Abstract
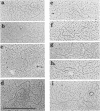
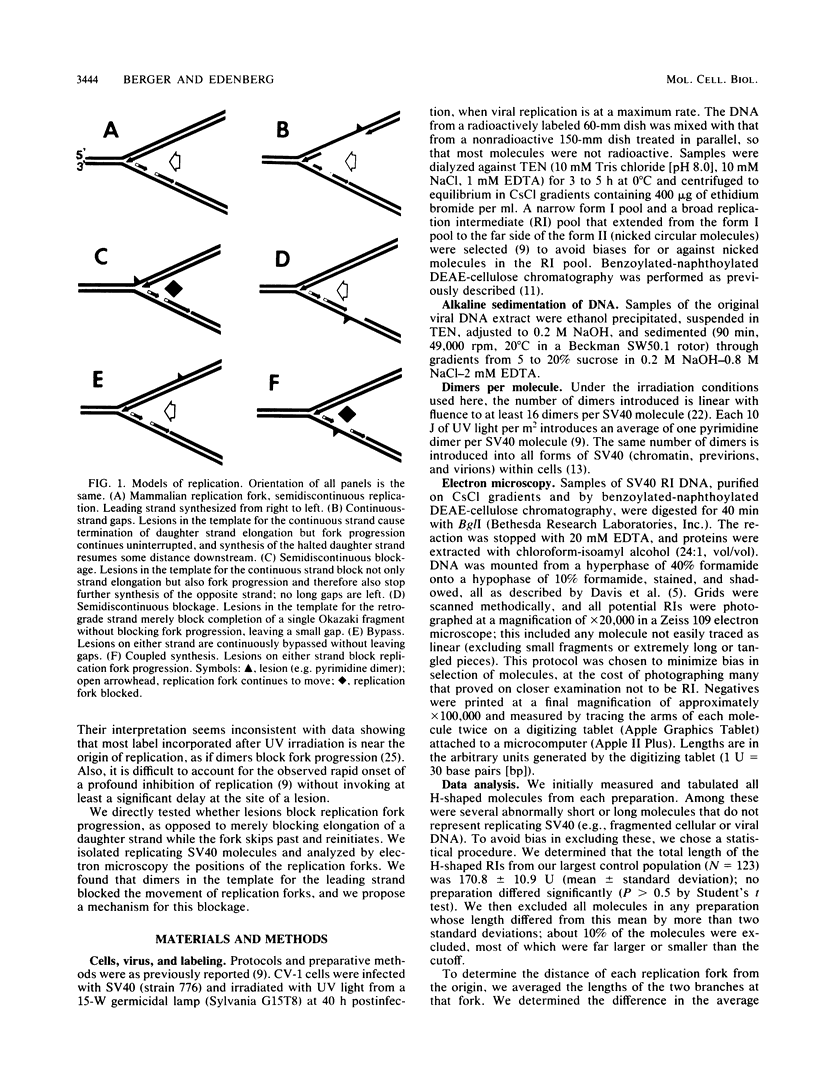
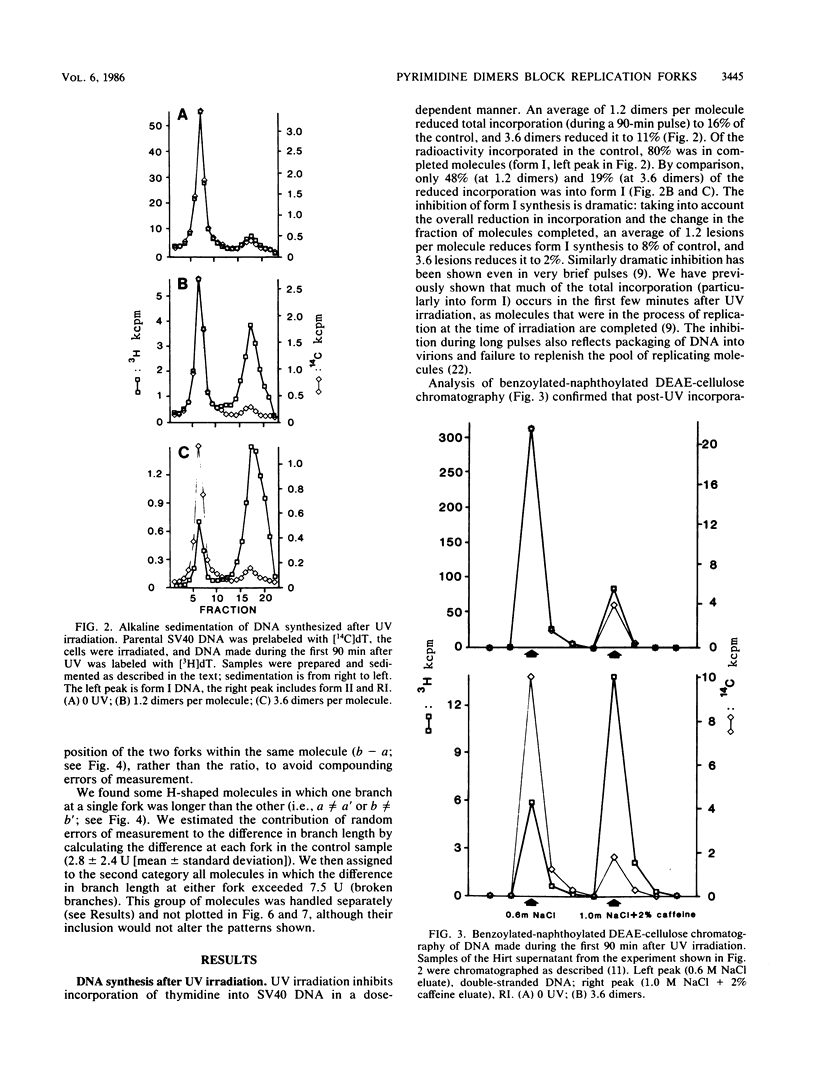
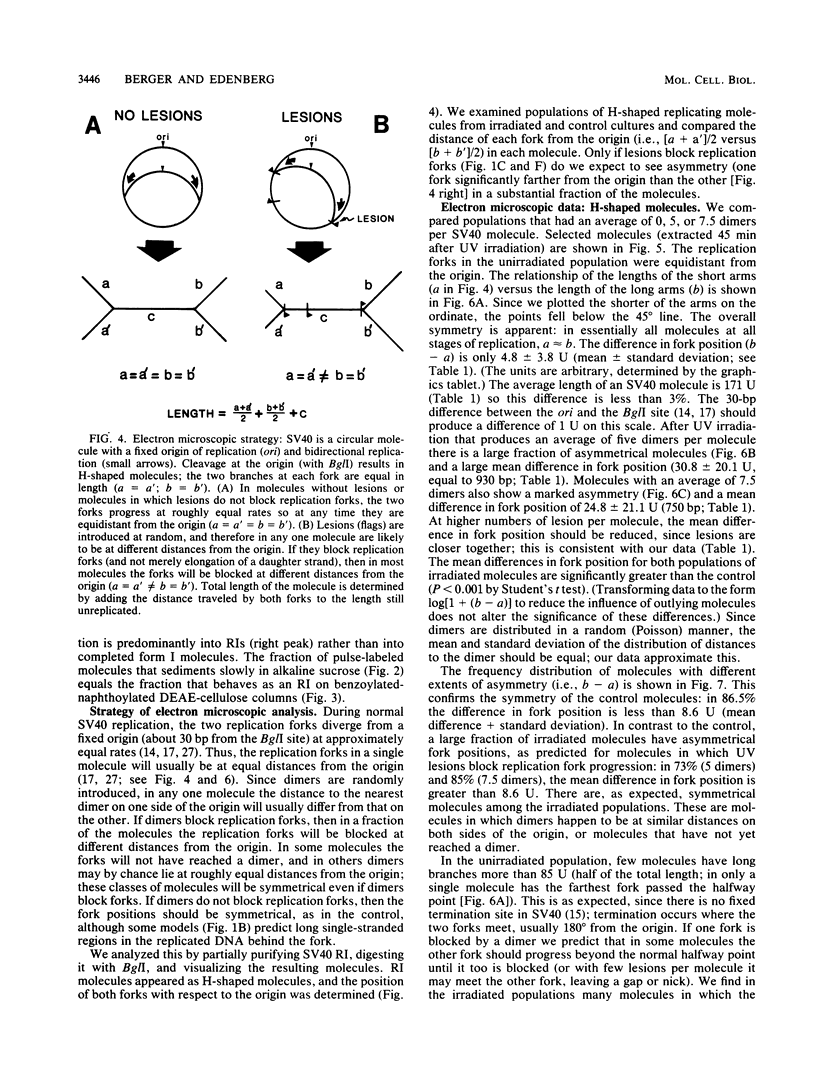
UV light produces lesions, predominantly pyrimidine dimers, which inhibit DNA replication in mammalian cells. The mechanism of inhibition is controversial: is synthesis of a daughter strand halted at a lesion while the replication fork moves on and reinitiates downstream, or is fork progression itself blocked for some time at the site of a lesion? We directly addressed this question by using electron microscopy to examine the distances of replication forks from the origin in unirradiated and UV-irradiated simian virus 40 chromosomes. If UV lesions block replication fork progression, the forks should be asymmetrically located in a large fraction of the irradiated molecules; if replication forks move rapidly past lesions, the forks should be symmetrically located. A large fraction of the simian virus 40 replication forks in irradiated molecules were asymmetrically located, demonstrating that UV lesions present at the frequency of pyrimidine dimers block replication forks. As a mechanism for this fork blockage, we propose that polymerization of the leading strand makes a significant contribution to the energetics of fork movement, so any lesion in the template for the leading strand which blocks polymerization should also block fork movement.
