Abstract
Our previous studies showed that an extract from Camellia sinenesis (green tea), which contains several polyphenols, attenuates nephrotoxicity caused by cyclosporine A (CsA). Since polyphenols are stimulators of mitochondrial biogenesis (MB), this study investigated whether stimulation of MB plays a role in green tea polyphenol protection against CsA renal toxicity. Rats were fed a powdered diet containing green tea polyphenolic extract (0.1%) starting 3 days prior to CsA treatment (25 mg/kg, i.g. daily for 3 weeks). CsA alone decreased renal nuclear DNA-encoded oxidative phosphorylation (OXPHOS) protein ATP synthase-β (AS-β) by 42%, mitochondrial DNA (mtDNA)-encoded OXPHOS protein NADH dehydrogenase-3 (ND3) by 87% and their associated mRNAs. Mitochondrial DNA copy number was also decreased by 78% by CsA. Immunohistochemical analysis showed decreased cytochrome c oxidase subunit IV (COX-IV), an OXPHOS protein, in tubular cells. Peroxisome proliferator-activated receptor-γ coactivator (PGC)-1α, the master regulator of MB, and mitochondrial transcription factor-A (Tfam), the transcription factor that regulates mtDNA replication and transcription, were 42% and 90% lower, respectively, in the kidneys of CsA-treated than in untreated rats. These results indicate suppression of MB by chronic CsA treatment. Green tea polyphenols alone and following CsA increased AS-β, ND3, COX-IV, mtDNA copy number, PGC-1α mRNA and protein, decreased acetylated PGC-1α, and increased Tfam mRNA and protein. In association with suppressed MB, CsA increased serum creatinine, caused loss of brush border and dilatation of proximal tubules, tubular atrophy, vacuolization, apoptosis, calcification, and increased neutrophil gelatinase-associated lipocalin expression, leukocyte infiltration, and renal fibrosis. Green tea polyphenols markedly attenuated CsA-induced renal injury and improved renal function. Together, these results demonstrate that green tea polyphenols attenuate CsA-induced kidney injury, at least in part, through the stimulation of MB.
Introduction
Cyclosporin A (CsA) is an important immunosuppressive agent. Even with the development of new immunosuppressants, CsA is still widely used after organ transplantation and for treatment of autoimmune diseases [1]–[3]. Immunosuppressive therapy with CsA is always long-term and results in a number of side effects, the most frequent and severe being nephrotoxicity (e.g. renal dysfunction in up to 30% of patients) [4]–[7].
The mechanisms by which CsA causes nephrotoxicity are not well understood but are thought in part due to calcineurin inhibition [8]. CsA causes acute reversible nephrotoxicity as well as chronic, irreversible nephrotoxicity [7]. Acute CsA renal toxicity is linked to increased renal vascular resistance due to increased vasoconstrictors, decreased vasodilators, activation of renal nerves, and mesangial cell contraction, hypoxia/reperfusion (I/R) and free radical production [5], [7], [9]–[13]. Upregulation of toll-like receptors (TLR) and TNF-α is also involved in CsA nephrotoxicity [14]. Chronic CsA causes decreases of glomerular filtration rates, tubulointerstitial injury, apoptosis, tubular microcalcification, arteriolar hyalinosis, fibrosis, and focal glomerular sclerosis [7]. Mechanisms of CsA chronic damage are less clear compared to the acute nephrotoxicity [7]. Cyclosporine A also upregulates TGF-β expression [15].
Energy supply is essential for cell survival and function. Mitochondrial dysfunction is a common cause of drug/toxicant-induced organ injury and CsA has profound effects on mitochondria. At low concentrations/doses, CsA inhibits the opening of the mitochondrial permeability transition (MPT) pores by binding to cyclophilin D in the matrix and the inner membrane of mitochondria, protecting against I/R injury [16]–[18]. However, at high concentrations/doses, CsA inhibits mitochondrial respiration and decreases ATP production in vivo and in vitro [19]–[21]. It is suspected that poor adaptation to altered mitochondrial energy metabolism is linked to organ vulnerability to CsA toxicity [19]. Mitochondrial biogenesis (MB) is an important adaptation, counteracting mitochondrial dysfunction/toxicity. Calcineurin plays an important role in the expression of peroxisome proliferator-activated receptor-γ coactivator (PGC)-1α [22]–[24], the master regulator of MB. Whereas CsA is a potent calcineurin inhibitor. it is possible that CsA suppresses MB to induce nephrotoxicity.
