Abstract
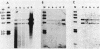
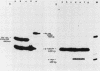
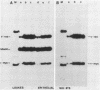
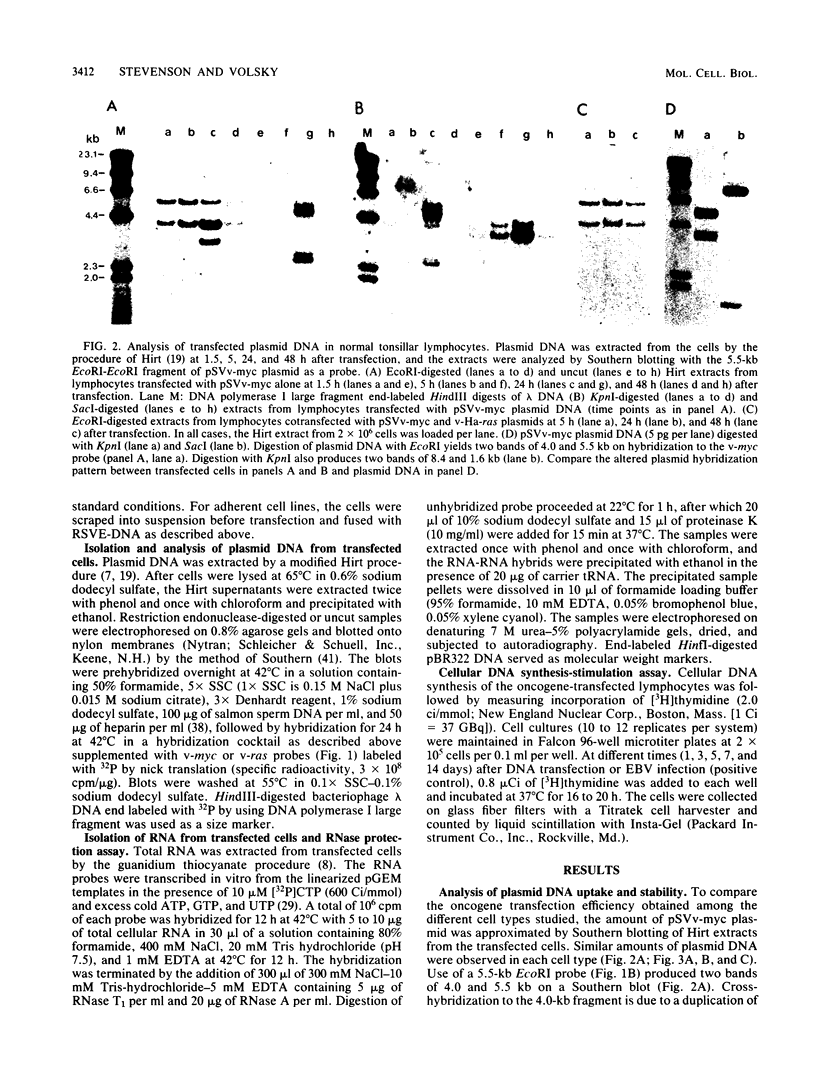
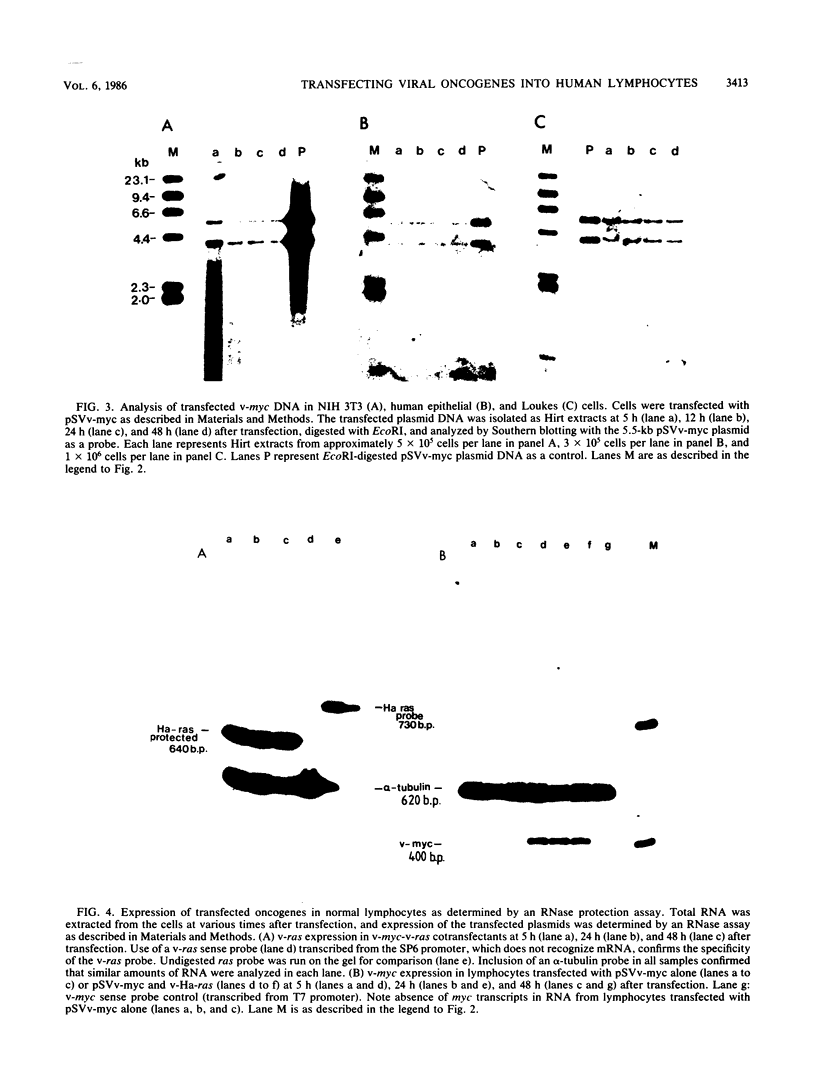
Activated v-myc (pSV v-myc) and v-Ha-ras (GT10) oncogenes were introduced into normal human lymphocytes, NIH 3T3 fibroblasts, B-lymphoblastoid cells, and human epithelial cells, using a reconstituted Sendai virus envelope-mediated gene transfer technique. Efficient transfer of the plasmid in each cell type was demonstrable within 1.5 h of transfection by Southern blotting of extrachromosomal DNA extracts, which unexpectedly revealed that v-myc plasmid DNA was unstable in normal lymphocytes but not in the other cell types. The v-myc plasmid was stabilized when cotransfected into lymphocytes together with v-Ha-ras. The transfected v-Ha-ras plasmid was stable in all the cell types tested. v-myc plasmid expression was clearly detectable by 5 h in all cell types except human lymphocytes. Lymphocytes expressed v-myc when transfected together with v-Ha-ras. Transfected ras oncogene was efficiently expressed in all the cell types tested. Expression of the transfected genes increased at 24 and 48 h after transfection. Even though plasmid stability and expression were achieved in myc-ras-cotransfected lymphocytes, no effects on cellular DNA synthesis or immortalization were observed, in contrast to efficient transformation of NIH 3T3 fibroblasts by the same procedure. Our data suggest that efficient expression of transfected myc and ras oncogenes in normal quiescent human lymphocytes is not sufficient for the induction of cell growth and immortalization.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C. Transient gene expression control: effects of transfected DNA stability and trans-activation by viral early proteins. Mol Cell Biol. 1985 May;5(5):1034–1042. doi: 10.1128/mcb.5.5.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J., Melchers F. T cell-dependent activation of resting B cells: requirement for both nonspecific unrestricted and antigen-specific Ia-restricted soluble factors. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2497–2501. doi: 10.1073/pnas.78.4.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armelin H. A., Armelin M. C., Kelly K., Stewart T., Leder P., Cochran B. H., Stiles C. D. Functional role for c-myc in mitogenic response to platelet-derived growth factor. Nature. 1984 Aug 23;310(5979):655–660. doi: 10.1038/310655a0. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. Viral oncogenes. Cell. 1985 Aug;42(1):23–38. doi: 10.1016/s0092-8674(85)80098-2. [DOI] [PubMed] [Google Scholar]