Full text
PDF
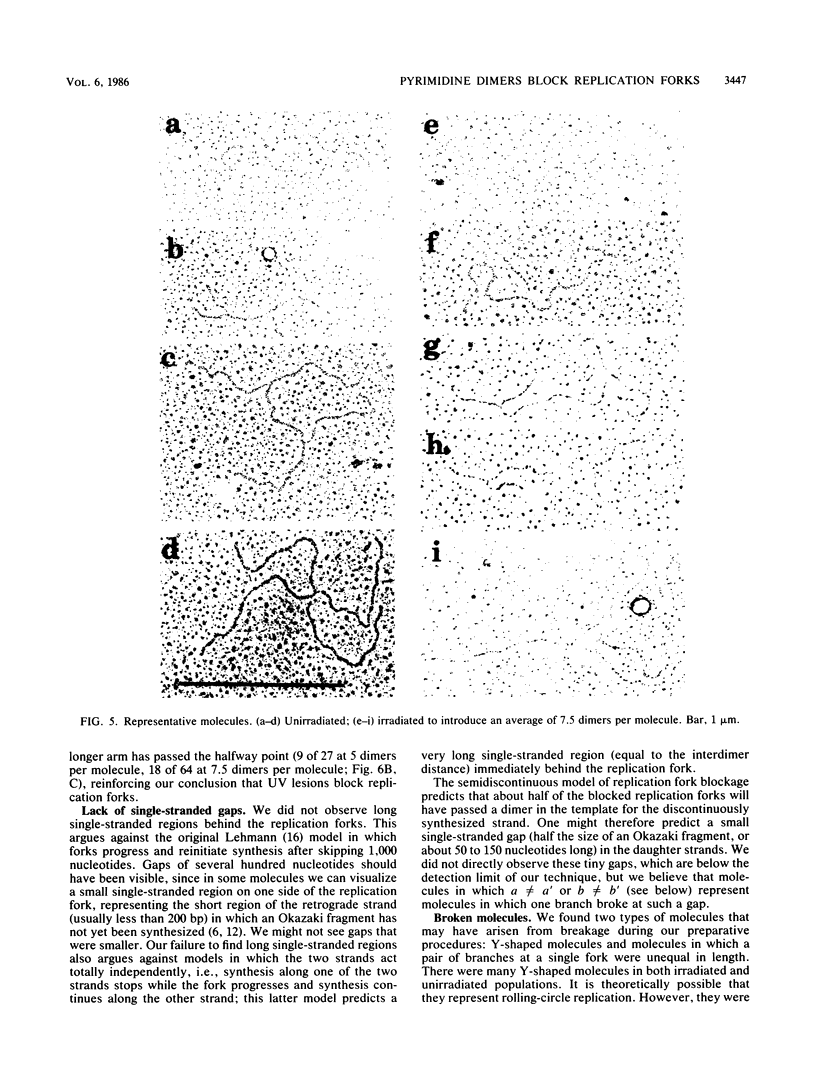
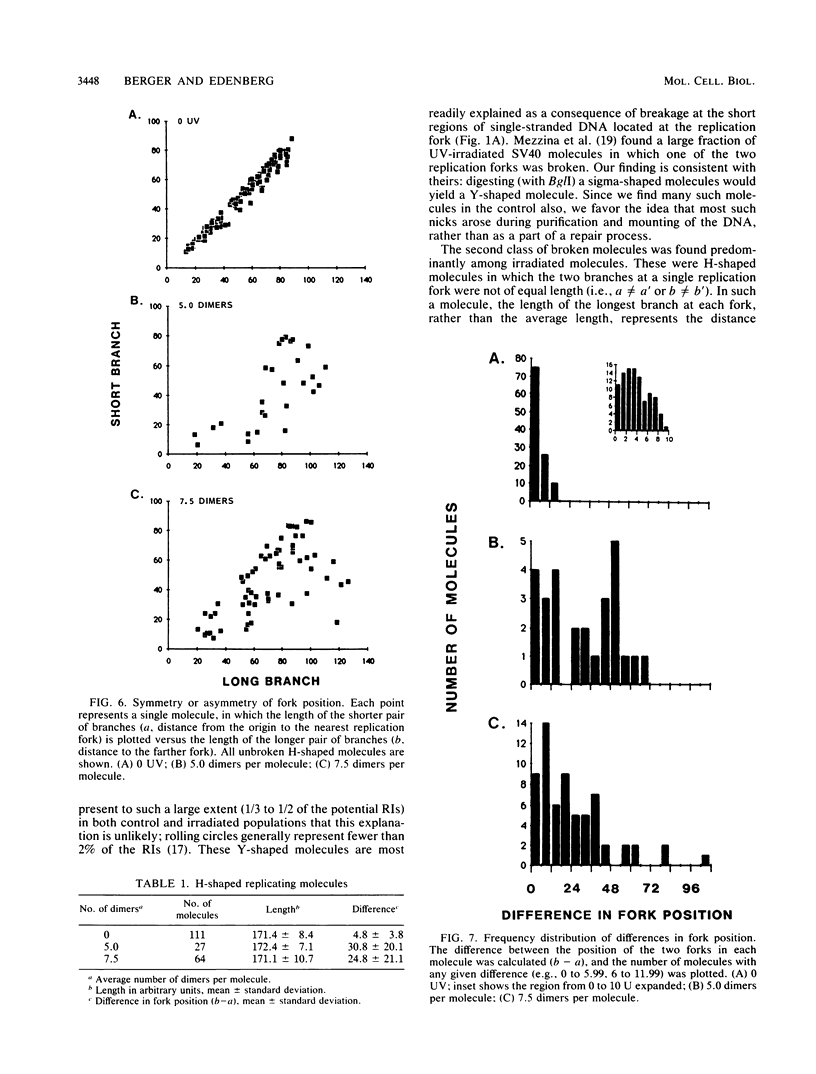


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts B. M. The DNA enzymology of protein machines. Cold Spring Harb Symp Quant Biol. 1984;49:1–12. doi: 10.1101/sqb.1984.049.01.003. [DOI] [PubMed] [Google Scholar]
- Barnett S. W., Landaw E. M., Dixon K. Test of models for replication of simian virus 40 DNA following ultraviolet irradiation. Biophys J. 1984 Sep;46(3):307–321. doi: 10.1016/S0006-3495(84)84027-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J. M., Hanawalt P. C. Replicative intermediates in UV-irradiated simian virus 40. Mutat Res. 1984 Jul-Aug;132(1-2):1–14. doi: 10.1016/0167-8817(84)90061-0. [DOI] [PubMed] [Google Scholar]
- Dahle D., Griffiths T. D., Carpenter J. G. Inhibition and recovery of DNA synthesis in UV-irradiated Chinese hamster V-79 cells. Photochem Photobiol. 1980 Aug;32(2):157–165. doi: 10.1111/j.1751-1097.1980.tb04003.x. [DOI] [PubMed] [Google Scholar]
