Abstract
This study evaluated the in vitro cytotoxicity of poly(propylene fumarate) (PPF). PPF is an aliphatic biodegradable polymer that has been well characterized for use in bone tissue engineering scaffolds. Four different cell types, human mesenchymal stem cells (hMSC), fibroblasts (L929), pre-osteoblasts (MC3T3), and canine mesenchymal stem cells (cMSC), were used to evaluate the cytotoxicity of PPF. These cell types represent the tissues that PPF would interact with in vivo as a bone tissue scaffold. The sol fraction of the PPF films was measured and then utilized to estimate crosslinking density. Cytotoxicity was evaluated using XTT assay and fluorescence imaging. Results showed that PPF supported similar cell metabolic activities of hMSC, L929, MC3T3 and cMSC compared to the non-cytotoxic control, high density polyethylene (HDPE) and were statistically different than those cultured with the cytotoxic control, a polyurethane film containing 0.1% zinc diethyldithiocarbamate (ZCF). Results showed differing cellular responses to ZCF, the cytotoxic control. The L929 cells had the lowest cell metabolic activity levels after exposure to ZCF compared to the cell metabolic activity levels of the MC3T3, hMSC or cMSC cells. Qualitative verification of the results using fluorescence imaging demonstrated no change in cell morphology, vacuolization, or detachment when cultured with PPF compared to HDPE or blank media cultures. Overall the cytotoxicity response of the cells to PPF was demonstrated to be similar to the cytotoxic response of cells to known non-cytotoxic materials (HDPE).
Keywords: cytotoxicity, poly(propylene fumarate), hMSC, MC3T3
Introduction
Cell and tissue response are key factors in the design and application of successful biomaterials. One method to evaluate cell and tissue response is to measure in vitro cytotoxicity, or its quality of being toxic to a cell. Cell toxicity is determined by cell lysis (death) or the inhibition of cell proliferation. Prior to investigating a material in vivo, cytotoxicity can provide insight to any potential issues with the local tissue response.
For bone tissue regeneration key factors in designing ideal biomaterials include mechanical strength, biocompatibility, and consistent mechanical performance during degradation1. Poly(propylene fumarate) (PPF) is a well characterized polymer that has been demonstrated to fit these characteristics2,3. PPF is an aliphatic polyester consisting of a carbon-carbon double bond, flanked by two ester groups (Figure 1). Covalent crosslinking of PPF occurs through the unsaturated carbon bond on the fumarate functional group either by thermal- or photo-initiation. Hydrolytic degradation of the ester bond produces fumaric acid and propylene glycol as byproducts4. As fumaric acid is a known byproduct of the Kreb’s cycle and propylene glycol is commonly used as a food additive, both of these degradation products are thought to be nontoxic in low concentrations1.
Figure 1. A Schematic of Poly(propylene fumarate).
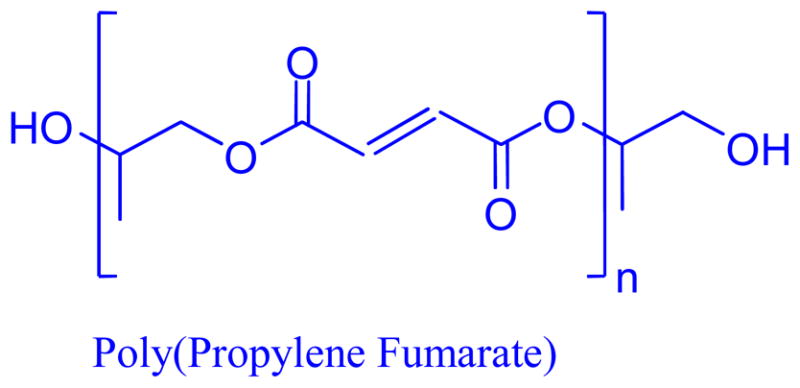
Poly(propylene fumarate) contains a repeating unit of two ester groups flanking a carbon-carbon double bond.
Previous studies have evaluated the cell and tissue response and degradability of thermally crosslinked PPF and have found it to be acceptable for in vivo implantation with responses ranging from a lack of an inflammatory response to a mild inflammatory response5–7. Although previous studies have evaluated the toxicity of thermally crosslinked PPF they were performed either using in vivo models or when using an in vitro model, they did not implement the previously developed standards for in vitro cytotoxicity. With the further development of PPF as a photocrosslinkable polymer, many studies have evaluated the use of PPF as a coating for cortical bone implants, a scaffold to repair critical sized bone defects, and as a delivery method for signaling factors8–11. Additional studies have evaluated the in vitro degradation of photocrosslinked PPF12. In vivo studies of photocrosslinked PPF have identified it as having a mild tissue response initially following implantation but after 8 weeks a reduction in this response was observed13. Previous work has also identified that un-crosslinked PPF co-polymers (PPF/PPF-diacrylate (PPF/PPF-DA)) are highly cytotoxic (viability <3%), compared to crosslinked networks; whereas crosslinked PPF networks had cell viabilities >80%14. This study investigates the in vitro cytotoxicity of PPF that has been photocrosslinked using the photoinitiator bis(2,4,6-trimethylbenzoyl) phenylphosphine oxide (BAPO) using the ISO 10993-5 standards.
We hypothesized that PPF will have a low cytotoxic response as its degradation byproducts are nontoxic, and previous research has demonstrated biocompatibility using other crosslinking methods. To test this we investigated the cellular response of four cell types: fibroblasts (L929), pre-osteoblasts (MC3T3) and mesenchymal stem cells (human and canine) (hMSC, cMSC) to PPF. The cell types studied where chosen to represent the many tissues that PPF will interact with in vivo during bone regeneration.
Experimental Section: Materials and methods
Poly(propylene fumarate) synthesis and film fabrication
Poly(propylene fumarate) was synthesized in a two-step process as described previously15. Briefly, propylene glycol and diethyl fumarate were combined in a 3:1 molar ratio. Zinc chloride and hydroquinone were added in a 0.01:0.002 molar ratio to act as catalyst and radical inhibitor, respectively. The solution was reacted under a flow of nitrogen gas producing ethanol as a byproduct and bis(hydroxypropyl) as the intermediate. The second step is a transesterification of the intermediate, performed under a vacuum, to produce PPF with propylene glycol as a byproduct. Gel permeation chromatography was used to calculate the number average molecular weight (Mn) and polydispersity index (PDI) of the purified PPF. For the 3 hour UV crosslinked PPF (180M PPF) Mn = 1100g/mol and PDI = 2.7; for the PPF films crosslinked using 30 minute UV exposure (30M PPF) the Mn = 1290g/mol and PDI = 2.01. Thin films of PPF were photocrosslinked using BAPO as an initiator according to previously reported methods16. A solution of 4g BAPO in 10 mL methylene chloride was prepared. The PPF mixture was spread evenly onto a glass plate and placed into the oven to spread for 2 minutes. A glass plate was depressed on top of the PPF mixture to create a thin film. The two plates were then placed in a UV cross-linking light box for 3 hours (180M) or 30 minutes (30M). The films were then washed in phosphate buffered saline (PBS) for 15 minutes to remove surface debris followed by a 30 minutes wash in acetone to remove soluble components and then washed twice, 15 minutes in PBS to remove any remaining acetone. One group was left un-washed to evaluate the soluble components of the 30M film (UN-30M).