- Cheah M. S., Ley T. J., Tronick S. R., Robbins K. C. fgr proto-oncogene mRNA induced in B lymphocytes by Epstein-Barr virus infection. Nature. 1986 Jan 16;319(6050):238–240. doi: 10.1038/319238a0. [DOI] [PubMed] [Google Scholar]
- Chinsky J., Soeiro R. Fv-1 host restriction of Friend leukemia virus: analysis of unintegrated proviral DNA. J Virol. 1981 Oct;40(1):45–55. doi: 10.1128/jvi.40.1.45-55.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cowan N. J., Dobner P. R., Fuchs E. V., Cleveland D. W. Expression of human alpha-tubulin genes: interspecies conservation of 3' untranslated regions. Mol Cell Biol. 1983 Oct;3(10):1738–1745. doi: 10.1128/mcb.3.10.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar R., Ellis R. W., Shih T. Y., Oroszlan S., Shapiro B., Maizel J., Lowy D., Scolnick E. Nucleotide sequence of the p21 transforming protein of Harvey murine sarcoma virus. Science. 1982 Sep 3;217(4563):934–936. doi: 10.1126/science.6287572. [DOI] [PubMed] [Google Scholar]
- Eick D., Piechaczyk M., Henglein B., Blanchard J. M., Traub B., Kofler E., Wiest S., Lenoir G. M., Bornkamm G. W. Aberrant c-myc RNAs of Burkitt's lymphoma cells have longer half-lives. EMBO J. 1985 Dec 30;4(13B):3717–3725. doi: 10.1002/j.1460-2075.1985.tb04140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman V. H., Shin S. I. Cellular tumorigenicity in nude mice: correlation with cell growth in semi-solid medium. Cell. 1974 Dec;3(4):355–359. doi: 10.1016/0092-8674(74)90050-6. [DOI] [PubMed] [Google Scholar]
- Gilmer T. M., Annab L. A., Oshimura M., Barrett J. C. Neoplastic transformation of normal and carcinogen-induced preneoplastic Syrian hamster embryo cells by the v-src oncogene. Mol Cell Biol. 1985 Jul;5(7):1707–1713. doi: 10.1128/mcb.5.7.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glanville N. Unstable expression and amplification of a transfected oncogene in confluent and subconfluent cells. Mol Cell Biol. 1985 Jun;5(6):1456–1464. doi: 10.1128/mcb.5.6.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Greig R. G., Koestler T. P., Trainer D. L., Corwin S. P., Miles L., Kline T., Sweet R., Yokoyama S., Poste G. Tumorigenic and metastatic properties of "normal" and ras-transfected NIH/3T3 cells. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3698–3701. doi: 10.1073/pnas.82.11.3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross T. G., Sakai K., Volsky D. J. Transfer of the Epstein-Barr virus (EBV) DNA fragment coding for EBNA-1, the putative transforming antigen of EBV, into normal human lymphocytes: gene expression without cell transformation. Biochem Biophys Res Commun. 1986 Feb 13;134(3):1260–1268. doi: 10.1016/0006-291x(86)90386-4. [DOI] [PubMed] [Google Scholar]
