Abstract
Background
Few clinical data are available on the relationship between genospecies and outcome of Acinetobacter bacteremia, and the results are inconsistent. We performed this study to evaluate the relationship between genospecies and the outcome of Acinetobacter bacteremia.
Methods
Clinical data from 180 patients who had Acinetobacter bacteremia from 2003 to 2010 were reviewed retrospectively. The genospecies were identified by rpoB gene sequence analysis. The clinical features and outcomes of 90 patients with A. baumannii bacteremia were compared to those of 90 patients with non-baumannii Acinetobacter bacteremia (60 with A. nosocomialis, 17 with Acinetobacter species “close to 13 TU”, 11 with A. pittii, and two with A. calcoaceticus).
Results
A. baumannii bacteremia was associated with intensive care unit-onset, mechanical ventilation, pneumonia, carbapenem resistance, and higher APACHE II scores, compared to non-baumannii Acinetobacter bacteremia (P<0.05). In univariate analyses, age, pneumonia, multidrug resistance, carbapenem resistance, inappropriate empirical antibiotics, higher APACHE II scores, and A. baumannii genospecies were risk factors for mortality (P<0.05). Multivariate analysis revealed A. baumannii genospecies (OR, 3.60; 95% CI, 1.56–8.33), age, pneumonia, and higher APACHE II scores to be independent risk factors for mortality (P<0.05).
Conclusion
A. baumannii genospecies was an independent risk factor for mortality in patients with Acinetobacter bacteremia. Our results emphasize the importance of correct species identification of Acinetobacter blood isolates.
Introduction
The spread of multidrug-resistant (MDR) Acinetobacter strains among critically ill, hospitalized patients, and subsequent epidemics, have become an increasing cause for concern [1], [2]. These organisms are one of the most common causes of infection in patients in the intensive care unit (ICU) and are associated with a greater risk of hospital death by causing catheter-related infection, pneumonia, urinary tract infection, wound infection, and primary bacteremia in patients with critical illness [3]–[6]. The organisms’ ability to accumulate diverse mechanisms of resistance limits therapeutic agents and makes the infection difficult to treat [7].
To date, more than 30 Acinetobacter species have been described [8]. Among these, Acinetobacter baumannii (genotype 2) is the most commonly found in clinical specimens. However, recently, infection caused by other Acinetobacter species belonging to the A. calcoaceticus-A. baumannii (ACB) complex, including A. nosocomialis (known also as 13 TU), A. calcoaceticus (genotype 1), A. pittii (genotype 3), and Acinetobacter genomic species “close to 13 TU”, has caused concern [9], [10]. Although these are increasingly reported as human pathogens with the introduction of genotyping, it is impossible to distinguish these from A. baumannii by automation systems or phenotypic tests [9], [11].
For these reasons, it is important to know whether or not the clinical features, risk factors, and outcomes differ according to species. To date, few studies have been published that evaluate this matter using genotypic assays in patients with Acinetobacter bacteremia [12]–[14]; however, among these reports, the influence of genospecies on outcome is inconsistent. Thus, we compared the clinical features, antimicrobial resistance, and outcome of bacteremia caused by A. baumannii versus non-baumannii ACB complex strains.
Patients and Methods
Ethics
This study was approved by the Institutional Review Board of Chonnam National University Hospital. A waiver of consent was granted given the retrospective nature of the project.
Patients
All adult patients (age, ≥16 years) with bacteremia caused by ACB complex who were admitted to Chonnam National University Hospital (1000 beds; Gwang-ju, Republic of Korea) from January 2003 to December 2010 were included. Only patients with their first episode of Acinetobacter bacteremia were included.
Data Collection
Demographic and clinical data were collected by reviewing the electronic medical records of the patients and included comorbid conditions, duration of ICU and hospital stay, use of a ventilator, and use of central venous catheters at the time of bacteremia onset. The severity of patient infection was evaluated using Acute Physiology and Chronic Health Evaluation (APACHE) II scores within 24 h of bacteremia onset.
