concentration, or [Ca2+]i, in unstimulated T and B cells is kept at ~ 50–100 nM, which is ~ 104-fold lower than the [Ca2+] in the serum. Following antigen binding to the T cell receptor (TCR) or B cell receptor (BCR), [Ca2+]i can rise to ~ 1 μM 1. Several ion channels have been identified in lymphocytes that mediate Ca2+ influx 1 (Fig. 1, Table 1). In the following sections, we discuss Ca2+ release-activated Ca2+ (CRAC) channels, P2X purinoreceptor channels, transient receptor potential (TRP) channels and voltage-gated Ca2+ (Cav) channels.
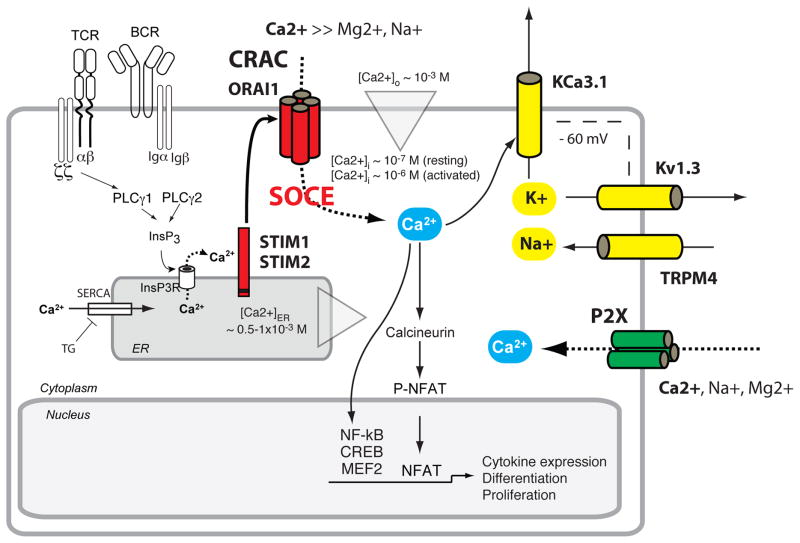
Figure 1. Ion channels regulating Ca2+ signalling in lymphocytes.
CRAC channels are activated following antigen receptor (TCR, BCR) engagement, which results in the activation of phospholipase Cγ (PLCγ), production of inositol 1,4,5 triphosphate (InsP3) and release of Ca2+ from ER Ca2+ stores 1, 6, 17. The ensuing activation of STIM1 and STIM2 results in the opening of ORAI1 CRAC channels and store-operated Ca2+ entry (SOCE) (for details see Fig. 2, 3). Sustained Ca2+ influx through CRAC channels leads to the activation of Ca2+-dependent enzymes and transcription factors, including calcineurin and NFAT. P2X receptors such as P2X4 and P2X7 are non-selective Ca2+ channels activated by extracellular ATP originating, for instance, from autocrine ATP release through pannexin hemichannels (Panx1) 52. Ca2+ influx in lymphocytes depends on the gradient between Ca2+ concentrations in the extracellular (~ 1 mM) and intracellular (~ 0.1 μM) compartments and on an electrical gradient established by two K+ channels, Kv1.3 and KCa3.1, and the Na+-permeable channel TRPM4 76, 92. Abbreviations: SERCA, sarco/endoplasmic reticulum Ca2+ ATPase.
Table 1. Properties and functions of ion channels and transporters in lymphocytes.
This table includes the majority of ion channels and transporters reported to be functional or expressed in lymphocytes. Some molecules such as CRAC channels and K+ channels are well-studied and widely recognized to mediate important roles in lymphocycte function. By contrast, our understanding of the properties and roles of other channels (including TRPC, Cav and Cl− channels as well as Zn2+ transporters) is still in infancy and requires further clarification. Nevertheless, the data to date illustrate that lymphocytes use a diverse set of ion transport mechanisms to fine-tune the overall immune response.
| Channel | Selectivity | Activation | Function in lymphocytes | Associated channelopathies |
|---|---|---|---|---|
| Calcium | ||||
| CRAC | ||||
| ORAI1 | Ca2+ | Antigen receptor stimulation, depletion of ER Ca2+ stores by IP3, activation of STIM1 and STIM2. | T, B and NK cells in vitro: proliferation, cytokine production, cytotoxicity. In vivo: immunity to infection, T cell-mediated autoimmunity and inflammation, allogeneic T cell responses; Treg development. | CRAC channelopathy with immuno-deficiency and autoimmunity caused by mutations in STIM1 and ORAI1. |
| ORAI2, ORAI3 | Ca2+ | (see ORAI1) | t.b.d. | |
| TRP | ||||
| TRPC | Ca2+, Na+ | t.b.d. | t.b.d. | |
| TRPM2 | Ca2+, Na+ | ADPR, cADRP, H2O2, NAADP | t.b.d. | |
| TRPM4 | Na+ | Intracellular Ca2+ | Depolarization of Vm. Cytokine production. | |
| Cav | ||||
| Cav1.2, 1.3, 1.4 | Ca2+ | Activation mechanism following TCR stimulation unknown. Activation not mediated by depolarization. Cav function inhibited by STIM1. | Cytokine production, CD8 T cell survival, CD8 T cell immunity to infection, Th2 function in asthma. | |
| P2X | ||||
| P2X7 | Ca2+, Na+, other | Extracellular ATP | T cell proliferation, cytokine production, promotes Th17 and inhibits Treg differentiation. | |
| P2X1,4 | Ca2+, Na+ | Extracellular ATP | T cell proliferation, cytokine production; thymocyte apoptosis. | |
| Magnesium | ||||
| TRPM7 | Ni2+ > Zn2+ > Mg2+, Ca2+ | Upstream cellular activation mechanism unknown. Regulators include intracellular Mg2+, PIP2 and extracellular pH | Thymocyte development, production of thymocyte growth factors. Proliferation and survival of DT40 B cells. | |
| MagT1 | Mg2+ | TCR stimulation. Activation mechanism unknown. | CD4+ T cell development and activation. Immunity to infection (EBV). | XMEN syndrome caused by X-linked mutations in MAGT1. |
| Zinc | ||||
| ZIP3, ZIP6, ZIP8 | Zn2+ permeable | Activation mechanism unknown. TCR stimulation (ZIP6). | T cell activation (ZIP6). T cell development (ZIP3)? | Acrodermatitis enteropathica with immunodeficiency caused by mutations in intestinal ZIP4 transporter. |
| ZnT | Zn2+ permeable | t.b.d. | t.b.d. | |
| Potassium | ||||
| Kv1.3 | K+ | Membrane depolarization | Regulation of Vm. T cell activation (Th17, TEM), cytokine production, T cell-mediated autoimmunity and inflammation. | |
| KCa3.1 | K+ | Intracellular Ca2+ | Hyperpolarization of Vm. T cell activation (Th1, Th2, TCM), cytokine production, autoimmune colitis. | |
| Chloride | ||||
| Clswell | Cl− (I−, Br−) | Molecular identity of channel unknown. Cell swelling activates Clswell currents. | Apoptosis in T cells. | |
| CFTR | Cl− | cAMP | Cytokine production by T cells ? | |
| GABAA | Cl− | Extracellular GABA | Inhibition of T cell proliferation, cytokine production, cytotoxicity and T cell-mediated autoimmunity. |
Abbreviations: ADPR, ADP ribose; cADPR, cyclic ADP ribose; cAMP, cyclic adenosine monophosphate; Cav, voltage-gated Ca2+ channel; CFTR, Cystic fibrosis transmembrane conductance regulator; CRAC, Ca2+ release activated Ca2+ channel; GABA, γ-aminobutyric acid; Kv, voltage-gated K+ channel; KCa, Ca2+ gated K+ channel; MagT, Mg2+ transporter; NAADP, nicotinic acid adenine dinucleotide phosphate; PLC, phospholipase C; STIM, stromal interaction molecule; t.b.d., to be determined; TRP, transient receptor potential; Vm, membrane potential; XMEN, X-linked immunodeficiency with Mg2+ defect and EBV infection and neoplasia; ZIP, Zrt-Irt like protein; ZnT, Zinc transporter.
CRAC channels
Antigen binding by the TCR and BCR is coupled – via protein tyrosine kinases – to the activation of PLCγ1 in T cells and PLCγ2 in B cells and the generation of the lipid metabolite InsP3. InsP3 promotes the release of Ca2+ from ER stores, resulting in Ca2+ influx across the plasma membrane, a process termed store-operated Ca2+ entry (SOCE) (Fig. 1,2) 2. The store-operated Ca2+ (SOC) channels of T cells, known as CRAC channels, have been extensively characterized 3, 4 and are distinguished by an extremely high selectivity for Ca2+ and low conductance 5 (Table 1). CRAC channels are activated through the binding of the ER Ca2+ sensors, stromal interaction molecule 1 (STIM1) and STIM2 to the CRAC channel proteins, ORAI1-ORAI3 (also known as CRACM1-CRACM3) 6.
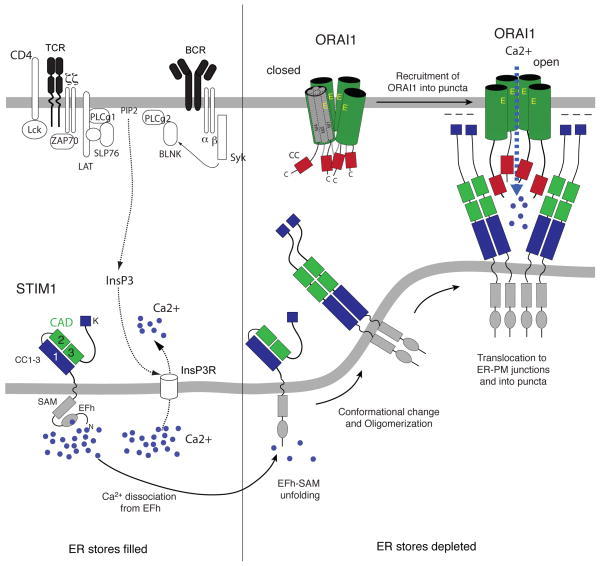
Figure 2. The molecular choreography of CRAC channel activation.
In resting lymphocytes, ER Ca2+ stores are filled with Ca2+ bound to the EF hand Ca2+ binding domain in the N-terminus of STIM1. Antigen receptor stimulation causes the activation TCR/BCR-proximal signalling cascades and the production of InsP3, resulting in the release of Ca2+ from the ER through InsP3 receptors, which are non-selective ion channels. The fall in ER Ca2+ concentration leads to the dissociation of Ca2+ from the EF hand domain in STIM1, unfolding of the STIM1 N-terminus and the multimerization of STIM1 proteins 6. STIM1 multimers translocate to junctional ER sites in which the ER membrane is juxtaposed to the plasma membrane. STIM1 multimers form large clusters (or puncta) into which they recruit ORAI1 CRAC channels. A minimal CRAC channel activation domain (variously referred to as the CAD, SOAR, OASF or CCb9 domain) in the C terminus of STIM1 (green boxes) is necessary and sufficient for ORAI1 binding, CRAC channel activation, and SOCE 29, 184, 186, 187. This domain contains two coiled (CC) domains, which interact with a CC domain in the C-terminus (red boxes) and additional domains in the N-terminus (not shown) of ORAI1 27. Abbreviations: SAM, sterile-alpha motif.
Identification of ORAI1 proteins
An important milestone in the identification of ORAI1 as the prototypic CRAC channel was the discovery that human patients with a severe form of combined immunodeficiency (CID) lack functional CRAC channels and SOCE in T cells 7–11. ORAI1 (or CRACM1) was identified nearly simultaneously by three laboratories as the gene encoding the CRAC channel by linkage analysis in CID patients and RNAi screens for regulators of SOCE and NFAT function 12–14. ORAI1 is a widely expressed surface glycoprotein with four predicted transmembrane domains, intracellular N- and C- termini (Fig. 2, Box 1), and no sequence homology to other ion channels. CID arises from a single point mutation in ORAI1 (R91W) that abrogates CRAC channel activity 12. All three ORAI isoforms (ORAI1-3) form Ca2+ channels with broadly similar functional properties when ectopically expressed, although they differ in their inactivation characteristics, pharmacological properties and tissue expression 15, 16. ORAI1 remains the best-studied CRAC channel homologue and appears to be the predominant isoform mediating SOCE in lymphocytes 6, 17. By contrast, there is no direct functional or genetic evidence for a role of ORAI2 and ORAI3 channels in immune cells yet.
Box 1. Molecular structure of the CRAC channel components ORAI1 and STIM1.