Green tea polyphenols are free radical and singlet oxygen scavengers. Beneficial effects of green tea polyphenols in the prevention/treatment of cardiovascular, hepatic, renal, neural, pulmonary and intestinal diseases, cancer, diabetes, arthritis, shock, and decreases in ischemia/reperfusion injury and drug/chemical toxicity in various organs/tissues have been widely reported and many of these effects are presumably due to their antioxidant and anti-inflammatory properties [25]–[40]. Our previous study showed that a Camellia sinenesis (green tea) extract, which contains high levels of plant polyphenols (e.g. epigallocatechin gallate, epigallocatechin, epicatechin, and catechin), attenuated CsA nephrotoxicity, in part, by scavenging free radicals [31]. Recent studies showed that isoflavones are effective MB stimulators and improve mitochondrial function after renal I/R injury, diabetes, chronic heart failure, and aging [41]–[45]. Stimulation of MB increases mitochondrial proteins and mass, improves function, and is an attractive strategy for promoting cell repair and regeneration, preserving organ function and treating a number of pathologies resulting from damage/inhibition of mitochondrial function [42]–[50]. Thus, this study was designed to explore the effects of green tea polyphenols on renal MB after chronic CsA treatment.
Materials and Methods
Cyclosporin A and Polyphenol Treatments
CsA (Sandimmune oral solution) was obtained from Novartis (Basel, Switzerland). Green tea extracts, produced by Taiyo Kagaku Co. (Yokkaichi, Mie, Japan), contained 85% polyphenols by weight. Components of polyphenols in the extract was determined by high performance liquid chromatography (HPLC) as described previously [31] and included epigallocatechin gallate (47.2% of total polyphenols), epigallocatechin (11.0%), gallocatechin gallate (11.0%), epicatechin gallate (10.8%), gallocatechin (8.6%), epicatechin (8.4%), and catechin (3.0%).
Male Sprague-Dawley rats (200–250 g) were fed a semi-synthetic powdered diet (AIN 76, Dyets Inc. Bethlehem, PA) containing 0% or 0.1% green tea polyphenolic extract starting 3 days prior to and lasting throughout CsA or vehicle treatment. Previous studies showed that 0.1% green tea extract in the diet blunted CsA renal toxicity, hepatic I/R injury, cholestatic liver fibrosis and inhibited development of renal cell tumors in rats [29], [31], [40], [51]. Daily food consumption was not different between the groups with or without green tea polyphenolic extract addition. Average polyphenolic extract intake calculated by the daily food consumption and polyphenolic content in the diet was ∼80 mg/kg/day. CsA oral solution or its vehicle (Cremophor EL 20 mg/ml in 12.5% dehydrated alcohol) was further diluted in olive oil. Previous studies have shown that higher doses of CsA are required in rats to cause renal damages that are similar to the damages observed in humans [6], [11], [12]. Therefore, CsA (25 mg/kg, 0.25 mL/100 g body weight) or an equivalent volume of vehicles was gavaged daily for 21 days.
Serum Creatinine, Renal Histology and Immunohistological Staining
At 21 days after CsA treatment, rats were anesthetized with pentobarbital (50 mg/kg, i.p), and blood was collected from the vena cava. Serum creatinine was determined using a kit from Sigma-Aldrich Co. (St. Louis, MO). The left kidney was rinsed with 5 ml normal saline, perfusion-fixed with 10% formaldehyde in phosphate buffered saline, then removed and placed in the same fixative for 48 h. Sections were stained with hematoxylin-eosin (H&E) and analyzed microscopically for pathological changes. Apoptosis was assessed by terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) using an in situ cell death detection kit from Roche Diagnostics (Penzberg, Germany) [52]. Tubules in the cortex with and without injury (vacuolization, loss of brush border, dilatation, necrosis, atrophy, and calcification), leukocytes, and TUNEL-positive cells were counted in a blinded manner in 10 randomly selected fields per slide under a Nikon Optiphot-2 microscope (Nikon Instruments Inc., Melville, NY) using a 40x objective lens after H&E and TUNEL staining, respectively. Renal fibrosis was detected using the Masson’s Trichrome staining. Immunohistological staining of cytochrome c oxidase subunit IV (COX-IV), a nuclear DNA (nDNA)-encoded mitochondrial oxidative phosphorylation (OXPHOS) protein, was performed as described elsewhere [53] using a specific antibody against COX-IV at a dilution of 1∶200.