- Hayward W. S., Neel B. G., Astrin S. M. Activation of a cellular onc gene by promoter insertion in ALV-induced lymphoid leukosis. Nature. 1981 Apr 9;290(5806):475–480. doi: 10.1038/290475a0. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Imperiale M. J., Feldman L. T., Nevins J. R. Activation of gene expression by adenovirus and herpesvirus regulatory genes acting in trans and by a cis-acting adenovirus enhancer element. Cell. 1983 Nov;35(1):127–136. doi: 10.1016/0092-8674(83)90215-5. [DOI] [PubMed] [Google Scholar]
- Keath E. J., Caimi P. G., Cole M. D. Fibroblast lines expressing activated c-myc oncogenes are tumorigenic in nude mice and syngeneic animals. Cell. 1984 Dec;39(2 Pt 1):339–348. doi: 10.1016/0092-8674(84)90012-6. [DOI] [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Lacy J., Sarkar S. N., Summers W. C. Induction of c-myc expression in human B lymphocytes by B-cell growth factor and anti-immunoglobulin. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1458–1462. doi: 10.1073/pnas.83.5.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Cellular oncogenes and multistep carcinogenesis. Science. 1983 Nov 18;222(4625):771–778. doi: 10.1126/science.6356358. [DOI] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983 Aug 18;304(5927):596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- Loyter A., Vainstein A., Graessmann M., Graessmann A. Fusion-mediated injection of SV40-DNA. Introduction of SV40-DNA into tissue culture cells by the use of DNA-loaded reconstituted Sendai virus envelopes. Exp Cell Res. 1983 Feb;143(2):415–425. doi: 10.1016/0014-4827(83)90068-x. [DOI] [PubMed] [Google Scholar]
- Maguire R. T., Robins T. S., Thorgeirsson S. S., Heilman C. A. Expression of cellular myc and mos genes in undifferentiated B cell lymphomas of Burkitt and non-Burkitt types. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1947–1950. doi: 10.1073/pnas.80.7.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbold R. F., Overell R. W. Fibroblast immortality is a prerequisite for transformation by EJ c-Ha-ras oncogene. Nature. 1983 Aug 18;304(5927):648–651. doi: 10.1038/304648a0. [DOI] [PubMed] [Google Scholar]