- DePamphilis M. L., Wassarman P. M. Replication of eukaryotic chromosomes: a close-up of the replication fork. Annu Rev Biochem. 1980;49:627–666. doi: 10.1146/annurev.bi.49.070180.003211. [DOI] [PubMed] [Google Scholar]
- Edenberg H. J. Altered structure of ultraviolet-irradiated DNA: evidence for unwinding. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):379–382. doi: 10.1101/sqb.1983.047.01.044. [DOI] [PubMed] [Google Scholar]
- Edenberg H. J., Huberman J. A. Eukaryotic chromosome replication. Annu Rev Genet. 1975;9:245–284. doi: 10.1146/annurev.ge.09.120175.001333. [DOI] [PubMed] [Google Scholar]
- Edenberg H. J. Inhibition of DNA replication by ultraviolet light. Biophys J. 1976 Aug;16(8):849–860. doi: 10.1016/S0006-3495(76)85735-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg H. J. Inhibition of DNA synthesis by ultraviolet light. Basic Life Sci. 1975;5B:631–633. doi: 10.1007/978-1-4684-2898-8_34. [DOI] [PubMed] [Google Scholar]
- Edenberg H. J. Inhibition of simian virus 40 DNA replication by ultraviolet light. Virology. 1983 Jul 30;128(2):298–309. doi: 10.1016/0042-6822(83)90257-x. [DOI] [PubMed] [Google Scholar]
- Edenberg H. J., Roman A. Introduction of pyrimidine dimers into different intracellular forms of simian virus 40. Photochem Photobiol. 1983 Mar;37(3):297–299. doi: 10.1111/j.1751-1097.1983.tb04476.x. [DOI] [PubMed] [Google Scholar]