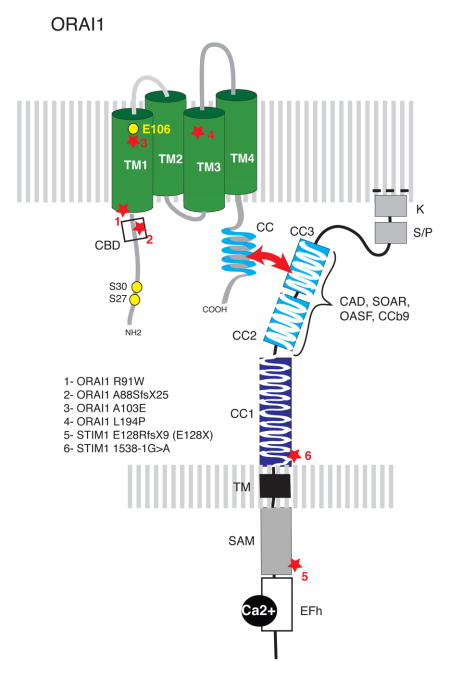
ORAI1 is localized in the plasma membrane and constitutes the pore-forming subunit of the CRAC channel. The channels is formed by assembly of four ORAI1 subunits 6, of which the first transmembrane domain (TM1) line the channel pore 178, 179. The selectivity filter of the CRAC channel is formed by a quartet of glutamate (E) 106 residues that form a high-affinity Ca2+ binding site to furnish the CRAC channel with high Ca2+ selectivity 180–183. Analysis of other pore-lining residues in TM1 indicate that the CRAC channel pore is narrow 178, 179, potentially explaining its low conductance (i.e. a small number of Ca2+ ions passing through it), which limits the increase in [Ca2+]i following channel opening. The intracellular C-terminus of ORAI1 features a coiled-domain (CC) domain that comprises a STIM1 binding site. The ORAI1 N-terminus contains a calmodulin binding domain (CBD) and two inhibitory phosphorylation sites (S27, S30) 184, 185. STIM1 and STIM2 (not shown) are single-pass membrane proteins in the ER. Their N-terminus is located in the lumen of the ER and contains an EF-hand Ca2+ binding domain that allows them to sense the ER Ca2+ concentration. Mutations in the EF-hand domain of STIM1 result in impaired Ca2+ binding and constitutive CRAC channel activation independently of ER Ca2+ store depletion 18, 21. The second and third coiled-coil domains (CC2, CC3) in the C-terminus of STIM1 are part of a minimal CRAC channel activation domain (CAD, also called SOAR, OASF, CCb9 29, 184, 186, 187), which binds directly to ORAI1 to activate CRAC channels. Autosomal recessive mutations in ORAI1 and STIM1 genes identified in patients with CRAC channelopathy are indicated by stars 12, 35–38. These mutations abolish CRAC channel function and SOCE, either by eliminating channel function (1) or by abolishing ORAI1 and STIM1 protein expression (2–6). SAM, sterile alpha motif.
Activation of CRAC channels
Activation of ORAI CRAC channels involves a complex series of coordinated steps during which STIM proteins fulfill two critical roles: first, they sense the depletion of ER Ca2+ stores, and second, they communicate store depletion to CRAC channels 18–20 (Fig .2). In resting cells with replete Ca2+ stores, STIM proteins are diffusely distributed throughout the ER membrane 18, 21. Upon depletion of Ca2+ stores, STIM1 is activated, oligomerizes and redistributes into discrete puncta located in junctional ER sites that are in close proximity to the plasma membrane 22–25. In these puncta, STIM1 co-localizes with and interacts directly with the C- and N-termini of ORAI1 to activate CRAC channels 26. The formation of overlapping STIM1-ORAI1 puncta involves direct binding of a cytoplasmic domain of STIM1 to the C- and N-termini of ORAI1 27–29. Lymphocytes express two closely related STIM isoforms, STIM1 and STIM2, and both mediate SOCE in T and B cells 30, 31. Like STIM1, STIM2 also binds to and activates ORAI1-CRAC channels, but it does so upon smaller decreases in [Ca2+]ER and with slower kinetics compared to STIM1 32, 33. This and the higher expression levels of STIM1 compared to STIM2 in naive mouse T cells may explain why STIM2-deficient T cells have initially normal Ca2+ levels after TCR stimulation but fail to sustain Ca2+ influx. By contrast, STIM1-deficient T cells display a near-complete lack of SOCE 31.
Control of lymphocyte function by CRAC channels
Genetic studies in patients with mutations in ORAI1 or STIM1 genes and in mice lacking functional Orai1, Stim1 and/or Stim2 genes have established important and non-redundant roles for CRAC channels in lymphocytes and other immune cells (reviewed in 34). Autosomal recessive mutations in human ORAI1 (12q24) and STIM1 (11p15) abolish CRAC channel function and Ca2+ influx in T cells, B cells and NK cells, resulting in CID with increased susceptibility to severe infections with viruses (especially herpes viruses 35, 36), bacteria and fungal pathogens (Candida albicans 36, 37) (Box 1, Table 1). The combination of CID with associated non-immunological clinical symptoms is referred to as CRAC channelopathy 38, 39.
CD4+ and CD8+ T cells from ORAI1- and STIM1-deficient patients and mice show defective production of many cytokines including interleukin-2 (IL-2), IL-4, interferon-γ (IFNγ), tumour necrosis factor (TNF)α and IL-17 7, 40, which is partly due to the impaired activation of the Ca2+-dependent transcription factor nuclear factor of activated T cells (NFAT) 7, 31. SOCE-deficient human T cells also fail to proliferate in response to TCR or mitogen stimulation 8, 10, 36, 37. This dependence of lymphocyte effector functions on SOCE is not limited to T cells. NK cells from an ORAI1-deficient patient showed impaired production of IFNγ, TNFα and CC-chemokine ligand 2 (CCL2) and failed to degranulate and kill tumour target cells 41. B cells of mice lacking ORAI1 or STIM1/STIM2 exhibit diminished BCR-induced (but not anti-CD40- or LPS-dependent) proliferative responses 30, 42. SOCE is also required for the production of IL-10, especially by CD1dhi CD5+ regulatory B cells. Impaired expression of this anti-inflammatory cytokine in mice with B cell-specific deletion of Stim1 and Stim2 genes was associated with exacerbated autoimmune CNS inflammation in the EAE model of multiple sclerosis 30. By contrast, CRAC channels do not play a major role in antibody production despite profoundly impaired SOCE in B cells from ORAI1- and STIM1-deficient patients and mice. Serum immunoglobulin levels in patients are not reduced, and T-dependent and T-independent antibody responses following immunization were normal in Stim1−/− 43 and Stim1fl/fl Stim2fl/fl Mb1Cre mice 30. It is noteworthy however that in some ORAI1- and STIM1-deficient patients titers of specific antibodies against the recall antigens diphtheria and tetanus toxoid (DT) were absent 44. Collectively, these studies emphasize the critical importance of CRAC channels for T cell- (and to a lesser degree B cell) mediated immunity.
Immunopathologies resulting from CRAC channel deficiencies
CRAC channels in T cells are not only critical for host defense to infection, but also for T cell-mediated hypersensitivity, allotransplant rejection and autoimmune inflammation. CD4+ T cell-dependent skin contact hypersensitivity responses are abolished in ORAI1-deficient mice and these mice also failed to efficiently reject MHC-mismatched skin allografts 45. Likewise, CD4+ T cells from Stim1−/− mice exhibit slower and attenuated acute graft versus host disease (GvHD) compared to wildtype T cells when transferred to fully allogeneic mice 43. When investigated for their ability to mediate autoimmunity in animal models of multiple sclerosis (experimental autoimmune encephalomyelitis, EAE) and inflammatory bowel disease (IBD), CRAC-deficient T cells from ORAI1-, STIM1- and STIM2-deficient mice failed to induce disease 45–47. Th1 and Th17 cells are critical mediators of inflammation in these models as in their human disease counterparts 48, 49. Accordingly, IFNγ and IL-17 production by CRAC-deficient T cells isolated from central nervous system (CNS)-draining lymph nodes and mesenteric lymph nodes of mice was severely impaired, indicating that SOCE is required for the function of Th1 and Th17 cells 45–47. Disease severity correlated with residual SOCE in T cells as STIM1-deficient mice were completely protected from developing EAE, whereas mice lacking STIM2 showed either delayed onset 47 or reduced severity of disease 46.
In humans, immunodeficiency in STIM1-deficient (and to a lesser degree ORAI-deficient) patients is associated with autoimmunity characterized by hemolytic anemia, thrombocytopenia and lymphoproliferative disease. Autoimmunity is most likely due to reduced numbers of CD25+ Foxp3+ regulatory T (TReg) cells found in these patients 37. A more profound reduction in TReg numbers is observed in the thymus and secondary lymphoid organs of mice with combined T cell-specific deletion of Stim1 and Stim2 31. STIM1/2-deficient Treg cells in addition show severely impaired suppressive function 31. Accordingly, these mice develop massive splenomegaly, lymphadenopathy and pulmonary inflammation. The dependence of TReg cell development and function on SOCE is likely explained by the Ca2+-dependent activation of NFAT and its role in FOXP3 expression 50, 51. By contrast, the development and suppressive function of TReg cells from ORAI1- and STIM1-deficient mice were only moderately impaired 43, 45, indicating that residual Ca2+ influx, likely mediated by ORAI2/3 and STIM2 respectively, is sufficient for TReg cell development and function. Taken together, CRAC channels emerge as important regulators of T cell-dependent self-tolerance and autoimmunity.
Other Ca2+ permeable ion channels
P2X purinoreceptor channels
P2X receptors are a family of non-selective ion channels (Fig. 3) that are activated by extracellular ATP and allow the influx of Na+, Ca2+ and other cations (reviewed in 52). At least three different P2X receptors have been implicated in Ca2+ influx in human T cells: P2X1, P2X4 53 and P2X7 54. Their opening, especially that of P2X7, causes Ca2+ influx and activation of downstream signalling molecules such as calcineurin, resulting in the proliferation of T and B cells 55, 56 and IL-2 production 53, 57. RNAi-mediated depletion of P2X1, P2X4 and P2X7 or their pharmacological inhibition with P2X receptor antagonists results in impaired Ca2+ influx, NFAT activation and IL-2 production following TCR stimulation in Jurkat T cells and human CD4+ T cells 53, 54. Potential sources of ATP required for P2X receptor activation include T cells themselves, which are reported to release ATP in an autocrine manner through pannexin-1 hemichannels that colocalize with P2X7 receptors at the immunological synapse (IS) 53, 58 (Fig. 3). It has been suggested that autocrine ATP signalling in T cells via P2X receptors serves to amplify weak TCR signals, gene expression and T cell effector functions 52.
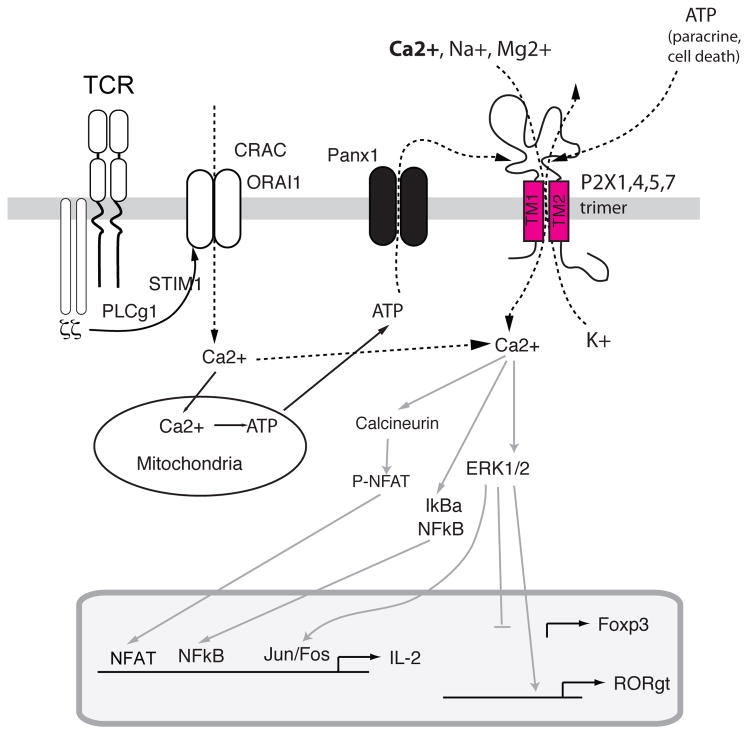
Figure 3. P2X receptors are non-selective Ca2+ channels mediating T cell activation.
P2X receptors are homotrimeric ion channels located in the plasma membrane of lymphocytes. They form non-selective ion channels that allow influx of Ca2+, Na+ and other cations 52, 55. P2X1, P2X4 and P2X7 are activated by extracellular ATP, for which they have distinct affinities 52. P2X7 is unusual among P2X receptors, as it functions as a non-selective cation channel at low extracellular [ATP], but forms large pores following prolonged exposure to high extracellular [ATP]. In addition, it was reported to mediate K+ efflux required for NLRP3 inflammasome activation in innate immune cells 188. The ATP required for P2X receptor opening in T cells originates from dying cells, ATP secreting cells (paracrine) or T cells themselves (autocrine). T cells were shown to release ATP through pannexin 1 hemichannels following TCR stimulation and mitochondrial ATP production 58. Opening of P2X receptors results in Ca2+ influx that has been suggested to synergizes with SOCE to activate Ca2+ dependent signalling molecules and transcription factors resulting in enhanced cytokine expression. P2X7 dependent ERK1/2 activation was shown to repress FOXP3 transcription in favor of RORγt expression, thereby promoting the differentiation of CD4 T cells into Th17 cells 59.
Several lines of evidence suggest that P2X receptors regulate T cell immune responses in vivo. Inhibition of all P2X receptors with oxidized ATP (oATP) protects mice from diabetes following adoptive transfer of pancreatic β-cell-specific T cells and colitis in an adoptive T cell transfer model of IBD 58. Protection was associated with impaired production of IL-17, IFNγ and TNFα, suggesting that P2X7 signalling is required for the function of pro-inflammatory T cells 58. Further analysis revealed that P2X7 also controls the function of CD4+ CD25+ TReg cells. Stimulation of TReg cells with the P2X7 agonist BzATP inhibited expression of FOXP3 but enhanced the levels of the Th17 cell-specific transcription factor RORγt 59. A similar ATP-dependent conversion into Th17 cells was not observed in TReg cells from P2x7−/− mice. P2X7 signalling in T cells therefore appears to be pro-inflammatory by mediating the differentiation and function of Th17 cells and by inhibiting the stability of TReg cells 59. The effects of P2X7 on adaptive immune responses are not always this unambiguous as several studies using P2x7−/− mice have alternatively shown an increased 60 or decreased 61 susceptibility to autoimmune CNS inflammation in the EAE model of multiple sclerosis. The cause of these discrepancies is not known. Future studies will need to carefully address which P2X receptors contribute to the influx of Ca2+ and other cations in T cells at physiological ATP concentrations and and which P2X receptors regulate adaptive immune responses in vivo, taking advantage of the various P2X receptor knock-out mice that have been generated 62–64.