- Nishikura K., ar-Rushdi A., Erikson J., Watt R., Rovera G., Croce C. M. Differential expression of the normal and of the translocated human c-myc oncogenes in B cells. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4822–4826. doi: 10.1073/pnas.80.15.4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope J. H., Horne M. K., Scott W. Transformation of foetal human keukocytes in vitro by filtrates of a human leukaemic cell line containing herpes-like virus. Int J Cancer. 1968 Nov 15;3(6):857–866. doi: 10.1002/ijc.2910030619. [DOI] [PubMed] [Google Scholar]
- Rabbitts P. H., Forster A., Stinson M. A., Rabbitts T. H. Truncation of exon 1 from the c-myc gene results in prolonged c-myc mRNa stability. EMBO J. 1985 Dec 30;4(13B):3727–3733. doi: 10.1002/j.1460-2075.1985.tb04141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruley H. E. Adenovirus early region 1A enables viral and cellular transforming genes to transform primary cells in culture. Nature. 1983 Aug 18;304(5927):602–606. doi: 10.1038/304602a0. [DOI] [PubMed] [Google Scholar]
- Schwab M., Alitalo K., Klempnauer K. H., Varmus H. E., Bishop J. M., Gilbert F., Brodeur G., Goldstein M., Trent J. Amplified DNA with limited homology to myc cellular oncogene is shared by human neuroblastoma cell lines and a neuroblastoma tumour. Nature. 1983 Sep 15;305(5931):245–248. doi: 10.1038/305245a0. [DOI] [PubMed] [Google Scholar]
- Schwab M., Varmus H. E., Bishop J. M. Human N-myc gene contributes to neoplastic transformation of mammalian cells in culture. Nature. 1985 Jul 11;316(6024):160–162. doi: 10.1038/316160a0. [DOI] [PubMed] [Google Scholar]
- Shapiro I. M., Klein G., Volsky D. J. Epstein-Barr virus co-reconstituted with Sendai virus envelopes infects Epstein-Barr virus-receptor negative cells. Biochim Biophys Acta. 1981 Aug 5;676(1):19–24. doi: 10.1016/0304-4165(81)90004-0. [DOI] [PubMed] [Google Scholar]
- Shapiro I. M., Stevenson M., Sinangil F., Volsky D. J. Transfection of lymphoblastoid cells using DNA-loaded reconstituted Sendai virus envelopes: expression of transfected DNA and selection of transfected cells. Somat Cell Mol Genet. 1986 Jul;12(4):351–356. doi: 10.1007/BF01570729. [DOI] [PubMed] [Google Scholar]
- Singh L., Jones K. W. The use of heparin as a simple cost-effective means of controlling background in nucleic acid hybridization procedures. Nucleic Acids Res. 1984 Jul 25;12(14):5627–5638. doi: 10.1093/nar/12.14.5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slamon D. J., deKernion J. B., Verma I. M., Cline M. J. Expression of cellular oncogenes in human malignancies. Science. 1984 Apr 20;224(4646):256–262. doi: 10.1126/science.6538699. [DOI] [PubMed] [Google Scholar]