- Hay R. T., DePamphilis M. L. Initiation of SV40 DNA replication in vivo: location and structure of 5' ends of DNA synthesized in the ori region. Cell. 1982 Apr;28(4):767–779. doi: 10.1016/0092-8674(82)90056-3. [DOI] [PubMed] [Google Scholar]
- Lai C. J., Nathans D. Non-specific termination of simian virus 40 DNA replication. J Mol Biol. 1975 Sep 5;97(1):113–118. doi: 10.1016/s0022-2836(75)80026-x. [DOI] [PubMed] [Google Scholar]
- Lehmann A. R. Postreplication repair of DNA in ultraviolet-irradiated mammalian cells. J Mol Biol. 1972 May 28;66(3):319–337. doi: 10.1016/0022-2836(72)90418-4. [DOI] [PubMed] [Google Scholar]
- Martin R. G., Setlow V. P. The initiation of SV40 DNA synthesis is not unique to the replication origin. Cell. 1980 Jun;20(2):381–391. doi: 10.1016/0092-8674(80)90624-8. [DOI] [PubMed] [Google Scholar]
- Meneghini R. Gaps in DNA synthesized by ultraviolet light-irradiated WI38 human cells. Biochim Biophys Acta. 1976 Apr 2;425(4):419–427. doi: 10.1016/0005-2787(76)90006-x. [DOI] [PubMed] [Google Scholar]
- Mezzina M., Gentil A., Sarasin A. Simian virus 40 as a probe for studying inducible repair functions in mammalian cells. J Supramol Struct Cell Biochem. 1981;17(2):121–131. doi: 10.1002/jsscb.380170203. [DOI] [PubMed] [Google Scholar]