Voltage-gated Ca2+ (CaV) channels
CaV channels are highly Ca2+ selective channels that mediate Ca2+ influx in response to depolarization of excitable cells such as myocytes, cardiomyocytes and neurons 65. All members of the L-type family of CaV channels (CaV1.1, 1.2, 1.3 and 1.4) and their regulatory β3 and β4 subunits were found to be expressed in human and mouse T cells with several studies reporting the presence of truncated or alternatively spliced CaV isoforms 66–68. Recent genetic studies in mice have implicated Cav channels in T cell function. CD4+ and CD8+ T cells from mice with mutations in β3 and β4 subunits had partially reduced Ca2+ influx in response to TCR stimulation and impaired IL-4, IFNγ and TNFα production 66, 69. Impaired Ca2+ influx in β3-deficient CD8+ T cells was associated with absent CaV1.4 protein expression 69. Likewise, naive CD4+ and CD8+ T cells from CaV1.4-deficient (Cacna1f−/−) mice had impaired TCR-induced Ca2+ influx. Cacna1f−/− mice failed to mount an effective T cell response to infection with Listeria monocytogenes 70 that was associated with reduced cytotoxic function of CD8+ T cells 70. CaV1.4-deficient T cells also showed enhanced cell death 70 consistent with a role of the β3 subunit in CD8+ T cell survival reported previously 69. In addition to CaV1.4, the RNAi-mediated depletion of CaV1.2 and CaV1.3 in T cells reduced TCR-induced Ca2+ influx in Th2 cells, attenuated IL-4 production and reduced airway inflammation in mouse model of allergic asthma 71.
Despite these intriguing findings, the role of Cav channels in lymphocytes remains highly controversial. A major gap in our understanding of the role of L-type CaV channels in lymphocytes is whether they function as Ca2+ channels or facilitate Ca2+ influx by other mechanisms. Cell depolarization, the canonical mechanism to activate Cav channels, fails to evoke typical CaV channel currents in the hands of most investigators. Although it is theoretically possible that Cav channels in T cells are activated by other, depolarization-independent pathways, this remains speculative. Complicating the picture further are recent studies that report the inhibition of CaV channels by STIM1 72, 73, raising the possibility that the function of CRAC and CaV channels might be reciprocally regulated. Evidence against a significant role of Cav channels in T cells comes from loss-of-function mutations in CaV channels in human patients, which are not associated with an overt immunological phenotype 74 and pharmacological inhibitors of L-type Cav channels such as nifedipine and verapamil, which are in wide clinical use for cardiovascular diseases, yet have no reported effects on immune function. In the absence of a thorough validation of Cav channel currents by patch-clamp measurements and a molecular mechanism of Cav channel activation in T cells, the effects of genetic deletion of Cav channel components on T cell function and immune responses remain difficult to interpret.
Channels controlling membrane potential and Ca2+ influx
Ca2+ influx in lymphocytes is dependent on a negative membrane potential (Vm) that provides the electrical driving force for Ca2+ entry 75. Two classes of channels regulate Vm in lymphocytes: K+ channels and TRPM4 channels.
K+ channels
K+ channels protect against membrane depolarization by mediating the efflux of K+ to hyperpolarize the plasma membrane 76. The best-studied K+ channels that predominately regulate Vm in lymphocytes are the voltage-activated K+ channel Kv1.3 and the Ca2+-activated K+ channel KCa3.1 (or KCNN4, IKCa2+ and SK4). Kv1.3 is a homotetramer of four α-subunits, each composed of six transmembrane segments (S1–S6) and is activated by membrane depolarization 77. Depolarization of the membrane potential is sensed by four arginine residues localized in the S4 segment, which results in a conformational change causing channel opening 78. KCa3.1, by contrast, is a Ca2+-activated K+ channel that has a similar membrane topology and pore architecture to Kv1.3. However, rather than containing a voltage sensor, the carboxy-terminus of KCa3.1 is constitutively bound to calmodulin and channel opening occurs after Ca2+ binding to calmodulin 79. KCa3.1 channels powerfully hyperpolarize the membrane following [Ca2+]i elevations and help to sustain the driving force for Ca2+ entry. In addition to Ca2+, KCa3.1 channel activity depends on the class II phosphatidylinositol 3-kinase (PI3K) PI3K-C2β, which increases the plasma membrane PI(3)P concentration. This allows the histidine kinase nucleoside diphosphate kinase B (NDPK-B, also known as nm23H2) to activate KCa3.1 by phosphorylating histidine 358 in the C-terminus of KCa3.1 80, 81. In agreement with the finding that both PI(3)P and histidine phosphorylation of KCa3.1 are critical for activation, the PI(3)P phosphatase myotubularin related protein 6 and the histidine phosphatase phosphohistidine phosphatase 1 inhibit KCa3.1, TCR-stimulated Ca2+ influx and proliferation of activated naïve human CD4+ T cells by dephosphorylating PI(3)P and KCa3.1, respectively 80, 82. In addition, the E3 ubiquitin ligase tripartite motif-containing protein 27 inhibits KCa3.1 and TCR-stimulated Ca2+ influx and cytokine production by ubiquitylating and inhibiting PI3K-C2β 83.
The relative contribution of Kv1.3 and KCa3.1 in lymphocyte Ca2+ influx is determined primarily by their expression levels, which is related to lymphocyte subsets and their state of activation. Under resting conditions, CCR7+CD45RA+ naïve human T cells predominantly express Kv1.3 channels and depend on Kv1.3 for activation 84. Following activation, naïve human T cells upregulate KCa3.1 85 and KCa3.1 inhibition in pre-activated T cells inhibits TCR-stimulated Ca2+ influx and proliferation86, 87. Furthermore, mouse Th1 and Th2 cells predominantly express KCa3.1 and depend on KCa3.1 for TCR-stimulated Ca2+ influx and cytokine production, whereas Th17 cells mainly express Kv1.3 and depend on Kv1.3 for activation and production of IL-17 88. Differential use of K+ channels is also observed in effector (TEM) and central (TCM) memory T cells 76, 80, 89, 90. TEM cells (CCR7−CD62LlowCD45RA−) activated at sites of inflammation produce cytokines including IFNγ, IL-4 and IL-5 and exclusively upregulate Kv1.3. By contrast, TCM cells (CCR7+CD62LhiCD45RA−) present in lymph nodes and mucosal lymphoid organs upregulate KCa3.1 upon activation. As a result, Kv1.3 blockers are effective inhibitors of TEM cells, whereas KCa3.1 blockers are effective at inhibiting TCM cells.
Given their prominent role in regulating Ca2+ signalling, Kv1.3 and KCa3.1 have emerged as important drug targets 91, 92. Several potent peptide toxins, such as ShK derived from sea anenome venom, and oral small molecule inhibitors, such as Psora-4 and PAP-1, specifically inhibit Kv1.3 93–95. Several specific inhibitors of KCa3.1 channels have also been developed and include TRAM34 and ICA-17043 96, 97. Inhibitors of Kv1.3 and KCa3.1 have been very useful to study the role of K+ channels for immune responses in vivo, especially as Kv1.3 knockout mice showed no overt T cell defect due to the upregulation of a Cl− channel that compensates for the loss of Kv1.3 98.
The finding that Kv1.3 and KCa3.1 function to activate distinct lymphocyte subsets provides an opportunity to more selectively target lymphocyte subsets for therapeutic purposes. Studies in a rat model of multiple sclerosis reveal that Kv1.3 expression is upregulated and required for the proliferation of encephalogeneic T cells, and treatment of rats with Kv1.3 blockers in models of EAE markedly ameliorated disease89. The relevance of these findings to humans was demonstrated by findings of high levels of Kv1.3 expression on myelin-reactive T cells isolated from patients with multiple sclerosis 99. Similar studies have shown an increase in disease-associated TEM cells in patients with type 1 diabetes, rheumatoid arthritis and psoriasis, and treatment of these diseases with Kv1.3 blockers ShK or PAP1 led to the amelioration of disease 90, 100–102. Consistent with a role for Kv1.3 in the activation of Th17 cells 88, Kv1.3 blockers may have a therapeutic benefit in autoimmune diseases driven by Th17 cells103, 104. By contrast, Th1 and Th2 cells depend on KCa3.1 for their activation 88. Inhibition of KCa3.1 protected mice from developing colitis in two mouse models of IBD88, suggesting that KCa3.1 may be a novel therapeutic target to treat patients with Crohn’s disease or ulcerative colitis.
TRPM4 channels
TRPM4 channels are expressed by T cells and many other immune cells. Unlike most other TRP channels, their role in lymphocyte function is well documented. TRPM4 channels mainly conduct Na+ and K+ and, in contrast to other TRP channels, are only weakly permeable to Ca2+ 105. Activation of TRPM4 channels – accomplished by increases in [Ca2+]i – results in Na+ influx, membrane depolarization and a reduction in the electrical driving force for Ca2+ influx. TRPM4 channels thus provide a negative feedback mechanism for the regulation of SOCE and were proposed to prevent Ca2+ overload of cells. Given that activation of TRPM4 and Kv channels elicit opposing effects on Vm, it remains to be elucidated precisely how TRPM4 works together with Kv1.3 and KCa3.1 channels to regulate changes in Vm and intracellular Ca2+ levels. Overexpression of a dominant-negative mutant of TRPM4 or depletion of TRPM4 by RNAi in Jurkat T cells resulted in enhanced Ca2+ signalling and increased IL-2 production 106. Similar effects were observed in mouse Th2 cells, in which TRPM4 regulates Ca2+ levels, motility and the production of IL-2 and IL-4 by controlling the nuclear translocation of NFAT 107. Mast cells from Trpm4−/− mice showed increased Ca2+ influx, degranulation and histamine release after FcεRI stimulation; accordingly these mice had a more severe IgE-mediated acute passive cutaneous anaphylactic response 108. How enhanced Ca2+ influx in the absence of TRPM4 affects lymphocyte-dependent immune responses in vivo remains to be elucidated.
TRP channels
TRP channels form a large superfamily of 28 cation channels in humans, which can be divided into 7 subfamilies 109. T cells predominantly express channels belonging to the TRPC and TRPM subfamilies including TRPC1, TRPC3, TRPC5, TRPM2, TRPM4 and TRPM7 110 (Table 1). Most TRP channels are non-selective and permeable to several cations including Ca2+ and Na+ 111, 112. We will briefly discuss the role of TRPC and TRPM2 channels; TRPM7 channels will be discussed further below in the context of Mg2+ signaling.
TRPC channels
TRPC1-7 form non-selective cation channels whose activation is generally linked to stimulation of plasma membrane receptors coupled to PLCγ 111, 113. Prior to the identification of ORAI1 as the CRAC channel 12–14, there was avid interest in the possibility that TRPC channels contribute to SOCE in T cells. A recent study showed that RNAi-mediated depletion of TRPC3 has a moderate effect on SOCE and T cell proliferation 110. Expression of TRPC5 was reported to increase after activation of mouse CD4+ and CD8+ T cells and to mediate Ca2+ influx after crosslinking of GM1 ganglioside with the B subunit of cholera toxin 114; whether TRPC5 mediates TCR-induced Ca2+ influx has not been examined. While these studies were generally interpreted as supporting a role for TRPC channels in SOCE, recent evidence has questioned whether TRPC channels are activated by store depletion 115. Overall, the biophysical and immunological evidence for a significant role of TRPC channels in lymphocytes and adaptive immune responses awaits further evaluation.
TRPM2 channels
TRPM2 is a non-selective Ca2+ permeable channel that is activated by intracellular adenosine diphosphate ribose (ADPR) and regulated by several intracellular factors including Ca2+, cyclic ADPR (cADPR), H2O2, NAADP and AMP 116, 117. TRPM2 channels mediate stress induced Ca2+signals in a diverse group of immune cells including myeloid cells and T cells 116, 117. In T cells, TRPM2 expression was found to increase after TCR stimulation 110 and endogenous TRPM2 currents could be activated by cADPR, ADPR and NAADP 118. Although there is no direct evidence that TRPM2 is required for Ca2+ influx in lymphocytes and T cell function, TCR stimulation has been reported to evoke the release of cADPR from the ER 119, thus potentially activating TRPM2 in T cells. Studies in myeloid cells indicate that cADPR and hydrogen peroxide (H2O2)synergize in the activation of TRPM2 (reviewed in 116, 117). Since H2O2 is produced by several immune cell types under inflammatory conditions, Ca2+ influx through TRPM2 has been investigated as a potential mediator of reactive oxygen species (ROS)-induced pathologies 120, 121. TRPM2-deficient mice were largely resistant to dextran sulphate sodium (DSS)-induced colitis due to impaired Ca2+ influx, nuclear factor-κB (NF-κB) activation and production of CXCL2 by monocytes 122. Conversely, TRPM2 inhibited ROS production in phagocytic cells by attenuating NADPH oxidase function and prevented endotoxin-induced lung inflammation in mice 120. Whether TRPM2 channels are modulated by ROS in T cells and regulate T cell responses during inflammation in vivo remains to be elucidated.
Magnesium channels and transporters
Mg2+ is the most abundant divalent cation in eukaryotic cells. It binds to and regulates the function of many polyphosphate-containing molecules such as DNA, RNA and ATP. While > 90% of all cellular Mg2+ is in the form of Mg-ATP 123, ~ 5% is free and can potentially function as a second messenger similar to Ca2+. Mg2+ is required for the proliferation of mitogen-stimulated T cells 124, 125, and stimulation of T cells through the TCR results in a transient increase in [Mg2+]i 126. Recent studies provide evidence for an important role of TRPM7, a Mg2+ permeable channel, and MagT1, a Mg2+ transporter, in T cell function and development 126, 127.
