Abstract
Studies have shown that N-methyl-d-aspartate (NMDA) receptors play a critical role in pain processing at different levels of the central nervous system. In this study, we used cortex-specific NR1 knockout mice (C57BL/6 strain) to elucidate the role of cortical NMDA receptors in pain processes. On post-natal day 20, paw withdrawal latency (PWL) to a noxious thermal stimulus was measured in male and female knockout (KO), control (Ctrl), and C57BL/6 (C57) mice. Twenty-four hours later, the same mice were tested in the formalin-pain assay (20 μl of 5% formalin injected into one hind-paw). The results show that KO mice (both male and female) have significantly reduced pain responses during both early and late phases of formalin test, as compared with Ctrl and C57 mice (p < 0.01). By contrast, no differences among groups were found in PWL to a noxious thermal stimulus. Taken together, these results demonstrate dissociation in the role of cortical NMDA receptors in mediating different types of pain.
Keywords: Pain, Analgesia, NMDA receptor, Knockout, Formalin test, Noxious thermal stimuli
N-methyl-d-aspartate (NMDA) receptors are suggested to play a critical role in pain and analgesia [see [20] for review]. For example, following subcutaneous injection of formalin, there is an up-regulation in the levels of excitatory amino acids (EAA) in the spinal cord, and administration of NMDA antagonists into both spinal [5] and supraspinal sites [27] have been shown to reduce formalin-induced pain. Therefore, given that NMDA receptors activation is involved in the development of pain after tissue damage and nerve injury [5,8,15], antagonists that target NMDA receptors may have potential therapeutic benefits [10,13,22].
The present research was designed to elucidate further the role of NMDA receptors in mediating different types of pain by using NR1–NMDA cortex-specific KO mice [6,7,16]. Western blot and in situ hybridization analysis reveals that, compared to controls, these mice have a 95% reduction of NR1 subunit levels in cortex and hippocampus [16]. Moreover, x-gal staining verified the spatial restriction of Cre recombination at cortical levels [16]. Therefore, an examination of the pain response in these mice, as compared with WT controls, can provide valuable insights into the role of cortical NMDA in pain processes. In addition, for further delineation of the role of NMDA receptors in mediating different types of pain we compared both models of tonic pain (formalin test) [9] and phasic pain (paw withdrawal to thermal stimuli) [12].
Iwasato et al. [16] originally generated cortex-specific NR1 KO mice by inserting the Cre recombinase gene downstream from the Emx1 promoter via homologous recombination in embryonic stem cells. Emx1 is a homeobox gene expressed only in dorsal telencephalon from embryonic stages to adulthood. Emx1Cre/Cre mice were crossed with heterozygous NR1 null mutant mice (NR1+/−), then further crossed with homozygous floxed NR1 mice (NR1flox/flox). This breeding scheme generated one cortex-specific NR1KO genotype (Emx1Cre/+NR1flox/−) and three control genotypes. For the present study we adopted a modified breeding strategy in which half of the progeny are KO mice (of the same genotype described above) and the other half are of one of the three original control genotypes, the genotype that differs by only one allele from the KOs. These mice were used in the present study. For additional control, we included genetically unmodified C57BL/6 mice (the background strain). Genotype was determined by the polymerase chain reaction method (PCR) on genomic DNA purified from tail biopsies.
Male and female cortex-specific NR1KO mice, genetically modified mice with normal NR1 expression, and C57BL/6 mice were bred, housed and maintained in the animal care facilities of the Louisiana State University Health Sciences Center according to PHS guidelines. Animals were housed in light/dark cycles of 12 h × 12 h (light onset at 06:00), with food and water available ad libitum. Behavioral testing was performed when mice were P20 (Hargreaves test) and P21 (formalin test) days of age. This range in age was chosen because of upper and lower time constraints. NR1 KO mice have a very short lifespan, most dying between 30 and 40 days of age; moreover, pain responses reach full maturity by post-natal day 20, but not sooner [3,25].