Sol Fraction and Crosslinking Density
To assess the crosslinking density the sol fraction was measured per the previously described method2. Samples of the photocrosslinked film were weighed (Wi) prior to incubation in acetone, the solvent. The samples were then submerged in the solvent for 24 h. After incubation, samples were dried overnight and weighed again (Wd). Sol fraction was calculated using the formula
Crosslinking density (q) was then approximated using the Charlesby-Pinner equation and the relationship between crosslinking coefficient (δ) and the weight average degree of polymerization (Xw). Sol fraction and crosslinking coefficient are related by the Charlesby-Pinner equation which assumes the following: a high degree of crosslinking without main chain scission, the initial molecular weight distribution is random (PDI ≈ 2), that the structure of the polymer does not affect crosslinking or main chain scission, and that the degree of crosslinking and main-chain scission is proportional to the radiation dose. From the crosslinking coefficient, the weight average degree of polymerization (Xw), derived from Mw, and Mo, the molecular weight of the monomer unit (156.19Da), the crosslinking density was approximated using the following formula28,17
Material Preparation
Tests were performed using either a 12 well or 24 well tissue culture polystyrene plate (Corning, Corning, NY) with surface areas of 3.8cm2 or 1.9cm2, respectively. High-density polyethylene (HDPE) (U.S. Plastic Corp, Lima, OH) and polyurethane film containing 0.1% zinc diethyldithiocarbamate (ZCF) (Hatano Research Institute, Food and Drug Safety Center, Kanagawa Japan) samples were measured to a minimum of 38mm2 or 20mm2, for the 12 well or 24 well tests, respectively, to ensure that at least 10% of the surface area of the well was covered by the material. After washing and drying, the PPF was apportioned using a calculation of the density of PPF ρ, (ρ = 1.3g/cm3), film thickness (t), and the required area of sample (A) using the formula
This formula ensured that the surface area of each sample was at least 10% of the total well surface area. Each material used was sterilized at 121°C for 15 minutes prior to use in cell culture. For extract studies the method used is the same, but the required sample mass is halved because both sides of the sample are exposed to media.
Cell Culture
Four cell types were evaluated: L929 (ATCC, Manassas, VA), MC3T3 (ATCC), hMSC (Lonza, Walkersville, MD), and cMSC (Case Western Reserve University, Cleveland, OH). L929, mouse fibroblasts, are suggested for use per ISO Standard 10993-5. L929 cells were cultured per the manufacturer’s specifications with Minimum Essential Medium (αMEM) (Life Technologies, Frederick, MD) and 10% horse serum (Life Technologies). MC3T3, a mouse osteoblast precursor cell, were cultured per the manufacturer’s specifications with alpha Minimum Essential Medium (aMEM) (Life Technologies) containing ribonucleosides, deoxyribonucleosides, 2 mM L-glutamine (Life Technologies), 1 mM sodium pyruvate (Life Technologies), and 10% fetal bovine serum (FBS) (Life Technologies). The hMSCs were cultured as previously described and per the manufacturer’s protocol, with Dulbeccos Modified Eagle Medium (DMEM) (Life Technologies), supplemented with 10% fetal bovine serum (Life Technologies), 1.0% v/v penicillin/streptomycin (Life Technologies), 0.1mM non-essential amino acids (Life Technologies), and 4mM L-glutamine (Life Technologies)18,19. The cMSCs were cultured with low glucose DMEM (Life Technologies) containing 10ng/mL of fibroblast growth factor and 10% FBS. Cells were plated and grown to ~80% confluency prior to initiating the assays.
Cytotoxicity Assays
For all assays HDPE (U.S. Plastic Corp.) was used as a negative, or non-cytotoxic, control. Cells cultured under normal, or blank conditions and without any material were used as a blank control (blank). For the direct and indirect testing a polyurethane film containing 0.1% zinc diethyldithiocarbamate (ZCF) was used as a positive, or a cytotoxic, control which has been shown to provide a reproducible cytotoxic response20,21. For the extract testing a 70% dilution of methanol was used as a cytotoxic control.
Following ISO standard 10993-5 three different culturing methods were implemented to evaluate if there is a cytotoxic response to PPF: direct contact, indirect contact and extract tests. For the direct contact test (Figure 2A) cells were plated and cultured per the methods described above. The direct contact test allows for the physical interaction of the cells and the material. The test was initiated by placing the material onto the cell monolayer. The material was incubated at 37°C and 5% CO2 for 24 h after which the cytotoxicity of the material was evaluated qualitatively with fluorescence microscopy and quantitatively through the XTT cell metabolic activity assay (Roche, Mainheim, Germany). To reduce disrupting the cell monolayer the materials were removed using a Pasteur pipet attached to a vacuum line so that the material and the media were removed simultaneously.
Figure 2. A Schematic of the Cytotoxicity Tests.
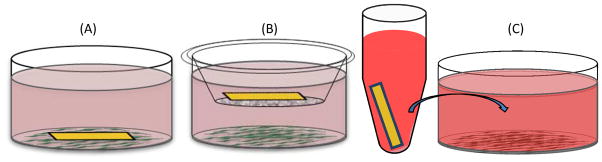
(A) Direct contact test where cells are seeded and the material is placed directly on top of cell sheet. (B) Indirect contact test where the material is placed into a transwell insert, which is cultured with cells seeded on the bottom of the well plate. (C) Extract test where the material is incubated in the appropriate culture media for 24 h and then the media is transferred to replace the culture media on cells seeded in a well plate.
The indirect contact test (Figure 2B) allows for the interaction of any leachable byproducts to interact with the cell monolayer without direct contact of the material. The materials were placed into a transwell microplate membrane insert (3.0μm size exclusion) (Corning, Corning, NY) above the cell surface and submerged in the culture media. The treatment groups were incubated at 37°C and 5% CO2 for 24 h prior to cytotoxic evaluation with XTT cell metabolic activity assay and fluorescence microscopy.
The extract test (Figure 2C) evaluates the cytotoxicity of any leachable byproducts from the material by the simulation of clinical application. Cells were plated and grown to 80% confluency prior to initiating the assay. The materials (PPF and HDPE) were incubated with the appropriate culture media at a concentration of 3cm2/mL for 24 h. After 24 h, the cell culture media was removed and replaced with the extract media. Cells were then incubated at 37°C and 5% CO2 for 24 h prior to cytotoxic evaluation with XTT cell metabolic activity assay and fluorescence microscopy. For the cytotoxic control the culture media was removed and cells were incubated with 70% methanol for 30 minutes prior to evaluation of cytotoxicity.
