Abstract
Several lines of evidence indicate that excess iron may play an etiologically significant role in neurodegenerative disorders. This idea is supported, for example, by experimental studies in animals demonstrating significant neuroprotection by iron chelation. Here, we tested whether this effect might be related to a functional link between iron and the endogenous excitotoxin quinolinic acid (QUIN), a presumed pathogen in several neurological disorders. In particular, the present in vitro study was designed to examine the effects of Fe2+, a known co-factor of oxygenases, on the activity of QUIN’s immediate biosynthetic enzyme, 3-hydroxyanthranilic acid dioxygenase (3HAO), in the brain. In crude tissue homogenate, addition of Fe2+ (2–40 μM) stimulated 3HAO activity 4–6-fold in all three species tested (mouse, rat and human). The slope of the iron curve was steepest in rat brain where an increase from 6 to 14 μM resulted in a more than 5-fold higher enzyme activity. In all species, the Fe2+-induced increase in 3HAO activity was dose-dependently attenuated by the addition of ferritin, the main iron storage protein in the brain. The effect of iron was also readily prevented by N,N′-bis(2-hydroxybenzyl) ethylenediamine-N,N′-diacetic acid (HBED), a synthetic iron chelator with neuroprotective properties in vivo. All these effects were reproduced using neostriatal tissue obtained postmortem from normal individuals and patients with end-stage Huntington’s disease. Our results suggest that QUIN levels and function in the mammalian brain might be tightly controlled by endogenous iron and proteins that regulate the bioavailability of iron.
Keywords: 3-Hydroxyanthranilic acid dioxygenase, Ferritin, HBED, Huntington’s disease, Neuroprotection
Introduction
Excitotoxicity, i.e. excessive stimulation of glutamate receptors leading to “axon-sparing” nerve cell death (Olney et al 1975), likely plays a central role in the pathogenesis of several neurological disorders including epilepsy, stroke and chronic neurodegenerative diseases (Schwarcz et al 1984). The cascade of neurodestructive events might be triggered by glutamate itself or by a number of other endogenous excitotoxins. The most potent of these agents is quinolinic acid (QUIN), a selective NMDA receptor agonist and product of the kynurenine pathway (KP) of tryptophan degradation. Endogenous QUIN has been most comprehensively linked to the pathophysiology of Huntington’s disease (HD) but there is also credible evidence for its role in Alzheimer’s disease, amyotrophic lateral sclerosis and other catastrophic CNS diseases (see Németh et al 2006, Chen and Guillemin 2009, and Schwarcz et al 2010, for recent reviews). The metabolic fate of QUIN, especially the mechanism(s) regulating its disposition and function in the brain, have therefore attracted significant attention from biochemists and neuroscientists alike.
In the brain as in peripheral organs, QUIN biosynthesis is catalyzed by 3-hydroxyanthranilic acid 3,4-dioxygenase (3HAO) (Long et al 1954; Foster et al 1986). 3HAO transforms the KP metabolite 3-hydroxyanthranilic acid (3HANA) into an unstable intermediate (2-amino-3-carboxymuconic acid semialdehyde; Wiss and Bettendorf 1956), which rearranges spontaneously to yield QUIN. In the brain, the formation of QUIN from 3HANA, which has been verified in vivo (Speciale et al 1989), takes place preferentially in microglial cells (Alberati-Giani et al 1996; Lehrmann et al 2001). 3HAO activity – and QUIN production – is therefore increased in situations where microglial cells are activated, for example following immune stimulation, during ongoing neuronal deterioration or as a consequence of neuronal loss (Schwarcz et al 1988, 1989; Saito et al 1993; Hutton et al 2008).
Microglial cells are increasingly viewed as active participants in neurodegenerative phenomena, and several cellular and molecular mechanisms have been implicated (Block and Hong 2005; Sugama et al 2009). One of the most persuasive arguments has been made for a critical role of iron. An excess of free iron in microglia, by catalyzing the Fenton reaction, leads to the generation of highly reactive free radical species, oxidative stress, and subsequent lipid peroxidation and neuronal damage (Saleppico et al 1996; Sian-Hülsmann et al 2011). Activated microglial cells are less efficient in maintaining iron homeostasis, so that iron-induced oxidative damage is amplified (Shoham and Youdim 2000). This dysregulation has been traced to the iron storage protein ferritin, which is responsible for the intracellular sequestration of iron (Yoshida et al 1995, 1998). Notably, brain ferritin is abundant in microglial cells (Kaneko et al 1989), and dystrophic microglial cells in HD and Alzheimer’s disease are associated with ferritin immunoreactivity (Simmons et al 2007; Lopes et al 2008). These findings, together with the fact that abnormally high iron levels are seen – and seen early – in vulnerable brain regions in HD and other neurodegenerative diseases (Dexter et al 1991; Götz et al 2004; Bartzokis et al 2007), led to the hypothesis that aberrant regulation of microglial iron disposition may be crucial to the disease process. Furthermore, several studies in animals showed remarkable neuroprotection by iron chelation in relevant disease models, revealing promising therapeutic implications of the “iron hypothesis” (Zecca et al 2004; Li et al 2011).
As Fe2+ is a co-factor of 3HAO (Long et al 1954), fluctuations in microglial iron levels might determine enzyme activity and, by extension, control the function of QUIN in the brain under both physiological and pathological conditions. We therefore decided to study the Fe2+ dependence of 3HAO in detail in vitro, using tissue homogenates obtained from mouse, rat and human brain. In all cases, including assessment of tissue from HD patients, we also evaluated the effects of ferritin and the synthetic iron chelator N,N′-bis(2-hydroxybenzyl) ethylenediamine-N,N′-diacetic acid (HBED), which provides effective neuroprotection in vivo (Bergeron et al 1999; Liang et al 2008).