Microbiological Tests
Acinetobacter was identified using the automated system Vitek 2 (bioMérieux, Marcy l’Etoile, France). Acinetobacter species were determined on the basis of partial rpoB gene sequences (468 bp) detected using the primers Ac1055F (GTGATAARATGGCBGGTCGT) and Ac1598R (CGBGCRTGCATYTTGTCRT), as described by us previously [15], [16]. In vitro susceptibility testing was conducted using Vitek 2 (bioMérieux).
Definitions
Multidrug resistance (MDR) was defined if an isolate showed resistance to more than one of the following five antimicrobial agents: imipenem, ciprofloxacin, amikacin, cefepime, and piperacillin/tazobactam, in accordance with Paterson’s suggestion [17]. Appropriate empirical antibiotics was defined as the use of at least one antibiotic to which the Acinetobacter strain was susceptible within 48 days from the onset of bacteremia. Mortality was defined as Acinetobacter-related in the absence of another definite cause of death [18].
Statistical Analysis
Categorical variables were compared using Fisher’s exact test or the Pearson χ2 test, and continuous variables were compared using Student’s t-test or Mann-Whitney rank sum test, as appropriate. Variables with P values ≤0.10 in the univariate analysis were included in the multivariate analysis. Multivariate analyses were performed using a logistic regression model in the backward stepwise conditional manner. All tests of significance were two-tailed, and P values ≤0.05 were deemed to indicate statistical significance. Statistical analyses of the data were performed using SPSS software (version 19.0; SPSS Inc., Chicago, IL, USA).
Results
Study Population and Genospecies Distribution
During the study period, we identified 180 isolates of the ACB complex, including 90 (50%) A. baumannii, 60 (33%) A. nosocomialis, 17 (9%) Acinetobacter genomic species “close to 13 TU”, 11 (6%) A. pittii, and two (1%) A. calcoaceticus.
Comparison of the Clinical Features of Bacteremia Caused by A. baumannii versus Non-baumannii ACB Complex
The clinical features of bacteremia caused by A. baumannii (n = 90) and non-baumannii ACB complex (n = 90) bacteria are shown in Table 1. Demographic data were not different between the two groups. ICU onset, mechanical ventilation, and underlying chronic liver disease were risk factors for A. baumannii bacteremia, compared to non-baumannii Acinetobacter bacteremia (P<0.05). Pneumonia, MDR, and carbapenem resistance were more commonly found in A. baumannii bacteremia, while primary bacteremia was more commonly caused by non-baumannii Acinetobacter (P<0.05). No significant difference in clinical features was observed between bacteremia caused by A. nosocomialis, Acinetobacter species “close to 13 TU”, and A. pittii (data not shown).
Table 1. Comparison of the clinical features of bacteremia caused by A. baumannii (n = 90) or non-baumannii ACB complex (n = 90).