XTT Assay
The Cell Proliferation Kit II (XTT) (Roche, Mainheim, Germany) was used to quantitatively evaluate cell metabolic activity. XTT (2,3-bis-(2- methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide) was used according to the manufacturer’s protocols. The electron coupling and XTT labeling reagents were thawed and immediately combined in a 1μl:50μL ratio. Then the XTT solution was added to the cell culture wells, 500μl or 1mL for a 24 well or a 12 well plate, respectively. Absorbance was measured after 4 hours of incubation at 37°C with a M5 SpectraMax plate reader (Molecular Devices, Sunnyvale, CA). Net absorbance was calculated (A450–A650) for each sample of the three biological replicates. Relative cell metabolic activity was normalized to the mean of the blank culture media. Samples were evaluated, the mean cell metabolic activity and standard deviations are reported (n=5).
Osmolality
The osmolality of the cell culture media was measured using the Advanced™ Micro Osmometer (Advanced Instruments, Inc, Norwood, MA) using freezing point depression. The osmolality of the cell culture media measured after 24 hours of direct contact with the material, per the direct contact test. A 20μL sample was used to measure the total molar concentration of dissolved solids, three samples were used per treatment group (n=3).
Fluorescence Imaging
Live/dead imaging was performed to qualitatively evaluate cell viability as described previously22. A live/dead solution was prepared with 4μM of calcein AM (Invitrogen, Carlsbad, CA) and 2μM of ethidium homodimer (Invitrogen, Carlsbad, CA) in PBS. Prior to the addition of the live/dead stain, cells were washed with PBS to remove any remaining culture media and FBS. Cells were incubated with the live/dead solution in dark conditions for 30 minutes prior to imaging. For the positive, or cytotoxic, control, cells were incubated with 70% methanol 30 minutes prior to the addition of the live/dead solution. Images were obtained with a fluorescence microscope (Axiovert 40CFL, filter set 23, Zeiss, Thornwood, NY) fitted with a digital camera (SPOT Insight 1120, or SPOT Idea 2920, Diagnostics Instruments, Sterling Heights, MI) and with an inverted TE2000-E microscope (Nikon, Melville, NY) outfitted with a CoolSnap HQ2 (Photometrics, Tucson, AZ) digital camera.
Statistics
Statistical analysis was performed using ANOVA and Tukey’s multiple pairwise comparison (p<0.05). All tests were performed in triplicate (n=3) unless otherwise specified. Values provided are mean ± standard deviation. Please note that only relevant statistical relationships are denoted on figures.
Experimental Section: Results
The objective of this work was to evaluate if there is a cytotoxic response to PPF. For each of the three cytotoxicity tests (direct, indirect, and extract) the cell metabolic activities of the cells exposed to 180M PPF were found to be statistically different than those exposed to the cytotoxic control, ZCF, and not statistically different from the cells exposed to HDPE, and blank culture media. Additionally, no changes in cell viability, morphology, vacuolization or detachment were observed in the cells exposed to 180M PPF, HDPE or blank culture media.
Sol fraction was measured and used to estimate the crosslinking density of the PPF films used for cytotoxicity evaluation. For the 180M PPF films the sol fraction was found to be 3% ±2% (n=7) (Table 1) and the crosslinking density was estimated to be 58% ±25% (n=7) (Table 2). The sol fraction for sterilized 180M PPF, 4% ± 3% (n=4), and pre-sterilized 180M PPF, 3% ± 0% (n=3), were found to be statistically similar (Table 1). The sol fraction for 30M PPF films was found to be 53% ± 4% (n=3) (Table 1) and the crosslinking density was calculated to be 10%± 0% (n=3) (Table 2). The crosslinking densities and the sol fractions of the 180M and 30M films were found to be statistically different (p<0.05).
Table 1. Sol Fraction of PPF.
Sol fraction was measured to calculate crosslinking density. The 180M PPF films were evaluated pre-sterilization and post sterilization to ensure that sterilization did not have an impact on the sol fraction. These groups were found to be statistically similar, and therefore it was determined that sterilization did not have an impact on the sol fraction. The 30M PPF films have a sol fraction significantly greater than the 180M PPF films (p<0.05)
| Sol Fraction of PPF | |
|---|---|
| 180M PPF (n=7) | 3% ± 2% |
|
| |
| Pre-sterilization PPF (n=3) | 3% ± 0% |
| Sterilized PPF (n=4) | 4% ± 3% |
|
| |
| 30M PPF (n=3) | 53% ± 4% |
Table 2. Crosslinking Density of PPF.
Crosslinking density was then calculated from the sol fraction using the Charlesby-Pinner equation, crosslinking coefficient, the weight average degree of polymerization (Xw). as described previously17,27,28. The difference in crosslinking density between the 180M PPF and the 30M PPF films was found to be statistically significant (p<0.05)
| Crosslinking Density of PPF | |
|---|---|
| 180M Crosslinked PPF (n=7) | 58% ± 25% |
| 30M Crosslinked PPF (n=3) | 10% ± 0% |
The cytotoxicity of 30M, UN-30M, and 180M PPF films was investigated using the direct contact test. There was a statistical difference in the cell metabolic activities of L929 cells cultured with 30M, 180M, and UN-30M PPF films, and the blank culture media when compared to the cell metabolic activity of the cells cultured with the cytotoxic control, ZCF (Figure 3A). The cell metabolic activities of the UN-30M PPF and the blank culture media were found to be statistically different (Figure 3A). The cell metabolic activities were 100.0 ± 8.6% (blank) 95.4 ± 8.7% (180M), 90.2 ± 17.6% (30M PPF), and 75.2 ± 24.7% (UN-30M PPF). Qualitative verification showed an increase in cell death with a large number of detached cells and dead cells in the UN-30M PPF treatment group (Figure 3B).
Figure 3. Cytotoxicity of 30M PPF. A: Cell Metabolic Activity.
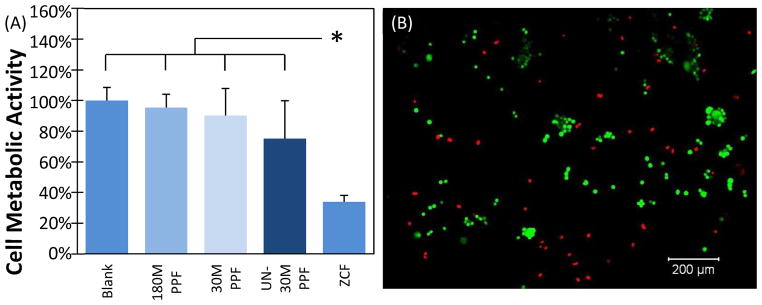
Cell metabolic activity for L929 cells cultured with 180M PPF, 30M PPF, UN-30M PPF and those cultured with only the culture media (Blank) were found to be statistically different from those cultured with the cytotoxic control (p<0.05). The (*) symbol represent a statistical difference between ZCF and all other groups (p<0.05). B: Fluorescent images of L929 cells. Calcein AM (green) represents live cells, and ethidium homodimer (red) represents dead cells. Cells incubated with UN-30M PPF showed increased cell detachment and cell death.
