Abstract
Nocardia brasiliensis is an important etiologic agent of mycetoma. These bacteria live as a saprobe in soil or organic material and enter the tissue via minor trauma. Mycetoma is characterized by tumefaction and the production of fistula and abscesses, with no spontaneous cure. By using mass sequencing, we determined the complete genomic nucleotide sequence of the bacteria. According to our data, the genome is a circular chromosome 9,436,348-bp long with 68% G+C content that encodes 8,414 proteins. We observed orthologs for virulence factors, a higher number of genes involved in lipid biosynthesis and catabolism, and gene clusters for the synthesis of bioactive compounds, such as antibiotics, terpenes, and polyketides. An in silico analysis of the sequence supports the conclusion that the bacteria acquired diverse genes by horizontal transfer from other soil bacteria, even from eukaryotic organisms. The genome composition reflects the evolution of bacteria via the acquisition of a large amount of DNA, which allows it to survive in new ecological niches, including humans.
Introduction
Actinobacteria are gram-positive organisms that are ecologically important in nature as re-cyclers of organic matter, including cellulose from plants and chitin from insects. Many actinobacteria are branched and may produce exospores. Actinobacteria are important in medicine because they produce many biological active compounds. Since Waksman described actinomycin in 1940, many antibiotic, cytostatic and immunosuppressive compounds have been obtained from these organisms [1]. One of the subgroups, Corynebacterineae, is characterized by the production of mycolic acids that provide strength to the bacterial cell wall. Included in this sub-order are the families Corynebacteriaceae, Dietziaceae, Gordoniaceae, Mycobacteriaceae, Nocardiaceae, Tsukamurellaceae, and Williamsiaceae, which include specialized human pathogens, such as Mycobacterium tuberculosis, M. leprae, and Corynebacterium diphtheriae [2]. The Nocardiaceae family includes the genera Nocardia and Rhodococcus. The latter is an animal pathogen, particularly found in horses and immunodepressed human patients [3], [4].
Nocardia species produce pulmonary, cutaneous and subcutaneous human diseases [5]. The most commonly isolated species include N. brasiliensis, N. farcinica, N. cyriacigeorgica, and N. nova [6], [7], [8]. Pulmonary nocardiosis has been reported particularly in patients with debilitating underlying conditions, such as organ transplant, leukemia, and diabetes. Mycetoma is a subcutaneous infection with differential histological and clinical characteristics [9]. There is an increase of the volume of the region affected, generally the limbs, and may affect muscle and fascia; in old and extended lesions bone destruction can be produced [Fig. 1]. The subcutaneous infection drains through the skin via fistulae discharging a serous purulent liquid. Generally, mycetoma occurs though a minor trauma with thorns, exposure of cutaneous lesions to soil, implantation of wood in the back, or even car accidents [9], [10]. Histologically, microcolonies of the agent, composed of a tightly branched mass of filaments, are observed in microabscesses surrounded by fibrous tissue. Patients do not report pain and are generally immunocompetent. Actinomycetes producing mycetoma include species of Nocardia, Actinomadura, Streptomyces and Nocardiopsis. Because they are soil bacteria, the etiologic species distribution depends on the geographical region. A. madurae and A. pelletieri are more commonly reported in Africa and India [9]. In America, Nocardia, particularly Nocarda brasiliensis, is the most abundant etiologic agent. In Mexico, N. brasiliensis is responsible for approximately 86% of cases.
Figure 1. Mycetoma of the foot from Nocardia brasiliensis showing the characteristic triad of tumefaction, fistulae and microcolonies.
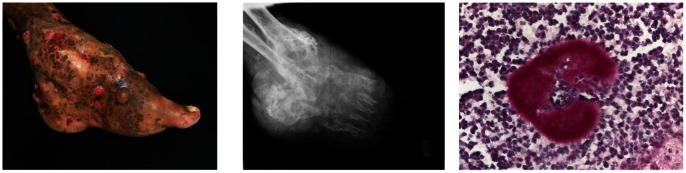
Central image, an X-ray analysis of the ankle and foot region showing the severe destruction of bones. Right image, a microcolony of N. brasiliensis stained with PAS surrounded by a PMN infiltrate.
The immunological mechanisms involved in actinomycetoma, as well as the virulence factors of Nocardia, are poorly understood, primarily because of a lack of tools to study them. Molecular genetic techniques have proved to be an excellent means of studying phylogenetic relationships, as well as the biological and pathogenic properties of bacteria, for both human pathogens and industrial organisms [11]. To elucidate the virulence mechanisms and biological properties of N. brasiliensis, we previously determined the complete genome sequence of N. brasiliensis HUJEG-1 (ATCC 700358), a strain used by our group in many immunological and antimicrobial assays [12], [13], [14]. The WGS was annotated in GenBank under the number NZ_AIHV00000000.1 and comprises 53 contigs [15]. Now, we have prepared the complete physical map by obtaining an optical map (OpGen Inc., Gaithersburg, Maryland) using pulse field electrophoresis, fixation and digestion with BglII and labeling of cut fragments with several fluorescence tags in order to obtain a restriction map. The contigs were aligned using this restriction map using MapSolver™ software and deposited in GenBank under the reference number NC_018681.1. Herein, we present an in silico analysis comparing the N. brasiliensis HUJEG-1 genome sequence with other available actinobacteria genomes, including other Nocardia spp.
Results
General Characteristics of the N. brasiliensis ATCC700358 Genome
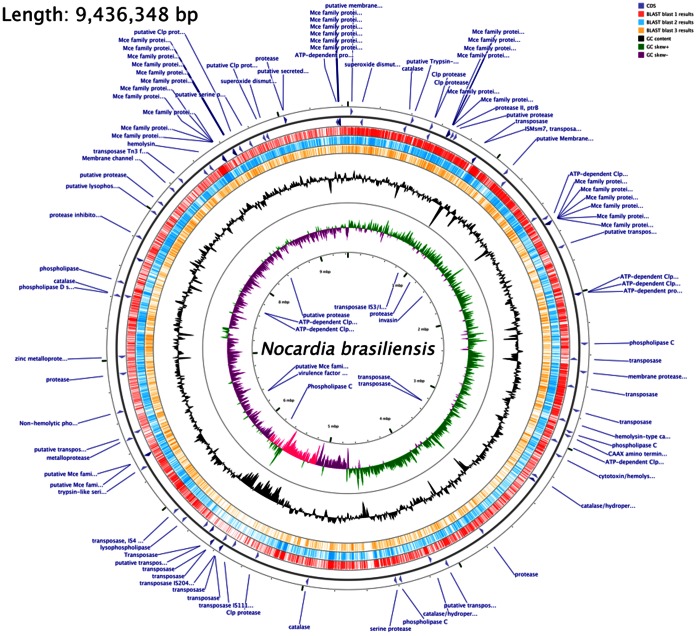
In Figure 2, we show the physical map of the complete genome. Only one large contig (NZ_AIHV000025; 59,711-bp) could not be found in the optical map, and therefore, it is believed to be of extra-chromosomal origin. The total genome size is 9,436,348-bp, with a G+C% of 68. The genome encodes 51 tRNA, three copies of the 16S-23S-5S rRNA operon, and 8,414 predicted protein-coding sequences. Hypothetical proteins were predominant (5,745/8,414 proteins). In addition, 2,888 of the ORFs could be annotated using the BLAST program. Interestingly, there is a zone of about 600,000-bp starting at about nucleotide 5,126,00 to nucleotide 5,800,000 with a lower G+C % (63–65%). When analyzing this DNA stretch by using the internet program BLAST, very little homology with any gene in the GenBank library was observed, and we observed less than 10 genes that were similar in two other complete Nocardia genomes, N. farcinica IFM102 and N. cyriacigerogica GUH-2. Recently, the complete WGS of 26 Nocardia species, including one N. brasiliensis isolate, were released to GenBank. Surprisingly, this fragment was not observed in any of these genome sequences. It is possible that this fragment was acquired by N. brasiliensis HUJEG-1 by horizontal transfer, which has been observed in soil bacteria [16], although a transferred fragment this large has not been reported.
Figure 2. Physical map of the N. brasiliensis chromosome and gene clusters for putative virulence genes.
The outer scale is numbered in megabases starting from the dnaA gene. The outermost two circles represent the position and strand direction of putative virulence genes and transposases. Circles 3, 4, and 5 represent a BLAST comparison with the complete genome of Nocardia farcinica IFM 10152 (NC_6361.1, red), Rhodococcus equi 103S (NC_014659.1, cyan) and Mycobacterium tuberculosis H37Rv (NC_000962.2, yellow), respectively. Circle 6, GC content; circle 7, GC bias. In magenta, we show the fragment with different G+C% (between nucleotide 5,126,000 and nucleotide 5,800,000).