Methods
Animals
FVB/N mice (Taconic, Hudson, NY) and Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) were housed in a temperature-controlled, AAALAC-approved animal facility at the Maryland Psychiatric Research Center on a 12hr/12hr light-dark cycle with free access to food and water. Animals were killed according to protocols approved by the Institutional Animal Care and Use Committee of the University of Maryland School of Medicine. Brains were then rapidly removed, dissected, and frozen on dry ice.
Human brain tissue
Cortical tissue specimens were obtained from the Maryland Brain Collection, a repository of post-mortem tissue maintained in cooperation with the Office of the Chief Medical Examiner of the State of Maryland and located at the Maryland Psychiatric Research Center. Brain donors (n = 3) were aged 40.0 ± 11.9 years and free of neurological or psychiatric disorders at the time of death. The postmortem interval was 18.0 ± 6.0 hr (both means ± SEM).
Striatal tissue from grade 4 Huntington’s Disease (HD) patients and matched controls were received from the Harvard Brain Tissue Resource Center at McLean Hospital (n = 4 per group). Brain donors were aged 47.8 ± 9.4 years (controls) and 51.8 ± 11.1 years (HD) at the time of death. The postmortem interval was 20.0 ± 2.2 hr (controls) and 19.1 ± 1.3 hr (HD) (all means ± SEM).
All human tissues were obtained and used in accordance with the Ethical Principles for Medical Research Involving Human Subjects (Declaration of Helsinki).
Cortical and striatal tissue blocks were stored at −80°C until analysis was performed.
Chemicals
Ferritin (containing both the L- and the H-subunit; cf. Discussion) derived from human liver was purchased from EMD Chemicals (Gibbstown, NJ). HBED was obtained from Strem chemicals (Newburyport, MA). 4-Chloro-3-hydroxyanthranilic acid was provided by Drs. W. P. Todd and B. K. Carpenter (Department of Chemistry, Cornell University, Ithaca, NY). All other chemicals were purchased from various commercial suppliers and were of the highest available purity. Custom-synthesized [1-14C]-3-hydroxyanthranilic acid (6 mCi/mmol) was obtained from DuPont/New England Nuclear (Boston, MA). 1-[14C]-Glutamic acid (55 mCi/mmol) was purchased from Sigma (St. Louis, MO).
Enzyme assays
3-Hydroxyanthranilic acid 3,4-dioxygenase (3HAO)
Tissues were homogenized 1:5 (wt/vol) by sonication in ice-cold 60 mM [2-(N-morpholino)ethanesulfonic acid] (MES) buffer (pH 6.0), and the homogenate was further diluted in the same buffer to a final dilution of 1:25 (rat and mouse) for use in the assay. The final dilution of human tissue homogenate was 1:50 or, in the experiment described in Fig. 4a, 1:250. One hundred μL of the tissue preparation were then incubated for 1 hr at 37°C in an aqueous solution containing 0.01% ascorbic acid and 7.2 μM (8.2 nCi) [1-14C]-3-hydroxyanthranilic acid. Fe(NH4)2(SO4)3 (“Fe2+”), ferritin, or HBED were added in small aliquots as needed. The final incubation volume was 200 μL. The enzyme reaction was terminated by the addition of 50 μL of 6% perchloric acid, and the product, 14C-QUIN, was recovered by ion-exchange chromatography (Dowex 50, H+-form) and quantitated by liquid scintillation spectrometry (Foster et al 1986). Blanks were obtained using 10 μM 4-chloro-3-hydroxyanthranillic acid, a specific inhibitor of 3HAO activity (Parli et al 1980).
Figure 4.
a: Striatal 3HAO and GAD activities in controls (CTR) and HD patients (n = 4 per group). 3HAO activity was determined in the presence of 150 μM Fe2+. Data are the mean + SEM. *p < 0.05 vs. controls (unpaired Student’s t-test); b: Ferritin and HBED attenuate the Fe2+-induced increase of 3HAO activity in the human striatum. Data (means + SEM) are from 4 control and 4 HD brains and are expressed as a percentage of enzyme activity in the presence of 30 μM Fe2+ (control; C). Dotted lines represent 3HAO activity in the absence of exogenously supplied Fe2+ (see text for absolute activities). *p < 0.05, **p < 0.01, ***p < 0.001 vs. enzyme activity in the presence of 30 μM Fe2+ (repeated measures one-way ANOVA followed by Bonferroni’s post-hoc analysis).
Glutamic acid decarboxylase (GAD)
Tissues were homogenized 1:5 (wt/vol) by sonication in ultrapure water and further diluted in 100 mM Tris-HCl buffer, pH 7.4, containing 0.2% Triton X-100 for a final tissue dilution of 1:10. Twenty-five μL of the homogenate were then incubated for 1 hr at 37°C in 100 mM phosphate buffer, pH 6.9, containing 1 mM disodium EDTA, 50 μM mercaptoethanol, 100 μM pyridoxal-5′-phosphate, and 0.045 μCi [14C]-glutamic acid in 13 mM monosodium glutamate. The reaction was terminated by the addition of 200 μL of 30% trichloroacetic acid. The 14CO2 generated by the enzyme reaction was trapped using filter paper moistened with 200 μL benzethonium hydroxide, and radioactivity was quantified by liquid scintillation spectrometry. Blanks were obtained by adding 25 μL of the phosphate buffer containing disodium EDTA in place of the tissue homogenate.
Protein measurement
Protein was determined by the method of Lowry et al (1951), using bovine serum albumin as a standard.