Pain assays were performed in Plexiglas boxes (10 cm × 18 cm) following a 40 min habituation period. All testing was performed under “blind” conditions (i.e., genotyping of mice was performed after behavioral testing was completed). On post-natal day 20, paw withdrawal latency (PWL) to thermal stimuli was measured according to the techniques previously described [12]. Briefly, PWL from a focused beam of light applied to the ventral paw surface was measured using an anal-gesiometer (IITC Life Sciences, Woodland Hills, CA), and a 10 s cutoff was imposed to prevent tissue damage. The mean of four PWL (two left and two right) measured at 3–5 min intervals was recorded for each mouse. Twenty-four hours later (post-natal day 21) formalin testing was performed in the same mice, as previously described [9,14]. Briefly, mice were injected s.c. into the plantar surface of the right hind-paw with 20 μl of 5% formalin. The mice were observed for a period of one h following injection and the amount of time spent licking/biting the formalin-injected paw was recorded. The pain scores (licking/biting) were determined for blocks of 5 min. All behavioral testing was performed between 07:00 and 12:00.
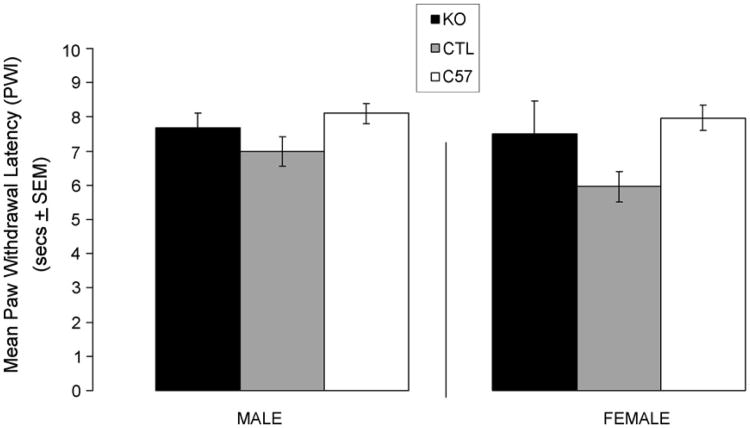
Fig. 1 shows the mean PWL. Analysis of Variance (ANOVA) revealed no significant difference in paw withdrawal latencies between KO, Ctrl, and C57 mice of either gender (male: F(2, 39) = 2.003, p = 0.149, n.s.; female: F(2, 31) = 3.519, p = 0.042, n.s.).
Fig. 1.
Paw withdrawal latency (PWL) to thermal stimuli in cortex-specific NR1 knockout (KO), genetic control (Ctrl), and C57BL/6 (C57) mice. Data are expressed as mean time (sec ± S.E.M.) of PWL after application of a heat stimulus to the hind-paw. No significant differences were found between KO, Ctrl and C57 groups (male F(2, 39)= 2.003, p = 0.149, n.s.; female F(2, 31) = 3.519, p = 0.042, n.s.
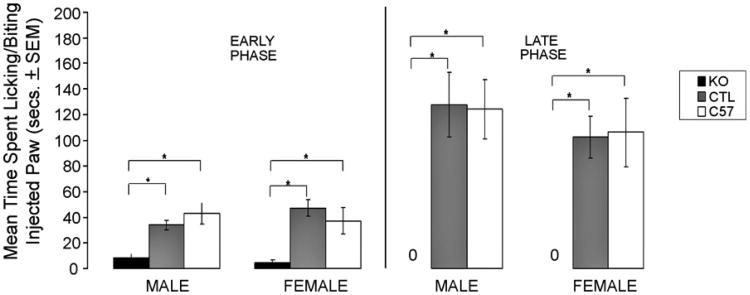
The formalin test produces a biphasic profile of pain response that is characterized by an “early phase” during the first 5min after formalin injection that is followed by a “late phase” pain response from 10 to 60 min [9]. Fig. 2 shows the total time spent licking/biting the formalin-injected paw during both the early and late phases. ANOVA revealed a significant decrease in pain response in both male and female KO mice as compared with genetic and C57 controls, during both the early phase (male: F(2, 38) = 15.220, p< 0.001; female: F(2, 29) = 9.215, p < 0.001) and late phases (male: F(2, 38) = 14.013, p < 0.001; female: F(2, 29) = 9.678, p < 0.001). In general, the KO group only showed pain responses during the first 5 min, after which there was virtually no pain behavior observed (see Fig. 2). Genetic and C57 mice did not differ from each other.