At the time of the release of the N. brasiliensis HUJEG-1 WGS sequence, only two Nocardia genomes had been published, N. farcinica IFM 10142 and N. cyriacigeorgica GUH-2, both belonging to the previously named N. asteroides complex [17], [18]. The reported sizes were approximately 6 MB (Table 1). Thus, we were surprised to find a 3.4-Mb larger genome. When comparing the complete genomes of pathogenic versus non-pathogenic bacteria, it has been observed that organisms that are more adapted to humans tend to have a smaller genome size because the bacteria eliminate those genes that are not needed for their parasitic lifestyle, such as the reduction in size of the genomes of the human pathogen Mycobacterium leprae and the filarial symbiont Wolbachia spp. [19], [20]. In our case, we found the opposite: a human pathogen with a large genome. When we compared the genome size among Nocardia spp., we observed sizes from 6.96 Mb for N. asteroides NBRC 15531 to 10.45 Mb for N. jiangxiensis NBRC 101359. N. brasiliensis NBRC 14402 (ATCC 19296) has a genome size of 8.9 Mb, which is similar to that of our strain. It appears that N. brasiliensis is not yet a specialized human pathogen and, instead, is a soil bacteria that occasionally affects humans.
Table 1. Comparison of genomic features of Nocardia brasiliensis and other bacteria.
| GenBank number | Size (Mb) | GC% | CDS | rRNA | tRNA | Genes | |
| Mycobacterium leprae TN | NC_002677.1 | 3.27 | 57.8 | 1,605 | 3 | 45 | 2,770 |
| Mycobacterium tuberculosis H37Rv | NC_000962.2 | 4.41 | 65.6 | 4,003 | 3 | 45 | 4,062 |
| Mycobacterium abscessus ATCC 19977 | NC_010397.1 | 5.07 | 64.1 | 4,920 | 3 | 47 | 4,970 |
| Mycobacterium smegmatis str. MC2 155 | NC_008596.1 | 6.99 | 67.4 | 6,717 | 6 | 47 | 6,938 |
| Nocardia farcinica IFM 10152 | NC_006361.1 | 6.29 | 70.7 | 5,934 | 9 | 53 | 5,998 |
| Nocardia cyriacigeorgica GUH-2 | NC_016887.1 | 6.19 | 68.4 | 5,477 | 9 | 49 | 5,560 |
| Nocardia brasiliensis HUJEG-1 | NC_018681.1 | 9.44 | 68 | 8,414 | 6 | 51 | 8,471 |
| Rhodococcus equi ATCC 33707 | NZ_CM001149.1 | 5.26 | 68.7 | 5,030 | 15 | 52 | 5,105 |
| Streptomyces griseus subsp. griseus NBRC 13350 | NC_010572.1 | 8.55 | 72.2 | 7,136 | 18 | 66 | 7,224 |
| Micromonospora aurantiaca ATCC 27029 | NC_014391.1 | 7.03 | 72.8 | 6,222 | 9 | 52 | 6,361 |
| Amycolatopsis mediterranei U32 | NC_014318.1 | 10.24 | 71.3 | 9,228 | 12 | 52 | 9,292 |
| Escherichia coli O157:H7 str. EC4115 | NC_011353.1 | 5.57 | 50.5 | 5,315 | 22 | 110 | 5,891 |
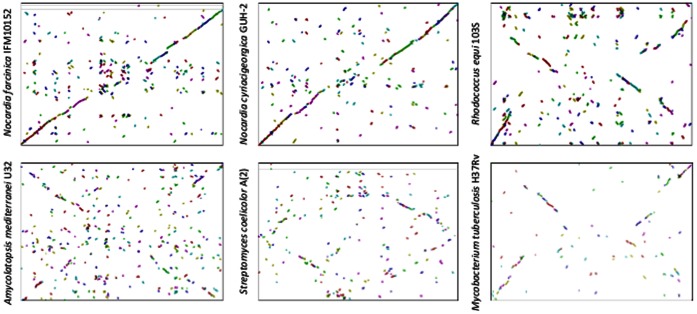
To attain a macro view of the genetic relationships with other bacteria, we compared the N. brasiliensis genome sequence with those of other actinomycetes, including N. farcinica 10152, N. cyriacigeorgica GUH-2, Rhodococcus equi 103S, Amycolatopsis mediterranei U32 (former Nocardia mediterranei, a rifampicin producer), Streptomyces coelicolor A(2) (antibiotic producer) and Mycobacterium tuberculosis, a recognized human pathogen. In Figure 3, we show a syntenic dot plot of the genomes of these species. A close relationship was observed with N. cyriacigeorgica and N. farcinica, with a higher density particularly at the end and the beginning of the chromosome (DNA core). As expected, there was more homology with another Nocardiaceae (Rhodococcus) than with the other bacteria, although a higher homology of the A. mediterranei and S. coelicolor genomes was observed than with another Corynebacterineae, such as M. tuberculosis. This finding may be explained by the fact that M. tuberculosis has evolved to be an almost exclusively human pathogen and has lost many of its soil inhabitant characteristics.
Figure 3. Syntenic dot plot of N. brasiliensis ATCC 700358 genome against Nocardia farcinica IFM10152 (a), Nocardia cyriacigeorgica GUH-2 (b), Rhodococcus equi 103S, Amycolatopsis mediterranei U32, Streptomyces coelicolor A(2), and Mycobacterium tuberculosis H37Rv genomes.
Dots represent a reciprocal best match by BLASTP comparison. The x-axis corresponds to the N. brasiliensis genome plotted against the rest of the genomes (y-axis). Inclination to the right corresponds to ORFs in same direction. An inclination to the left corresponds to an opposite direction. The highest homology in the Nocardia species was found at about the dnaA site. In each case, genome coverage was 30, 30, 11, 7, 5 and 4%.
Putative Virulence Factors
There have been several experimentally described virulence factors of Nocardia, including catalase, superoxide dismutase, cell-wall lipids, and proteases, as well as some immunodominant antigens, using mainly N. cyriacigerogica GUH-2 (formerly N. asteroides) and N. brasiliensis HUJEG-1 [21], [22], [23], [24], [25].
Catalases have been proved, in N. cyriacigeorgica and in other microorganisms, to be important in detoxifying the H202 produced by phagocytes. In N. brasiliensis, a catalase was described as the target of the humoral response in patients suffering mycetoma. At that time, the catalase was named P61 or katN [24]. katN is encoded by O3I_001640, and the closest ortholog proteins are found in N. cyriacigeorgica GUH-2 (87%) and in M. abscessus (85%). N. farcinica IFM 10152 has a lower-homology ortholog, nfa27070 (katE, 41%). Four more catalases were observed (Table 2), one of them very similar to katG of M. tuberculosis (77% identity, O3I_014530). The catalase gene O3I_032795 appears to be the most specific for N. brasiliensis, with a protein homology of 71% with N. transvalensis and of <30% with N. farcinica and N. cyriacigeorgica (Table S1).
Table 2. Distribution of putative virulence factors among actinomycetes with complete genome sequence.
| Catalase | Superoxide dismutase | Phospholipase C | Hemolysin | Protease | Chitinase | |
| Mycobacterium leprae TN | 0 | 2 | 0 | 0 | 0 | 0 |
| Mycobacterium tuberculosis H37Rv | 1 | 2 | 4 | 0 | 0 | 1 |
| Mycobacterium abscessus ATCC 19977 | 4 | 3 | 2 | 1 | 23 | 0 |
| Mycobacterium smegmatis str. MC2 155 | 5 | 1 | 0 | 0 | 0 | 0 |
| Nocardia farcinica IFM 10152 | 4 | 2 | 0 | 2 | 29 | 0 |
| Nocardia cyriacigeorgica GUH-2 | 3 | 2 | 0 | 2 | 35 | 0 |
| Nocardia brasiliensis HUJEG-1 | 5 | 2 | 5 | 4 | 32 | 3 |
| Rhodococcus equi ATCC 33707 | 4 | 4 | 4 | 4 | 25 | 4 |
| Streptomyces griseus subsp. griseus NBRC 13350 | 4 | 2 | 5 | 0 | 52 | 10 |
| Micromonospora aurantiaca ATCC 27029 | 2 | 4 | 3 | 2 | 10 | 1 |
| Amycolatopsis mediterranei U32 | 2 | 1 | 7 | 3 | 64 | 13 |
| Escherichia coli O157:H7 str. EC4115 | 3 | 3 | 0 | 0 | 0 | 0 |
As an external control we used Escherichia coli.
Superoxide dismutases are enzymes that are important for destroying deleterious superoxide and singlet O− 2 ions that are produced during intracellular killing by phagocytes. This function has been demonstrated using N. cyriacigeorgica GUH-2 [22]. N. brasiliensis possesses two SODs: O3I_000385, which is very similar to N. farcinica and N. cyriacigeorgica GUH-2 sodA (97%), and other nocardial and mycobacterial species. The SOD gene O3I_039690 encodes for a protein that is similar only to N. farcinica and N. cyriacigeorgica GUH-1 (74 and 78% homology, respectively).