Results
Fe2+-dependency of brain 3HAO activity
Absolute enzyme activities in cortical tissue in the absence of exogenously supplied Fe2+ were 5.8 ± 0.3 (rat), 3.9 ± 0.0 (mouse) and 26.9 ± 6.9 (human) pmoles/h/mg tissue, respectively. As illustrated in Fig. 1, addition of Fe2+ to the reaction mixture substantially increased brain 3HAO activity in all three species examined, attaining maxima of 450–600% of control values. In rat brain tissue homogenate, enzyme activity reached more than 500% of control values following the addition of 14 μM Fe2+, a steep rise from the essentially unchanged 3HAO activity seen in the presence of 6 μM exogenous Fe2+ in the incubation medium. Fe2+-dependent increases in 3HAO activity in mouse and human brain tissue were somewhat shallower than in rat brain. In the mouse brain, the dose-response curve for Fe2+ was shifted to the right, and the maximal effect was seen at approximately 40 μM Fe2+. Stimulation of 3HAO activity by Fe2+ in the human brain more closely resembled the rat brain (maximal effect approx. 450% of control values).
Figure 1.
Fe2+-dependency of rat, mouse and human 3HAO activity in brain tissue homogenate (cortex). Data are expressed as a percentage of activity in the absence of exogenously supplied Fe2+ and are the means ± SEM of three separate experiments for each species. See text for absolute enzyme activities. *p < 0.05, **p < 0.01, ***p<0.001 vs. activity in the absence of added Fe2+ (repeated measures one-way ANOVA followed by Bonferroni’s post-hoc analysis).
Repeated measures one-way ANOVA revealed an overall significance of p < 0.0001 in all three species. Higher concentrations of Fe2+ resulted in significantly elevated 3HAO activity compared to control activity measured in the absence of exogenous Fe2+ (Fig. 1).
Effects of ferritin
Next, we examined the effects of ferritin on 3HAO activity in the absence or presence of exogenously supplied Fe2+. As shown in Table 1, ferritin, added at 1, 3, 10, 30 and 100 μg/assay tube in the absence of supplemental Fe2+, reduced 3HAO activity up to 60%. Although the data did not attain statistical significance in the mouse cortex, the results were significant in rat (10–100 μg ferritin) and human (1–100 μg ferritin) brain tissue homogenate (p < 0.005-0.0001; repeated measures one-way ANOVA).
Table 1.
Effect of ferritin on 3HAO activity in mouse, rat and human brain (cortex)
| Rat | Mouse | Human | |
|---|---|---|---|
|
| |||
| Control | 100.0 ± 13.6 | 100.0 ± 17.5 | 100.0 ± 7.9 |
|
| |||
| 1 μg Ferritin | 82.3 ± 6.4 | 73.3 ± 7.6 | 71.7 ± 1.9 ** |
| 3 μg Ferritin | 67.7 ± 2.0 | 63.7 ± 4.4 | 61.3 ± 1.3 *** |
|
| |||
| 10 μg Ferritin | 59.0 ±3.1 * | 60.0 ± 10.4 | 59.3 ± 0.3 *** |
| 30 μg Ferritin | 55.7 ±3.2 * | 61.0 ± 13.8 | 54.3 ± 2.3 *** |
|
| |||
| 100 μg Ferritin | 58.3 ±3.8 * | 56.3 ± 11.5 | 43.7 ± 2.6 *** |
Data are expressed as a percentage of activity in the absence of exogenously supplied Fe2+ and are the means ± SEM of three separate experiments for each species.
p < 0.05,
p < 0.01,
p<0.001 vs. controls in the absence of added Fe2+ (repeated measures one-way ANOVA followed by Bonferroni’s post-hoc analysis). See text for absolute enzyme activities in the absence of ferritin (100%).
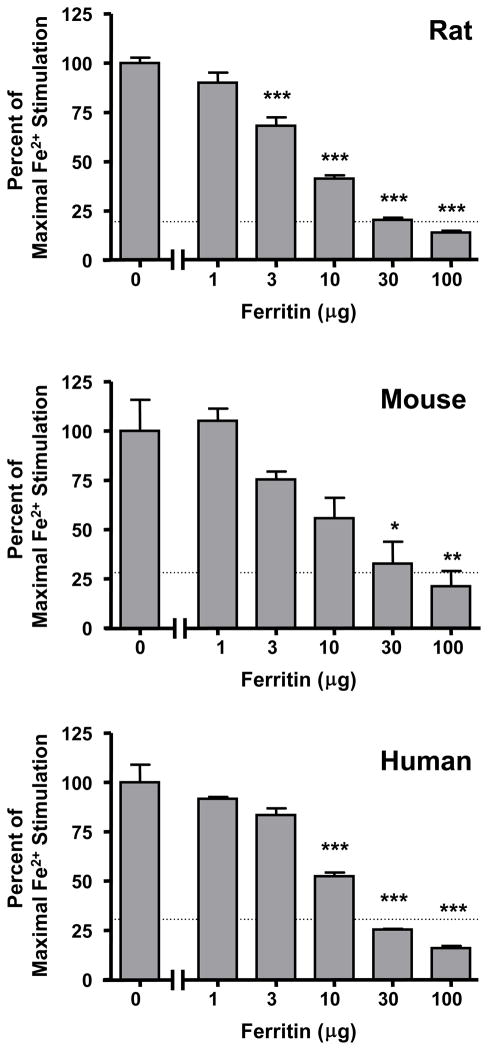
Ferritin also attenuated the stimulation of 3HAO activity by exogenously supplied Fe2+. Tested against maximally effective doses of Fe2+ (cf. Fig. 1; 30 μM in rat and human, 40 μM in mouse), the addition of ferritin dose-dependently reduced 3HAO stimulation in all three species. Although moderate quantitative differences were seen between rat, mouse and human enzyme, 30 μg ferritin caused complete reversal to control activity, and 100 μg ferritin resulted in enzyme activities below control levels in all cases (Fig. 2). Repeated measure one-way ANOVAs revealed an overall significance of p < 0.001 in all three species.
Figure 2.