TRPM7 channels
TRPM7 is a ubiquitously expressed nonselective cation channel that exhibits nearly equal permeabilities for Mg2+ and Ca2+ 128 (Fig. 4). TRPM7 channels are believed to regulate cellular and whole body Mg2+ homeostasis because of their high Mg2+ permeability. This is supported by evidence indicating that mutations in the closely related TRPM6 channel cause hypomagnesemia due to impaired renal and intestinal Mg2+ absorption 129–131. Direct evidence for a role of TRPM7 in immune function came from genetic deletion of TRPM7 in DT-40 chicken B cells, which failed to proliferate, showed increased cell death and had reduced total cellular Mg2+ 132. These defects could partially be rescued by growing cells in medium containing high extracellular Mg2+ (10 mM) 132.
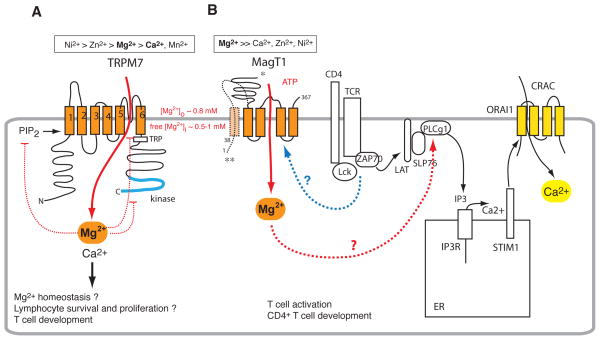
Figure 4. Mg2+ channels and transporters in lymphocytes.
A, TRPM7 is a Mg2+-permeable channel that is a “chanzyme” because it functions as both an ion channel and an enzyme through its C-terminal serine/threonine kinase domain. As with other TRP channels, its ion channel pore is located between transmembrane (TM) domains 5 and 6. TRPM7 is a non-selective cation channel and conducts Mg2+ and Ca2+ with near equal permeabilities. One of the defining features of TRPM7 channels is inhibition by intracellular Mg2+ but the mechanism of Mg2+ regulation is incompletely understood 76. TRPM7 function further depends on PIP2 and is regulated by extracellular pH 133. B, MagT1 belongs to a family of recently identified Mg2+ transporters. It is highly selective for Mg2+ compared to Ca2+, Zn2+, Ni2+ and other divalent cations 134. MagT1 opening in response to TCR stimulation results in a global increase in [Mg2+]i, activation of PLCγ1 and Ca2+ influx, presumably via CRAC channels. The mechanisms by which TCR signalling causes MagT1 to open and how Mg2+ activates PLCγ1 are not understood. Two MagT1 isoforms have been described: a short one (335 aa) with a confirmed tetraspanning membrane topology (*) 135 and a longer version (367 aa) 126 predicted to contain five TM domains and an intracellular N terminus (**), which may facilitate TCR-dependent activation of MagT1.
An important role for TRPM7 in T cell development was shown using mice with T cell-specific deletion of TRPM7. Trpm7fl/− Lck-Cre mice had a severe block in T cell development at the CD4−CD8− double negative stage, resulting in reduced numbers of CD4+ and DP T cells in the thymus and CD3+ T cells in the spleen 127. Lack of TRPM7 in T cells was associated with impaired expression of growth factors such as FGF7, FGF13 and midkine, and consequently a progressive loss of medullary thymic epithelial cells (mTECs) 127. It remains unclear, however, whether the observed defects are intrinsic to TRPM7-deficient T cells or secondary to the loss of mTECs. Another question is whether the primary role of TRPM7 in T cells is in Mg2+ homeostasis. Although Mg2+ currents in thymocytes from Trpm7fl/−Lck-Cre mice were markedly reduced, Mg2+ influx and total cellular Mg2+ content were normal, suggesting that TRPM7 may not be required for Mg2+ influx in T cells 127. How does TRPM7 then control T cell development and function? Possible explanations are that Mg2+ influx through TRPM7 may cause highly localized increases in [Mg2+]i close to the mouth of the channel resulting in the activation of signal transduction (Fig. 4) or, alternatively, that the main function of TRPM7 in T cells is not to promote Mg2+ but Ca2+ influx consistent with the channel’s documented Ca2+ permeability 133. Finally, TRPM7 could regulate T cell development through its C terminal kinase domain independent of channel function 128. While the mechanisms by which TRPM7 controls lymphocyte function remain in debate, it is noteworthy that this is the only ion channel identified so far (with the exception of the Mg2+ transporter MagT1 discussed below) that is required for lymphocyte development.
MagT1
MagT1 is a Mg2+ transporter essential for Mg2+ signalling in T cells (Fig. 4). It was discovered in two independent screens and has little sequence similarity to other ion channels or transporters 134, 135. MagT1 is highly selective for Mg2+ and does not conduct Ca2+, Zn2+, Ni2+ or other divalent cations when expressed in Xenopus oocytes 134. It mediates Mg2+ influx in T cells and RNAi-mediated depletion of MagT1 resulted in a moderate decrease in cytoplasmic Mg2+ concentrations 135. The importance of MagT1 and Mg2+ signalling in T cells is emphasized by patients with inherited mutations in MAGT1 who suffer from a rare form of immunodeficiency (Table 1) 126. Patients with XMEN disease (for X-linked immunodeficiency with magnesium defect and EBV infection and neoplasia) suffer from CD4+ lymphocytopenia and increased susceptibility to viral infections, particularly with Epstein-Barr virus (EBV) due to abolished MagT1 protein expression and Mg2+ influx following TCR stimulation. In contrast to T cells, B cell development and function are normal in these patients, consistent with the lack of Mg2+ influx in control B cells stimulated by anti-IgM or anti-CD40 antibodies.
One of the main functions of Mg2+ influx by MagT1 is the activation of PLCγ1 as TCR crosslinking of MagT1-deficient T cells resulted in delayed activation of PLCγ1 and abolished SOCE 126. By contrast, proximal TCR signalling events such as the phosphorylation of CD3ε, ζ-chain-associated protein kinase of 70 kDa (ZAP70) and linker for activation of T cells (LAT) occurred normally 126. The mechanisms by which Mg2+ influx through MagT1 regulates PLCγ1 activation are not understood (Fig. 4). Another open question is how TCR stimulation activates MagT1 and thus Mg2+ influx, especially since MagT1 only contains two short intracellular domains available to interact with cytoplasmic signalling molecules (Fig. 4) 135. Despite these unresolved questions, the profound immunological effects of MagT1 deficiency validate the important role of Mg2+ ions in T cell function.
Zinc transporters
Zinc is an essential trace element and a structural component of numerous metalloproteins such as zinc finger-containing transcription factors through which it contributes to immune function (reviewed in 136–138). In addition, emerging evidence suggests that Zn2+ regulates lymphocyte function directly as a second messenger. The free [Zn2+]i in lymphocytes is very low (~ 0.35 nM) 139, whereas that in the serum is ~16 μM 140, establishing a > 104-fold gradient between extracellular and intracellular [Zn2+]. Stimulation of human and mouse T cells by IL-2 141 or incubation with DCs results in rapid increases in [Zn2+]i accompanied by T cell proliferation and cytokine production, suggesting a potentially important role for Zn2+ in lymphocyte signal transduction 141–143.
The role of Zn2+ in immunity is highlighted by the inherited Zn2+ malabsorption syndrome acrodermatitis enteropathica (AE) caused by impaired Zn2+ uptake through the zinc transporter Zrt-Irt like protein 4 (ZIP4) in the intestinal epithelium 144, 145. The AE phenotype includes immunodeficiency with recurrent infections in ~ 30% of patients. Immunodeficiency is associated with thymus atrophy and lymphopenia (146, 147 and references therein), which have been attributed to increased glucocorticoid production and apoptosis of immature T and B cells 147. Several in vitro studies have shown that Zn2+ is required for T cell functions, for instance proliferation 143, 148 and the production of cytokine like IL-2 and IFNγ 149. At higher concentrations, however, Zn2+ was shown to exert an inhibitory effect on the proliferation of mouse T cells 150 and the expression of cytokines by Jurkat T cells 151 and human CD4+ T cells 142. The molecular mechanisms underlying these concentration-dependent effects of Zn2+ are only partially understood. Fig. 5 shows some of the signalling pathways in lymphocytes that are either activated or inhibited by Zn2+ (reviewed in 136–138). For instance, increases in [Zn2+]i were reported to enhance activation of kinases such as Lck and PKC 152, but to inhibit the phosphatase calcineurin (Fig. 5) 153, 154. More recently, Zn2+ influx was reported to mediate T cell activation by enhancing the phosphorylation of ZAP70 and decreasing the recruitment of the tyrosine phosphatase SHP1 to the TCR, thereby prolonging Ca2+ influx 143.
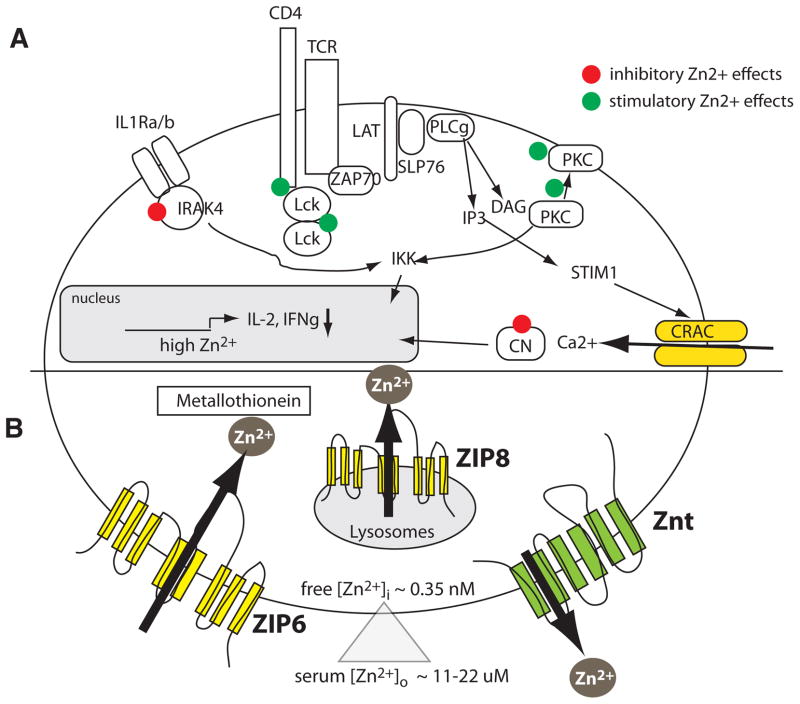
Figure 5. Zinc signalling and Zinc transporters in T cells.
A, Zn2+ ions have activating and inhibitory effects on signal transduction in T cells 136–138. Zn2+ mediates the recruitment of the src kinase Lck to CD4 and CD8 and promotes Lck dimerization, resulting in enhanced TCR signalling 152. Zn2+ also promotes protein kinase C (PKC) signalling, likely by recruiting PKC to the plasma membrane. By contrast, Zn2+ inhibits the activity of the phosphatase calcineurin, thus preventing nuclear translocation of the transcription factor NFAT 153, 154. Furthermore, Zn2+ inhibits the function of interleukin-1 receptor-associated kinase (IRAK) 4, thereby restraining signalling through the IL-1R and activation of NF-kB. Inhibitory effects of Zn2+ on both NFAT and NF-kB may explain the reduced production of cytokines such as IL-2 and IFNg in the presence of increasing extracellular [Zn2+]. B, Increases in intracellular [Zn2+] in lymphocytes are mediated by Zinc influx from the extracellular space or efflux from intracellular organelles that are mediated by Zrt-Irt like proteins (ZIP). These Zn2+ transporters contain eight transmembrane domains (TM) with an aqueous pore predicted to be formed by TM4 and TM5 189. Zn2+ is exported from the cytoplasm by ZnT transporters resulting in decreased intracellular [Zn2+]. In T cells, the Zinc transporters ZIP3, ZIP6 and ZIP8 have been implicated in Zn2+ influx 142, 143, 156, whereas the nature of ZnT proteins mediating Zn2+ efflux in lymphocytes are presently unknown. In addition to Zn2+ transport, intracellular Zn2+ levels are modulated by Zn2+ binding to metallothionein and other proteins.
The proteins that mediate Zn2+ levels in lymphocytes and their molecular regulation are still poorly defined. Two classes of Zn2+ transporters have been described to regulate intracellular Zn2+ concentrations: ZIP transporters (or solute carrier family 39, SLC39A) and Zinc transporters (ZnT or SLC30A). ZIP and ZnT proteins are localized either in the plasma membrane or intracellular organelles where they mediate Zn2+ influx (ZIP) or Zn2+ efflux (ZnT) into or from the cytoplasm respectively (Fig. 5) (reviewed in 155). 14 mammalian ZIP genes have been identified 137. ZIP3 is highly expressed in CD34+ human haematopoietic stem cells and genetic deletion of Zip3 in mice resulted in the depletion of CD4+CD8+ T cells in the thymus under zinc-limiting conditions. By contrast, the numbers of single-positive CD4+ or CD8+ thymocytes were increased suggesting accelerated T cell maturation 156. In T cells, two Zn2+ transporters, ZIP6 and ZIP8, were reported to mediate Zn2+ influx across the plasma membrane 143 and release from lysosomal Zn2+ stores 142, respectively. When primary human CD4+ T cells were stimulated by incubation with DCs, [Zn2+]i increased within 1 minute after formation of the IS between T cells and DCs. This increase in [Zn2+]i and the subsequent expression of CD25 and CD69 depended on ZIP6. Interestingly, increases in [Zn2+]i were spatially restricted to the IS 143, potentially due to rapid sequestration by zinc-binding proteins such as metallothionein. Another mechanism to limit increases in [Zn2+]i is provided by ZnT transporters that mediate uptake of Zn2+ into intracellular organelles or promote Zn2+ export across the plasma membrane. Of the 10 ZnT transporters in mammalian cells, only a few are known to be functional in immune cells. ZnT5 is required for mast cell function 157, 158, but the ZnT molecules controlling [Zn2+]i in lymphocytes remain to be elucidated. It is noteworthy that primary human T and B cells express ZnT1, ZnT4, ZnT6 and ZnT7 157 and that mRNA expression in T cells was strongly reduced following phytohaemagglutinin stimulation. Thus, downregulation of ZnT levels may be a means to maintain elevated [Zn2+]i during T cell activation. Despite these leads, the overall role of Zn2+ transporters in immune function, development and adaptive immunity remains poorly understood.