| Variables | No. (%) of patients with bacteremia caused by | P value | |
| Acinetobacter baumannii (n = 90) | Non- baumannii ACB complex (n = 90) | ||
| Demographic data | |||
| Agea | 60±17 | 58±18 | 0.46 |
| Male gender | 62 (69) | 53 (59) | 0.16 |
| Underlying illness | |||
| Ischemic heart disease | 25 (28) | 16 (18) | 0.11 |
| Trauma | 13 (14) | 16 (18) | 0.54 |
| Diabetes mellitus | 11 (12) | 14 (16) | 0.52 |
| Cancer | 8 (9) | 12 (13) | 0.34 |
| Cerebrovascular accident | 7 (8) | 8 (9) | 0.79 |
| Chronic liver disease | 14 (16) | 3 (3) | 0.01 |
| End-stage renal disease | 4 (4) | 4 (4) | >0.99 |
| Benign biliary disease | 7 (8) | 7 (8) | >0.99 |
| Primary site of infection | |||
| Vascular catheter | 27 (30) | 27 (30) | >0.99 |
| Pneumonia | 29 (32) | 16 (18) | 0.03 |
| Primary bacteremia | 8 (9) | 18 (20) | 0.03 |
| Intra-abdominal | 12 (13) | 9 (10) | 0.49 |
| Urinary tract | 3 (3) | 7 (8) | 0.33 |
| Soft tissue or wound | 7 (8) | 7 (8) | >0.99 |
| Other characteristics | |||
| Length of hospitalization (day)b, c | 12 (7, 21) | 11 (7, 22) | 0.90 |
| ICU stay at culture | 77 (86) | 57 (63) | 0.001 |
| Mechanical ventilation | 64 (71) | 44 (49) | 0.002 |
| MDR | 69 (77) | 29 (32) | <0.001 |
| Carbapenem-resistance | 51 (57) | 12 (16) | <0.001 |
| APACHE II scorea | 18±7 | 17±8 | 0.30 |
| Outcomes | |||
| 30-day mortality | 35 (39) | 14 (16) | <0.001 |
| Acinetobacter-related mortality | 33 (37) | 11 (12) | <0.001 |
ACB, A. calcoaceticus-A. baumannii; ICU, intensive care unit; MDR, multidrug resistance; APACHE, acute physiology and chronic health evaluation.
Continuous variables were expressed as means ± SDs a or medians (IQRs) b and were compared by the Student’s t test a or Mann-Whitney U test b.
Until the onset of Acinetobacter bacteremia.
Risk Factors for 30-day Mortality in Patients with Acinetobacter Bacteremia
Risk factors for 30-day mortality in the 180 patients with Acinetobacter bacteremia are shown in Table 2. In the univariate analysis, A. baumannii genospecies, age, pneumonia, MDR, carbapenem resistance, inappropriate empirical antibiotics, and APACHE II score were each associated with 30-day mortality (P<0.05). Multivariate analysis included the following variables: A. baumannii genospecies, APACHE II score, age, pneumonia, vascular catheter infection, inappropriate empirical antibiotics, MDR and carbapenem resistance. The independent risk factors for 30-day mortality identified in the multivariate logistic regression analysis included A. baumannii genospecies (OR, 3.60; 95% CI, 1.56–8.33), pneumonia, age, and APACHE II score (Table 2).
Table 2. Risk factors for 30-day mortality in 180 patients with Acinetobacter bacteremia.
| Variables | Univariate analysis | Multivariate analysis | |||
| No.(%) of patients | P value | Odds ratio (95% CI) | P value | ||
| Survivor(n = 131) | Non-survivor (n = 49) | ||||
| Agea | 55±18 | 69±12 | <0.001 | 1.07 (1.03–1.10) | <0.001 |
| Vascular catheter infection | 44 (34) | 10 (20) | 0.086 | ||
| Pneumonia | 23 (18) | 22 (45) | <0.001 | 2.72 (1.16–6.37) | 0.021 |
| APACHE II scorea | 16±7 | 22±7 | <0.001 | 1.12 (1.06–1.19) | <0.001 |
| MDR | 62 (47) | 36 (74) | 0.002 | ||
| Carbapenem resistance | 38 (29) | 27 (55) | 0.001 | ||
| Inappropriate empirical antibiotics | 65 (50) | 37 (76) | 0.002 | ||
| A. baumannii genospecies | 55 (42) | 35 (71) | <0.001 | 3.60 (1.56–8.33) | 0.003 |
APACHE, acute physiology and chronic health evaluation; MDR, multidrug resistance.
Continuous variables were expressed as means ± SDs and were compared by the Student’s t test.