Phospholipase C proteins can be important virulence factors in tissue-destroying organisms such as N. brasiliensis, as has been demonstrated for other microorganisms [26], [27], [28]. We observed four phospholipase C proteins in the genome. O3I_010265 is quite specific for this microorganism. The closest protein (54% similar) is found in Amycolatopsis mediterranei. When compared to Nocardia spp, only the genome of N. tenerifensis contains a similar orthologous protein (87%). The other Nocardia spp genomes contained proteins with less than 51% homology. The phospholipase C gene O3I_012930 is very similar to the orthologs found in Nocardia spp (up to 87%), as well as in some Gordonia and Rhodococcus species. Phospholipase C O3I_019520 and O3I_025065 are even more specific to N. brasiliensis, with low observed homology to orthologs in other Nocardia species (<37%, except N. transvalensis at 62%) and other Corynebacterineae. No proteins with a significant E value were observed in the genomes of N. farcinica IFM10152 or N. cyriacigeorgica GUH-2. It appears that these phospholipases are specific to N. brasiliensis and that they may play an important role in N. brasiliensis pathogenesis.
Hemolysins are toxic proteins important in bacterial pathogenesis [29], [30]. The N. brasiliensis HUJEG-1 genome encodes 4 hemolysins. O3I_012605 is exclusive to N. brasiliensis. The gene is not present in N. brasiliensis NBRC 14402 or in the rest of the Nocardia spp, except N. tenerifensis where a similar protein (72%) is observed. The hemolysin O3I_037730 is present in N. brasiliensis NBRC 14402, with similar proteins in N. tenerifensis (86%), and low-homology proteins are present in the rest of Nocardia spp and other soil bacteria (<45%). The other two hemolysins have similar proteins in Nocardia spp and other actinobacteria. Mycetoma cases differ in extent and dissemination; these differences could be explained by the presence of more destructive enzymes in some strains, obtained from other innocuous soil bacteria by horizontal transfer.
Invasin is a protein that is used by several microorganisms (including Yersinia pestis and Y. entreocolitica, Helycobacter jejuni, Plasmodium spp) [30], [31], [32] to attach and penetrate into host cells. Nocardia is an intracellular facultative microorganism and possesses an invasin gene, O3I_027570, with a similar protein in N. farcinica (72%) and N. cyriacigeorgica GUH-2 (73%). Because all Nocardia are intracellular facultative cells, they most likely use this protein to attach to cells.
N. brasiliensis is identified by conventional methods from other Nocardia spp by analyzing the differential hydrolysis of compounds, including tyrosine, hypoxanthine, adenine and casein [33]. Although the profiles may vary, N. brasiliensis is the only casein-positive species. Surprisingly, we found not one but 32 proteases and one protease inhibitor. Most of the proteases have orthologs in other nocardial or other actinomycetes species. Proteases encoded by O3I_030410 and O3I_002340 presented homologies of 85–96% in many Nocardia species orthologs. These genes may constitute highly conserved genes, particularly the latter, with a homology of 93% to the phylogenetically distant M. leprae. In contrast, the protease gene O3I_013280 is quite specific, particularly after amino acid 111, where the homology with other Nocardia species is close to zero. The closest proteins are from N. brasiliensis NBRC 14402 (94% identity) and N. tenerifensis (57% identity). When compared by BLAST with other bacteria, a similar finding was observed, with the highest similarity shown to Bifidobacterium angulatum (49%). It is possible that O3I_013280 (228-amino acids long) was generated by homologous recombination from the huge O3I_002340 (772-amino acids long), given their homology for the first 111 amino acids (48%). Although not yet proved, given its specificity, it is possible that O3I_002340 codes for the caseinase utilized to differentiate N. brasiliensis from other Nocardia spp.
Free-living bacteria need to process many materials, including oligo elements, metals, and nutrients. In addition, they also need to release cell-wall synthetic materials, toxic compounds or other components. ABC transporters are specialized proteins that perform these functions [34]. The N. brasiliensis HUJEG-1 genome encodes 516 of these proteins compared to 150 encoded by N. cyriacigeorgica GUH-2; 139, N. farcinica IFM 102; 217, Streptomyces coelicolor; 458, Amycolatopsis mediterranei U32; and <100, Mycobacterium tuberculosis. In this regard, N. brasiliensis more resembles a soil bacteria than a pathogenic bacteria.
Mammalian cell entry proteins (mce) are essential for M. tuberculosis virulence. Their importance was first demonstrated by transferring the Mce1 gene of M. tuberculosis to Escherichia coli, which produced an E. coli strain with the ability to attach and enter phagocytes, features not previously possessed [35], [36]. N. brasiliensis HUJEG-1 possesses 33 genes encoding mce proteins distributed in six operons, with four of them grouped in arrays of six ORFs (Table 3). All of these are also present in N. brasiliensis NBRC 14402 and have ortholog genes in many other Nocardia species and other Corynebacterineae, including Mycobacterium, Rhodococcus, Gordonia, and Tsukamurella. They have been demonstrated to be important in the virulence of Mycobacterium tuberculosis, as well as for transmembrane transportation [37].
Table 3. Distribution of important genome features among actinomycetes with complete genome sequence.
| Mce proteins | PE/PPE/PGRS | Cytochrome P450 | Protocatechuate dioxygenase | Homogentisate dioxygenase | |
| Mycobacterium leprae TN | 5 | 4 | 0 | 0 | 0 |
| Mycobacterium tuberculosis H37Rv | 21 | 176 | 20 | 0 | 0 |
| Mycobacterium abscessus ATCC 19977 | 44 | 12 | 25 | 0 | 1 |
| Mycobacterium smegmatis str. MC2 155 | 1 | 0 | 0 | 0 | 0 |
| Nocardia farcinica IFM 10152 | 36 | 0 | 26 | 0 | 0 |
| Nocardia cyriacigeorgica GUH-2 | 40 | 4 | 6 | 1 | 1 |
| Nocardia brasiliensis HUJEG-1 | 33 | 3 | 58 | 2 | 1 |
| Rhodococcus equi ATCC 33707 | 13 | 4 | 4 | 3 | 2 |
| Streptomyces griseus subsp. griseus NBRC 13350 | 6 | 0 | 26 | 0 | 1 |
| Micromonospora aurantiaca ATCC 27029 | 0 | 0 | 14 | 0 | 1 |
| Amycolatopsis mediterranei U32 | 0 | 0 | 54 | 3 | 0 |
| Escherichia coli O157:H7 str. EC4115 | 0 | 0 | 0 | 0 | 0 |
As an external control we used Escherichia coli.
PE (proline-glutamate) and PPE (proline-proline-glutamate) family proteins constitute approximately 10% of the M. tuberculosis genome [38]. These proteins possibly provide for a high level of antigenic variability. In the N. brasiliensis HUJEG-1 genome, we observed only three PPE genes, O3I_000480, O3I_023795 and O3I_023865, which were 394, 391 and 488 amino acids in length, respectively. The first PPE protein is identical in N. brasiliensis NBRC 14402 and has orthologs in other Nocardia species, including N. farcinica IFM 10152 and N. cyriacigeorgica GUH-2 (66 and 62% identity, respectively). Other bacteria having orthologs for this protein include Rhodococcus and Mycobacterium spp. Interestingly, in this protein, after amino acid 228, homology significantly decreases. In contrast, PE_PPE O3I_023795 is almost exclusively found in N. brasiliensis HUJEG-1 and in the first 251 amino acids has stretches of low homology to N. cyriacigeorgica and Mycobacterium spp. The rest of the sequence has no homology to any other protein according to a BLAST analysis. The sequence is not present in N. brasiliensis NBRC 14402. PPE O3I_023865 is also not present in N. brasiliensis NBRC 14402 and has zones of homology with proteins of several Nocardia spp only until amino acid 272 (up to 39% homology). The rest of the sequence presents only scarce homology. Only N. pneumonia and N. abscessus have homology throughout the entire sequence. The gene is exclusive to Nocardia spp, with no similar orthologs in any other bacteria according to a BLAST analysis. The division of these proteins into homology dominions indicates a possible recombination origin among them.
DNA Duplication
Gyrases are type II topoisomerases that help helicases to unwind double-stranded DNA. N. brasiliensis HUJEG-1 possesses one gyrA (O3I_000030) and one gyrB (O3I_000025) subunit, plus one additional gyrB subunit (O3I_029325). As in most bacterial species, in N. brasiliensis HUJEG-1, the gyrA and gyrB genes are situated close to the replication origin. The second B subunit gene is located at nucleotide 6,674,508. The three proteins exist also in N. brasiliensis NBRC 14402; gyrA and gyrB are very similar in other Nocardia spp (88–95%) and Mycobacterium (84–86%), including M. tuberculosis. The second B subunit is less similar among Nocardia and mycobacterial gyrB genes (approximately 74%). The presence of three gyrase genes in N. brasiliensis instead of two may explain the natural resistance of the bacteria to quinolones, such as ciprofloxacin [39]. Third-generation, more potent drugs, such gatifloxacin and moxifloxacin, are active against N. brasiliensis in vitro. In to regard the number of gyrase genes, other soil-inhabiting bacteria such as Amycolatopsis mediterranei possess four gyrase genes.