Fig. 2.
Pain responses in KO, Ctrl, and C57 mice (abbreviations as above) during the early (0–5 min) and late (10–60 min) phases of the formalin test. Data are expressed as mean time (sec ± S.E.M.) spent licking/biting the hind-paw after injection of 20 μl of 5% formalin. Significant differences were found between KO and Ctrl or C57 mice in both early (0–5 min) and late (10–60 min) phases. Ctrl and C57 mice did not differ from each other. *Male: early phase F(2, 38) = 15.220, p<0.001; late phase F(2, 38) = 14.013, p<0.001. Female: early phase F(2, 29) = 9.215, p<0.001; late phase: F(2, 29) = 9.678, p<0.001.
The results presented here demonstrate a dissociation of the role of cortical NMDA receptors in mediating different types of pain. In particular, a decreased pain response was observed in KO mice in the formalin test but not in their sensitivity to a thermal stimulus. The PWL measures a phasic response to threshold level thermal stimulus in which major cortical structures would not be expected to play a critical role [1,2,29]. By contrast, the formalin test produces inescapable pain due to tissue injury, which involves supraspinal structures, including the cortex and limbic system [1,2,21,29]. Indeed, the decrease in pain response in the formalin test observed in the KO mice supports the critical role of cortical structures in mediating this type of pain. Furthermore, it is unlikely that non-specific effects could explain these results as no deficit was observed in the PWL, which involves similar motor/behavioral responses that are observed after formalin injection (i.e., lifting of the hind-paw).
The transgenic mouse model used in this study has a knocking out of the NR1 subunit of the NMDA receptor, which is an essential functional subunit of the NMDA receptor [11]. As a consequence, these mice display non-functional NMDA receptors in the cerebral cortex, hippocampus and olfactory bulb, whereas NMDA receptors in the rest of the brain are fully functional and are expressed at normal levels [16]. There is evidence that the cingulate cortex is involved in the processing of the affective component of pain, and thus in the tonic pain responses in the formalin test [29,30]. Furthermore, there is a consensus between the present results and previous imaging studies done in rodents; specifically, there is a relationship between the levels of excitatory activity at supraspinal levels and the degree of pain behavior intensity. For instance, Chang and Shyu [4], showed a correlation between high levels of neural activity in cingulate cortex, somatosensory cortex, medial thalamus, and hypothalamus with increased levels of noxious stimulation. Similarly, Tuor et al. [26] reported a correlation between increased levels of neural activity in anterior cingulate cortex, frontal cortex and sensory-motor cortex with noxious chemical stimulation in the forepaw. These studies, along with the results of the present report, suggest that cortical excitatory activity is involved in the perception of long lasting noxious stimulation.
Previous studies have demonstrated sex-dependent differences in NMDA involvement in pain and analgesia [17,18,19]. For example, Mogil et al. [19], reported that sexually mature male and female mice have similar levels of analgesia after swim stress (swim stress induced analgesia, SSIA) at 15 °C; however, there are differences in the underlying mechanisms. Specifically, NMDA antagonists (dizocilpine; MK-801) blocked the analgesic response in male but not in female. However, sensitivity to dizocilpine/MK-801 reducing effects on analgesia was reinstated by ovariectomy, and after estrogen replacement the females recovered the dizocilpine/MK-801 insensitivity during analgesic response. Thus, it is suggested that these sex differences in NMDA involvement are hormonally mediated [19]. In the present study, no sex differences were found in the effect of knocking out the cortical NMDA receptor, as the transgenic modification reduced the formalin-induced pain response in both males and females. This may be explained by the lack of sexual maturity and its hormonal correlates at the age of testing (post-natal day 21).