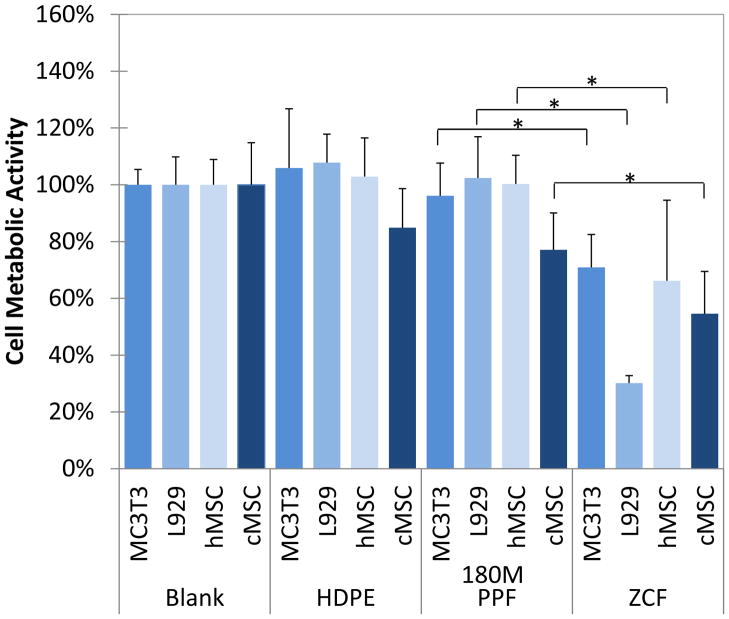
For the direct contact test, 180M PPF was shown to support a similar level of cell metabolic activity as HDPE, a material previously designated as non-toxic. Also, the cell metabolic activities of cells cultured with 180M PPF and to those cultured with blank culture media were found not to be statistically different (Figure 4). The L929 cells had the highest cell metabolic activity of 102.4 ± 14.6% when directly cultured with 180M PPF. The cell metabolic activities were found to be 96.1 ± 11.5% (MC3T3), 100.3± 10.1% (hMSC), and 77.1 ± 13.0% (cMSC). These results were statistically different (p<0.05) from the cells cultured with the cytotoxic control, ZCF.
Figure 4. Cell Metabolic Activity (Direct Contact).
Four different cell populations (MC3T3, L929, hMSC, and cMSC) were cultured in monolayer and in direct contact with HDPE, 180M PPF, ZCF, or nothing (Blank). Each cell type cultured in direct contact with 180M PPF was found to have significantly higher metabolic activity when compared to those in contact with the positive, cytotoxic control, ZCF (p<0.05, * designated a statistical difference between groups). There were no statistical differences found between cells cultured with 180M PPF, HDPE or under blank culture media.
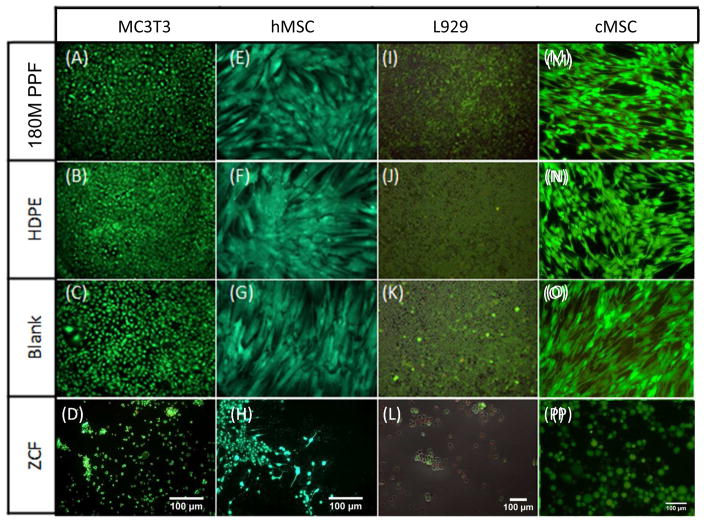
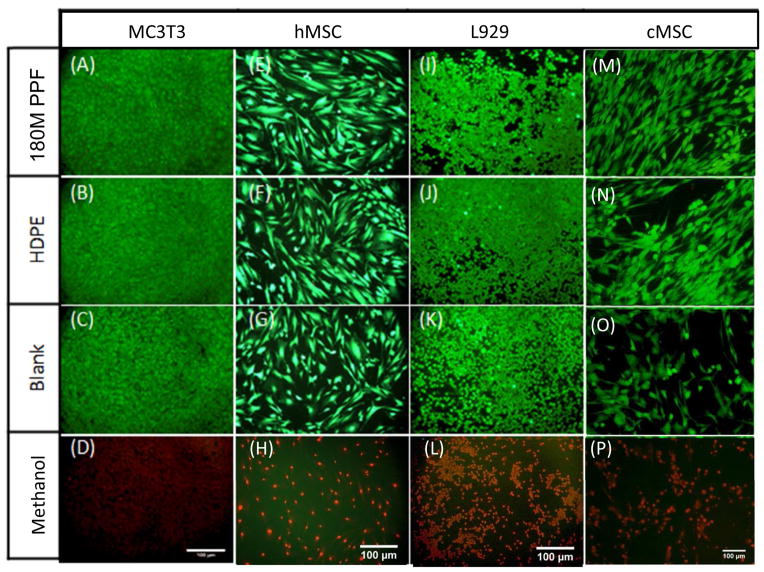
Fluorescence imaging was used to confirm the XTT assay results. No changes in cell morphologies were seen in cells cultured directly with 180M PPF (Figures 5A–5D). hMSCs that were directly exposed to 180M PPF were observed to have a spread, spindle-like morphology and appeared to be fully attached to the culture plate surface (Figure 5A). This spread, elongated morphology was consistent with cells that were directly exposed to HDPE (Figure 5E) and those that were incubated with blank culture media (Figure 5I). Imaging of L929 cells revealed that a normal, round morphology and confluent cell monolayer were maintained after direct incubation with 180M PPF (Figure 5C). The spread, confluent morphology that was observed per each cell type (Figures 5A–5L) was notably different than the robust amount of cell detachment and cell death that was observed for cells exposed to ZCF, the cytotoxic control. Cell detachment and morphological change was observed (Figures 5M–5P) during the direct contact test.
Figure 5. Direct Contact Test.
Fluorescent images of cells, where calcein AM (green) represents live cells, and ethidium homodimer (red) represents dead cells. 4A–4D: MC3T3 cells cultured with (A) 180M PPF (B) HDPE, (C) Blank media, (D) ZCF; 4E– 4H: hMSC cultured with (A) 180M PPF (B) HDPE, (C) Blank media, (D) ZCF; 4I–4L: L929 cells cultured with cultured with (A) 180M PPF (B) HDPE, (C) Blank media, (D) ZCF; 4M–4P: cMSC cells cultured with (A) 180M PPF (B) HDPE, (C) Blank media, (D) ZCF. Normal cell morphology was observed in the populations cultured with 180M PPF, HDPE and blank media.