Chloride channels
Several chloride channels that allow the influx of Cl− anions across the plasma membrane were reported tobe active in lymphocytes and control their function. Volume-regulated Cl− (or Clswell) channels open upon swelling of T cells in a hypotonic environment, resulting in the efflux of Cl− and, ultimately, water from the cell, and thus a return to normal cell volume 76, 159, 160. The osmotic activation of chloride channels in Jurkat T cells depends on the Src kinase Lck 161. Interestingly, the induction of apoptosis in T cells by crosslinking of Fas (CD95) induces Cl− currents in a Lck-dependent manner 162, suggesting that Cl− channels may regulate apoptosis in T cells. A further analysis of the physiological role of volume-regulated Cl− channels in lymphocytes is hampered, however, by the fact that their molecular identity is unknown (reviewed in 76).
Several studies have demonstrated the expression of γ-aminobutyric acid (GABA) receptors in human, mouse and rat T cells 163, 164. GABAA receptors are heteropentameric ligand-gated Cl− channels whose inhibitory role in neuronal function in the CNS is well established 165. GABA-activated Cl− currents were reported in mouse and rat T cells and macrophages 164, 166, 167. GABA administration inhibited T cell proliferation, the production of IL-2 and IFNγ as well as the cytotoxic function of CD8+ T cells in vitro 163, 164, 168, 169. In vivo, administration of GABA or GABAergic agents ameliorated disease outcome in a number of animal models of autoimmunity such as type 1 diabetes 163, rheumatoid arthritis 170 and multiple sclerosis 166, suggesting that GABAA receptors may inhibit the activation of T cells to protect GABA-secreting cells from T cell-mediated inflammatory tissue damage. How GABA receptor-mediated Cl− influx inhibits T cell function has not been elucidated. Unlike excitable cells, in which GABA receptors inhibit Cav channels through membrane hyperpolarization, this mechanism is unlikely to account for the effects of GABA on T cells.
Another chloride channel that has been reported to regulate T cell function is the cystic fibrosis transmembrane conductance regulator (CFTR), mutations of which cause cystic fibrosis (CF). cAMP-activated Cl− currents were originally reported in Jurkat T cells, CD4+ T cell clones and EBV-transformed B cells and shown to be defective in T and B cells from CF patients 171, 172. The effects of the (F508 CFTR mutation, the most common mutation in CF patients, on murine and human T cells were however very different. Whereas T cell clones from CF patients showed impaired production of IL-5 and IL-10 after stimulation with anti-CD3 and PMA171, CD4+ T cells from Cftr-F508 (Cftr−/−) mice showed increased IL-4 and IL-13 production when stimulated with congenic monocytes and OVA peptide 173. The cause of this discrepancy between human and mouse T cells is not clear. Further studies are required to corroborate a role for CFTR in lymphocyte function and to provide a better mechanistic understanding how CFTR may regulate T cell function.
Summary and perspectives
Lymphocytes express an abundance of ion channels that are critical for their development and function. While the importance of individual ion channels and transporters for T-cell effector function is now well-recognized, how different ion transport mechanisms interact with each other to fine-tune overall cellular responses for the most the most optimal outcome still remains poorly understood. It seems likely that interactions among the various ion transport mechanisms could help to generate complex signal transduction patterns and generate specificity by enhancing the dynamic range of the individual signaling pathways and by improving signal-to-noise. Examples of cross-talk include the regulation of Ca2+ influx by the MagT1 Mg2+ transport proteins 126, Zn2+ influx 143, and the well-known modulation of Ca2+ influx by K+ channels through control of the membrane potential. Such cross-talk could permit more finely tuned regulation of cell signalling than may be possible through the action of individual independent pathways. In most cases, the molecular foundations of cross-talk are unclear, but possible explanations include the colocalization of ion transport proteins, as suggested for CRAC (ORAI) and K+ channels 9 and CRAC channels and P2X receptors 53. It is tempting to speculate that different types of ion channel aggregate in signalling complexes in lymphocytes where they modulate each others function, but more in-depth studies are needed to investigate this possibility.
Many ion channels discussed here contribute to T cell-mediated autoimmune and/or inflammatory responses and therefore are attractive targets for pharmacological immune modulation. Whereas drugs acting on ion channels have successfully been used for the treatment of neurological and cardiovascular disorders 174, ion channels have not been systematically exploited as drug targets for immune therapy. Plasma membrane channels are readily accessible to small molecule compounds and biological reagents such as blocking antibodies and peptides. Inhibitory antibodies against TRPC5 and TRPM3 channels have been developed that target an extracellular loop in close proximity to the ion channel pore 175, 176. It is possible that these approaches could be extended to TRPM2 channels (given its pro-inflammatory role in monocytes 122) and ORAI Ca2+ channels. As described above, genetic deletion of ORAI1 and STIM1 in mice abolishes the expression of several pro-inflammatory cytokines 7, 46, 47 and protects mice from autoimmune CNS inflammation, colitis, allograft rejection and GvHD 43, 45–47. Inhibition of SOCE can be achieved by directly targeting ORAI1 CRAC channels, or indirectly by inhibiting the function of K+ channels. As discussed above, considerable progress has been made in developing K+ channel blockers 92, 177. Similarly, P2X7 receptor antagonists may provide a multipronged approach to anti-inflammatory therapy given the role of these channels in the pro-inflammatory function of lymphocytes and innate immune cells 58, 59. It will therefore be an important long-term goal to develop safe, selective and potent inhibitors of ion channels for the treatment of inflammation, autoimmunity, allergy and transplant rejection.
Acknowledgments
We thank Drs. Heike Wulff and Helen McBride for their critical reading of the manuscript and helpful suggestions. This work was supported in part by National Institutes of Health grants AI066128 (to S.F.), NS057499 (to M.P.) and GM084195 (to E.Y.S.).
GLOSSARY
- inositol 1,4,5 triphosphate (InsP3) receptor
a Ca2+-permeable channel located in the membrane of the endoplasmic reticulum (ER) which mediates the release of Ca2+ from ER stores upon binding by the second messenger InsP3
- ryanodine receptor (RyR)
a Ca2+-permeable channel located in the membrane of the sarcoplasmic reticulum (SR) and ER which mediates the release of Ca2+ from the SR/ER stores upon binding by the second messenger cyclic ADP ribose (cADPR) or Ca2+ itself
- sarco/endoplasmic reticulum Ca2+ ATPase (SERCA)
located in the membrane of the ER, is a Ca2+ pump that moves Ca2+ from the cytoplasm into the ER through hydrolysis of ATP
- plasma membrane Ca2+ ATPases (PMCA)
a family of ion transport ATPases located in the plasma membrane that export Ca2+ from the cytoplasm
- store-operated Ca2+ entry (SOCE)
Ca2+ influx process triggered by the depletion of ER Ca2+ stores and activation of plasma membrane ORAI calcium channels by STIM proteins
- Ca2+ release-activated Ca2+ (CRAC) channel
a highly Ca2+ selective ion channel located in the plasma membrane that is encoded by ORAI proteins
- ion selectivity
the specificity of the channel for a particular species of ions, for example Ca2+, Mg2+, Na+, K+ etc; non-selective channels do not discriminate between different types of ions
- conductance
a measure of the ability of an ion channel to carry electric charge, measured by the ratio of current divided by the potential difference (voltage); measured in siemens (S)
- (Severe) combined immunodeficiency (SCID and CID)
SCID is caused by inherited defects in T cell (and in some cases B cell) development, whereas CID is due to inherited defects in T cell function (but not T cell development). SCID and CID result in severe, often lethal infections in early infancy
- nuclear factor of activated T cells (NFAT)
a family of Ca2+-dependent transcription factors that are activated via dephosphorylation by the phosphatase calcineurin; mediate expression of many cytokine genes in lymphocytes
- CRAC channelopathy
CRAC channel dysfunction caused by autosomal recessive mutations in ORAI1 and STIM1 genes that results in a pathognomonic clinical combination of immunodeficiency, autoimmunity, congenital muscular hypotonia and ectodermal dysplasia with impaired dental enamel calcification and sweat gland dysfunction
- membrane potential
difference between the electrical potential between the inside and outside of a cell; typically −60 to −80 mV in resting cells
- ion channels
pore-forming transmembrane proteins that enable the flow of ions down an electrochemical gradient
- ion transporters
pore-forming transmembrane proteins that carry ions against a concentration gradient using energy, typically in the form of ATP
Footnotes
Conflict of interest disclosure: S.F. is a co-founder and scientific advisor to Calcimedica Inc.
Further information
ONLINE LINKS for gene expression data:
BioGPS: http://biogps.gnf.org
Immunological Genome Project: http://www.immgen.org.