The data reported in the present work are in agreement with previous reports suggesting a critical role of NMDA receptors system in pain processes [5,28]. It is important to note that in those studies different methods were used and different levels of the nervous system were examined. For instance, pharmacological studies described by Coderre and Melzack [5] demonstrate the importance of NMDA receptor system in the second phase of the formalin test. In fact, that group reports that up-regulation in the levels of EAA and intrathecal application of NMDA receptor agonists at the levels of the spinal cord are correlated with persistent nociception and central sensitization of the formalin test's second phase. In addition, conditional deletion of spinal cord NMDA receptors produces a reduction of 70% of the pain response in the second phase of the formalin test, suggesting that spinal cord NMDA receptors are involved in the pain response of the second phase of the formalin test [24]. In general, most research suggests a correlation between the decrease of the pain response in the second phase of the formalin test (tonic pain), and decreases in the activation of the NMDA receptor at different levels of the nervous system. It is important to note, however, that in the present study a significant reduction in pain response was observed in both the early and late phase of the formalin test. Therefore, unlike spinal NMDA, which appear to be restricted to the late phase response [24], the results of the present study suggest that cortical NMDA receptors are involved in mediated pain during both phases of the formalin test.
The results presented here are in agreement with similar pain studies in related knockout mice models. In particular, AC1 and AC8 knockout rodents [31] have been shown to display a reduction or abolition in the levels of sensitization to peripheral inflammatory stimuli. It is already known that AC1 and AC8 are highly expressed in the anterior cingulate cortex. In addition, both adenylyl cyclases are the molecular – signaling link between NMDA receptor activation, Ca2+ influx, CaM (calcium calmodulin), and the downstream activation of cAMP/CREB; this is related to tonic pain as shown in the formalin test second phase. Recapitulating, the data reported in the present study and in previous studies using similar transgenic mice demonstrate a reduction in the tonic pain response due to the blockade of the signaling pathways downstream of the NMDA receptor. It should be noted that the mouse model in the present study has a knockout condition restricted to cortex, hippocampus and olfactory bulb, in which the genetic modification is manifested from the beginning of the embryonic development. This model represents an advantage in comparison to the general NR1 knockout model, in which the mice die at post-natal day 0 (P0) [11]. Additionally, the model presented by South et al. [24] in which the genetic deletion is restricted in space and time represents a more precise approach that reduces the probability of non-controlled developmental deficits. In the future, it would therefore be helpful to perform a study disrupting cortical NMDA receptors, but adopting the South approach [24]. Specifically, to employ mice in which the NR1 gene is flanked by flox sites combined with the administration of a vector (with inserted Cre recombinase) to cingulate cortex.
In conclusion, the present work proposes that NMDA receptors in the cerebral cortex are an important element in the generation of the nociceptive response in tonic pain, but not in phasic pain. NMDA receptors have been the focus of studies related to the treatment of different pathological conditions including pain [23]. The present research suggests that cortical NMDA receptors have a significant influence in the development of inflammatory pain and that antagonists that target to cortical NMDA receptors (specifically NR1 subunit) could be an effective treatment for painful conditions.
Acknowledgments
This work was supported by National Institute of Health (NIH) NS 039050 (R.S.E.), LSUHSC Research Enhancement Fund, and IFARHU/SENACYT “Programa de Becas 2003 para formación de investigadores”, Republic of Panama. We thank Takuji Iwasato, PhD (RIKEN Brain Science Institute, Japan) for providing the transgenic mice, Ms. Barri King for breeding the mice, and Gerald J La Hoste, PhD (University of New Orleans) for his comments and help in the revised version.