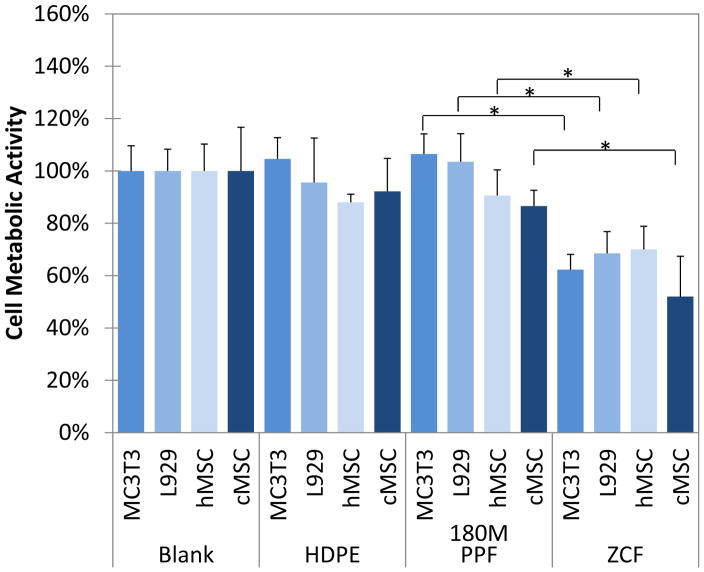
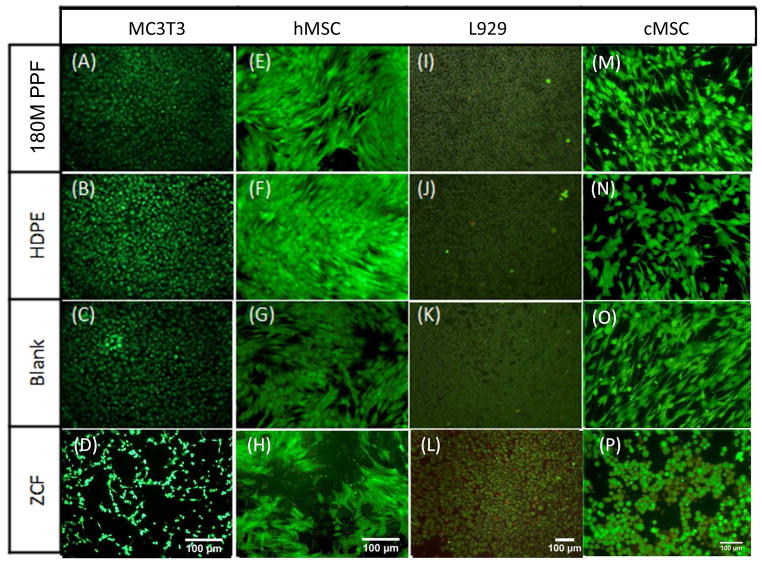
Similar results were documented for the indirect contact test. Cell metabolic activity levels were found to be statistically similar compared to those cultured with either 180M PPF, HDPE or under blank culture media for each cell type. The highest levels of cell metabolic activity were seen in the MC3T3 cells when cultured with 180M PPF with 106.5± 7.7%. The other cell metabolic activities, when cultured indirectly with 180M PPF, were 103.5 ± 10.8% (L929), 90.6 ± 9.8% (hMSC), and 86.6 ± 6.0% (cMSC) (Figure 6). The lack of cytotoxic response to indirect culturing with 180M PPF was visually confirmed, no documented changes in morphology were observed compared to the non-cytotoxic control or the blank culture media(Figures 7A–7E). Confluent, normal morphology was observed for cells exposed to 180M PPF, HDPE and blank culture media for each cell type (Figures 7A–7C, 7E–7G, 7I–7K, 7M–7O). A confluent monolayer was observed for each cell type indirectly exposed to 180M PPF. The hMSCs were elongated, spread, and maintained a characteristic spindle shape (Figure 7E). MC3T3s and L929s cultured with 180M PPF (Figures 7A and 7I) had similar confluency, morphology, viability, and had no noticeable cell detachment or abnormal morphology when compared to those cultured with HDPE or under blank culture media (Figures 7C, 7G, 7K and 7O). Cells exposed to the cytotoxic control, ZCF, were less spread compared to cells in the blank control. Detachment of the cell monolayer was also observed for the cells exposed to ZCF (Figures 7D, 7H, 7L and 7P). A significant change in morphology was observed in the MC3T3s exposed to the cytotoxic control (Figure 7D), cells became spherical and detached from the monolayer surface as compared to MC3T3 cells exposed to 180M PPF.
Figure 6. Cell Metabolic Activity (Indirect Contact).
Four different cell populations (MC3T3, L929, hMSC, and cMSC) were cultured in monolayer under indirect contact with HDPE, 180M PPF, ZCF, or nothing (Blank). Each cell type cultured under indirect contact with 180M PPF was found to have significantly higher metabolic activity when compared to those cultured with the positive, cytotoxic control, ZCF (p<0.05, * designated a statistical difference between groups). There were no statistical differences found between cells cultured with 180M PPF, HDPE or under blank culture media.
Figure 7. Indirect Contact Test.
Fluorescent images of cells, calcein AM (green) represents live cells, and ethidium homodimer (red) represents dead cells. 6A–6D: MC3T3 cells cultured with (A) 180M PPF (B) HDPE, (C) Blank media, (D) ZCF; 6E– 6H: hMSC cultured with (A) 180M PPF (B) HDPE, (C) blank media, (D) ZCF; 6I–6L: L929 cells cultured with cultured with (A) 180M PPF (B) HDPE, (C) blank media, (D) ZCF; 6M–6P: cMSC cells cultured with (A) 180M PPF (B) HDPE, (C) blank media, (D) ZCF. Normal morphology was seen in the treatment groups cultured with 180M PPF, HDPE and blank media.
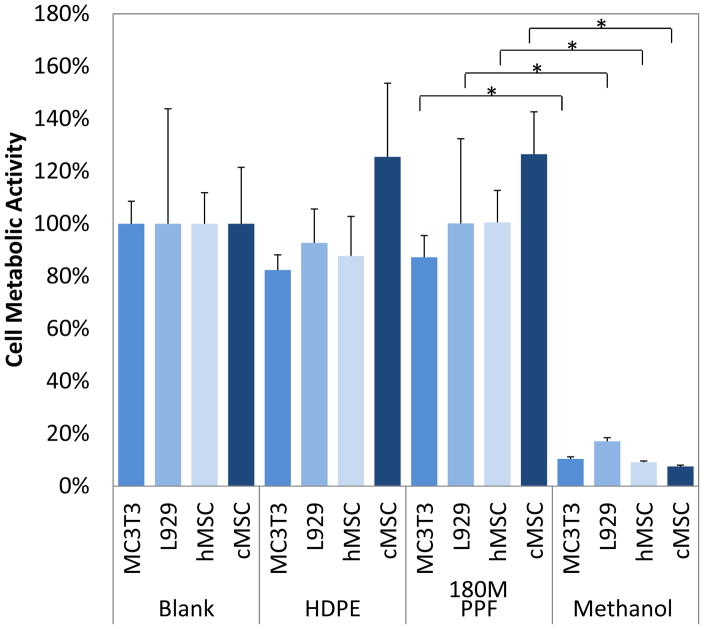
As with the indirect and direct contact tests, the extract test revealed that all cells cultured with HDPE extract or blank media had statistically similar cell metabolic activities compared to those cultured with 180M PPF extract. The cell metabolic activity of cells cultured with 180M PPF were found to be statistically different (p<0.05) to those of the cells cultured with the cytotoxic control, 70% methanol (Figure 8). When cultured with the 180M PPF extract the cell metabolic activity levels were found to be 126.5 ± 16.2% (cMSC), 87.2 ± 8.2% (MC3T3), 100.1 ± 32.3% (L929), and 100.5 ± 12.2% (hMSCs). Fluorescence imaging was used to qualitatively verify cell viability. For all four cell types, no significant morphological changes were observed in cell populations that were incubated with 180M PPF, HDPE and blank media (Figures 9A–9C, 9E–9G, 9I–9K, 9M–9O). All cells exposed to 70% methanol appeared red indicating a significant decrease in viability of the entire population per cell type (Figures 9D, 9H, 9L, 9P).