Secondary Metabolites
Non-ribosomal peptides are secondary metabolites with cyclic or branched structures, which are not synthesized by ribosomal mRNA but instead by non-ribosomal peptide synthetases. These proteins synthesize only one specific peptide, and they can include D-amino acids and catalyze chemical changes such as glycosylations and acylations. These peptides have a wide spectrum of biological activities, including functions as immunosuppressants, toxins, fluorescent pigments, and cytostatics, such as garamycin, vancomycin, and cyclosporin [40]. N. brasiliensis HUJEG-1 possesses approximately 30 genes encoding for non-ribosomal peptide synthetases, including some with very high calculated molecular weights, such as 03I_037910 (1,570,367 Da). The genes 03I_007380 and 03I_007385 code for the synthesis of a compound similar to pyoverdin, the typical green pigment of Pseudomonas aeruginosa. The produced product is similar to pyoverdin from Frankia (47%) and similar to peptides from bacteria belonging to the Pseudomonadaceae family, e.g., Aeromonas, Burkholderia and Pseudomonas (approximately 36% homology) [41].
Polyketides are secondary metabolites that are produced by the decarboxylative condensation of malonyl-CoA derived extender units. Polyketides possess a wide variety of biological properties as antibiotics, anti-cancer compounds, insecticides, and so forth [42]. Among these compounds are macrolides, such as anthramycin; polyene antifungals, such as amphotericin B; and toxic compounds, such as aflatoxins. N. brasiliensis HUJEG-1 encodes 20 related polyketide synthase genes. Some of these genes are very similar to proteins in N. cyriacigeorgica GUH-2 and N. farcinica IFM10152. In contrast, the polyketide synthase gene O3I_007465 presents very low homology to nfa17160 of N. farcinica IF 10152 and N. cyriacigeorgica GUH-2 pknG (approximately 18% coverage and 36% identity in both cases). It also encodes a polyketide synthase that is highly homologous (80%) to a protein from Streptomyces venezuelae involved in the synthesis of jadomycin, a polyketide antibiotic. Nocardia have been the source of many bioactive substances [43], and it will be important to determine the role of these enzymes in the synthesis of N. brasiliensis polyketide compounds.
Terpenes are derived biosynthetically from units of isoprene and were originally isolated from the resin of the turpentine tree [44]. Terpene compounds in Nocardia have been found to have antibiotic and cytostatic properties [44], [45]. N. brasiliensis HUJEG-1 encodes for 4 terpene synthases with no orthologs in N. cyriacigeorgica GUH-2 or N. farcinica IFM10152 but with orthologs in other actinobacteria genera, such as Saccharopolyspora, Streptomyces and Amycolatopsis.
The N. brasiliensis HUJEG-1 genome encodes nine genes that are involved in antibiotic synthesis, most of them with orthologs in N. cyriacigeorgica GUH-2 and N. farcinica IFM 10152. The antibiotic synthesis genes O3I_005645, O3I_014615, and O3I_014620 are not found in N. cyriacigeorgica GUH-2 or N. farcinica IFM 10152. In contrast, they have orthologs in Bacillus, Paenibacillus or Brevibacillus. Other antibiotic biosynthesis genes that are present in the N. brasiliensis HUJEG-1 genome include those involved in the synthesis of erythromycin, hygromycin, puromycin, saframycin, streptomycin and tetracenomycin.
When analyzing the genomes of N. brasiliensis HUJEG-1, N. farcinica and N. cyriacigeorgica using the Antibiotics and Secondary Metabolites Analysis Shell (antismash) software [http://antismash.secondarymetabolites.org], which searches genomes looking for secondary metabolite gene clusters, we found 47 clusters in N. brasiliensis HUJEG-1, 16 in N. farcinica IFM 10152 and 21 in N. cyriacigeorgica GUH-2. N. brasiliensis uses approximately a quarter of its genome to synthesize secondary metabolites (2,157,079-bp). In contrast, N. farcinica IFM 10152 uses 833,872 bp and N. cyriacigeorgica GUH-2 uses 985,767 bp of their respective genomes to encode for these metabolites. M. tuberculosis H37Rv uses 778,422 bp. When analyzing the cluster locations in the genome, half of them are situated between nucleotide 3,000,000 and nucleotide 5,800,000 (Fig. S1). In this zone, only 1 out of 21 clusters have orthologs found in N. farcinica or N. cyriacigeorgica. In contrast, in the clusters found in the “DNA core” zone of the genome (approximately three megabases before and after the dnaA gene), half of the genes have orthologs in either N. farcinica or N. cyriacigeorgica, or in both (13 out of 26).
Oxidative Pathways
Cytochromes P450 (CYPs) are important proteins that catalyze the oxidation of many substrates. CYPs are hemoproteins, enzymes containing a heme prosthetic group, and thus these proteins have a characteristic red color. N. brasiliensis HUJEG-1 possesses abundant CYPs (57 genes), and some of them have many orthologs, such as O3I_016325, which has similar proteins in N. cyriacigeorgica GUH-2 and N. farcinica IFM 10152, with homology of approximately 68 and 78% respectively. This CYP has also many homologs distributed in Streptomyces spp., such as S. coelicolor (61%). Other CYPs are more specific to N. brasiliensis, with a homology of less than 30% with N. cyriacigeorgica (coverage 37%) and with a high similarity to CYPs in other actinobacteria, such as A. mediterranei (91% homology, 57% coverage). The high abundance of CYP reflects its large metabolic capacity, and homology with other soil bacterial proteins indicates its possible acquisition via horizontal transfer. This abundance of CYPs also may explain the natural resistance of N. brasiliensis to most azolic compounds, which mainly target the cytochrome P450 enzymes homologues to 14-alfa-sterol demethylases [46], [47]. For instance, Candida albicans possesses only two cytochrome P450 proteins and is highly susceptible to azoles.
We also observed genes encoding antioxidants, such as thioredoxins (seven genes), and low-molecular weight non-heme iron proteins, such as rubredoxin and rubrerythrin, in the genome of N. brasiliensis.
DNA Elements
Transposases are enzymes that catalyze the movement of a transposon from one location in a chromosome to another via a copy and paste system [48]. Transposases can provide important plasticity and variability to bacterial genomes [49]. The Nocardia brasiliensis HUJEG-1 genome contains 21 genes encoding transposases. Some of them, such as O3I_014660, are shared with N. cyriacigeorgica GUH-2 (82%) and N. farcinica IFM 10152 (85%) and are similar to proteins found in other actinomycetes, such as IS994 of Renibacterium salmoninarum (68%) and IS6110 of Mycobacterium tuberculosis (75%). In contrast, O3I_024350, a transposase of the IS204/IS1001/IS1096/IS1165 family, is not present in either N. cyriacigeorgica GUH-2 or N. farcinica IFM 10152 but is similar to proteins in Streptomyces violaceusniger Tu 4113 (51%), which supports an external origin for these insertional elements. Insertion sequences have been used widely to subtype bacterial species, but unfortunately, in this strain, we only observed single copies of these elements, thus eliminating a possible use for subtyping, unless other N. brasiliensis strains have a variable number, as has been observed for IS6110 of M. tuberculosis, where the number of copies can range from 0 to 25 [48]. Interestingly, eight of the transposases are present in a hot spot (Fig. 2) that is located in a stretch (mentioned above) from nucleotide 5,126,00 to nucleotide 5,800,000, in the fragment with different G+C content, but are not present in N. brasiliensis ATCC 19296. These transposases may have been acquired by horizontal transference. The abundance of transposase may partially explain the acquisition of this fragment.
During phage infection, some of the virus enters a lysogenic cycle, and the phage DNA is integrated into the bacterial chromosome [50]. In the N. brasiliensis HUJEG-1 genome, at least six genes associated with phages are present. In comparison with M. tuberculosis (two prophages) or N. farcinica (three prophages), where these genes are found in clusters, including the ORFs for each viral function, in N. brasiliensis, these genes are dispersed in the genome, and only two sequences encoding phage integrases are adjacently located. This may be explained by the elimination of the remaining phage genes or the fact that the genes have very low homology to other sequences reported in GenBank.
In response to external DNA invasion, either by plasmids or phages, bacteria have developed a protective system based on the recognition of foreign DNA using Clustered Regularly Interspaced Short Palindromic Repeat (CRISPR) sequences together with CRISPR-associated proteins (CAS) [51]. N. brasiliensis HUJEG-1 possesses one gene encoding for a Cas5E protein (O3I_023695) with orthologs in N. farcinica IFM 10152, N. cyriacigeorgica GUH-2 and other soil bacteria.