References
- 1.Lewis RS. Calcium signaling mechanisms in T lymphocytes. Annu Rev Immunol. 2001;19:497–521. doi: 10.1146/annurev.immunol.19.1.497. [DOI] [PubMed] [Google Scholar]
- 2.Parekh AB, Putney JW. Store-operated calcium channels. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 3.Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992;355:353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- 4.Zweifach A, Lewis RS. Mitogen-regulated Ca2+ current of T lymphocytes is activated by depletion of intracellular Ca2+ stores. Proc Natl Acad Sci USA. 1993;90:6295–6299. doi: 10.1073/pnas.90.13.6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prakriya M. The molecular physiology of CRAC channels. Immunol Rev. 2009;231:88–98. doi: 10.1111/j.1600-065X.2009.00820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hogan PG, Lewis RS, Rao A. Molecular basis of calcium signaling in lymphocytes: STIM and ORAI. Annu Rev Immunol. 2010;28:491–533. doi: 10.1146/annurev.immunol.021908.132550. Reference 6 provides an excellent overview of the molecular regulation and function of CRAC channels in lymphocytes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feske S, Giltnane J, Dolmetsch R, Staudt LM, Rao A. Gene regulation mediated by calcium signals in T lymphocytes. Nat Immunol. 2001;2:316–324. doi: 10.1038/86318. [DOI] [PubMed] [Google Scholar]
- 8.Feske S, et al. Severe combined immunodeficiency due to defective binding of the nuclear factor of activated T cells in T lymphocytes of two male siblings. Eur J Immunol. 1996;26:2119–2126. doi: 10.1002/eji.1830260924. [DOI] [PubMed] [Google Scholar]
- 9.Feske S, Prakriya M, Rao A, Lewis RS. A severe defect in CRAC Ca2+ channel activation and altered K+ channel gating in T cells from immunodeficient patients. J Exp Med. 2005;202:651–662. doi: 10.1084/jem.20050687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le Deist F, et al. A primary T-cell immunodeficiency associated with defective transmembrane calcium influx. Blood. 1995;85:1053–1062. [PubMed] [Google Scholar]
- 11.Partiseti M, et al. The calcium current activated by T cell receptor and store depletion in human lymphocytes is absent in a primary immunodeficiency. J Biol Chem. 1994;269:32327–32335. [PubMed] [Google Scholar]
- 12.Feske S, et al. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 13.Vig M, et al. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang SL, et al. Genome-wide RNAi screen of Ca(2+) influx identifies genes that regulate Ca(2+) release-activated Ca(2+) channel activity. Proc Natl Acad Sci U S A. 2006;103:9357–9362. doi: 10.1073/pnas.0603161103. References 12–14 describe the cloning of ORAI1 (CRACM1) as the gene encoding the CRAC channel and show (ref 12) that a single point mutation in ORAI1 abolishes CRAC channel function in T cells and causes combined immunodeficiency (CID) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lis A, et al. CRACM1, CRACM2, and CRACM3 are store-operated Ca2+ channels with distinct functional properties. Curr Biol. 2007;17:794–800. doi: 10.1016/j.cub.2007.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeHaven WI, Smyth JT, Boyles RR, Putney JW. Calcium inhibition and calcium potentiation of Orai1, Orai2, and Orai3 calcium release-activated calcium channels. J Biol Chem. 2007;282:17548–17556. doi: 10.1074/jbc.M611374200. [DOI] [PubMed] [Google Scholar]
- 17.Feske S. Calcium signalling in lymphocyte activation and disease. Nat Rev Immunol. 2007;7:690–702. doi: 10.1038/nri2152. [DOI] [PubMed] [Google Scholar]
- 18.Liou J, et al. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roos J, et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. References 18–19 describe the cloning of STIM1 as the ER Ca2+ sensor and activator of CRAC channels. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cahalan MD. STIMulating store-operated Ca(2+) entry. Nat Cell Biol. 2009;11:669–677. doi: 10.1038/ncb0609-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang SL, et al. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437:902–905. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luik RM, Wang B, Prakriya M, Wu MM, Lewis RS. Oligomerization of STIM1 couples ER calcium depletion to CRAC channel activation. Nature. 2008;454:538–542. doi: 10.1038/nature07065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stathopulos PB, Li GY, Plevin MJ, Ames JB, Ikura M. Stored Ca2+ depletion-induced oligomerization of stromal interaction molecule 1 (STIM1) via the EF-SAM region: An initiation mechanism for capacitive Ca2+ entry. J Biol Chem. 2006;281:35855–35862. doi: 10.1074/jbc.M608247200. [DOI] [PubMed] [Google Scholar]
- 24.Stathopulos PB, Zheng L, Li GY, Plevin MJ, Ikura M. Structural and mechanistic insights into STIM1-mediated initiation of store-operated calcium entry. Cell. 2008;135:110–122. doi: 10.1016/j.cell.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Wu MM, Buchanan J, Luik RM, Lewis RS. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J Cell Biol. 2006;174:803–813. doi: 10.1083/jcb.200604014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luik RM, Wu MM, Buchanan J, Lewis RS. The elementary unit of store-operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. J Cell Biol. 2006;174:815–825. doi: 10.1083/jcb.200604015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muik M, et al. Dynamic coupling of the putative coiled-coil domain of ORAI1 with STIM1 mediates ORAI1 channel activation. J Biol Chem. 2008;283:8014–8022. doi: 10.1074/jbc.M708898200. [DOI] [PubMed] [Google Scholar]
- 28.Navarro-Borelly L, et al. STIM1-Orai1 interactions and Orai1 conformational changes revealed by live-cell FRET microscopy. J Physiol. 2008;586:5383–5401. doi: 10.1113/jphysiol.2008.162503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park CY, et al. STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell. 2009;136:876–890. doi: 10.1016/j.cell.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsumoto M, et al. The Calcium Sensors STIM1 and STIM2 Control B Cell Regulatory Function through Interleukin-10 Production. Immunity. 2011;34 doi: 10.1016/j.immuni.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 31.Oh-Hora M, et al. Dual functions for the endoplasmic reticulum calcium sensors STIM1 and STIM2 in T cell activation and tolerance. Nat Immunol. 2008;9:432–443. doi: 10.1038/ni1574. Reference 31 describes that STIM1 and STIM2 mediate Ca2+ influx in T cells and that complete deletion of STIM1 and STIM2 in mouse T cells interferes with the development and function of Treg cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brandman O, Liou J, Park WS, Meyer T. STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ levels. Cell. 2007;131:1327–1339. doi: 10.1016/j.cell.2007.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stathopulos PB, Zheng L, Ikura M. Stromal Interaction Molecule (STIM) 1 and STIM2 Calcium Sensing Regions Exhibit Distinct Unfolding and Oligomerization Kinetics. J Biol Chem. 2009;284:728–732. doi: 10.1074/jbc.C800178200. [DOI] [PubMed] [Google Scholar]
- 34.Shaw PJ, Feske S. Physiological and pathophysiological functions of SOCE in the immune system. Front Biosci (Elite Ed) 2012;4:2253–2268. doi: 10.2741/540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Byun M, et al. Whole-exome sequencing-based discovery of STIM1 deficiency in a child with fatal classic Kaposi sarcoma. J Exp Med. 2010;207:2307–2312. doi: 10.1084/jem.20101597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCarl CA, et al. ORAI1 deficiency and lack of store-operated Ca2+ entry cause immunodeficiency, myopathy, and ectodermal dysplasia. J Allergy Clin Immunol. 2009;124:1311–1318. e1317. doi: 10.1016/j.jaci.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Picard C, et al. STIM1 mutation associated with a syndrome of immunodeficiency and autoimmunity. N Engl J Med. 2009;360:1971–1980. doi: 10.1056/NEJMoa0900082. Reference 37 describes the first patients with immunodeficiency due to a mutation of STIM1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feske S. CRAC channelopathies. Pflugers Arch. 2010;460:417–435. doi: 10.1007/s00424-009-0777-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feske S. Immunodeficiency due to defects in store-operated calcium entry. Ann N Y Acad Sci. 2011;1238:74–90. doi: 10.1111/j.1749-6632.2011.06240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feske S. ORAI1 and STIM1 deficiency in human and mice: roles of store-operated Ca2+ entry in the immune system and beyond. Immunol Rev. 2009;231:189–209. doi: 10.1111/j.1600-065X.2009.00818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maul-Pavicic A, et al. ORAI1-mediated calcium influx is required for human cytotoxic lymphocyte degranulation and target cell lysis. Proc Natl Acad Sci U S A. 2011;108:3324–3329. doi: 10.1073/pnas.1013285108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gwack Y, et al. Hair loss and defective T- and B-cell function in mice lacking ORAI1. Mol Cell Biol. 2008;28:5209–5222. doi: 10.1128/MCB.00360-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beyersdorf N, et al. STIM1-Independent T Cell Development and Effector Function In Vivo. The Journal of Immunology. 2009;182:3390–3397. doi: 10.4049/jimmunol.0802888. [DOI] [PubMed] [Google Scholar]
- 44.Feske S, Picard C, Fischer A. Immunodeficiency due to mutations in ORAI1 and STIM1. Clin Immunol. 2010;135:169–182. doi: 10.1016/j.clim.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCarl CA, et al. Store-operated Ca2+ entry through ORAI1 is critical for T cell-mediated autoimmunity and allograft rejection. J Immunol. 2010;185:5845–5858. doi: 10.4049/jimmunol.1001796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma J, McCarl CA, Khalil S, Luthy K, Feske S. T-cell-specific deletion of STIM1 and STIM2 protects mice from EAE by impairing the effector functions of Th1 and Th17 cells. Eur J Immunol. 2010;40:3028–3042. doi: 10.1002/eji.201040614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schuhmann MK, et al. Stromal interaction molecules 1 and 2 are key regulators of autoreactive T cell activation in murine autoimmune central nervous system inflammation. J Immunol. 2010;184:1536–1542. doi: 10.4049/jimmunol.0902161. References 46–47 show that STIM1 and STIM2 are required for the proinflammatory function of Th1 and Th17 cells and the induction of experimental autoimmune encephalomyelitis (EAE) [DOI] [PubMed] [Google Scholar]
- 48.Stromnes IM, Cerretti LM, Liggitt D, Harris RA, Goverman JM. Differential regulation of central nervous system autoimmunity by T(H)1 and T(H)17 cells. Nat Med. 2008;14:337–342. doi: 10.1038/nm1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.El-behi M, Rostami A, Ciric B. Current views on the roles of Th1 and Th17 cells in experimental autoimmune encephalomyelitis. J Neuroimmune Pharmacol. 2010;5:189–197. doi: 10.1007/s11481-009-9188-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oh-hora M. Calcium signaling in the development and function of T-lineage cells. Immunol Rev. 2009;231:210–224. doi: 10.1111/j.1600-065X.2009.00819.x. [DOI] [PubMed] [Google Scholar]
- 51.Tone Y, et al. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat Immunol. 2008;9:194–202. doi: 10.1038/ni1549. [DOI] [PubMed] [Google Scholar]
- 52.Junger WG. Immune cell regulation by autocrine purinergic signalling. Nat Rev Immunol. 2011;11:201–212. doi: 10.1038/nri2938. Reference 52 provides an excellent overview of signaling by P2X and other purinergic receptors in immune cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woehrle T, et al. Pannexin-1 hemichannel-mediated ATP release together with P2X1 and P2X4 receptors regulate T-cell activation at the immune synapse. Blood. 2010;116:3475–3484. doi: 10.1182/blood-2010-04-277707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yip L, et al. Autocrine regulation of T-cell activation by ATP release and P2X7 receptors. The FASEB Journal. 2009;23:1685–1693. doi: 10.1096/fj.08-126458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baricordi OR, et al. An ATP-activated channel is involved in mitogenic stimulation of human T lymphocytes. Blood. 1996;87:682–690. [PubMed] [Google Scholar]
- 56.Padeh S, Cohen A, Roifman CM. ATP-induced activation of human B lymphocytes via P2-purinoceptors. J Immunol. 1991;146:1626–1632. [PubMed] [Google Scholar]
- 57.Adinolfi E, et al. Basal activation of the P2X7 ATP receptor elevates mitochondrial calcium and potential, increases cellular ATP levels, and promotes serum-independent growth. Mol Biol Cell. 2005;16:3260–3272. doi: 10.1091/mbc.E04-11-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schenk U, et al. Purinergic control of T cell activation by ATP released through pannexin-1 hemichannels. Science Signaling. 2008;1:ra6. doi: 10.1126/scisignal.1160583. [DOI] [PubMed] [Google Scholar]
- 59.Schenk U, et al. ATP inhibits the generation and function of regulatory T cells through the activation of purinergic P2X receptors. Sci Signal. 2011;4:ra12. doi: 10.1126/scisignal.2001270. [DOI] [PubMed] [Google Scholar]
- 60.Ratner D, Mueller C. Immune responses in Cystic Fibrosis; are they intrinsically defective? Am J Respir Cell Mol Biol. 2012 doi: 10.1165/rcmb.2011-0399RT. [DOI] [PubMed] [Google Scholar]
- 61.Sharp AJ, et al. P2x7 deficiency suppresses development of experimental autoimmune encephalomyelitis. J Neuroinflammation. 2008;5:33. doi: 10.1186/1742-2094-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mulryan K, et al. Reduced vas deferens contraction and male infertility in mice lacking P2X1 receptors. Nature. 2000;403:86–89. doi: 10.1038/47495. [DOI] [PubMed] [Google Scholar]
- 63.Solle M, et al. Altered cytokine production in mice lacking P2X(7) receptors. J Biol Chem. 2001;276:125–132. doi: 10.1074/jbc.M006781200. [DOI] [PubMed] [Google Scholar]
- 64.Yamamoto K, et al. Impaired flow-dependent control of vascular tone and remodeling in P2X4-deficient mice. Nat Med. 2006;12:133–137. doi: 10.1038/nm1338. [DOI] [PubMed] [Google Scholar]
- 65.Tsien RW, Hess P, McCleskey EW, Rosenberg RL. Calcium channels: mechanisms of selectivity, permeation, and block. Annu Rev Biophys Biophys Chem. 1987;16:265–290. doi: 10.1146/annurev.bb.16.060187.001405. [DOI] [PubMed] [Google Scholar]