Figure 8. Cell Metabolic Activity (Extract).
Four different cell populations (MC3T3, L929, hMSC, and cMSC) were cultured in monolayer with extract media of HDPE, 180M PPF, ZCF, or nothing (Blank). Each cell type cultured with 180M PPF extract media was found to have significantly higher metabolic activity when compared to those cultured with the positive, cytotoxic control, methanol (p<0.05, * designated a statistical difference between groups). There were no statistical differences found between cells exposed to extract media from 180M PPF, HDPE or under blank culture media.
Figure 9. Extract Test.
Fluorescent images of cells, calcein AM (green) represents live cells, and ethidium homodimer (red) represents dead cells. 8A–8D: MC3T3 cells cultured with (A) 180M PPF (B) HDPE, (C) blank media, (D) 70% methanol; 8E– 8H: hMSC cultured with (A) 180M PPF (B) HDPE, (C) blank media, (D) 70% methanol; 8I–8L: L929 cells cultured with cultured with (A) 180M PPF (B) HDPE, (C) blank media, (D) 70% methanol; 8M–8P: cMSC cells cultured with (A) 180M PPF (B) HDPE, (C) blank media, (D) 70% methanol. Normal morphology was seen in the treatment groups cultured with 180M PPF, HDPE and blank media.
Experimental Section: Discussions
Cytotoxic effects can hinder the natural assimilation process that is required for successful in vivo integration of a biomaterial. The ideal in vitro test mimics the in vivo physiological environment. This study therefore chose cells to represent tissues that PPF will interact with in vivo in various bone tissue engineering therapies along with the cell line suggested per ISO 10993-523,24. The use of the ISO Standard 10993 allows for the comparison of the biocompatibility of PPF to other biomaterials. Other ISO Standard 10993-compliant cytotoxicity studies have evaluated implanted biomaterials such as electrospun collagen/chitosan nanofibers, poly (ε-caprolactone)/calcium sulfate and hydroxyapatite–ethylene vinyl acetate co-polymer25–27. Overall, our study demonstrated that 180M PPF has the same cytotoxic response as a known non-cytotoxic material when cultured with fibroblasts, preosteoblasts and mesenchymal stem cells.
Cellular response to a biomaterial can be impacted by both the crosslinked material and the soluble monomers that may leach out. For PPF, previous studies identified that uncrosslinked monomers of PPF based polymers have low cell viability14. We also determined that samples with a high sol fraction with leachable components remaining in the network impacted cell viability negatively. This was primarily seen when these films were not washed with acetone prior to evaluation (UN-30M). The acetone removes the soluble components of the polymer films, leaving only the fully crosslinked network. To evaluate the cytotoxicity of PPF films with high sol fractions, a direct contact test using L929 was performed to compare the 30M, UN-30M, and the 180M PPF films (Figure 3). The cell metabolic activities of the UN-30M PPF and the blank culture media were found to be statistically different (Figure 3A). With increasing sol fraction and therefore decreasing crosslinking density, a trend of decreasing cell metabolic activity was observed (Figure 3A). Cell viability was qualitatively confirmed using live/dead fluorescent imaging. The UN-30M PPF treatment group showed some cell death (Figure 3B). To ensure that the cytotoxicity of the crosslinked polymer network was evaluated, and not impacted by the leachable components, the 180M PPF films were used for the remainder of the direct, indirect and extract tests.
The sol fraction of the 30M PPF films was determined to be 53% compared to the 180M PPF films that had a sol fraction of 3% (Table 1). Assuming that the Charlesby-Pinner equation is a representative model of the crosslinking during UV irradiation for PPF, the crosslinking densities were 10% (30M) and 58% (180M)28,17. Previous studies have shown that although photocrosslinking of PPF is initiated with BAPO, the crosslinking rate can be augmented with heat28. To ensure that sterilization, autoclaving at 121°C, had no impact on sol fraction, the sol fraction was measured pre and post-sterilization. Sterilization was found to have no impact, as the sol fraction for the sterilized and pre-sterilized 180M PPF were found to be statistically similar (Table 1).
All three cytotoxicity tests demonstrated that the cell metabolic activity of cells exposed to 180M PPF directly (Figure 4), indirectly (Figure 6), or as an extract (Figure 8), were statistically different from cells exposed to the cytotoxic controls. Parallel tests using HDPE and blank culture media showed similar results as to the 180M PPF and were confirmed visually using fluorescence imaging. The greatest cell metabolic activity, a representative of cell viability, was seen in the extract tests; with values as large as 126.5 ± 16. 2% (Figure 8) for the cMSCs cultured with PPF extract. Cell metabolic activity levels were normalized using the blank culture media allowing for the possibility of metabolic levels greater than 100% to be achieved. The general trend of cell metabolic activities was lower in the indirect culture test (Figure 6) than in the extract test and lowest in the direct culture test (Figure 4). These results were as expected, as they followed a general trend of increasing interaction with the materials. The extract test and indirect culture tests provide for no physical interaction of the material with the cell monolayer, whereas the direct culture test allows for the material to be placed directly adjacent to the cell monolayer. The direct contact test also allows for the physical disruption of the cell monolayer, which may increase the cytotoxic impact. These results were acutely present for the tests using ZCF, the cytotoxic control.
Cell death and detachment was greatest in the direct contact tests when cultured with ZCF (Figure 5D, 5H, 5L, 5P) compared to the indirect culture test with ZCF (Figure 7D, 7H, 7L, 7P). We suggest that this is due to the culture method. There may be some concern that degrading materials may impact cell viability through an increase osmotic pressure. To rule out increasing osmotic pressure as the reason for decreased cell viability during the tests, the osmolality of the direct culture test was measured. The blank media was found to have the highest osmolality (342 ± 5 mOSM) and was statistically different from all other medias. Since there was no documented increase in osmotic pressure after the direct contact test, we believe that the cytotoxicity of the ZCF is due to the direct contact with the material itself, and less the soluble factors released by the ZCF. During the direct contact test the most prominent sites of cell detachment were observed where the cytotoxic material was placed. However, cell morphological changes, detachment, and death were present throughout each cell culture for cells exposed to ZCF.
Comparatively, the cellular response to indirect incubation with ZCF did not elicit massive cell sheet detachment as with the direct contact test. The greatest cytotoxic impact of ZCF was seen directly below the transwell insert. These results are consistent with the expected response due to the localized increased concentration of soluble factors. Direct contact would have a greater concentration of cytotoxic material when compared with indirect contact, where a smaller localized concentration is observed. In indirect contact experiments, the transwell inserts allow for ZCF to sit above the cell monolayer and not contiguous to the cell layer. These results are consistent with the fact that the direct contact test is cited as most sensitive of the three tests utilized20,21,31.