Antimicrobial Resistance
N. brasiliensis actinomycetoma is difficult to treat, in part because of the natural resistance of this microorganism to many drugs. As described above, N. brasiliensis HUJEG-1 possesses many ABC transporters that may facilitate the elimination of toxic compounds, including drugs and metabolite derivatives. In addition, N. brasiliensis is resistant to most beta-lactams, even after adding strong anti-beta lactam inhibitors, such as tazobactam or clavulanic acid [52], [53]. This resistance may be explained by the large amount of beta-lactamases that are encoded in the N. brasiliensis genome (n = 29). In contrast, N. cyriacigeorgica has 12 such genes, and N. farcinica has only one. Some N. brasiliensis beta-lactamases, such as O3I_003795, are highly conserved among Corynebacterineae, with 76% homology to N. cyriacigeorgica GUH-2 and Rhodococcus (77%) orthologs. Some others genes, such as O3I_003205, display a lower similarity to N. farcinica (35%) than to Streptosporangium roseum (69%) orthologs, suggesting that some of these beta-lactamase genes were acquired by horizontal transfer from other soil bacteria. The presence of extra genes that are the target of antimicrobials may be the basis for the resistance of N. brasiliensis. For instance, the presence of a second gyrase B and an extra copy of rpoB can be associated with antimicrobial resistance to quinolones and rifampin.
Metabolism
Environmental microorganisms may use simple organic compounds such as alkanes or even substrates containing aromatic rings as nutrients [54], [55]. These compounds may be degraded using the protocatechuate and/or the homogentisate pathways, producing succinate-acetyl-CoA and fumarate-acetoacetate, which enter the general metabolism pathways for use in the catabolism or synthesis of compounds. N. brasiliensis can use both systems because the bacteria possess genes that encode both enzymes: protocatechuate 3,4-dioxygenase, in the alpha and beta subunits (O3I_021760 and O3I_021765), and a homogentisate 2,3-dioxygenase (O3I_039745). The protocatechuate 3,4-dioxygenase is very similar to other proteins from Geodermatophilus or Saccharomonospora (up to 74%). In contrast, they are not observed or have orthologs with very low homology to some of these genes in N. cyriacigeorgica GUH-2 and N. farcinica IFM 10152. The homogentisate 1,2 dioxygenase of N. brasiliensis is quite similar to an ortholog in N. cyriacigeorgica GUH-2 and N. farcinica IFM 10152 (89 and 83% homology). This enzyme is also conserved in other Nocardiaceae.
Degrading Enzymes Production
Chitinases are enzymes that degrade glycosidic bonds in chitin and are a very useful enzyme for soil bacteria and fungi for degrading dead insects [56]. N. brasiliensis HUJEG-1 possesses three genes encoding chitinases, with no significant orthologs in N. cyriacigeorgica GUH-2 or N. farcinica IFM152. The chitinase genes O3I_019530 and O3I_029865 have very similar orthologs in Streptomyces and Kitasatospora (up to 80% homology). Interestingly, a third gene, O3I_036765, the closest homologous protein according to the BLAST analysis (37%), is from Nasonia vitripennis, a small wasp. N. brasiliensis has not been isolated from insects, and some insects may use antibiotics produced by symbiotic actinomycetes to keep their eggs free of bacterial infection [57]. There is only one report of the production of mycetoma after the sting of a yellow wasp. It would be interesting to study insects as a putative ecological niche or substrate for N. brasiliensis [58].
Plants leaves are protected by an external polyester layer that is composed of hydroxy and hydroxyl epoxy fatty acids named cutin [59]. Fungi and other plant eater organisms possess cutinases, enzymes that can degrade this polymer. N. brasiliensis HUJEG-1 has three genes, O3I_014370, O3I_015280 and O3I_017645, that encode cutinases. Orthologs are found in N. cyriacigeorgica GUH-2 and N. farcinica IFM 10152. The gene O3I_017645 is 43% homologous to the cutinase of Phytophthora sojae, an oomycete producing plant infestations, such as the famous potato epidemic in Ireland, which resulted in massive emigration to the U.S. circa 1845. Mycobacterium tuberculosis possesses a cutinase, similar to O3I_014370 (27 coverage, 34% homology), probably as a result of its history of being a soil living bacteria.
General Metabolism
It is apparent from general genome inspection that N. brasiliensis possesses the complete pathways for glycolysis, pentose phosphate processing, and the tricarboxylic acid and glyoxylate cycles. The presence of a high number of oxidoreductases (approximately 150), dehydrogenases (n = 335), and oxygenases (approximately 150, including CYPs) reflects the aerobic metabolism of N. brasiliensis. Using these proteins, ATP is produced by NADH, and the ubiquinone chain is present. Even if Nocardia are typically an aerobic organism, genes for nitrate reductase (two operons), fumarate reductase and nitrite reductase, which are important enzymes in anaerobic phosphorylation, are also present. These genes might support growth in low vascularized abscessed tissue with reduced redox potential.
A large chromosome, such as the genome of N. brasiliensis, that contains the genetic information necessary to adapt to different environments, such as soil and possible insect and human hosts, requires an extensive regulatory system. Approximately 17 sigma factors, 11 anti-sigma factors, and 21 RNA polymerase sigma factors are predicted to exist in the N. brasiliensis genome. In a similar manner to M. tuberculosis, which possesses 11 genes for two-component histidine kinase sensors, N. brasiliensis also has a small number of these genes (n = 7), compared to >30 present in E. coli. N. brasiliensis also has 78 two-component system genes. This environmental signal transduction system is complemented by the presence of 30 predicted serine/threonine protein kinases, which are important in regulating apoptosis and cell division through the phosphorylation of specific proteins.
LuxR regulators are a group of bacterial helix-turn-helix (HTH) transcription factors that are involved in the regulation of many bacterial quorum-sensing (QS) mechanisms [60]. N. brasiliensis has a LuxR system, including a putative two-component system response regulator of the LuxR family protein together with 23 transcriptional regulators. These LuxR systems conduct a census of their own bacterial population, which is important for soil-inhabiting bacteria, as a great variety of species co-exist in the soil.
Lipid Metabolism
Organisms belonging to the Corynebacterineae are characterized by possessing large amounts of lipids in their cell wall, including, fatty acids, lipo-oligosaccharides, phenolthiocerols, mycolic acids, and lipoarabinomannans. This trait is a hallmark of this suborder. In the N. brasiliensis genome, there is an abundance of enzymes that are involved in lipid catabolic and anabolic pathways, including 15 acyl-CoA synthetases, 6 long chain acyl-CoA synthetases, 12 enoyl-CoA hydratase/isomerases, twelve acetyl-CoA acetyltransferases and FadA/FadB beta-oxidation complex proteins (O3I_003935 and O3I_001385 genes), which complete the beta-oxidation of the fatty acids.
Nocardia, as with all Corynebacterineae, has an abundant amount of mycolic acids, (beta-hydroxy-alpha branched fatty acids) which can account for up to 60% dry weight in some species. The biosynthesis of mycolic acids precursors in Corynebacterineae requires two systems: a unique polypeptide multifunctional enzyme denominated FAS I and a FAS-II system composed of several enzymes [61], [62]. A FAS-I homolog is encoded in N. brasiliensis by O3I_007715, a 3,125-amino acid polyfunctional protein that is highly conserved in N. cyriacigeorgica GUH-2 (86% ), N. farcinica IFM 102 (86% ) and M. tuberculosis (63%). The products of FAS I serve as substrates for the FAS II system. In M. tuberculosis, the FAS II genes are clustered in two transcriptional units: the mtfabD-acpM-kasA-kasB-accD6 and the mabA-inhA clusters. Malonyl-CoA:ACP transacylase (O3I_025190 in N. brasiliensis) transforms malonyl-CoA into malonyl-ACP. Beta-ketoacyl-ACP synthase III (fabH in MTB) condenses the acetyl-CoA that is produced by FAS I with malonyl-ACP to elongate fatty acids. In N. brasiliensis, there are 4 of these enzymes. The O3I_039605 and O3I_040210 genes encode for proteins homologous to fabH (55 and 51% homology, respectively). In a second step of the elongation, the beta-hydroxyacyl-ACP intermediate is dehydrated to form trans-2-enoyl-ACP by the ketoacyl-ACP reductase (mabA in MTB). N. brasiliensis possesses four of these ketoacyl-ACP reductases. O3I_027550 encodes a protein similar to mabA in M. tuberculosis (67%). The other three proteins have no significant homology with M. tuberculosis proteins. O3I_027550 is very conserved in N. farcinica IFM 10152 and N. cyriacigeorgica GUH-2 (85 and 73%, respectively). The final elongation step is carried out by the inhA gene, an NADH-dependent enoyl-ACP reductase. The equivalent in N. brasiliensis is an enoyl-(acyl carrier protein) reductase (O3I_027545), which is very conserved in N. cyriacigeorgica GUH-2 and N. farcinica IFM 10152 (90 and 91%), with less homology to MTB (59%).