Ferritin dose-dependently attenuates the Fe2+-induced increase of 3HAO activity in rat, mouse and human brain tissue (cortex). Data are expressed as a percentage of 3HAO activity under conditions of maximal enzyme activity, i.e. in the presence of 30 μM (rat, human) or 40 μM (mouse) Fe2+ (control), and are the means + SEM of three separate experiments for each species. Dotted lines represent 3HAO activity in the absence of exogenously supplied Fe2+. Control enzyme activities in the presence of Fe2+ were 38.7 ± 5.5 (rat), 19.2 ± 5.7 (mouse), 113.4 ± 11.4 (human) pmoles/h/mg tissue, respectively. *p < 0.05, **p < 0.01, ***p<0.001 vs. enzyme activity in the presence of 30 μM (rat, human) or 40 μM (mouse) Fe2+, respectively (repeated measures one-way ANOVA followed by Bonferroni’s post-hoc analysis).
Effects of HBED
An identical experimental protocol was used to examine the ability of the synthetic iron chelator HBED to attenuate the increase in cortical 3HAO activity caused by 30 μM (rat, human) or 40 μM (mouse) Fe2+. In contrast to ferritin (see above), addition of HBED up to a concentration of 100 μM in the absence of exogenously supplied Fe2+ failed to reduce 3HAO activity in any species (data not shown). However, addition of HBED (1–100 μM) caused steep, dose-dependent reductions in Fe2+-stimulated 3HAO. The effect was very similar in all three species, with 30 and 100 μM HBED providing significant attenuation (Fig. 3, insets). Repetition of the experiments using six HBED concentrations within a narrow range, i.e. between 10 and 30 μM, gave similar results in rat, mouse and human brain homogenates (Fig. 3). Statistical analysis of the effect showed an overall significance of p < 0.001 (repeated measures one-way ANOVA).
Figure 3.
HBED dose-dependently attenuates the Fe2+-induced increase of 3HAO activity in rat, mouse and human brain tissue (cortex). Data are expressed as a percentage of 3HAO activity under conditions of maximal enzyme activity, i.e. in the presence of 30 μM (rat, human) or 40 μM (mouse) Fe2+ (control) and are the means ± SEM of three separate experiments for each species. Insets depict a wider range of HBED concentrations. Dotted lines represent 3HAO activity in the absence of exogenously supplied Fe2+. Control enzyme activities in the presence of Fe2+ were 30.4 ± 3.3 (rat), 22.2 ± 1.2 (mouse) and 114.9 ± 10.8 (human) pmoles/h/mg tissue, respectively. *p < 0.05, **p < 0.01, ***p<0.001 vs. enzyme activity in the presence of 30 μM (rat, human) or 40 μM (mouse) Fe2+, respectively (repeated measures one-way ANOVA followed by Bonferroni’s post-hoc analysis).
Fe2+-dependency of 3HAO activity in the human neostriatum: normal controls and HD
Since abnormal disposition of iron and/or QUIN may be linked to the pathophysiology of HD (Bartzokis et al 2007; Schwarcz et al 2010), we next determined the Fe2+-dependence of 3HAO using neostriatal tissue (used in the assay at a final dilution of 1:250 to avoid a ceiling effect) obtained postmortem from individuals with end-stage (grade 4) HD and normal controls (n = 4 each). Compared to control specimens, HD tissue showed the expected large decrease in GAD activity (Fig. 4a; p < 0.05, unpaired t-test), confirming massive neuronal degeneration (Bird and Iversen 1974). In the same tissues, 3HAO activity in the presence of 150 μM Fe2+, i.e. the concentration routinely used to obtain optimal enzyme activity in vitro (Schwarcz et al 1988), was higher in HD (375.0 ± 132.7 vs. 122.0 ± 22.9 pmoles/h/mg protein), though the group difference did not attain statistical significance (Fig. 4a; p = 0.11, unpaired t-test).
Next, we assessed striatal 3HAO activity in the same tissues in the absence and presence of externally supplied Fe2+ (30 μM; cf. Fig. 1). In the absence of added Fe2+, 3HAO activities were 74.0 ± 15.7 (control) and 84.7 ± 24.5 (HD) pmoles/h/mg tissue. Addition of 30 μM Fe2+ caused an approximately 3-fold increase in enzyme activity in both control and HD tissue (Fig. 4b).
In the presence of 30 μM Fe2+, addition of ferritin or HBED had similar effects in both HD and control tissue (Fig. 4b). Thus, whereas neither 10 μg of ferritin or 10 μM HBED affected the stimulation of 3HAO activity by Fe2+ significantly in either case, the chelators had a substantive effect at 100 μg or 100 μM, respectively, reducing 3HAO activity to control levels (absence of added Fe2+) or below in both groups. With the exception of 100 μg HBED in HD tissue, all these effects attained statistical significance (repeated measures one-way ANOVA followed by Bonferroni’s post-hoc analysis). Change scores, i.e. the effect of the iron chelators relative to the respective control value (30 μM Fe2+), did not reveal differences between normal control and HD tissue (Student’s t-test).
Discussion
Using crude tissue homogenates and a conventional radiochemical assay procedure (Foster et al 1986), the present in vitro study was designed to investigate in detail the potential of Fe2+ to regulate 3HAO activity in the brain. Our results revealed that even relatively minor fluctuations in Fe2+ concentration in the assay mixture had significant effects on 3HAO in all three species tested (rat, mouse and human), suggesting that Fe2+ is capable of fine tuning cerebral QUIN production. The slope of the iron curve was steepest in rat brain where a fairly modest increase – from 6 to 14 μM – resulted in a more than 5-fold stimulation of enzyme activity. In all species, the Fe2+-induced increase in 3HAO activity was dose-dependently attenuated by the addition of ferritin, the main iron storage protein in the brain. Moreover, the effect of iron was also readily prevented by micromolar concentrations of N,N′-bis(2-hydroxybenzyl) ethylenediamine-N,N′-diacetic acid (HBED), a cell membrane-permeable, experimental iron chelator with impressive neuroprotective properties in vivo (Liang et al 2008). Finally, we compared the effects of iron and iron chelation on 3HAO activity in neostriatal specimens obtained from HD patients and normal controls. This attempt to relate our study to the roles of both Fe2+ and QUIN in the pathophysiology of HD failed to reveal significant abnormalities in the diseased tissue.