Of particular interest were the differing responses to the cytotoxic control seen in the four cell types studied. The use of ZCF as cytotoxic control has been established previously for multiple cell types with varying cytotoxic responses20,21,31,32. L929 cells had the greatest cytotoxic response to ZCF when compared to the MC3T3, hMSC and cMSC cells. Robust cell death, as well as lifting of the cell sheet, was seen most prominently in the L929 cells after incubation with ZCF. Previous studies have identified the L929 cell line as having a relatively high sensitivity compared to other fibroblasts, epithelial cells and astrocytes20,21. We expected that both mesenchymal stem cell populations (hMSC, cMSC) would have similar responses to the ZCF as they both are MSC populations from mammals. For both tests using the ZCF the cMSC had a lower cell metabolic activity indicating a stronger cellular response than the hMSCs. Other similarities were expected between the MC3T3 and L929 cell lines, as previous studies established that both MC3T3 and L929 cells had similar cytotoxic responses33. However this was not the case for our study. The cytotoxic response during the direct contact test of the L929 cells was statistically different (p>0.05) than the MC3T3 cells. For both the indirect contact and extract tests the cell metabolic activities of L929 and MC3T3 are not statistically different. For the indirect culture and extract tests there were no distinctive variation by cell type in responses to the cytotoxic control.
Conclusion
Cytotoxicity testing, consisting of direct contact, indirect contact and extract testing on multiple cell types was performed to determine the cytotoxic response of cells exposed to PPF in bone tissue engineering applications. For all cell types and cytotoxicity tests, the cell metabolic activity of cells exposed to 180M PPF were found to be statistically different (p<0.05) from the cytotoxic control, ZCF, as well as not statistically different when compared to blank culture media and cells recommended by the ISO 10993-5 standard when exposed to the negative control, high-density polyethylene (HDPE). To confirm these results qualitatively, cell morphology, viability, vacuolization and detachment were evaluated. These results confirmed that there was little to no cytotoxic response of cells exposed to PPF. Therefore PPF appeared to not elicit a cytotoxic response under all of the experimental conditions for which it was evaluated. These results demonstrate that PPF has a similar cytotoxic profile to a known non-cytotoxic material (HDPE). Additionally, previous studies have demonstrated that PPF scaffolds can be used for the culture and osteoblastic differentiation of MSCs3,16,34. We suggest that PPF is a suitable material for bone tissue engineering as it showed a lack of cytotoxic response through its ability to support cell metabolic activity at similar levels compared to HDPE, a known non-toxic material.
Acknowledgments
This research was supported by the National Institutes of Health (R01-DE013740), a Howard Hughes Medical Institute award, and the Maryland Technology Enterprise Institute (Mtech) ASPIRE program as well as through a Food and Drug Administration Center of Excellence in Regulatory Science and Innovation (CERSI) grant. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Food and Drug Administration. We would like to acknowledge Helim Aranda-Espinoza, Ph.D., and the Cell Biophysics Laboratory, for the use of their osmometer. The ZCF was generously provided by Dr. Ronald P. Brown at the Food and Drug Administration. The cMSCs were prepared by David Dean, Ph.D., Donald Lennon, D.D.S., and Vaijayantee Belle, M.Ch., D.N.B. in the Animal Resource Center and the Skeletal Research Center at Case Western Reserve University (CWRU), Cleveland, OH. We are grateful to Dr. James Anderson, Department of Pathology, CWRU for his guidance and helpful suggestions on this study.
References
- 1.Rezwan K, Chen Q, Blaker J, Boccaccini AR. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials. 2006;27:3413–3431. doi: 10.1016/j.biomaterials.2006.01.039. [DOI] [PubMed] [Google Scholar]
- 2.Fisher JP, Dean D, Mikos AG. Photocrosslinking characteristics and mechanical properties of diethyl fumarate/poly(propylene fumarate) biomaterials. Biomaterials. 2002;23:4333–4343. doi: 10.1016/s0142-9612(02)00178-3. [DOI] [PubMed] [Google Scholar]
- 3.Kim K, Dean D, Mikos AG, Fisher JP. Effect of Initial Cell Seeding Density on Early Osteogenic Signal Expression of Rat Bone Marrow Stromal Cells Cultured on Cross-Linked Poly(propylene fumarate) Disks. Biomacromolecules. 2009;10:1810–1817. doi: 10.1021/bm900240k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He S, Yaszemski MJ, Yasko AW, Engel PS, Mikos AG. Injectable biodegradable polymer composites based on poly(propylene fumarate) crosslinked with poly(ethylene glycol)-dimethacrylate. Biomaterials. 2000;21:2389–2394. doi: 10.1016/s0142-9612(00)00106-x. [DOI] [PubMed] [Google Scholar]
- 5.Peter SJ, Miller ST, Zhu G, Yasko AW, Mikos AG. In vivo degradation of a poly(propylene fumarate)/β-tricalcium phosphate injectable composite scaffold. Journal of Biomedical Materials Research. 1998;41:1–7. doi: 10.1002/(sici)1097-4636(199807)41:1<1::aid-jbm1>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 6.Yaszemski MJ, Payne RG, Hayes WC, Langer R, Mikos AG. In vitro degradation of a poly(propylene fumarate)-based composite material. Biomaterials. 1996;17:2127–2130. doi: 10.1016/0142-9612(96)00008-7. [DOI] [PubMed] [Google Scholar]
- 7.Timmer MD, Ambrose CG, Mikos AG. In vitro degradation of polymeric networks of poly(propylene fumarate) and the crosslinking macromer poly(propylene fumarate)-diacrylate. Biomaterials. 2003;24:571–577. doi: 10.1016/s0142-9612(02)00368-x. [DOI] [PubMed] [Google Scholar]
- 8.Kempen DH, Lu L, Hefferan TE, Creemers LB, Maran A, Classic KL, Dhert WJ, Yaszemski MJ. Retention of in vitro and in vivo BMP-2 bioactivities in sustained delivery vehicles for bone tissue engineering. Biomaterials. 2008;29:3245–3252. doi: 10.1016/j.biomaterials.2008.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisher JP, Holland TA, Dean D, Engel PS, Mikos AG. Synthesis and properties of photocross-linked poly(propylene fumarate) scaffolds. Journal of Biomaterials Science, Polymer Edition. 2001;12:673–687. doi: 10.1163/156856201316883476. [DOI] [PubMed] [Google Scholar]