The modification of the fatty acids includes the introduction of cis double bonds. In S. pneumoniae, a combination of fabZ and fabM is used to introduce a double bond in the nascent acyl chain. N. brasiliensis possesses two genes, O3I_026205 and O3I_026230, that encode a beta-hydroxyacyl-(acyl-carrier-protein) dehydratase (FabZ) with low homology with proteins from N. cyriacigeorgica/N. farcinica (<30%), Halanaerobium hydrogeniformans (32%) and Nitratireductor aquibiodomus (37%). No fabZ- or fabA-like proteins exist in M. tuberculosis. Instead, M. tuberculosis has three potential aerobic desaturases encoded by desA1, desA2 and desA3. N. brasiliensis has 15 desaturases, including a phytoene (40-carbon carotene synthesis intermediate) desaturase. O3I_016590 is a homolog of MTB desA1(55%).O3I_034520 is similar to erg3, an MTB desataurase (Rv1814) (47%). The genes O3I_007130, O3I_035030 and O3I_035040 encode proteins similar to a linoleoyl-CoA desaturase (Rv3229c) (about 60%) of MTB. The other genes have no similarity to MTB proteins. Most of these desaturase enzymes have highly similar orthologs in N. farcinica IFM 10152 and N. cyriacigeorgica GUH-2 (>75%). Desaturase genes O3I_022675 and O3I_029100 have no significant orthologs in N. farcinica IFM 10152 or N. cyriacigeorgica GUH-2; the highest homology found was with proteins of Streptomyces bingchenggensis (49%) and Micromonospora sp. ATCC 39149 (63%), strongly suggesting that these desaturase genes were acquired by horizontal transfer.
Mycolic acids differ among the Corynebacterineae not only in the length of the fatty acids but also in modifications such as the presence of oxygenated functions, cyclopropanes, or double bonds. N. brasiliensis encodes for 7 cyclopropane fatty acid synthases. O3I_001400 is present in some MTB strains, such as CDC1551 (68% protein identity), but is not present in H37Rv. O3I_008300 is an ortholog (58%) of cmaA1 of MTB. O3I_029080 is an ortholog (33%) of cmaA2, and O3I_034505 is an ortholog of ufaA1 (54%). The other genes have homology to genes in soil bacteria and other Nocardiaceae. O3I_008300, in particular, has many orthologs among the Mycobacterium species with a protein homology close to 60%. The loss of cyclopropane rings has been associated with a loss of virulence in MTB [63]. Interestingly, the Nocardia species that affect most immunocompromised patients, such as N. farcinica and N. cyriacigeorgica, have genomes with only one cyclopropane fatty synthase.
S-adenosylmethionine (SAM) is used as a methyl donor to a cis-ethylenic precursor to produce cyclopropanes and a methyl branch adjacent to a trans double bond or a trans cyclopropane. N. brasiliensis encodes for four S-adenosylmethionine-dependent methyltransferases, all of which have similar orthologs in N. farcinica IFM 10152 and N. cyriacigeorgica GUH-2. Two of these methyltransferases, O3I_000195 and O3I_006220, have orthologs in MTB (hypothetical proteins Rv0329c [34%] and Rv1300 [51%]).
In MTB, the introduction of keto- or methoxy- groups is mediated by the enzyme methoxy mycolic acid synthase Hma (MmaA4). In N. brasiliensis, the equivalent ortholog gene is O3I_008300, which encodes for a protein that is annotated as a cyclopropane-fatty-acyl-phospholipid synthase and that is also identified as an SAM-dependent methyl transferase, with a higher homology to MTB proteins (49%) and other Mycobacterium species than to N. farcinica IFM 10152 and N. cyriacigeorgica GUH-2 proteins (34% in both cases).
Condensation is the final step in mycolic acids biosynthesis. This process is carried out in Corynebacterium and Mycobacterium by a polyketide synthase, pks13, together with the activation of the meromycolic chain (acyl-AMP ligase) and that carboxylation of the alpha branch (by an acyl-CoA carboxylase). In N. brasiliensis, we found an orthologous gene, O3I_000755, similar to MTB pks13 (53%), with orthologs in N. farcinica IFM 10152 and N. cyriacigeorgica GUH-2 (78% in both cases). In MTB, fadD32 catalyzes the production of acyl-AMP using free fatty acid as a substrate. N. brasiliensis has a fadD32 ortholog, O3I_000750, which is 61 and 60% similar to fadD32 of MTB and M. leprae, respectively. O3I_000750 is also highly conserved in Nocardiaceae, with 92% homology to orthologs in N. farcinica IFM 10152 and N. cyriacigeorgica GUH-2. In a similar manner to fadD32, which is located beside pk13 in MTB, O3I_000750 is adjacent to O3I_000755 (an ortholog of pk13), demonstrating a highly conserved similarity in these essential pathways in the Corynebacterineae. The activation of the alpha branch in the mycolic acids is carried out by an acyl-CoA carboxylase (accdD4) in Corynebacterium and Mycobacterium spp, which is the final step before condensation is carried out by pk13. The ortholog of accD4 in N. brasiliensis is an acyl-CoA carboxylase that is encoded by O3I_000760, which is 71% homologous to accD4 of MTB and 94% homologous to those of N. farcinica IFM 10152 and N. cyriacigeorgica GUH-2. This gene is located immediately after the pks13 ortholog, suggesting that these three enzymes have common transcriptional regulators.
After condensation, the mycolic acids must be transported to their final location in the cell wall. In MTB, this step is carried out by mycolyl-transferases called fbp proteins or Antigen85 complex [64]. The N. brasiliensis genome encodes 10 mycolyl-transferases. Three of these genes, O3I_000710, O3I_000715 and O3I_000720, are arranged in tandem. N. cyriacigeorgica GUH-2 has 5 mycolyl transferases, and N. farcinica IFM 10152 has three of them. The N. brasiliensis O3I_000720, O3I_015965 and O3I_000980 genes are orthologs of fbpC of MTB (approximately 40% homology). No orthologs of the MTB genes fbpA, fbpB or fbpD were observed. One of the N. brasiliensis mycolyl-transferases, O3I_027065, has many orthologs in Corynebacterineae but only for the first 256 amino acids. The rest of the sequence is similar only to an ortholog in Corynebacterium variabile and Corynebacterium ulcerans, indicating that to N. brasiliensis acquired this sequence by horizontal transfer from Corynebacterium spp.
Discussion
The in silico analysis of the N. brasiliensis HUJEG-1 genome demonstrates the transfer of genomic components among environmental bacteria, such as Corynebacterium, Bacillus, and Streptomyces, and even DNA from an insect origin, varying in size from very large fragments (approximately 600,000-bp) to small stretches of DNA. As a result, the bacteria are able to infect human hosts, but unlike the elimination of DNA observed in professional intracellular actinobacteria, such as M. leprae, N. brasiliensis has one of the largest bacterial genomes reported. Bacterial evolution seems to produce not only organisms with complex DNA to evolve to larger organisms, such as plants or animals, but also organisms with reduced DNA that are highly dependent on their hosts, such as parasites or symbionts.
The study of the genomic properties that N. brasiliensis shares with other mycetoma producing actinobacteria, such as Actinomadura and Streptomyces, will allow us to separate the part of the genome that is involved in environmental survival and those genes that allow these bacteria to infect human hosts.
Materials and Methods
Genome Sequencing and Assembly
Nocardia brasiliensis HUJEG-1 was deposited in the American Type Culture Collection Institute and designated as ATCC700358. The bacteria used for genome sequencing were isolated from a single colony-purified stock that was kept at −70°C, and the genomic DNA was extracted directly from the expanded culture. The genome sequence was determined using the Roche/454 GS (FLX Titanium) sequencing platform (8-kb library). A total of 786,647 reads were obtained, providing about 27-fold genome coverage. The Roche/454 GS reads were assembled using Newbler 2.5.3 software (Roche Diagnostics, Branford, CT). The unclosed draft genome of N. brasiliensis HUJEG-1 was composed of 53 contigs, for a total 10.8 Mbp, with 68% G+C content. The physical map was constructed in part by comparison with another clone of N. brasiliensis HUJEG-1 and a WGS available in GenBank, N. brasiliensis ATCC 19296. The final assembly was performed using pulse field electrophoresis followed by fluorescence labeling of the cut fragments. An optical map of the separated and labeled DNA fragments was prepared using BglII digestion (OpGen, Gaithersburg, Maryland). The contigs were aligned using this restriction map using the MapSolver™ software. The physical map image (Fig. 2) was prepared by Jason Grant from the Department of Agricultural, Food and Nutritional Science (AFNS) Edmonton, Alberta, Canada using the CG view program [65]. The final sequence was deposited in GenBank under the reference number NC_018681.1.
Genome Annotation and Analysis
The genome annotation was conducted with the NCBI Prokaryotic Genomes Automatic Annotation Pipeline (PGAAP). According to the NCBI, the PGAAP combines HMM-based gene prediction methods with a sequence similarity-based approach that combines a comparison of the predicted gene products to the non-redundant protein database, Entrez Protein Clusters, the Conserved Domain Database, and the COGs (Clusters of Orthologous Groups) [66]. Gene predictions were performed using a combination of the GeneMark and Glimmer programs [67], [68]. A short step resolving conflicts of start sites was conducted at this point. Ribosomal RNAs were predicted by sequence similarity searching using BLAST against an RNA sequence database and/or using Infernal and Rfam models. Transfer RNAs were predicted using tRNAscan-SE [69]. To detect missing genes, a complete six-frame translation of the nucleotide sequence was performed, and predicted proteins (generated above) were masked. All predictions were then searched using BLAST against all proteins from complete microbial genomes. Annotation was based on comparison to protein clusters and on the BLAST results. The Conserved Domain Database and Cluster of Orthologous Group information was then added to the annotation.