References
- 1.Abbott FV, Melzack R. Brainstem lesions dissociate neural mechanisms of morphine analgesia in different kinds of pain. Brain Res. 1982;251:149–151. doi: 10.1016/0006-8993(82)91282-3. [DOI] [PubMed] [Google Scholar]
- 2.Abbott FV, Melzack R, Samuel C. Morphine analgesia in the tail-flick and formalin pain tests is mediated by different neural systems. Exp Neurol. 1982;75:644–651. doi: 10.1016/0014-4886(82)90031-0. [DOI] [PubMed] [Google Scholar]
- 3.Barr GA. Maturation of the biphasic behavioral and heart rate response in the formalin test. Pharmacol Biochem Behav. 1998;60:329–335. doi: 10.1016/s0091-3057(97)00602-3. [DOI] [PubMed] [Google Scholar]
- 4.Chang C, Shyu BC. A fMRI study of brain activation during non-noxious and noxious electrical stimulation of the sciatic nerve of rats. Brain Res. 2001;897:71–81. doi: 10.1016/s0006-8993(01)02094-7. [DOI] [PubMed] [Google Scholar]
- 5.Coderre TJ, Melzack R. The contribution of excitatory amino acids to central sensitization and persistent nociception after formalin induced tissue injury. J Neurosci. 1992;12:3665–3670. doi: 10.1523/JNEUROSCI.12-09-03665.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Datwani A, Iwasato T, Itohara S, Erzurumlu RS. NMDA receptor-dependent pattern transfer from afferents to postsynaptic cells and dendritic differentiation in the barrel cortex. Mol Cell Neurosci. 2002 Nov 21;3:477–492. doi: 10.1006/mcne.2002.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Datwani A, Iwasato T, Itohara S, Erzurumlu RS. Lesion-induced thalamocortical axonal plasticity in the S1 cortex is independent of NMDA receptor function in excitatory cortical neurons. J Neurosci. 2002;22(21):9171–9175. doi: 10.1523/JNEUROSCI.22-21-09171.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doubell TJ, Mannion RJ, Woolf CJ. The dorsal horn: state-dependent sensory processing, plasticity and the generation of pain. In: Wall PD, Melzack R, editors. Textbook of Pain. fourth. Churchill Livingstone; London: 1999. pp. 165–181. [Google Scholar]
- 9.Dubuisson D, Dennis SG. The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain. 1977;4(2):161–174. doi: 10.1016/0304-3959(77)90130-0. [DOI] [PubMed] [Google Scholar]
- 10.Fisher K, Coderre TJ, Hagen NA. Targeting N-methyl-d-aspartate receptor for chronic pain management: preclinical animals studies, recent clinical experience and future research directions. J Pain Symptom Manage. 2000;20:358–373. doi: 10.1016/s0885-3924(00)00213-x. [DOI] [PubMed] [Google Scholar]
- 11.Forrest D, Yuzaki M, Soares HD, Ng L, Luk DC, Sheng M, Stewart CL, Morgan JI, Connor JA, Curran T. Targeted disruption of NMDA receptor 1 gene abolishes NMDA response and results in neonatal death. Neuron. 1994;13:325–338. doi: 10.1016/0896-6273(94)90350-6. [DOI] [PubMed] [Google Scholar]
- 12.Hargreaves K, Dubner R, Brown C, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 13.Hewitt DJ. The use of NMDA-receptor antagonists in the treatment of chronic pain. Clin J Pain. 2000;16:73–79. doi: 10.1097/00002508-200006001-00013. [DOI] [PubMed] [Google Scholar]
- 14.Hunskaar S, Fasmer OB, Hole K. Formalin test in mice, a useful technique for evaluating mild analgesics. J Neurosci Methods. 1985;14(1):69–76. doi: 10.1016/0165-0270(85)90116-5. [DOI] [PubMed] [Google Scholar]
- 15.Hunter JC, Singh L. Role of excitatory amino acid receptors in the mediation of the nociceptive response to formalin in the rat. Neurosci Lett. 1994;174:217–221. doi: 10.1016/0304-3940(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 16.Iwasato T, Datwani A, Wolf AM, Nishiyama I, Taguchi Y, Tone-gawa S, Knopfel T, Erzurumlu RS, Itohara S. Cortex-restricted disruption of NMDAR1 impairs neuronal patterns in the barrel cortex. Nature. 2000;406:726–731. doi: 10.1038/35021059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kavaliers M, Choleris E. Sex differences in N-methyl-d-aspartate involvement in kappa opioid and non-opioid predator-induced analgesia in mice. Brain Res. 1997;768(1–2):30–36. doi: 10.1016/s0006-8993(97)00569-6. [DOI] [PubMed] [Google Scholar]