Iron homeostasis is critical for normal brain function, and several mechanisms have evolved to control the disposition of the metal in cerebral tissue. Iron uptake from the blood, its storage and release within the brain, as well as iron efflux from the brain, are tightly regulated by a host of proteins, including divalent metal transporters, transferrin and its receptors, and a number of additional iron-binding proteins. Although the biochemistry and cell biology of several of these proteins are reasonably well understood individually, their joint role in controlling intracellular trafficking and physiological functions of iron in the brain is highly complex and has not been clarified (see Burdo and Connor 2003, and Ke and Qian 2007 for reviews). Notably, however, the ambient cytosolic concentration of free iron in the normal rat brain is approximately 30 μM, i.e. in the range used experimentally to modulate 3HAO in the present study (Erikson et al 1997).
In the brain as elsewhere in the body, ferritin is the major protein involved in the storage and release of intracellular iron and therefore controls the many functions of iron, ranging from oxygen transport to DNA synthesis (Ponka et al 1998). By effectively sequestering iron as soluble, “mineralized” ferrihydrite, ferritin protects the cell against the production of destructive oxygen radicals and at the same time provides ferrous ions for biological functions. Widely distributed within the cell, ferritin consists of a protein shell that is made of two distinct polypeptide subunits [H (heavy) and L (light)] and encloses a hollow interior where bioavailable iron is stored. The two chains fulfill distinct, yet synergistic roles in the handling of intracellular iron, with the H-subunit accounting for the rapid accumulation and subsequent oxidation of Fe2+ to Fe3+, and the L-subunit serving a conventional storage function (Koorts and Viljoen 2007). Of interest in the present context, microglia express mostly L-ferritin (Connor et al 1994), possibly affecting access of endogenous reducing agents such as cysteine, glutathione and ascorbic acid, and the subsequent transport of ferrous ions to various intracellular sites (see Koorts and Viljoen 2007, for review). Thus, while ferritin can be used experimentally as an iron chelator, its biological role is clearly far more multi-faceted and versatile.
Not unexpectedly in light of its central role in the sequestration of brain iron, abnormal ferritin has detrimental effects on brain structure and function. This was originally shown by Curtis et al (2001), who reported the occurrence of extrapyramidal features similar to HD in affected individuals from a family with a mutation in the gene coding for L-ferritin. Similar features of a hereditary “ferritinopathy” were described in a different pedigree (Kubota et al 2009), and the phenotype was duplicated in mice expressing a mutant form of L-ferritin and traced to iron mishandling on the molecular level (Vidal et al 2008; Baraibar et al 2010; Luscieti et al 2010). However, abnormal iron function has also been associated with the genes of H-ferritin and several other proteins involved in iron metabolism and trafficking. Clinical implications of these gene findings have so far focused primarily on HD and, in particular, Alzheimer’s and Parkinson’s disease (see Zecca et al 2004 and Rhodes and Ritz 2008, for review).
The present study, demonstrating a tight regulation of QUIN neosynthesis by Fe2+ concentrations in the endogenous range, suggests additional, underappreciated mechanisms by which impaired intracellular iron disposition might lead to brain dysfunction and neurodegeneration. Thus, while the Fe2+ dependency of 3HAO is well characterized (Long et al 1954; Koontz and Shiman 1976; Dilović et al 2009), direct interactions between free iron and QUIN, too, might be causally involved in pathology. In addition to its ability to selectively activate the NMDA subtype of glutamate receptors (see Introduction), QUIN potently stimulates lipid peroxidation (Rios and Santamaria 1991), and this effect, which is strictly dependent on the presence of ferrous ions (Stípek et al 1997), potentiates QUIN-induced excitotoxicity (Santamaria et al 2001). Notably, the generation of reactive oxygen species by QUIN is secondary to the formation and slow, pH-dependent autooxidation of Fe2+-QUIN complexes and can be readily prevented by iron chelation (Pláteník et al 2001; Dairam et al 2008). Finally, to complicate matters further, endogenous iron also appears to directly control the binding of QUIN to the NMDA receptor (St’astny et al 1999). By inference, our present results imply that 3HAO, by factually determining the ratio between free iron and QUIN in the cell, plays a central role in controlling the highly complex interplay between Fe2+ and QUIN in the mammalian brain. The physiological significance of the balance of free iron and QUIN in the brain has so far not been interrogated in detail, however.
Microglial cells may hold the key to the puzzle since they contain 3HAO and are richly endowed with ferritin (Kaneko et al 1989; Alberati-Giani et al 1996; Lehrmann et al 2001). Following nerve cell loss or an immune challenge, activated or dystrophic microglia show intense ferritin immunoreactivity in both animals and humans (Gorter et al 2005; Zhang et al 2005; Simmons et al 2007; Lopes et al 2008). Similarly, and coincident with increased 3HAO activity measured in tissue homogenate (Schwarcz et al 1989), microglial QUIN immunoreactivity is dramatically enhanced in the excitotoxically lesioned rat striatum, which models neostriatal HD pathology (Lehrmann et al 2001). In spite of these remarkable changes, our present in vitro experiments revealed neither qualitative nor quantitative differences between the effects of iron or iron chelation on neostriatal 3HAO activity in tissue from normal controls and HD patients (Fig. 4). Ongoing in vivo experiments in our laboratory are designed to elaborate the dynamics between microglial iron and QUIN in greater depth and, in particular, to determine the potential relevance of these interactions for neuronal dysfunction and death.