Detection of Mitochondrial DNA (mtDNA) Copy Number, ATP Synthase-β (AS-β), NADH Dehydrogenase-3 (ND3), PGC-1α, and Mitochondrial Transcription Factor A (Tfam) mRNAs by Quantitative Real-time PCR (qPCR)
Relative quantities of mtDNA content in the kidney were determined using qPCR [46]. Total genomic DNA was extracted using a DNeasy Blood and Tissue kit (Qiagen, Valencia, CA). Mitochondrial DNA copy number was assessed by quantification of mtDNA-encoded NADH dehydrogenase-1 (ND1) gene using a CFX96 Real Time-PCR Detection System (Bio-Rad, Hercules, CA) and normalized against the nuclear-encoded POU class 5 homeobox 1 (Pou5f1) gene. Primer sequences used are listed in Table 1.
Table 1. Real-Time PCR Primers.
| DNAs/mRNAs | Primers | |
| AS-β | Forward: | 5′- TTG CTG AGG TCT TCA CAG GTC ACA-3′ |
| Reverse: | 5′- CAG CCT TTG CCA CAG CTT CTT CAA-3′ | |
| ND1 | Forward: | 5′- TTA ATT GCC ATG GCC TTC CTC ACC-3′ |
| Reverse: | 5′- TGG TTA GAG GGC GTA TGG GTT CTT-3′ | |
| ND3 | Forward: | 5′- CAA CAA GTT CTG CAC GCC TTC CTT-3′ |
| Reverse: | 5′- TTG TTT GAA TCG CTC ATG GGA GGG-3′ | |
| Tfam | Forward: | 5′- GAT GAG TCA CCT CAA GGG AAA TTG-3′ |
| Reverse: | 5′- GTC ATC TAG TAA AGC CCG GAA GGT-3′ | |
| Pou5f1 | Forward: | 5′- AGG TGT TCA GCC AGA CAA CCA TCT-3′ |
| Reverse: | 5′- TCT CGT TGT TGT CAG CTT CCT CCA-3′ | |
| HPRT | Forward | 5′- TCG AAG TGT TGG ATA CAG GCC AGA-3′ |
| Reverse: | 5′-TAC TGG CCA CAT CAA CAG GAC TCT-3′ |
AS-β, ATP synthase-β; ND1, NADH dehydrogenase-1; ND3, NADH dehydrogenase-3; Tfam, mitochondrial transcription factor-A; Pou5f1, POU class 5 homeobox 1; HPRT, hypoxanthine phospho-ribosyl-transferase.
Quantitative real-time PCR of mRNAs was performed as described elsewhere [54]. After total RNA isolation from kidney tissue with Trizol (Invitrogen, Grand Island, NY), single stranded cDNAs were synthesized from RNA (2 µg) using a Bio-Rad iScript cDNA Synthesis kit (Bio-Rad, Hercules, CA). Quantitative real-time PCR was conducted using the primer sequences in Table 1. The abundance of mRNAs was normalized against hypoxanthine phospho-ribosyl-transferase (HPRT) using the ΔΔCt method.
Immunoprecipitation of PGC-1α
Immunoprecipitation was performed as described elsewhere [55]. Kidneys were homogenized and extracted in ice-cold lysing buffer. Immunoprecipitations were carried out with protein lysate (500 µg protein as determined by the Bradford assay) and PGC-1α antibody (5 µg) using a Catch and Release v2.0 Reversible Immunoprecipitation System (Millipore, Billerica, MA). Protein content in the immunoprecipitates was determined by the Bradford assay. Acetylated lysine residues and PGC-1α were determined by immunoblotting [54] using corresponding specific antibodies (Cell Signaling Technology, Danvers, MA and Santa Cruz Biotech., Santa Cruz, CA, respectively).