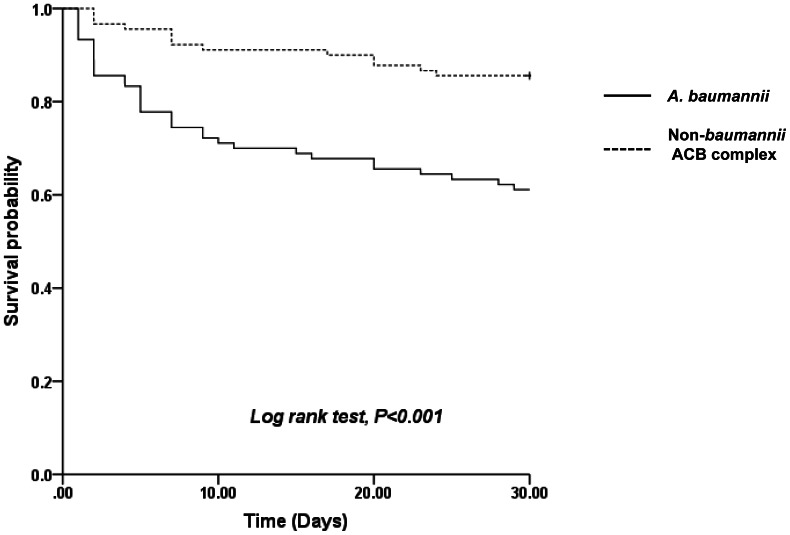
The Kaplan-Meier survival curves for Acinetobacter bacteremia caused by A. baumannii and non-baumannii ACB complex strains are shown in Figure 1. The 30-day survival rate was 84% (72/90) in patients with non-baumannii ACB complex bacteremia compared to 61% (55/90) in those with A. baumannii bacteremia (log-rank test; P<0.001). No significant difference in survival was identified between patients with A. nosocomialis bacteremia (83%; 50/60), Acinetobacter species “close to 13 TU” bacteremia (88%; 15/17), and A. pittii bacteremia (91%; 10/11).
Figure 1. Kaplan-Meier curves of survival in 90 patients with A. baumannii bacteremia and 90 patients with non-baumannii ACB complex bacteremia.
Discussion
In this study, we found that genospecies A. baumannii was independently associated with poor outcome in patients with ACB complex bacteremia.
Non-baumannii ACB complex are increasingly reported as a cause of bacteremia because of the introduction of genospecies identification. Recent studies showed that A. nosocomialis and A. pittii comprised 27% and 9% of ACB complex bacteremia in Taiwan [13] and 21% and 8% in the USA [14], respectively, which is less prevalent than A. baumannii. However, A. nosocomialis and A. pittii were sevenfold more prevalent causes of bacteremia than A. baumannii in Norway [19]. In our study, the proportion of non-baumannii species was 50%, and Acinetobacter genomic species “close to 13 TU”, newly defined as a member of the ACB complex [9], comprised a higher proportion, in contrast to previous reports. This finding suggests that the distribution of prevalent genospecies differs geographically.
Previous studies reported the differences in clinical features between A. baumannii bacteremia and non-baumannii ACB complex bacteremia [12]–[14]. ICU onset, mechanical ventilation, higher Pitt bacteremia scores, and chronic obstructive pulmonary disease were factors associated with A. baumannii bacteremia, compared to non-baumannii ACB complex bacteremia in those studies. MDR, carbapenem resistance, and pneumonia were more commonly found in A. baumannii bacteremia, while primary bacteremia itself was more commonly caused by non-baumannii ACB complex bacteria. Similar results were found in our study. MDR was more commonly found in A. baumannii than non-baumannii ACB complex blood isolates, in agreement with our previous studies [15]. Additionally, we found chronic liver disease, which is prevalent in Korea, as another risk factor for A. baumannii bacteremia.
The relationship between genospecies and outcome of Acinetobacter bacteremia was evaluated in some studies; however, the results are inconsistent. In previous studies, which did not identify ACB complex bacteria to the species level with genotyping assays and did not include genotype as a confounding factor in analysis, carbapenem resistance was revealed as an independent risk factor for mortality in ACB complex bacteremia [20]. However, Chuang et al. reported that A. baumannii species, and not carbapenem resistance, was an independent predictor of mortality in ICU patients with Acinetobacter bacteremia in Taiwan [12]. However, another study performed at the same hospital during a similar period reported that bacteremia due to MDR strains, but not A. baumannii per se, appeared to be associated with a poor outcome [13].