- 10.Lewandrowski KU, Bondre S, Hile DD, Thompson BM, Wise DL, Tomford WW, Trantolo DJ. Porous poly(propylene fumarate) foam coating of orthotopic cortical bone grafts for improved osteoconduction. Tissue Engineering. 2002;8:1017–1027. doi: 10.1089/107632702320934119. [DOI] [PubMed] [Google Scholar]
- 11.Vehof JW, Fisher JP, Dean D, van der Waerden JP, Spauwen PH, Mikos AG, Jansen JA. Bone formation in transforming growth factor beta-1-coated porous poly(propylene fumarate) scaffolds. Journal of Biomedical Materials Research. 2002;60:241–251. doi: 10.1002/jbm.10073. [DOI] [PubMed] [Google Scholar]
- 12.Fisher JP, Holland TA, Dean D, Mikos AG. Photoinitiated cross-linking of the biodegradable polyester poly(propylene fumarate). Part II. In vitro degradation. Biomacromolecules. 2003;4:1335–1342. doi: 10.1021/bm0300296. [DOI] [PubMed] [Google Scholar]
- 13.Fisher JP, Vehof JW, Dean D, van der Waerden JP, Holland TA, Mikos AG, Jansen JA. Soft and hard tissue response to photocrosslinked poly(propylene fumarate) scaffolds in a rabbit model. Journal of Biomedical Materials Research. 2002;59:547–556. doi: 10.1002/jbm.1268. [DOI] [PubMed] [Google Scholar]
- 14.Timmer MD, Shin H, Horch RA, Ambrose CG, Mikos AG. In vitro cytotoxicity of injectable and biodegradable poly(propylene fumarate)-based networks: unreacted macromers, cross-linked networks, and degradation products. Biomacromolecules. 2003;4:1026–1033. doi: 10.1021/bm0300150. [DOI] [PubMed] [Google Scholar]
- 15.Kasper FK, Tanahashi K, Fisher JP, Mikos AG. Synthesis of poly(propylene fumarate) Nature Protocols. 2009;4:518–525. doi: 10.1038/nprot.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim K, Dean D, Lu A, Mikos AG, Fisher JP. Early osteogenic signal expression of rat bone marrow stromal cells is influenced by both hydroxyapatite nanoparticle content and initial cell seeding density in biodegradable nanocomposite scaffolds. Acta Biomaterialia. 2011;7:1249–1264. doi: 10.1016/j.actbio.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charlesby A, Pinner S, Pinner S. Analysis of the solubility behaviour of irradiated polyethylene and other polymers. Proceedings of the Royal Society of London. Series A.Mathematical and Physical Sciences. 1959;249:367–386. [Google Scholar]
- 18.Yeatts AB, Geibel EM, Fears FF, Fisher JP. Human mesenchymal stem cell position within scaffolds influences cell fate during dynamic culture. Biotechnology and Bioengineering. 2012;109:2381–2391. doi: 10.1002/bit.24497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Betz MW, Yeatts AB, Richbourg WJ, Caccamese JF, Coletti DP, Falco EE, Fisher JP. Macroporous hydrogels upregulate osteogenic signal expression and promote bone regeneration. Biomacromolecules. 2010;11:1160–1168. doi: 10.1021/bm100061z. [DOI] [PubMed] [Google Scholar]
- 20.Park JC, Park BJ, Lee DH, Suh H, Kim DG, Kwon OH. Evaluation of the cytotoxicity of polyetherurethane (PU) film containing zinc diethyldithiocarbamate (ZDEC) on various cell lines. Yonsei Medical Journal. 2002;43:518. doi: 10.3349/ymj.2002.43.4.518. [DOI] [PubMed] [Google Scholar]
- 21.Tsuchiya T. Studies on the standardization of cytotoxicity tests and new standard reference materials useful for evaluating the safety of biomaterials. Journal of Biomaterials Applications. 1994;9:138–157. doi: 10.1177/088532829400900204. [DOI] [PubMed] [Google Scholar]
- 22.Betz MW, Modi PC, Caccamese JF, Coletti DP, Sauk JJ, Fisher JP. Cyclic acetal hydrogel system for bone marrow stromal cell encapsulation and osteodifferentiation. Journal of Biomedical Materials Research A. 2008;86:662–670. doi: 10.1002/jbm.a.31640. [DOI] [PubMed] [Google Scholar]
- 23.Oliva A, Della Ragione F, Salerno A, Riccio V, Tartaro G, Cozzolino A, D’Amato S, Pontoni G, Zappia V. Biocompatibility studies on glass ionomer cements by primary cultures of human osteoblasts. Biomaterials. 1996;17:1351–1356. [PubMed] [Google Scholar]
- 24.International Organization for Standardization. ISO 10993: parts 1–12. ISO; Geneva, Switzerland, Various: [Google Scholar]
- 25.Chen J-P, Chang G-Y, Chen J-K. Electrospun collagen/chitosan nanofibrous membrane as wound dressing. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2008;313–314:183–188. [Google Scholar]
- 26.Velayudhan S, Anilkumar TV, Kumary TV, Mohanan PV, Fernandez AC, Varma HK, Ramesh P. Biological evaluation of pliable hydroxyapatite–ethylene vinyl acetate copolymer composites intended for cranioplasty. Acta Biomaterialia. 2005;1:201–209. doi: 10.1016/j.actbio.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 27.La Gatta A, De Rosa A, Laurienzo P, Malinconico M, De Rosa M, Schiraldi C. A Novel Injectable Poly (ε-caprolactone)/Calcium Sulfate System for Bone Regeneration: Synthesis and Characterization. Macromolecular Bioscience. 2005;5:1108–1117. doi: 10.1002/mabi.200500114. [DOI] [PubMed] [Google Scholar]
- 28.Timmer MD, Carter C, Ambrose CG, Mikos AG. Fabrication of poly(propylene fumarate)-based orthopaedic implants by photo-crosslinking through transparent silicone molds. Biomaterials. 2003;24:4707–4714. doi: 10.1016/s0142-9612(03)00364-8. [DOI] [PubMed] [Google Scholar]
- 29.McNaught AD, Wilkinson A. Compendium of chemical terminology : IUPAC recommendations. 2. Blackwell Science; 1997. International Union of Pure and Applied Chemistry. [Google Scholar]
- 30.Alger MSM. Polymer Science Dictionary. Vol. 69. Elsevier Applied Science; 1989. p. 94. [Google Scholar]
- 31.Tsuchiya T, Ikarashi Y, Arai T, Ohhashi J, Isama K, Nakamura A. In vivo tissue/biomaterials toxic responses: Correlation with cytotoxic potential but not cell attachment. Clinical materials. 1994;16:1–8. [Google Scholar]
- 32.Tsuchiya T, Ikarashi Y, Hata H, Toyoda K, Takahashi M, Uchima T, Tanaka N, Sasaki T, Nakamura A. Comparative studies of the toxicity of standard reference materials in various cytotoxicity tests and in vivo implantation tests. Journal of Applied Biomaterials. 1993;4:153–156. doi: 10.1002/jab.770040206. [DOI] [PubMed] [Google Scholar]
- 33.Yamamoto A, Honma R, Sumita M. Cytotoxicity evaluation of 43 metal salts using murine fibroblasts and osteoblastic cells. Journal of Biomedical Materials Research. 1998;39:331–340. doi: 10.1002/(sici)1097-4636(199802)39:2<331::aid-jbm22>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 34.Kim K, Dean D, Wallace J, Breithaupt R, Mikos AG, Fisher JP. The influence of stereolithographic scaffold architecture and composition on osteogenic signal expression with rat bone marrow stromal cells. Biomaterials. 2011;32:3750–3763. doi: 10.1016/j.biomaterials.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]