Immunoblotting
Proteins in renal tissue extracts were detected by immunoblot analysis as previously described [54] using primary antibodies specific for AS-β, neutrophil gelatinase-associated lipocalin (NGAL), Tfam (GenWay Biotech, San Diego, CA), cleaved caspase-3 (Cell Signaling Technology, Danvers, MA), ND3 and PGC-1α (Santa Cruz Biotech., Santa Cruz, CA) at concentrations of 1∶100 to 1000, and actin (ICN, Costa Mesa, CA) at a concentration of 1∶3000 at 4°C over night, respectively. Horseradish peroxidase-conjugated secondary antibodies were applied afterwards, and detection was by chemiluminescence (Pierce Biotec., Rockford, IL).
Statistical Analysis
Groups were compared using ANOVA plus a Student-Newman-Keuls posthoc test. There were 4 rats per group for all parameters. Data shown are means±S.E.M. Differences were considered significant at p<0.05.
Ethics Statement
All animals were given humane care in compliance with institutional guidelines using protocols approved by the Institutional Animal Care and Use Committee of the University of North Carolina. All surgery was performed under sodium pentobarbital anesthesia (50 mg/kg, i.p.).
Results
CsA Decreases mtDNA Copy Number in the Kidney: Reversal by Green Tea Polyphenols
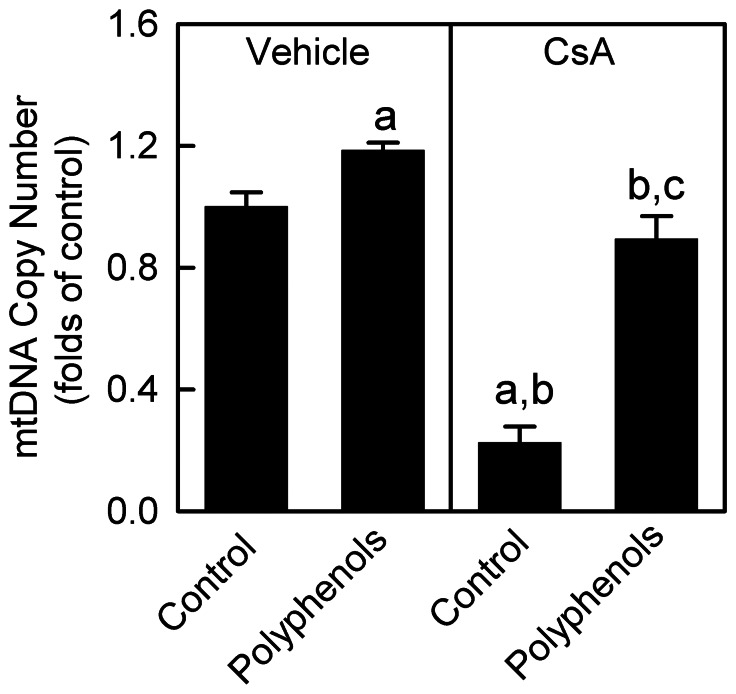
CsA treatment causes changes in high-energy phosphate homeostasis in tissues [19]–[21]. Mitochondrial DNA is responsible for synthesis of crucial mitochondrial OXPHOS proteins, and proper function of mitochondrial respiration requires an adequate copy number of mtDNA per cell [49], [56]. Therefore, we examined the alterations in mtDNA in the kidney after vehicle and CsA treatment. Renal mtDNA copy number decreased by 78% after chronic CsA treatment (Fig. 1). Polyphenols increased mtDNA copy number by 19% in rats treated with the vehicle and recovered mtDNA copy number to ∼90% of control levels after chronic CsA treatment (Fig. 1).
Figure 1. Decreases of mitochondrial DNA copy numbers by cyclosporin A (CsA): reversal by green tea polyphenols.
Rats were fed semi-synthetic powdered diets containing 0% (control) or 0.1% polyphenol extracts (polyphenols) starting 3 days prior to CsA treatment (25 mg/kg, i.g. for 3 weeks). Renal mitochondrial DNA copy numbers were determined by qPCR. a, p<0.05 vs. control diet+vehicle; b, p<0.05 vs. polyphenol-containing diet+vehicle; c, p<0.05 vs. control diet+CsA (n = 4 per group).