In the current study, we also found that A. baumannii species, rather than antibiotic resistance, mainly affected mortality, in accordance with a previous [12] and a more recent study performed in the USA [14]. Recently, Peleg et al. suggested that in vitro and in vivo virulence characteristics differed among individual strains of the ACB complex [21], which provides further evidence of the impact of genospecies on the outcome of Acinetobacter bacteremia. Our data also suggest that genotype might be a more significant predictive factor for mortality than carbapenem resistance and that genospecies should be included as a confounding factor in any analysis of risk factors for mortality in patients with Acinetobacter infections, because genospecies is significantly associated with MDR and carbapenem resistance [22], [23] and can affect the outcome. Moreover, our data provide more evidence of the importance of correct genospecies identification, which can predict the outcome of Acinetobacter bacteremia.
Our study had several limitations. First, since it was retrospective in design, the factors influencing the physicians’ choice of antibiotics were not determined, and they may have influenced our results as unmeasured confounding factors in the analysis. Second, the current study was performed in a single center; for this reason, factors such as the rate of referrals or surgery for complex disorders could have impacted the results.
In conclusion, our data showed that A. baumannii genospecies is an independent predictor of mortality in patients with ACB complex bacteremia, emphasizing the importance of genotyping for correct species identification and prognosis prediction.
Funding Statement
The authors have no support or funding to report.
References
- 1. Dijkshoorn L, Nemec A, Seifert H (2007) An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat Rev Microbiol 5: 939–951. [DOI] [PubMed] [Google Scholar]
- 2. Peleg AY, Seifert H, Paterson DL (2008) Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev 21: 538–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, et al. (2009) International study of the prevalence and outcomes of infection in intensive care units. JAMA 302: 2323–2329. [DOI] [PubMed] [Google Scholar]
- 4. Tabah A, Koulenti D, Laupland K, Misset B, Valles J, et al. (2012) Characteristics and determinants of outcome of hospital-acquired bloodstream infections in intensive care units: the EUROBACT International Cohort Study. Intensive Care Med 38: 1930–1945. [DOI] [PubMed] [Google Scholar]
- 5. Ntusi NB, Badri M, Khalfey H, Whitelaw A, Oliver S, et al. (2012) ICU-Associated Acinetobacter baumannii Colonisation/Infection in a High HIV-Prevalence Resource-Poor Setting. PLoS One 7: e52452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shields RK, Clancy CJ, Gillis LM, Kwak EJ, Silveira FP, et al. (2012) Epidemiology, Clinical Characteristics and Outcomes of Extensively Drug-Resistant Acinetobacter baumannii Infections among Solid Organ Transplant Recipients. PLoS One 7: e52349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cai Y, Chai D, Wang R, Liang B, Bai N (2012) Colistin resistance of Acinetobacter baumannii: clinical reports, mechanisms and antimicrobial strategies. J Antimicrob Chemother 67: 1607–1615. [DOI] [PubMed] [Google Scholar]
- 8. Nemec A, Musilek M, Maixnerova M, De Baere T, van der Reijden TJ, et al. (2009) Acinetobacter beijerinckii sp. nov. and Acinetobacter gyllenbergii sp. nov., haemolytic organisms isolated from humans. Int J Syst Evol Microbiol 59: 118–124. [DOI] [PubMed] [Google Scholar]