- Moore P. D., Bose K. K., Rabkin S. D., Strauss B. S. Sites of termination of in vitro DNA synthesis on ultraviolet- and N-acetylaminofluorene-treated phi X174 templates by prokaryotic and eukaryotic DNA polymerases. Proc Natl Acad Sci U S A. 1981 Jan;78(1):110–114. doi: 10.1073/pnas.78.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter R. B. Inhibition and recovery of DNA synthesis in human cells after exposure to ultraviolet light. Mutat Res. 1985 Jan-Mar;145(1-2):63–69. doi: 10.1016/0167-8817(85)90041-0. [DOI] [PubMed] [Google Scholar]
- Roman A., Edenberg H. J. Ultraviolet irradiation inhibits encapsidation of simian virus 40 chromatin. J Virol. 1981 Dec;40(3):729–734. doi: 10.1128/jvi.40.3.729-734.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp W. D., Howard-Flanders P. Discontinuities in the DNA synthesized in an excision-defective strain of Escherichia coli following ultraviolet irradiation. J Mol Biol. 1968 Jan 28;31(2):291–304. doi: 10.1016/0022-2836(68)90445-2. [DOI] [PubMed] [Google Scholar]
- Rupp W. D., Wilde C. E., 3rd, Reno D. L., Howard-Flanders P. Exchanges between DNA strands in ultraviolet-irradiated Escherichia coli. J Mol Biol. 1971 Oct 14;61(1):25–44. doi: 10.1016/0022-2836(71)90204-x. [DOI] [PubMed] [Google Scholar]