CsA Decreases Mitochondrial OXPHOS Proteins in the Kidney: Reversal by Green Tea Polyphenols
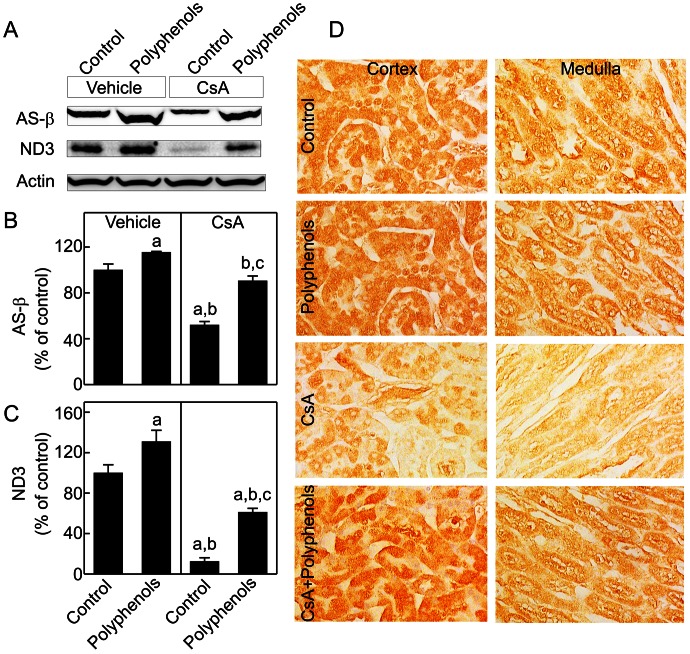
The majority of mitochondrial proteins are encoded by nuclear DNA (nDNA) [56], [57]. We examined a subunit of F0F1ATPase, AS-β, that is encoded by nDNA after chronic CsA treatment. CsA decreased AS-β by 48% (Fig. 2A,B). Polyphenols increased AS-β 15% above control levels in the kidneys from vehicle-treated rats and recovered AS-β to ∼91% of control levels in the kidneys from CsA-treated rats. ND3, a mtDNA-encoded mitochondrial OXPHOS protein, decreased 88% after chronic CsA treatment (Fig. 2A,C). Polyphenols increased ND3 to 31% above control levels in the kidneys of vehicle-treated rats and recovered ND3 to 61% of control levels in the kidneys of CsA-treated rats (Fig. 2A,C).
Figure 2. Decreases of mitochondrial oxidative phosphorylation proteins by CsA: reversal by green tea polyphenols.
Rats were fed semi-synthetic powdered diets containing 0% (Control) or 0.1% polyphenol extracts (Polyphenols) starting 3 days prior to CsA treatment (25 mg/kg, i.g. for 3 weeks). Renal mitochondrial oxidative phosphorylation proteins ATP synthase-β (AS-β, 55 kDa) and NADH dehydrogenase-3 (ND3, 24 kDa) were determined by immunoblotting and representative images are shown in A. AS-β (B) and ND3 (C) were quantified by densitometry. a, p<0.05 vs. control diet+vehicle; b, p<0.05 vs. polyphenol-containing diet+vehicle; c, p<0.05 vs. control diet+CsA (n = 4 per group). Cytochrome c oxidase subunit IV was detected by immunohistochemistry (D). Representative images of 4 rats per group are shown.
Expression of COX-IV, a nDNA-encoded mitochondrial OXPHOS protein, was observed in various renal cells by immunohistochemical staining (Fig. 2D). In control rats, immunoreactivity of COX-IV was localized throughout the tubular cells in kidney cortex and the medulla, particularly in the proximal tubules. COX-IV staining in tubular cells was punctate, consistent with mitochondrial localization. In contrast, immunoreactivity of COX-IV was low in glomeruli (data not shown). After CsA treatment, COX-IV immunoreactivity decreased substantially in tubular cells in the cortex and medulla. Polyphenols increased COX-IV immunoreactivity in tubular cells after CsA treatment.