Our study also pertains to the use of iron chelators as therapeutic agents in human brain diseases. Interestingly, the most impressive neuroprotective effects of iron chelation, including genetic up-regulation of ferritin (Kaur et al 2003), have so far been seen in animal models of Parkinson’s disease, Alzheimer’s disease and HD, i.e. disorders which have been linked to excitotoxic mechanisms and, specifically, to excess QUIN (Zecca et al 2004; Nguyen et al 2005; Németh et al 2006; Chen and Guillemin 2009; Schwarcz et al 2010; Li et al 2011). Our results with ferritin and HBED, a synthetic, membrane-permeable chelator of mitochondrial iron with striking in vivo potency (Bergeron et al 1999; Liang et al 2008), raise the possibility that dysregulation of iron storage mechanisms in the brain may contribute to the neuropathology of QUIN-related neurodegenerative disorders. Conversely, down-regulation of QUIN synthesis may contribute to the neuroprotective effects of iron chelation in vivo.
Acknowledgments
This study was supported by NIH grants NS057715 and AG022074.
Abbreviations
- 3HAO
3-hydroxyanthranilic acid dioxygenase
- HBED
N,N′-bis(2-hydroxybenzyl) ethylenediamine-N,N′-diacetic acid
- HD
Huntington’s disease
- QUIN
Quinolinic acid
References
- Alberati-Giani D, Ricciardi-Castagnoli P, Köhler C, et al. Regulation of the kynurenine metabolic pathway by interferon-gamma in murine cloned macrophages and microglial cells. J Neurochem. 1996;66:996–1004. doi: 10.1046/j.1471-4159.1996.66030996.x. [DOI] [PubMed] [Google Scholar]
- Baraibar MA, Muhoberac BB, Garringer HJ, et al. Unraveling of the E-helices and disruption of 4-fold pores are associated with iron mishandling in a mutant ferritin causing neurodegeneration. J Biol Chem. 2010;285:1950–1956. doi: 10.1074/jbc.M109.042986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G, Tishler TA, Shin IS, et al. Brain ferritin iron as a risk factor for age at onset in neurodegenerative diseases. Ann N Y Acad Sci. 2004;1012:224–236. doi: 10.1196/annals.1306.019. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Tishler TA, et al. Myelin breakdown and iron changes in Huntington’s disease: pathogenesis and treatment implications. Neurochem Res. 2007;32:1655–1664. doi: 10.1007/s11064-007-9352-7. [DOI] [PubMed] [Google Scholar]
- Bergeron RJ, Wiegand J, Brittenham GM. HBED: the continuing development of a potential alternative to deferoxamine for iron-chelating therapy. Blood. 1999;93:370–375. [PubMed] [Google Scholar]
- Bird ED, Iversen LL. Huntington’s chorea. Post-mortem measurement of glutamic acid decarboxylase, choline acetyltransferase and dopamine in basal ganglia. Brain. 1974;97:457–472. doi: 10.1093/brain/97.1.457. [DOI] [PubMed] [Google Scholar]
- Block ML, Hong JS. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol. 2005;76:77–98. doi: 10.1016/j.pneurobio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Burdo JR, Connor JR. Brain iron uptake and homeostatic mechanisms: an overview. Biometals. 2003;16:63–75. doi: 10.1023/a:1020718718550. [DOI] [PubMed] [Google Scholar]
- Chen Y, Guillemin GJ. Kynurenine pathway metabolites in humans: disease and healthy states. Int J Tryptophan Res. 2009;2:1–19. doi: 10.4137/ijtr.s2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor JR, Boeshore KL, Benkovic SA, et al. Isoforms of ferritin have a specific cellular distribution in the brain. J Neurosci Res. 1994;37:461–465. doi: 10.1002/jnr.490370405. [DOI] [PubMed] [Google Scholar]
- Curtis AR, Fey C, Morris CM, et al. Mutation in the gene encoding ferritin light polypeptide causes dominant adult-onset basal ganglia disease. Nat Genet. 2001;28:350–354. doi: 10.1038/ng571. [DOI] [PubMed] [Google Scholar]
- Dairam A, Fogel R, Daya S, et al. Antioxidant and iron-binding properties of circumin, capsaicin, and S-allylcysteine reduce oxidative stress in rat brain homogenate. J Agric Food Chem. 2008;56:3350–3356. doi: 10.1021/jf0734931. [DOI] [PubMed] [Google Scholar]
- Dexter DT, Carayon A, Javoy-Agid F, et al. Alterations in the levels of iron, ferritin and other trace metals in Parkinson’s disease and other neurodegenerative diseases affecting the basal ganglia. Brain. 1991;114:1953–1975. doi: 10.1093/brain/114.4.1953. [DOI] [PubMed] [Google Scholar]
- Dilović I, Gliubich F, Malpeli G, et al. Crystal structure of bovine 3-hydroxyanthranilate 3,4-dioxygenase. Biopolymers. 2009;91:1189–1195. doi: 10.1002/bip.21167. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Pinero DJ, Connor JR, et al. Regional brain iron, ferritin and transferrin concentrations during iron deficiency and iron repletion in developing rats. J Nutr. 1997;127:2030–2038. doi: 10.1093/jn/127.10.2030. [DOI] [PubMed] [Google Scholar]
- Foster AC, White RJ, Schwarcz R. Synthesis of quinolinic acid by 3-hydroxyanthranilic acid oxygenase in rat brain tissue in vitro. J Neurochem. 1986;47:23–30. doi: 10.1111/j.1471-4159.1986.tb02826.x. [DOI] [PubMed] [Google Scholar]