- Smeland E., Godal T., Ruud E., Beiske K., Funderud S., Clark E. A., Pfeifer-Ohlsson S., Ohlsson R. The specific induction of myc protooncogene expression in normal human B cells is not a sufficient event for acquisition of competence to proliferate. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6255–6259. doi: 10.1073/pnas.82.18.6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spandidos D. A., Wilkie N. M. Malignant transformation of early passage rodent cells by a single mutated human oncogene. Nature. 1984 Aug 9;310(5977):469–475. doi: 10.1038/310469a0. [DOI] [PubMed] [Google Scholar]
- Tarpley W. G., Temin H. M. The location of v-src in a retrovirus vector determines whether the virus is toxic or transforming. Mol Cell Biol. 1984 Dec;4(12):2653–2660. doi: 10.1128/mcb.4.12.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomassen D. G., Gilmer T. M., Annab L. A., Barrett J. C. Evidence for multiple steps in neoplastic transformation of normal and preneoplastic Syrian hamster embryo cells following transfection with Harvey murine sarcoma virus oncogene (v-Ha-ras). Cancer Res. 1985 Feb;45(2):726–732. [PubMed] [Google Scholar]
- Thompson C. B., Challoner P. B., Neiman P. E., Groudine M. Levels of c-myc oncogene mRNA are invariant throughout the cell cycle. 1985 Mar 28-Apr 3Nature. 314(6009):363–366. doi: 10.1038/314363a0. [DOI] [PubMed] [Google Scholar]
- Vennström B., Moscovici C., Goodman H. M., Bishop J. M. Molecular cloning of the avian myelocytomatosis virus genome and recovery of infectious virus by transfection of chicken cells. J Virol. 1981 Aug;39(2):625–631. doi: 10.1128/jvi.39.2.625-631.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volsky D. J., Gross T., Sinangil F., Kuszynski C., Bartzatt R., Dambaugh T., Kieff E. Expression of Epstein-Barr virus (EBV) DNA and cloned DNA fragments in human lymphocytes following Sendai virus envelope-mediated gene transfer. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5926–5930. doi: 10.1073/pnas.81.19.5926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ar-Rushdi A., Nishikura K., Erikson J., Watt R., Rovera G., Croce C. M. Differential expression of the translocated and the untranslocated c-myc oncogene in Burkitt lymphoma. Science. 1983 Oct 28;222(4622):390–393. doi: 10.1126/science.6414084. [DOI] [PubMed] [Google Scholar]
- van Santen V., Cheung A., Kieff E. Epstein-Barr virus RNA VII: size and direction of transcription of virus-specified cytoplasmic RNAs in a transformed cell line. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1930–1934. doi: 10.1073/pnas.78.3.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]