Supporting Information
Genomic location of the 47 gene clusters found in Nocardia brasiliensis HUJEG-1 using Antibiotics and Secondary Metabolites Analysis Shell (antismash) software [http://antismash.secondarymetabolites.org]. Besides the map we show a comparative analysis of cluster 1, which is highly conserved among the Nocardiaceae, located between nucleotides 1682661 – 1725128 nt, and cluster 38, which is quite specific of N. brasiliensis and is located between nucleotides 6591347–6636992
(PPTX)
Presence of ortholog genes of putative virulence factors of N. brasiliensis in other microorganisms.
(DOCX)
Acknowledgments
This paper is dedicated to those Mexican researchers (and friends) who have spent the last decades devoted to fighting this medical condition: O. Welsh, M.C. Salinas-Carmona, R. López-Martínez, and R. Arenas.
Funding Statement
CONACYT grant No. 155892. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Waksman SA, Schatz A, Reynolds DM (2010) Production of antibiotic substances by actinomycetes. Ann N Y Acad Sci 1213: 112–124. [DOI] [PubMed] [Google Scholar]
- 2. Stackebrandt E, Rainey FA, Ward-Rainey NL (1997) Proposal for a new hierarchic classification system, Actinobacteria classis nov. Int J Syst Bacteriol 47: 479–491. [Google Scholar]
- 3. Giguère S, Cohen ND, Chaffin MK, Hines SA, Hondalus MK, et al. (2011) Rhodococcus equi: clinical manifestations, virulence, and immunity. J Vet Intern Med 25: 1221–1230. [DOI] [PubMed] [Google Scholar]
- 4. Al Akhrass F, Al Wohoush I, Chaftari AM, Reitzel R, Jiang Y, et al. (2012) Rhodococcus bacteremia in cancer patients is mostly catheter related and associated with biofilm formation. PLoS One 7: e32945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wilson JW (2012) Nocardiosis: updates and clinical overview. Mayo Clin Proc 87: 403–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brown-Elliott BA, Brown JM, Conville PS, Wallace RJ Jr (2006) Clinical and laboratory features of the Nocardia spp. based on current molecular taxonomy. Clin Microbiol Rev 19: 259–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lai CC, Liu WL, Ko WC, Chen YH, Tan HR, et al. (2011) Multicenter study in Taiwan of the in vitro activities of nemonoxacin, tigecycline, doripenem, and other antimicrobial agents against clinical isolates of various Nocardia species. Antimicrob Agents Chemother 55: 2084–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Larruskain J, Idigoras P, Marimón JM, Pérez-Trallero E (2011) Susceptibility of 186 Nocardia sp. isolates to 20 antimicrobial agents. Antimicrob Agents Chemother 55: 2995–2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Welsh O, Vera-Cabrera L, Welsh E, Salinas MC (2012) Actinomycetoma and advances in its treatment. Clin Dermatol 30: 372–381. [DOI] [PubMed] [Google Scholar]
- 10. Welsh O, Morales-Toquero A, Vera-Cabrera L, Vazquez-Martinez O, Gómez-Flores M, et al. (2011) Actinomycetoma of the scalp after a car accident. Int J Dermatol 50: 854–857. [DOI] [PubMed] [Google Scholar]
- 11. Trost E, Blom J, Soares SdeC, Huang IH, Al-Dilaimi A, et al. (2012) Pangenomic study of Corynebacterium diphtheriae that provides insights into the genomic diversity of pathogenic isolates from cases of classical diphtheria, endocarditis, and pneumonia. J Bacteriol 194: 3199–3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vera-Cabrera L, Campos-Rivera MP, Gonzalez-Martinez NA, Ocampo-Candiani J, Cole ST (2012) In vitro activities of the new antitubercular agents PA-824 and BTZ043 against Nocardia brasiliensis . Antimicrob Agents Chemother 56: 3984–3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Almaguer-Chávez JA, Welsh O, Lozano-Garza HG, Said-Fernández S, Romero-Díaz VJ, et al. (2011) Decrease of virulence for BALB/c mice produced by continuous subculturing of Nocardia brasiliensis . BMC Infect Dis 26 11: 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Trevino-Villarreal JH, Vera-Cabrera L, Valero-Guillén PL, Salinas-Carmona MC (2012) Nocardia brasiliensis cell wall lipids modulate macrophage and dendritic responses that favor development of experimental actinomycetoma in BALB/c mice. Infect Immun 80: 3587–3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vera-Cabrera L, Ortiz-Lopez R, Elizondo-Gonzalez R, Perez-Maya AA, Ocampo-Candiani J (2012) Complete genome sequence of Nocardia brasiliensis HUJEG-1. J Bacteriol. 194: 2761–2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Doroghazi JR, Buckley DH (2010) Widespread homologous recombination within and between Streptomyces species. ISME J 4: 1136–1143. [DOI] [PubMed] [Google Scholar]
- 17. Ishikawa J, Yamashita A, Mikami Y, Hoshino Y, Kurita H, et al. (2004) The complete genomic sequence of Nocardia farcinica IFM 10152. Proc Natl Acad Sci USA 101: 14925–14930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zoropogui A, Pujic P, Normand P, Barbe V, Beaman B, et al. (2012) Genome sequence of the human- and animal-pathogenic strain Nocardia cyriacigeorgica GUH-2. J Bacteriol 194: 2098–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cole ST, Eiglmeier K, Parkhill J, James KD, Thomson NR, et al. (2001) Massive gene decay in the leprosy bacillus. Nature. 409: 1007–1011. [DOI] [PubMed] [Google Scholar]
- 20. Foster J, Ganatra M, Kamal I, Ware J, Makarova K, et al. (2005) The Wolbachia genome of Brugia malayi: endosymbiont evolution within a human pathogenic nematode. PLoS Biol 3: e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Revol A, Espinoza-Ruiz M, Medina-Villanueva I, Salinas-Carmona MC (2006) Expression of Nocardia brasiliensis superoxide dismutase during the early infection of murine peritoneal macrophages. Can J Microbiol 52: 1255–1260. [DOI] [PubMed] [Google Scholar]
- 22. Wu G, Nie L, Zhang W (2006) Predicted highly expressed genes in Nocardia farcinica and the implication for its primary metabolism and nocardial virulence. Antonie Van Leeuwenhoek 89: 135–146. [DOI] [PubMed] [Google Scholar]
- 23. Beaman BL, Black CM, Doughty F, Beaman L (1985) Role of superoxide dismutase and catalase as determinants of pathogenicity of Nocardia asteroides: importance in resistance to microbicidal activities of human polymorphonuclear neutrophils. Infect Immun 47: 135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Licón-Trillo A, Castro-Corona A, Salinas-Carmona MC (2003) Immunogenicity and biophysical properties of a Nocardia brasiliensis protease involved in pathogenesis of mycetoma. FEMS Immunol Med Microbiol 37: 37–44. [DOI] [PubMed] [Google Scholar]
- 25. Vera-Cabrera L, Johnson WM, Welsh O, Resendiz-Uresti FL, Salinas-Carmona MC (1999) Distribution of a Nocardia brasiliensis catalase gene fragment in members of the genera Nocardia, Gordona, and Rhodococcus. J Clin Microbiol. 37: 1971–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bryant AE, Chen RY, Nagata Y, Wang Y, Lee CH, et al. (2000) Clostridial gas gangrene. II. Phospholipase C-induced activation of platelet gpIIbIIIa mediates vascular occlusion and myonecrosis in Clostridium perfringens gas gangrene. J Infect Dis 182: 808–15. [DOI] [PubMed] [Google Scholar]
- 27. Wargo MJ, Gross MJ, Rajamani S, Allard JL, Lundblad LK, et al. (2011) Hemolytic phospholipase C inhibition protects lung function during Pseudomonas aeruginosa infection. Am J Respir Crit Care Med 184: 345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bakala N’goma JC, Schué M, Carrière F, Geerlof A, et al. (2010) Evidence for the cytotoxic effects of Mycobacterium tuberculosis phospholipase C towards macrophages. Biochim Biophys Acta1801: 1305–1313. [DOI] [PubMed] [Google Scholar]
- 29. Adhikari RP, Karauzum H, Sarwar J, Abaandou L, Mahmoudieh M, et al. (2012) Novel structurally designed vaccine for S. aureus α-hemolysin: protection against bacteremia and pneumonia. PLoS One 7: e38567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu H, Semino-Mora C, Dubois A (2012) Mechanism of H. pylori intracellular entry: an in vitro study. Front Cell Infect Microbiol 2: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Seo KS, Kim JW, Park JY, Viall AK, Minnich SS, et al. (2012) Role of a new intimin/invasin-like protein in Yersinia pestis virulence. Infect Immun 80: 3559–3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fairman JW, Dautin N, Wojtowicz D, Liu W, Noinaj N, et al. (2012) Crystal structures of the outer membrane domain of intimin and invasin from enterohemorrhagic E. coli and enteropathogenic Y. pseudotuberculosis. Structure. 20: 1233–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mishra SK, Gordon RE, Barnett DA (1980) Identification of nocardiae and streptomycetes of medical importance. J Clin Microbiol 11: 728–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Méndez C, Salas JA (2001) The role of ABC transporters in antibiotic-producing organisms: drug secretion and resistance. mechanisms152: 341–50. [DOI] [PubMed] [Google Scholar]