- 66.Badou A, et al. Critical role for the beta regulatory subunits of Cav channels in T lymphocyte function. Proc Natl Acad Sci U S A. 2006;103:15529–15534. doi: 10.1073/pnas.0607262103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kotturi MF, Jefferies WA. Molecular characterization of L-type calcium channel splice variants expressed in human T lymphocytes. Mol Immunol. 2005;42:1461–1474. doi: 10.1016/j.molimm.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 68.Stokes L, Gordon J, Grafton G. Non-voltage-gated L-type Ca2+ channels in human T cells: pharmacology and molecular characterization of the major alpha pore-forming and auxiliary beta-subunits. J Biol Chem. 2004;279:19566–19573. doi: 10.1074/jbc.M401481200. [DOI] [PubMed] [Google Scholar]
- 69.Jha MK, et al. Defective survival of naive CD8+ T lymphocytes in the absence of the beta3 regulatory subunit of voltage-gated calcium channels. Nat Immunol. 2009;10:1275–1282. doi: 10.1038/ni.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Omilusik K, et al. The Ca(V)1.4 Calcium Channel Is a Critical Regulator of T Cell Receptor Signaling and Naive T Cell Homeostasis. Immunity. 2011 doi: 10.1016/j.immuni.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 71.Cabral MD, et al. Knocking down Cav1 calcium channels implicated in Th2 cell activation prevents experimental asthma. Am J Respir Crit Care Med. 2010;181:1310–1317. doi: 10.1164/rccm.200907-1166OC. [DOI] [PubMed] [Google Scholar]
- 72.Park CY, Shcheglovitov A, Dolmetsch R. The CRAC channel activator STIM1 binds and inhibits L-type voltage-gated calcium channels. Science. 2010;330:101–105. doi: 10.1126/science.1191027. [DOI] [PubMed] [Google Scholar]
- 73.Wang Y, et al. The calcium store sensor, STIM1, reciprocally controls Orai and CaV1.2 channels. Science. 2010;330:105–109. doi: 10.1126/science.1191086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Striessnig J, Bolz HJ, Koschak A. Channelopathies in Cav1.1, Cav1.3, and Cav1.4 voltage-gated L-type Ca2+ channels. Pflugers Arch. 2010;460:361–374. doi: 10.1007/s00424-010-0800-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lewis RS, Cahalan MD. Potassium and calcium channels in lymphocytes. Annu Rev Immunol. 1995;13:623–653. doi: 10.1146/annurev.iy.13.040195.003203. [DOI] [PubMed] [Google Scholar]
- 76.Cahalan MD, Chandy KG. The functional network of ion channels in T lymphocytes. Immunol Rev. 2009;231:59–87. doi: 10.1111/j.1600-065X.2009.00816.x. Reference 81 is an excellent review from two of the pioneers studying ion channels in lymphocytes in which they describe the molecular properties and functions of ion channels in T cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cahalan MD, Chandy KG, DeCoursey TE, Gupta S. A voltage-gated potassium channel in human T lymphocytes. J Physiol. 1985;358:197–237. doi: 10.1113/jphysiol.1985.sp015548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bezanilla F. How membrane proteins sense voltage. Nat Rev Mol Cell Biol. 2008;9:323–332. doi: 10.1038/nrm2376. [DOI] [PubMed] [Google Scholar]
- 79.Xia XM, et al. Mechanism of calcium gating in small-conductance calcium-activated potassium channels. Nature. 1998;395:503–507. doi: 10.1038/26758. [DOI] [PubMed] [Google Scholar]
- 80.Srivastava S, et al. Phosphatidylinositol-3 phosphatase myotubularin-related protein 6 negatively regulates CD4 T cells. Mol Cell Biol. 2006;26:5595–5602. doi: 10.1128/MCB.00352-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Srivastava S, et al. The phosphatidylinositol 3-phosphate phosphatase myotubularin-related protein 6 (MTMR6) is a negative regulator of the Ca2+-activated K+ channel KCa3.1. Mol Cell Biol. 2005;25:3630–3638. doi: 10.1128/MCB.25.9.3630-3638.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Srivastava S, et al. Protein histidine phosphatase 1 negatively regulates CD4 T cells by inhibiting the K+ channel KCa3.1. Proc Natl Acad Sci USA. 2008;105:14442–14446. doi: 10.1073/pnas.0803678105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cai X, et al. Tripartite motif containing protein 27 negatively regulates CD4 T cells by ubiquitinating and inhibiting the class II PI3K-C2beta. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1111233109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Leonard RJ, Garcia ML, Slaughter RS, Reuben JP. Selective blockers of voltage-gated K+ channels depolarize human T lymphocytes: mechanism of the antiproliferative effect of charybdotoxin. Proc Natl Acad Sci U S A. 1992;89:10094–10098. doi: 10.1073/pnas.89.21.10094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ghanshani S, et al. Up-regulation of the IKCa1 potassium channel during T-cell activation. Molecular mechanism and functional consequences. J Biol Chem. 2000;275:37137–37149. doi: 10.1074/jbc.M003941200. [DOI] [PubMed] [Google Scholar]
- 86.Fanger C, Neben AL, Cahalan MD. Differential Ca2+ influx, KCa channel activity, and Ca2+ clearance distinguish Th1 and Th2 lymphocytes. J Immunol. 2000;164:1153–1160. doi: 10.4049/jimmunol.164.3.1153. [DOI] [PubMed] [Google Scholar]
- 87.Fanger CM, et al. Calcium-activated potassium channels sustain calcium signaling in T lymphocytes. Selective blockers and manipulated channel expression levels. J Biol Chem. 2001;276:12249–12256. doi: 10.1074/jbc.M011342200. [DOI] [PubMed] [Google Scholar]
- 88.Di L, et al. Inhibition of the K+ channel KCa3.1 ameliorates T cell-mediated colitis. Proc Natl Acad Sci U S A. 2010;107:1541–1546. doi: 10.1073/pnas.0910133107. Reference 94 shows diffential requirements for KCa3.1 and Kv1.3 for activation of Th1/Th2 and Th17 CD4 T helper cells and how targeting KCa3.1 can be used therapeutically to treat animal models of colitis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Beeton C, et al. Selective blockade of T lymphocyte K(+) channels ameliorates experimental autoimmune encephalomyelitis, a model for multiple sclerosis. Proc Natl Acad Sci U S A. 2001;98:13942–13947. doi: 10.1073/pnas.241497298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Beeton C, et al. Kv1.3 channels are a therapeutic target for T cell-mediated autoimmune diseases. Proc Natl Acad Sci U S A. 2006;103:17414–17419. doi: 10.1073/pnas.0605136103. Reference 90 demonstates that autoreactive CD4 T cells from patients with rheumatoid arthritis and type 1 diabetes melitus are T effector memory cells that express high levels of Kv1.3 channels and whose activation can be inhibited by Kv1.3 blockers in vitro and in animal models of these diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Castle NA. Pharmacological modulation of voltage-gated potassium channels as a therapeutic strategy. Expert Opin Ther Pat. 2010;20:1471–1503. doi: 10.1517/13543776.2010.513384. [DOI] [PubMed] [Google Scholar]
- 92.Chandy GK, et al. K+ channels as targets for specific immunomodulation. Trends Pharmacol Sci. 2004;25:280–289. doi: 10.1016/j.tips.2004.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Baell JB, et al. Khellinone derivatives as blockers of the voltage-gated potassium channel Kv1.3: synthesis and immunosuppressive activity. J Med Chem. 2004;47:2326–2336. doi: 10.1021/jm030523s. [DOI] [PubMed] [Google Scholar]
- 94.Kalman K, et al. ShK-Dap22, a potent Kv1.3-specific immunosuppressive polypeptide. J Biol Chem. 1998;273:32697–32707. doi: 10.1074/jbc.273.49.32697. [DOI] [PubMed] [Google Scholar]
- 95.Vennekamp J, et al. Kv1.3-blocking 5-phenylalkoxypsoralens: a new class of immunomodulators. Mol Pharmacol. 2004;65:1364–1374. doi: 10.1124/mol.65.6.1364. [DOI] [PubMed] [Google Scholar]
- 96.Wulff H, et al. Design of a potent and selective inhibitor of the intermediate-conductance Ca2+-activated K+ channel, IKCa1: a potential immunosuppressant. Proc Natl Acad Sci U S A. 2000;97:8151–8156. doi: 10.1073/pnas.97.14.8151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stocker JW, et al. ICA-17043, a novel Gardos channel blocker, prevents sickled red blood cell dehydration in vitro and in vivo in SAD mice. Blood. 2003;101:2412–2418. doi: 10.1182/blood-2002-05-1433. [DOI] [PubMed] [Google Scholar]
- 98.Koni PA, et al. Compensatory anion currents in Kv1.3 channel-deficient thymocytes. J Biol Chem. 2003;278:39443–39451. doi: 10.1074/jbc.M304879200. [DOI] [PubMed] [Google Scholar]
- 99.Wulff H, et al. The voltage-gated Kv1.3 K(+) channel in effector memory T cells as new target for MS. J Clin Invest. 2003;111:1703–1713. doi: 10.1172/JCI16921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fasth AE, Cao D, van Vollenhoven R, Trollmo C, Malmstrom V. CD28nullCD4+ T cells--characterization of an effector memory T-cell population in patients with rheumatoid arthritis. Scand J Immunol. 2004;60:199–208. doi: 10.1111/j.0300-9475.2004.01464.x. [DOI] [PubMed] [Google Scholar]
- 101.Friedrich M, et al. Flow cytometric characterization of lesional T cells in psoriasis: intracellular cytokine and surface antigen expression indicates an activated, memory/effector type 1 immunophenotype. Arch Dermatol Res. 2000;292:519–521. doi: 10.1007/s004030000167. [DOI] [PubMed] [Google Scholar]
- 102.Gilhar A, Bergman R, Assay B, Ullmann Y, Etzioni A. The beneficial effect of blocking Kv1.3 in the psoriasiform SCID mouse model. J Invest Dermatol. 2011;131:118–124. doi: 10.1038/jid.2010.245. [DOI] [PubMed] [Google Scholar]
- 103.Jager A, Kuchroo VK. Effector and regulatory T-cell subsets in autoimmunity and tissue inflammation. Scand J Immunol. 2010;72:173–184. doi: 10.1111/j.1365-3083.2010.02432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sallusto F, Lanzavecchia A. Human Th17 cells in infection and autoimmunity. Microbes Infect. 2009;11:620–624. doi: 10.1016/j.micinf.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 105.Vennekens R, Nilius B. Insights into TRPM4 function, regulation and physiological role. Handb Exp Pharmacol. 2007:269–285. doi: 10.1007/978-3-540-34891-7_16. [DOI] [PubMed] [Google Scholar]
- 106.Launay P, et al. TRPM4 regulates calcium oscillations after T cell activation. Science. 2004;306:1374–1377. doi: 10.1126/science.1098845. Reference 106 shows that TRPM4 mediates Na+ influx in Jurkat T cells, thereby inducing membrane depolarization and decreasing the driving force for Ca2+ entry. [DOI] [PubMed] [Google Scholar]
- 107.Weber KS, Hildner K, Murphy KM, Allen PM. Trpm4 differentially regulates Th1 and Th2 function by altering calcium signaling and NFAT localization. J Immunol. 2010;185:2836–2846. doi: 10.4049/jimmunol.1000880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vennekens R, et al. Increased IgE-dependent mast cell activation and anaphylactic responses in mice lacking the calcium-activated nonselective cation channel TRPM4. Nat Immunol. 2007;8:312–320. doi: 10.1038/ni1441. [DOI] [PubMed] [Google Scholar]
- 109.Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wenning AS, et al. TRP expression pattern and the functional importance of TRPC3 in primary human T-cells. Biochim Biophys Acta. 2011;1813:412–423. doi: 10.1016/j.bbamcr.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 111.Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Annu Rev Physiol. 2006;68:619–647. doi: 10.1146/annurev.physiol.68.040204.100431. [DOI] [PubMed] [Google Scholar]
- 112.Owsianik G, Talavera K, Voets T, Nilius B. Permeation and selectivity of TRP channels. Annu Rev Physiol. 2006;68:685–717. doi: 10.1146/annurev.physiol.68.040204.101406. [DOI] [PubMed] [Google Scholar]
- 113.Nilius B, Mahieu F, Karashima Y, Voets T. Regulation of TRP channels: a voltage-lipid connection. Biochem Soc Trans. 2007;35:105–108. doi: 10.1042/BST0350105. [DOI] [PubMed] [Google Scholar]
- 114.Wang J, et al. Cross-linking of GM1 ganglioside by galectin-1 mediates regulatory T cell activity involving TRPC5 channel activation: possible role in suppressing experimental autoimmune encephalomyelitis. J Immunol. 2009;182:4036–4045. doi: 10.4049/jimmunol.0802981. [DOI] [PubMed] [Google Scholar]
- 115.DeHaven WI, et al. TRPC channels function independently of STIM1 and Orai1. J Physiol. 2009;587:2275–2298. doi: 10.1113/jphysiol.2009.170431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sumoza-Toledo A, Penner R. TRPM2: a multifunctional ion channel for calcium signalling. J Physiol. 2011;589:1515–1525. doi: 10.1113/jphysiol.2010.201855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yamamoto S, Takahashi N, Mori Y. Chemical physiology of oxidative stress-activated TRPM2 and TRPC5 channels. Prog Biophys Mol Biol. 2010;103:18–27. doi: 10.1016/j.pbiomolbio.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 118.Beck A, Kolisek M, Bagley LA, Fleig A, Penner R. Nicotinic acid adenine dinucleotide phosphate and cyclic ADP-ribose regulate TRPM2 channels in T lymphocytes. FASEB J. 2006;20:962–964. doi: 10.1096/fj.05-5538fje. [DOI] [PubMed] [Google Scholar]
- 119.Guse AH, et al. Regulation of calcium signalling in T lymphocytes by the second messenger cyclic ADP-ribose. Nature. 1999;398:70–73. doi: 10.1038/18024. [DOI] [PubMed] [Google Scholar]
- 120.Di A, et al. The redox-sensitive cation channel TRPM2 modulates phagocyte ROS production and inflammation. Nat Immunol. 2011 doi: 10.1038/ni.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hara Y, et al. LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol Cell. 2002;9:163–173. doi: 10.1016/s1097-2765(01)00438-5. [DOI] [PubMed] [Google Scholar]
- 122.Yamamoto S, et al. TRPM2-mediated Ca2+ influx induces chemokine production in monocytes that aggravates inflammatory neutrophil infiltration. Nat Med. 2008;14:738–747. doi: 10.1038/nm1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Scarpa A, Brinley FJ. In situ measurements of free cytosolic magnesium ions. Fed Proc. 1981;40:2646–2652. [PubMed] [Google Scholar]
- 124.Modiano JF, Kelepouris E, Kern JA, Nowell PC. Requirement for extracellular calcium or magnesium in mitogen-induced activation of human peripheral blood lymphocytes. J Cell Physiol. 1988;135:451–458. doi: 10.1002/jcp.1041350312. [DOI] [PubMed] [Google Scholar]
- 125.Abboud CN, Scully SP, Lichtman AH, Brennan JK, Segel GB. The requirements for ionized calcium and magnesium in lymphocyte proliferation. J Cell Physiol. 1985;122:64–72. doi: 10.1002/jcp.1041220111. [DOI] [PubMed] [Google Scholar]