- 18.Kavaliers M, Colwell DD, Choleris E. Sex differences in opioid and N-methyl-d-aspartate mediated non-opioid biting fly exposure induced analgesia in deer mice. Pain. 1998;77(2):163–171. doi: 10.1016/S0304-3959(98)00092-X. [DOI] [PubMed] [Google Scholar]
- 19.Mogil JS, Sternberg WF, Kest B, Marek P, Liebeskind JC. Sex differences in the antagonism of swim stress-induced analgesia: effects of gonadectomy and estrogen replacement. Pain. 1993;53(1):17–25. doi: 10.1016/0304-3959(93)90050-Y. [DOI] [PubMed] [Google Scholar]
- 20.Petrenko AB, Yamakura T, Baba H, Shimoji K. The role of N-methyl-d-aspartate (NMDA) receptors in pain: a review. Anesth Analg. 2003;97:1108–1116. doi: 10.1213/01.ANE.0000081061.12235.55. [DOI] [PubMed] [Google Scholar]
- 21.Ryan SM, Watkins LR, Mayer DJ, Maier SF. Spinal pain suppression mechanisms may differ for phasic and tonic pain. Brain Res. 1985;334:172–175. doi: 10.1016/0006-8993(85)90582-7. [DOI] [PubMed] [Google Scholar]
- 22.Salter MW. Cellular neuroplasticity mechanisms mediating pain persistence. J Orofac Pain. 2004;18(4):318–324. [PubMed] [Google Scholar]
- 23.Smith PF. Therapeutic N-methyl-d-aspartate antagonists: Will reality meet expectation? Curr Opin Invest Drugs. 2003;4(7):826–832. [PubMed] [Google Scholar]
- 24.South SM, Kohno T, Kaspar BK, Hegarty D, Vissel B, Drake CT, Ohata M, Jenab S, Sailer AW, Malkmus S, Masuyama T, Horner P, Bogulaysky J, Gage FH, Yaksh TL, Woolf CJ, Heinemann SF, Inturrisi CE. A conditional deletion of the NR1 subunit of the NMDA receptor in adult spinal cord dorsal horn reduces NMDA current and injury-induced pain. J Neurosci. 2003;23(12):5031–5040. doi: 10.1523/JNEUROSCI.23-12-05031.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teng CJ, Abbott FV. The formalin test: a dose-response analysis at three developmental stages. Pain. 1998;76:337–347. doi: 10.1016/S0304-3959(98)00065-7. [DOI] [PubMed] [Google Scholar]
- 26.Tuor UI, Malisza K, Foniok T, Papadimitropoulos R, Jarmasz M, Somorjai R, Kozlowski P. Functional magnetic resonance imaging in rats subjected to intense electrical and noxious chemical stimulation of the forepaw. Pain. 2000;87:315–324. doi: 10.1016/S0304-3959(00)00293-1. [DOI] [PubMed] [Google Scholar]
- 27.Vaccarino AL, Clemmons HR, Mader GJ, Magnusson JE. A role of periaqueductal grey NMDA receptors in mediating formalin-induced pain in the rat. Neurosci Lett. 1997;236(2):117–119. doi: 10.1016/s0304-3940(97)00770-2. [DOI] [PubMed] [Google Scholar]
- 28.Vaccarino AL, Marek P, Kest B, Weber E, Keana JF, Liebeskind JC. NMDA receptor antagonists, MK-801 and ACEA-1011, prevent the development of tonic pain following subcutaneous formalin. Brain Res. 1993;615:331–334. doi: 10.1016/0006-8993(93)90045-o. [DOI] [PubMed] [Google Scholar]
- 29.Vaccarino AL, Melzack R. Analgesia produced by injection of lidocaine into the anterior cingulum bundle of the rat. Pain. 1989;39(2):213–219. doi: 10.1016/0304-3959(89)90008-0. [DOI] [PubMed] [Google Scholar]
- 30.Vaccarino AL, Melzack R. Temporal processes of formalin pain: differential role of the cingulum bundle, fornix pathway and medial bulboreticular formation. Pain. 1992;49:257–271. doi: 10.1016/0304-3959(92)90150-A. [DOI] [PubMed] [Google Scholar]
- 31.Wei F, Qiu ChS, Kim SJ, Muglia L, Maas JW, Pineda VV, Xu HM, Chen ZF, Storm DR, Muglia LJ, Zhuo M. Genetic elimination of behavioral sensitization in mice lacking calmodulin-stimulated adenylyl cyclases. Neuron. 2002;36:713–726. doi: 10.1016/s0896-6273(02)01019-x. [DOI] [PubMed] [Google Scholar]