- Gorter JA, Mesquita AR, van Vliet EA, et al. Increased expression of ferritin, an iron-storage protein, in specific regions of the parahippocampal cortex of epileptic rats. Epilepsia. 2005;46:1371–1379. doi: 10.1111/j.1528-1167.2005.11505.x. [DOI] [PubMed] [Google Scholar]
- Götz ME, Double K, Gerlach M, et al. The relevance of iron in the pathogenesis of Parkinson’s disease. Ann NY Acad Sci. 2004;1012:193–208. doi: 10.1196/annals.1306.017. [DOI] [PubMed] [Google Scholar]
- Hutton LC, Castillo-Melendez M, Smythe GA. Microglial activation, macrophage infiltration, and evidence of cell death in the fetal brain after uteroplacental administration of lipopolysaccharide in sheep in late gestation. Am J Obstet Gynecol. 2008;198:117.e1–11. doi: 10.1016/j.ajog.2007.06.035. [DOI] [PubMed] [Google Scholar]
- Kaneko Y, Kitamoto T, Tateishi J, et al. Ferritin immunohistochemistry as a marker for microglia. Acta Neuropathol. 1989;79:129–136. doi: 10.1007/BF00294369. [DOI] [PubMed] [Google Scholar]
- Kaur D, Yantiri F, Rajagopalan S, et al. Genetic or pharmacological iron chelation prevents MPTP-induced neurotoxicity in vivo: a novel therapy for Parkinson’s disease. Neuron. 2003;37:899–909. doi: 10.1016/s0896-6273(03)00126-0. [DOI] [PubMed] [Google Scholar]
- Ke Y, Qian ZM. Brain iron metabolism: neurobiology and neurochemistry. Prog Neurobiol. 2007;83:149–73. doi: 10.1016/j.pneurobio.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Koontz WA, Shiman R. Beef kidney 3-hydroxyanthranilic acid oxygenase. Purification, characterization, and analysis of the assay. J Biol Chem. 1976;251:368–377. [PubMed] [Google Scholar]
- Koorts AM, Viljoen M. Ferritin and ferritin isoforms I: Structure-function relationships, synthesis, degradation and secretion. Arch Physiol Biochem. 2007;113:30–54. doi: 10.1080/13813450701318583. [DOI] [PubMed] [Google Scholar]
- Kubota A, Hida A, Ichikawa Y, et al. A novel ferritin light chain gene mutation in a Japanese family with neuroferritinopathy: description of clinical features and implications for genotype-phenotype correlations. Mov Disord. 2009;24:441–445. doi: 10.1002/mds.22435. [DOI] [PubMed] [Google Scholar]
- Lehrmann E, Molinari A, Speciale C, et al. Immunohistochemical visualization of newly formed quinolinate in the normal and excitotoxically lesioned rat striatum. Exp Brain Res. 2001;141:389–397. doi: 10.1007/s002210100887. [DOI] [PubMed] [Google Scholar]
- Li X, Jankovic J, Le W. Iron chelation and neuroprotection in neurodegenerative diseases. J Neural Transm. 2011;118:473–477. doi: 10.1007/s00702-010-0518-0. [DOI] [PubMed] [Google Scholar]
- Liang LP, Jarrett SG, Patel M. Chelation of mitochondrial iron prevents seizure-induced mitochondrial dysfunction and neuronal injury. J Neurosci. 2008;28:11550–11556. doi: 10.1523/JNEUROSCI.3016-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long CL, Hill HN, Weinstock IM, et al. Studies of the enzymatic transformation of 3-hydroxyanthranilate to quinolinate. J Biol Chem. 1954;211:405–417. [PubMed] [Google Scholar]
- Lopes KO, Sparks DL, Streit WJ. Microglial dystrophy in the aged and Alzheimer’s disease brain is associated with ferritin immunoreactivity. Glia. 2008;56:1048–1060. doi: 10.1002/glia.20678. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Luscieti S, Santambrogio P, Langlois d’Estaintot B, et al. Mutant ferritin L-chains that cause neurodegeneration act in a dominant-negative manner to reduce ferritin iron incorporation. J Biol Chem. 2010;285:11948–11957. doi: 10.1074/jbc.M109.096404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Németh H, Toldi J, Vécsei L. Kynurenines, Parkinson’s disease and other neurodegenerative disorders: preclinical and clinical studies. J Neural Transm Suppl. 2006;70:285–304. doi: 10.1007/978-3-211-45295-0_45. [DOI] [PubMed] [Google Scholar]
- Nguyen T, Hamby A, Massa SM. Clioquinol down-regulates mutant huntingtin expression in vitro and mitigates pathology in a Huntington’s disease mouse model. Proc Natl Acad Sci USA. 2005;102:11840–11845. doi: 10.1073/pnas.0502177102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney JW, Misra CH, de Gubareff T. Cysteine-S-sulfate: brain damaging metabolite in sulfite oxidase deficiency. J Neuropathol Exp Neurol. 1975;34:167–177. doi: 10.1097/00005072-197503000-00005. [DOI] [PubMed] [Google Scholar]
- Parli CJ, Krieter P, Schmidt B. Metabolism of 6-chlorotryptophan to 4-chloro-3-hydroxyanthranilic acid: a potent inhibitor of 3-hydroxyanthranilic acid oxidase. Arch Biochem Biophys. 1980;203:161–166. doi: 10.1016/0003-9861(80)90164-2. [DOI] [PubMed] [Google Scholar]
- Pláteník J, Stopka P, Vejrazka M, et al. Quinolinic acid-iron (II) complexes: slow autoxidation, but enhanced hydroxyl radical production in the Fenton reaction. Free Radic Res. 2001;34:445–459. doi: 10.1080/10715760100300391. [DOI] [PubMed] [Google Scholar]
- Ponka P, Beaumont C, Richardson DR. Function and regulation of transferrin and ferritin. Semin Hematol. 1998;35:35–54. [PubMed] [Google Scholar]