- 126.Li FY, et al. Second messenger role for Mg2+ revealed by human T-cell immunodeficiency. Nature. 2011;475:471–476. doi: 10.1038/nature10246. Reference 130 shows that a mutation in the Mg2+ transporter MagT1 impairs Mg2+ influx and indirectly Ca2+ influx in T cells resulting in CD4 lymphopenia and primary immunodeficiency (XMEN syndrome) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jin J, et al. Deletion of Trpm7 disrupts embryonic development and thymopoiesis without altering Mg2+ homeostasis. Science. 2008;322:756–760. doi: 10.1126/science.1163493. Reference 131 describes how conditional deletion of Trpm7 in T cells causes a block in thymocyte development and the depletion of thymic medullary cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bates-Withers C, Sah R, Clapham DE. TRPM7, the Mg(2+) inhibited channel and kinase. Adv Exp Med Biol. 2011;704:173–183. doi: 10.1007/978-94-007-0265-3_9. [DOI] [PubMed] [Google Scholar]
- 129.Ryazanova LV, et al. TRPM7 is essential for Mg(2+) homeostasis in mammals. Nat Commun. 2010;1:109. doi: 10.1038/ncomms1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Schlingmann KP, et al. Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat Genet. 2002;31:166–170. doi: 10.1038/ng889. [DOI] [PubMed] [Google Scholar]
- 131.Walder RY, et al. Mutation of TRPM6 causes familial hypomagnesemia with secondary hypocalcemia. Nat Genet. 2002;31:171–174. doi: 10.1038/ng901. [DOI] [PubMed] [Google Scholar]
- 132.Schmitz C, et al. Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell. 2003;114:191–200. doi: 10.1016/s0092-8674(03)00556-7. [DOI] [PubMed] [Google Scholar]
- 133.Bae CY, Sun HS. TRPM7 in cerebral ischemia and potential target for drug development in stroke. Acta Pharmacol Sin. 2011;32:725–733. doi: 10.1038/aps.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Goytain A, Quamme GA. Identification and characterization of a novel mammalian Mg2+ transporter with channel-like properties. BMC Genomics. 2005;6:48. doi: 10.1186/1471-2164-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhou H, Clapham DE. Mammalian MagT1 and TUSC3 are required for cellular magnesium uptake and vertebrate embryonic development. Proc Natl Acad Sci U S A. 2009;106:15750–15755. doi: 10.1073/pnas.0908332106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Haase H, Rink L. Functional significance of zinc-related signaling pathways in immune cells. Annu Rev Nutr. 2009;29:133–152. doi: 10.1146/annurev-nutr-080508-141119. [DOI] [PubMed] [Google Scholar]
- 137.Hirano T, et al. Roles of zinc and zinc signaling in immunity: zinc as an intracellular signaling molecule. Adv Immunol. 2008;97:149–176. doi: 10.1016/S0065-2776(08)00003-5. References 136–137 provide an excellent overview of the role of Zn2+ as an intracellular signaling molecule in immune cells. [DOI] [PubMed] [Google Scholar]
- 138.Honscheid A, Rink L, Haase H. T-lymphocytes: a target for stimulatory and inhibitory effects of zinc ions. Endocr Metab Immune Disord Drug Targets. 2009;9:132–144. doi: 10.2174/187153009788452390. [DOI] [PubMed] [Google Scholar]
- 139.Haase H, Hebel S, Engelhardt G, Rink L. Flow cytometric measurement of labile zinc in peripheral blood mononuclear cells. Anal Biochem. 2006;352:222–230. doi: 10.1016/j.ab.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 140.Rukgauer M, Klein J, Kruse-Jarres JD. Reference values for the trace elements copper, manganese, selenium, and zinc in the serum/plasma of children, adolescents, and adults. J Trace Elem Med Biol. 1997;11:92–98. doi: 10.1016/S0946-672X(97)80032-6. [DOI] [PubMed] [Google Scholar]
- 141.Kaltenberg J, et al. Zinc signals promote IL-2-dependent proliferation of T cells. Eur J Immunol. 2010;40:1496–1503. doi: 10.1002/eji.200939574. [DOI] [PubMed] [Google Scholar]
- 142.Aydemir TB, Liuzzi JP, McClellan S, Cousins RJ. Zinc transporter ZIP8 (SLC39A8) and zinc influence IFN-gamma expression in activated human T cells. J Leukoc Biol. 2009;86:337–348. doi: 10.1189/jlb.1208759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yu M, et al. Regulation of T cell receptor signaling by activation-induced zinc influx. J Exp Med. 2011;208:775–785. doi: 10.1084/jem.20100031. Reference 147 shows that TCR stimulation results in localized Zn2+ influx at the immune synapse between T cells and DC, and that Zn2+ influx and T cell activation depend on ZIP6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kury S, et al. Identification of SLC39A4, a gene involved in acrodermatitis enteropathica. Nat Genet. 2002;31:239–240. doi: 10.1038/ng913. [DOI] [PubMed] [Google Scholar]
- 145.Wang K, Zhou B, Kuo YM, Zemansky J, Gitschier J. A novel member of a zinc transporter family is defective in acrodermatitis enteropathica. Am J Hum Genet. 2002;71:66–73. doi: 10.1086/341125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ackland ML, Michalczyk A. Zinc deficiency and its inherited disorders -a review. Genes Nutr. 2006;1:41–49. doi: 10.1007/BF02829935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fraker PJ, King LE. Reprogramming of the immune system during zinc deficiency. Annu Rev Nutr. 2004;24:277–298. doi: 10.1146/annurev.nutr.24.012003.132454. [DOI] [PubMed] [Google Scholar]
- 148.Kirchner H, Ruhl H. Stimulation of human peripheral lymphocytes by Zn2+ in vitro. Exp Cell Res. 1970;61:229–230. doi: 10.1016/0014-4827(70)90284-3. [DOI] [PubMed] [Google Scholar]
- 149.Fraker PJ, Jardieu P, Cook J. Zinc deficiency and immune function. Arch Dermatol. 1987;123:1699–1701. [PubMed] [Google Scholar]
- 150.Wellinghausen N, Martin M, Rink L. Zinc inhibits interleukin-1-dependent T cell stimulation. Eur J Immunol. 1997;27:2529–2535. doi: 10.1002/eji.1830271010. [DOI] [PubMed] [Google Scholar]
- 151.Tanaka S, Akaishi E, Hosaka K, Okamura S, Kubohara Y. Zinc ions suppress mitogen-activated interleukin-2 production in Jurkat cells. Biochem Biophys Res Commun. 2005;335:162–167. doi: 10.1016/j.bbrc.2005.07.059. [DOI] [PubMed] [Google Scholar]
- 152.Kim PW, Sun ZY, Blacklow SC, Wagner G, Eck MJ. A zinc clasp structure tethers Lck to T cell coreceptors CD4 and CD8. Science. 2003;301:1725–1728. doi: 10.1126/science.1085643. [DOI] [PubMed] [Google Scholar]
- 153.Huang J, et al. An approach to assay calcineurin activity and the inhibitory effect of zinc ion. Anal Biochem. 2008;375:385–387. doi: 10.1016/j.ab.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 154.Takahashi K, et al. Zinc inhibits calcineurin activity in vitro by competing with nickel. Biochem Biophys Res Commun. 2003;307:64–68. doi: 10.1016/s0006-291x(03)01122-7. [DOI] [PubMed] [Google Scholar]
- 155.Lichten LA, Cousins RJ. Mammalian zinc transporters: nutritional and physiologic regulation. Annu Rev Nutr. 2009;29:153–176. doi: 10.1146/annurev-nutr-033009-083312. [DOI] [PubMed] [Google Scholar]
- 156.Dufner-Beattie J, Huang ZL, Geiser J, Xu W, Andrews GK. Generation and characterization of mice lacking the zinc uptake transporter ZIP3. Mol Cell Biol. 2005;25:5607–5615. doi: 10.1128/MCB.25.13.5607-5615.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Overbeck S, Uciechowski P, Ackland ML, Ford D, Rink L. Intracellular zinc homeostasis in leukocyte subsets is regulated by different expression of zinc exporters ZnT-1 to ZnT-9. J Leukoc Biol. 2008;83:368–380. doi: 10.1189/jlb.0307148. [DOI] [PubMed] [Google Scholar]
- 158.Nishida K, et al. Zinc transporter Znt5/Slc30a5 is required for the mast cell-mediated delayed-type allergic reaction but not the immediate-type reaction. J Exp Med. 2009;206:1351–1364. doi: 10.1084/jem.20082533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cahalan MD, Lewis RS. Role of potassium and chloride channels in volume regulation by T lymphocytes. Soc Gen Physiol Ser. 1988;43:281–301. [PubMed] [Google Scholar]
- 160.Lewis RS, Ross PE, Cahalan MD. Chloride channels activated by osmotic stress in T lymphocytes. J Gen Physiol. 1993;101:801–826. doi: 10.1085/jgp.101.6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lepple-Wienhues A, et al. The tyrosine kinase p56lck mediates activation of swelling-induced chloride channels in lymphocytes. J Cell Biol. 1998;141:281–286. doi: 10.1083/jcb.141.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Szabo I, et al. Tyrosine kinase-dependent activation of a chloride channel in CD95-induced apoptosis in T lymphocytes. Proc Natl Acad Sci U S A. 1998;95:6169–6174. doi: 10.1073/pnas.95.11.6169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tian J, et al. Gamma-aminobutyric acid inhibits T cell autoimmunity and the development of inflammatory responses in a mouse type 1 diabetes model. J Immunol. 2004;173:5298–5304. doi: 10.4049/jimmunol.173.8.5298. [DOI] [PubMed] [Google Scholar]
- 164.Mendu SK, et al. Increased GABA(A) channel subunits expression in CD8(+) but not in CD4(+) T cells in BB rats developing diabetes compared to their congenic littermates. Mol Immunol. 2011;48:399–407. doi: 10.1016/j.molimm.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 165.Carter CR, Kozuska JL, Dunn SM. Insights into the structure and pharmacology of GABA(A) receptors. Future Med Chem. 2010;2:859–875. doi: 10.4155/fmc.10.178. [DOI] [PubMed] [Google Scholar]
- 166.Bhat R, et al. Inhibitory role for GABA in autoimmune inflammation. Proc Natl Acad Sci U S A. 2010;107:2580–2585. doi: 10.1073/pnas.0915139107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bjurstom H, et al. GABA, a natural immunomodulator of T lymphocytes. J Neuroimmunol. 2008;205:44–50. doi: 10.1016/j.jneuroim.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 168.Tian J, Chau C, Hales TG, Kaufman DL. GABA(A) receptors mediate inhibition of T cell responses. J Neuroimmunol. 1999;96:21–28. doi: 10.1016/s0165-5728(98)00264-1. [DOI] [PubMed] [Google Scholar]
- 169.Bergeret M, et al. GABA modulates cytotoxicity of immunocompetent cells expressing GABAA receptor subunits. Biomed Pharmacother. 1998;52:214–219. doi: 10.1016/S0753-3322(98)80019-X. [DOI] [PubMed] [Google Scholar]
- 170.Tian J, Yong J, Dang H, Kaufman DL. Oral GABA treatment downregulates inflammatory responses in a mouse model of rheumatoid arthritis. Autoimmunity. 2011;44:465–470. doi: 10.3109/08916934.2011.571223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Moss RB, et al. Reduced IL-10 secretion by CD4+ T lymphocytes expressing mutant cystic fibrosis transmembrane conductance regulator (CFTR) Clin Exp Immunol. 1996;106:374–388. doi: 10.1046/j.1365-2249.1996.d01-826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chen JH, Schulman H, Gardner P. A cAMP-regulated chloride channel in lymphocytes that is affected in cystic fibrosis. Science. 1989;243:657–660. doi: 10.1126/science.2464852. [DOI] [PubMed] [Google Scholar]
- 173.Mueller C, et al. Lack of cystic fibrosis transmembrane conductance regulator in CD3+ lymphocytes leads to aberrant cytokine secretion and hyperinflammatory adaptive immune responses. Am J Respir Cell Mol Biol. 2011;44:922–929. doi: 10.1165/rcmb.2010-0224OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Camerino DC, Tricarico D, Desaphy JF. Ion channel pharmacology. Neurotherapeutics. 2007;4:184–198. doi: 10.1016/j.nurt.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 175.Naylor J, Milligan CJ, Zeng F, Jones C, Beech DJ. Production of a specific extracellular inhibitor of TRPM3 channels. Br J Pharmacol. 2008;155:567–573. doi: 10.1038/bjp.2008.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Xu SZ, et al. Generation of functional ion-channel tools by E3 targeting. Nat Biotechnol. 2005;23:1289–1293. doi: 10.1038/nbt1148. [DOI] [PubMed] [Google Scholar]
- 177.Chandy KG, DeCoursey TE, Cahalan MD, McLaughlin C, Gupta S. Voltage-gated potassium channels are required for human T lymphocyte activation. J Exp Med. 1984;160:369–385. doi: 10.1084/jem.160.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.McNally BA, Yamashita M, Engh A, Prakriya M. Structural determinants of ion permeation in CRAC channels. Proc Natl Acad Sci U S A. 2009;106:22516–22521. doi: 10.1073/pnas.0909574106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zhou Y, Ramachandran S, Oh-Hora M, Rao A, Hogan PG. Pore architecture of the ORAI1 store-operated calcium channel. Proc Natl Acad Sci U S A. 2010;107:4896–4901. doi: 10.1073/pnas.1001169107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Prakriya M, et al. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–233. doi: 10.1038/nature05122. References 180, 181 and 183 demonstrate that ORAI1 (CRACM1) is the pore-forming subunit of the CRAC channel by identifying a glutamate residue in the first transmembrane domain of ORAI1 as the selectivity filter of the CRAC channel. [DOI] [PubMed] [Google Scholar]
- 181.Vig M, et al. CRACM1 multimers form the ion-selective pore of the CRAC channel. Curr Biol. 2006;16:2073–2079. doi: 10.1016/j.cub.2006.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yamashita M, Navarro-Borelly L, McNally BA, Prakriya M. Orai1 mutations alter ion permeation and Ca2+-dependent inactivation of CRAC channels: evidence for coupling of permeation and gating. J Gen Physiol. 2007;130:525–540. doi: 10.1085/jgp.200709872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yeromin AV, et al. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443:226–229. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kawasaki T, Lange I, Feske S. A minimal regulatory domain in the C terminus of STIM1 binds to and activates ORAI1 CRAC channels. Biochem Biophys Res Commun. 2009;385:49–54. doi: 10.1016/j.bbrc.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mullins FM, Park CY, Dolmetsch RE, Lewis RS. STIM1 and calmodulin interact with Orai1 to induce Ca2+-dependent inactivation of CRAC channels. Proc Natl Acad Sci U S A. 2009;106:15495–15500. doi: 10.1073/pnas.0906781106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Muik M, et al. A Cytosolic Homomerization and a Modulatory Domain within STIM1 C Terminus Determine Coupling to ORAI1 Channels. J Biol Chem. 2009;284:8421–8426. doi: 10.1074/jbc.C800229200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Yuan JP, et al. SOAR and the polybasic STIM1 domains gate and regulate Orai channels. Nat Cell Biol. 2009;11:337–343. doi: 10.1038/ncb1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Tschopp J, Schroder K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat Rev Immunol. 2010;10:210–215. doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- 189.Liuzzi JP, Cousins RJ. Mammalian zinc transporters. Annu Rev Nutr. 2004;24:151–172. doi: 10.1146/annurev.nutr.24.012003.132402. [DOI] [PubMed] [Google Scholar]