- 35. Arruda S, Bomfim G, Knights R, Huima-Byron T, Riley LW (1993) Cloning of a Mycobacterium tuberculosis DNA fragment associated with entry and survival inside cells. Science 26: 1454–1457. [DOI] [PubMed] [Google Scholar]
- 36. Chitale S, Ehrt S, Kawamura I, Fujimura T, Shimono N, et al. (2001) Recombinant Mycobacterium tuberculosis protein associated with mammalian cell entry. Cell Microbiol 3: 247–54. [DOI] [PubMed] [Google Scholar]
- 37. Gioffré A, Infante E, Aguilar D, Santangelo MP, Klepp L, et al. 2005 Mutation in mce operons attenuates Mycobacterium tuberculosis virulence. Microbes Infect 7: 325–34. [DOI] [PubMed] [Google Scholar]
- 38. Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, et al. (1998) Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 393: 537–544. [DOI] [PubMed] [Google Scholar]
- 39. Lai CC, Liu WL, Ko WC, Chen YH, Tan HR, et al. (2011) Multicenter study in Taiwan of the in vitro activities of nemonoxacin, tigecycline, doripenem, and other antimicrobial agents against clinical isolates of various Nocardia species . Antimicrob Agents Chemother 55: 2084–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Strieker M, Tanović A, Marahiel MA (2010) Nonribosomal peptide synthetases: structures and dynamics. Curr Opin Struct Biol 20: 234–2340. [DOI] [PubMed] [Google Scholar]
- 41. Visca P, Imperi F, Lamont IL (2007) Pyoverdine siderophores: from biogenesis to biosignificance. Trends Microbiol. 15: 22–30. [DOI] [PubMed] [Google Scholar]
- 42. Hopwood DA, Sherman DH (1990) Molecular genetics of polyketides and its comparison to fatty acid biosynthesis. Annu Rev Genet 24: 37–66. [DOI] [PubMed] [Google Scholar]
- 43. Mikami Y (2010) [Recent progress in taxonomic studies on pathogenic nocardia and usefulness of the bacteria for the studies on secondary metabolites and antibiotic resistant mechanisms]. Nihon Ishinkin Gakkai Zasshi 51: 179–92. [DOI] [PubMed] [Google Scholar]
- 44.González-Burgos E, Gómez-Serranillos MP (2012) Terpene compounds in nature: A review of their potential antioxidant activity. Curr Med Chem. [DOI] [PubMed]
- 45. Hayashi Y, Matsuura N, Toshima H, Itoh N, Ishikawa J, et al. (2008) Cloning of the gene cluster responsible for the biosynthesis of brasilicardin A, a unique diterpenoid. J Antibiot (Tokyo) 61: 164–174. [DOI] [PubMed] [Google Scholar]
- 46. McLean KJ, Marshall KR, Richmond A, Hunter IS, Fowler K, et al. (2002) Azole antifungals are potent inhibitors of cytochrome P450 mono-oxygenases and bacterial growth in mycobacteria and streptomycetes. Microbiology 148: 2937–2949. [DOI] [PubMed] [Google Scholar]
- 47. Vera-Cabrera L, Campos-Rivera MP, Escalante-Fuentes WG, Pucci MJ, Ocampo-Candiani J, et al. (2010) In vitro activity of ACH-702, a new isothiazoloquinolone, against Nocardia brasiliensis compared with econazole and the carbapenems imipenem and meropenem alone or in combination with clavulanic acid. Antimicrob Agents Chemother 54: 2191–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Aziz RK, Breitbart M, Edwards RA (2010) Transposases are the most abundant, most ubiquitous genes in nature. Nucleic Acids Res. 38: 4207–4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Crawford JT (2003) Genotyping in contact investigations: a CDC perspective. Int J Tuberc Lung Dis. 7: S453–7. [PubMed] [Google Scholar]
- 50. Hatfull GF (2010) Mycobacteriophages: genes and genomes. Annu Rev Microbiol. 64: 331–56. [DOI] [PubMed] [Google Scholar]
- 51. Bhaya D, Davison M, Barrangou R (2011) CRISPR-Cas systems in bacteria and archaea: versatile small RNAs for adaptive defense and regulation. Annu Rev Genet (45) 273–97. [DOI] [PubMed] [Google Scholar]
- 52. Gomez-Flores A, Welsh O, Said-Fernández S, Lozano-Garza G, Tavarez-Alejandro RE, et al. (2004) In vitro and in vivo activities of antimicrobials against Nocardia brasiliensis. Antimicrob Agents Chemother. 48: 832–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wallace RJ Jr, Steele LC, Sumter G, Smith JM (1988) Antimicrobial susceptibility patterns of Nocardia asteroides . Antimicrob Agents Chemother 32: 1776–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ni B, Zhang Y, Chen DW, Wang BJ, Liu SJ (2012) Assimilation of aromatic compounds by Comamonas testosteroni: characterization and spreadability of protocatechuate 4,5-cleavage pathway in bacteria. Appl Microbiol Biotechnol. [DOI] [PubMed]
- 55. Pérez-Pantoja D, Donoso R, Agulló L, Córdova M, Seeger M, et al. (2012) Genomic analysis of the potential for aromatic compounds biodegradation in Burkholderiales. Environ Microbiol 14: 1091–1117. [DOI] [PubMed] [Google Scholar]
- 56. Ubhayasekera W, Karlsson M (2012) Bacterial and fungal chitinase chiJ orthologs evolve under different selective constraints following horizontal gene transfer. BMC Res Notes 5: 581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kaltenpoth M, Yildirim E, Gürbüz MF, Herzner G, Strohm E (2012) Refining the roots of the beewolf-Streptomyces symbiosis: antennal symbionts in the rare genus Philanthinus (Hymenoptera, Crabronidae). Appl Environ Microbiol 78: 822–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Martínez-Reyes A, Cruz-López H, Aburto-Sánchez MA, Isauro-García L, Camacho MA, et al. (2008) La avispa amarilla (Polistes mexicana): nuevo vector de actinomicetos en micetoma. Rev Med UV 8: 19–24. [Google Scholar]
- 59. Beisson F, Ohlrogge J (2012) Plants: Knitting a polyester skin. Nat Chem Biol. 8: 603–604. [DOI] [PubMed] [Google Scholar]
- 60. Santos CL, Correia-Neves M, Moradas-Ferreira P, Mendes MV (2012) A walk into the luxr regulators of actinobacteria: phylogenomic distribution and functional diversity. PLoS One 7: e46758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gebhardt H, Meniche X, Tropis M, Krämer R, Daffé M, et al. (2007) The key role of the mycolic acid content in the functionality of the cell wall permeability barrier in Corynebacterineae. Microbiology (153) 1424–34. [DOI] [PubMed] [Google Scholar]
- 62. Takayama K, Wang C, Besra GS (2005) Pathway to synthesis and processing of mycolic acids in Mycobacterium tuberculosis . Clin Microbiol Rev 18: 81–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Barkan D, Hedhli D, Yan HG, Huygen K, Glickman MS (2012) Mycobacterium tuberculosis lacking all mycolic acid cyclopropanation is viable but highly attenuated and hyperinflammatory in mice. Infect Immun. 80: 1958–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Brennan PJ (2003) Structure, function, and biogenesis of the cell wall of Mycobacterium tuberculosis . Tuberculosis (Edinb) 83: 91–97. [DOI] [PubMed] [Google Scholar]
- 65. Grant JR, Stothard P (2008) The CGView Server: a comparative genomics tool for circular genomes. Nucleic Acids Res 36: W181–W184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Borodovsky M, McIninch J (1993) GeneMark: Parallel Gene Recognition for both DNA Strands Comput Chem. 17: 123–133. [Google Scholar]
- 67. Delcher AL, Hormon D, Kasif S, White O, Salzberg SL (1999) Improved microbial gene identification with GLIMMER. Nucleic Acids Res 27: 4636–4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lukashin A, Borodovsky M (1998) GeneMark.hmm: new solutions for gene finding. Nucleic Acids Res 26: 1107–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lowe TM, Eddy SR (1997) tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucl Acids Res 25: 955–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Genomic location of the 47 gene clusters found in Nocardia brasiliensis HUJEG-1 using Antibiotics and Secondary Metabolites Analysis Shell (antismash) software [http://antismash.secondarymetabolites.org]. Besides the map we show a comparative analysis of cluster 1, which is highly conserved among the Nocardiaceae, located between nucleotides 1682661 – 1725128 nt, and cluster 38, which is quite specific of N. brasiliensis and is located between nucleotides 6591347–6636992
(PPTX)
Presence of ortholog genes of putative virulence factors of N. brasiliensis in other microorganisms.
(DOCX)