- Rhodes SL, Ritz B. Genetics of iron regulation and the possible role of iron in Parkinson’s disease. Neurobiol Dis. 2008;32:183–195. doi: 10.1016/j.nbd.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios C, Santamaria A. Quinolinic acid is a potent lipid peroxidant in rat brain homogenates. Neurochem Res. 1991;16:1139–1143. doi: 10.1007/BF00966592. [DOI] [PubMed] [Google Scholar]
- Santamaría A, Jiménez-Capdeville ME, Camacho A. In vivo hydroxyl radical formation after quinolinic acid infusion into rat corpus striatum. Neuroreport. 2001;28(12):2693–2696. doi: 10.1097/00001756-200108280-00020. [DOI] [PubMed] [Google Scholar]
- Saito K, Nowak TS, Jr, Suyama K, et al. Kynurenine pathway enzymes in brain: responses to ischemic brain injury versus systemic immune activation. J Neurochem. 1993;61:2061–2070. doi: 10.1111/j.1471-4159.1993.tb07443.x. [DOI] [PubMed] [Google Scholar]
- Saleppico S, Mazzolla R, Boelaert JR, et al. Iron regulates microglial cell-mediated secretory and effector functions. Cell Immunol. 1996;170:251–259. doi: 10.1006/cimm.1996.0159. [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Foster AC, French ED, et al. Excitotoxic models for neurodegenerative disorders. Life Sci. 1984;35:19–32. doi: 10.1016/0024-3205(84)90148-6. [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Okuno E, White RJ, et al. 3-Hydroxyanthranilic acid oxygenase activity is increased in the brains of Huntington disease victims. Proc Natl Acad Sci USA. 1988;85:4079–4081. doi: 10.1073/pnas.85.11.4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarcz R, Okuno E, White RJ. Basal ganglia lesions in the rat: effects on quinolinic acid metabolism. Brain Res. 1989;490:103–109. doi: 10.1016/0006-8993(89)90435-6. [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Guidetti P, Sathyasaikumar KV, et al. Of mice, rats and men: revisiting the quinolinic acid hypothesis of Huntington’s disease. Prog Neurobiol. 2010;90:230–245. doi: 10.1016/j.pneurobio.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoham S, Youdim MB. Iron involvement in neural damage and microgliosis in models of neurodegenerative diseases. Cell Mol Biol. 2000;46:743–760. [PubMed] [Google Scholar]
- Sian-Hülsmann J, Mandel S, Youdim MB, et al. The relevance of iron in the pathogenesis of Parkinson’s disease. J Neurochem. 2011 doi: 10.1111/j.1471-4159.2010.07132.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Simmons DA, Casale M, Alcon B, et al. Ferritin accumulation in dystrophic microglia is an early event in the development of Huntington’s disease. Glia. 2007;55:1074–1084. doi: 10.1002/glia.20526. [DOI] [PubMed] [Google Scholar]
- Speciale C, Ungerstedt U, Schwarcz R. Production of extracellular quinolinic acid in the striatum studied by microdialysis in unanesthetized rats. Neurosci Lett. 1989;104:345–350. doi: 10.1016/0304-3940(89)90601-0. [DOI] [PubMed] [Google Scholar]
- St’astny F, Hinoi E, Ogita K, et al. Ferrous iron modulates quinolinate-mediated [3H]MK-801 binding to rat brain synaptic membranes in the presence of glycine and spermidine. Neurosci Lett. 1999;262:105–108. doi: 10.1016/s0304-3940(99)00061-0. [DOI] [PubMed] [Google Scholar]
- Stípek S, St’astný F, Pláteník J, et al. The effect of quinolinate on rat brain lipid peroxidation is dependent on iron. Neurochem Int. 1997;30:233–237. [PubMed] [Google Scholar]
- Sugama S, Takenouchi T, Cho BP, et al. Possible roles of microglial cells for neurotoxicity in clinical neurodegenerative diseases and experimental animal models. Inflamm Allergy Drug Targets. 2009;8:277–284. doi: 10.2174/187152809789352249. [DOI] [PubMed] [Google Scholar]
- Vidal R, Miravalle L, Gao X, et al. Expression of a mutant form of the ferritin light chain gene induces neurodegeneration and iron overload in transgenic mice. J Neurosci. 2008;28:60–67. doi: 10.1523/JNEUROSCI.3962-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiss O, Bettendorf G. Die Isolierung und vorläufige Charakterisierung des primären Oxidationsproduktes der 3-Hydroxy-anthranilsäure. Hoppe-Seyler’s Z Physiol Chemie. 1956;306:145–153. [PubMed] [Google Scholar]
- Yoshida T, Tanaka M, Sotomatsu A, et al. Activated microglia cause superoxide-mediated release of iron from ferritin. Neurosci Lett. 1995;190:21–24. doi: 10.1016/0304-3940(95)11490-n. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Tanaka M, Sotomatsu A, et al. Activated microglia cause iron-dependent lipid peroxidation in the presence of ferritin. Neuroreport. 1998;9:1929–1933. doi: 10.1097/00001756-199806220-00003. [DOI] [PubMed] [Google Scholar]
- Zecca L, Youdim MB, Riederer P, Connor JR, Crichton RR. Iron, brain ageing and neurodegenerative disorders. Nat Rev Neurosci. 2004;5:863–873. doi: 10.1038/nrn1537. [DOI] [PubMed] [Google Scholar]
- Zhang J, Stanton DM, Nguyen XV, et al. Intrapallidal lipopolysaccharide injection increases iron and ferritin levels in glia of the rat substantia nigra and induces locomotor deficits. Neuroscience. 2005;135:829–838. doi: 10.1016/j.neuroscience.2005.06.049. [DOI] [PubMed] [Google Scholar]