- Sarasin A. R., Hanawalt P. C. Replication of ultraviolet-irradiated simian virus 40 in monkey kidney cells. J Mol Biol. 1980 Apr;138(2):299–319. doi: 10.1016/0022-2836(80)90288-0. [DOI] [PubMed] [Google Scholar]
- Stacks P. C., White J. H., Dixon K. Accommodation of pyrimidine dimers during replication of UV-damaged simian virus 40 DNA. Mol Cell Biol. 1983 Aug;3(8):1403–1411. doi: 10.1128/mcb.3.8.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapper D. P., DePamphilis M. L. Preferred DNA sites are involved in the arrest and initiation of DNA synthesis during replication of SV40 DNA. Cell. 1980 Nov;22(1 Pt 1):97–108. doi: 10.1016/0092-8674(80)90158-0. [DOI] [PubMed] [Google Scholar]
- White J. H., Dixon K. Gap filling and not replication fork progression is the rate-limiting step in the replication of UV-damaged simian virus 40 DNA. Mol Cell Biol. 1984 Jul;4(7):1286–1292. doi: 10.1128/mcb.4.7.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. I., Cleaver J. E. Perturbations in simian virus 40 DNA synthesis by ultraviolet light. Mutat Res. 1978 Dec;52(3):301–311. doi: 10.1016/0027-5107(78)90169-0. [DOI] [PubMed] [Google Scholar]