- 9. Nemec A, Krizova L, Maixnerova M, van der Reijden TJ, Deschaght P, et al. (2011) Genotypic and phenotypic characterization of the Acinetobacter calcoaceticus-Acinetobacter baumannii complex with the proposal of Acinetobacter pittii sp. nov. (formerly Acinetobacter genomic species 3) and Acinetobacter nosocomialis sp. nov. (formerly Acinetobacter genomic species 13TU). Res Microbiol 162: 393–404. [DOI] [PubMed] [Google Scholar]
- 10. Sahl JW, Gillece JD, Schupp JM, Waddell VG, Driebe EM, et al. (2013) Evolution of a pathogen: a comparative genomics analysis identifies a genetic pathway to pathogenesis in acinetobacter. PLoS One 8: e54287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gerner-Smidt P, Tjernberg I, Ursing J (1991) Reliability of phenotypic tests for identification of Acinetobacter species. J Clin Microbiol 29: 277–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chuang YC, Sheng WH, Li SY, Lin YC, Wang JT, et al. (2011) Influence of genospecies of Acinetobacter baumannii complex on clinical outcomes of patients with acinetobacter bacteremia. Clin Infect Dis 52: 352–360. [DOI] [PubMed] [Google Scholar]
- 13. Lee YC, Huang YT, Tan CK, Kuo YW, Liao CH, et al. (2011) Acinetobacter baumannii and Acinetobacter genospecies 13TU and 3 bacteraemia: comparison of clinical features, prognostic factors and outcomes. J Antimicrob Chemother 66: 1839–1846. [DOI] [PubMed] [Google Scholar]
- 14. Wisplinghoff H, Paulus T, Lugenheim M, Stefanik D, Higgins PG, et al. (2012) Nosocomial bloodstream infections due to Acinetobacter baumannii, Acinetobacter pittii and Acinetobacter nosocomialis in the United States. J Infect 64: 282–290. [DOI] [PubMed] [Google Scholar]
- 15. Ko KS, Suh JY, Kwon KT, Jung SI, Park KH, et al. (2007) High rates of resistance to colistin and polymyxin B in subgroups of Acinetobacter baumannii isolates from Korea. J Antimicrob Chemother 60: 1163–1167. [DOI] [PubMed] [Google Scholar]
- 16. Park YK, Jung SI, Park KH, Cheong HS, Peck KR, et al. (2009) Independent emergence of colistin-resistant Acinetobacter spp. isolates from Korea. Diagn Microbiol Infect Dis 64: 43–51. [DOI] [PubMed] [Google Scholar]
- 17. Paterson DL (2006) The epidemiological profile of infections with multidrug-resistant Pseudomonas aeruginosa and Acinetobacter species. Clin Infect Dis 43 Suppl 2S43–48. [DOI] [PubMed] [Google Scholar]
- 18. Jang HC, Kim SH, Kim KH, Kim CJ, Lee S, et al. (2009) Salvage treatment for persistent methicillin-resistant Staphylococcus aureus bacteremia: efficacy of linezolid with or without carbapenem. Clin Infect Dis 49: 395–401. [DOI] [PubMed] [Google Scholar]
- 19. Karah N, Haldorsen B, Hegstad K, Simonsen GS, Sundsfjord A, et al. (2011) Species identification and molecular characterization of Acinetobacter spp. blood culture isolates from Norway. J Antimicrob Chemother 66: 738–744. [DOI] [PubMed] [Google Scholar]
- 20. Kwon KT, Oh WS, Song JH, Chang HH, Jung SI, et al. (2007) Impact of imipenem resistance on mortality in patients with Acinetobacter bacteraemia. J Antimicrob Chemother 59: 525–530. [DOI] [PubMed] [Google Scholar]
- 21. Peleg AY, de Breij A, Adams MD, Cerqueira GM, Mocali S, et al. (2012) The success of acinetobacter species; genetic, metabolic and virulence attributes. PLoS One 7: e46984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Houang ET, Chu YW, Chu KY, Ng KC, Leung CM, et al. (2003) Significance of genomic DNA group delineation in comparative studies of antimicrobial susceptibility of Acinetobacter spp. Antimicrob Agents Chemother 47: 1472–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lim YM, Shin KS, Kim J (2007) Distinct antimicrobial resistance patterns and antimicrobial resistance-harboring genes according to genomic species of Acinetobacter isolates. J Clin Microbiol 45: 902–905. [DOI] [PMC free article] [PubMed] [Google Scholar]