CsA Decreases AS-β and ND3 mRNAs in the Kidney: Recovery by Green Tea Polyphenols
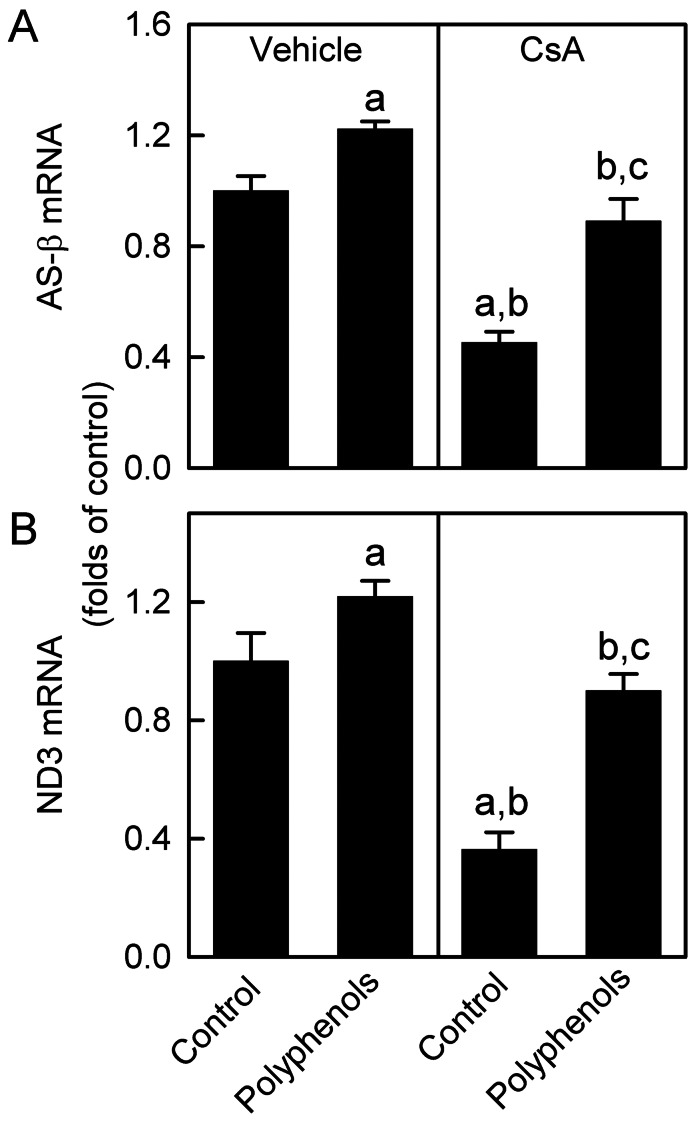
Since AS-β and ND3 were decreased by CsA, we investigated the mRNA levels of these proteins. AS-β mRNA decreased 55% by CsA treatment (Fig. 3A). Polyphenols increased AS-β mRNA 22% above control levels in the kidneys of vehicle-treated rats and recovered AS-β mRNA to 89% of control values in the kidneys of CsA-treated rats. Renal ND3 mRNA was 64% lower in CsA-treated rats compared to vehicle-treated rats (Fig. 3B). ND3 mRNA increased 21% in vehicle-treated rats and recovered to ∼90% of control values in CsA-treated rats. Together, we suggest that decreases in AS-β and ND3 proteins are due, at least in part, to suppression of their mRNAs and these effects were reversed by green tea polyphenols.
Figure 3. Decreases of ATP synthase-β and NADH dehydrogenase-3 mRNAs by CsA: reversal by green tea polyphenols.
Rats were fed semi-synthetic powdered diets containing 0% (Control) or 0.1% polyphenol extracts (Polyphenols) starting 3 days prior to CsA treatment (25 mg/kg, i.g. for 3 weeks). Renal ATP synthase-β (AS-β) and NADH dehydrogenase-3 (ND3) mRNAs determined by qPCR are shown in A and B, respectively. a, p<0.05 vs. control diet+vehicle; b, p<0.05 vs. polyphenol-containing diet+vehicle; c, p<0.05 vs. control diet+CsA (n = 4 per group).
Green Tea Polyphenols Increase PGC-1α mRNA and Activation after CsA Treatment
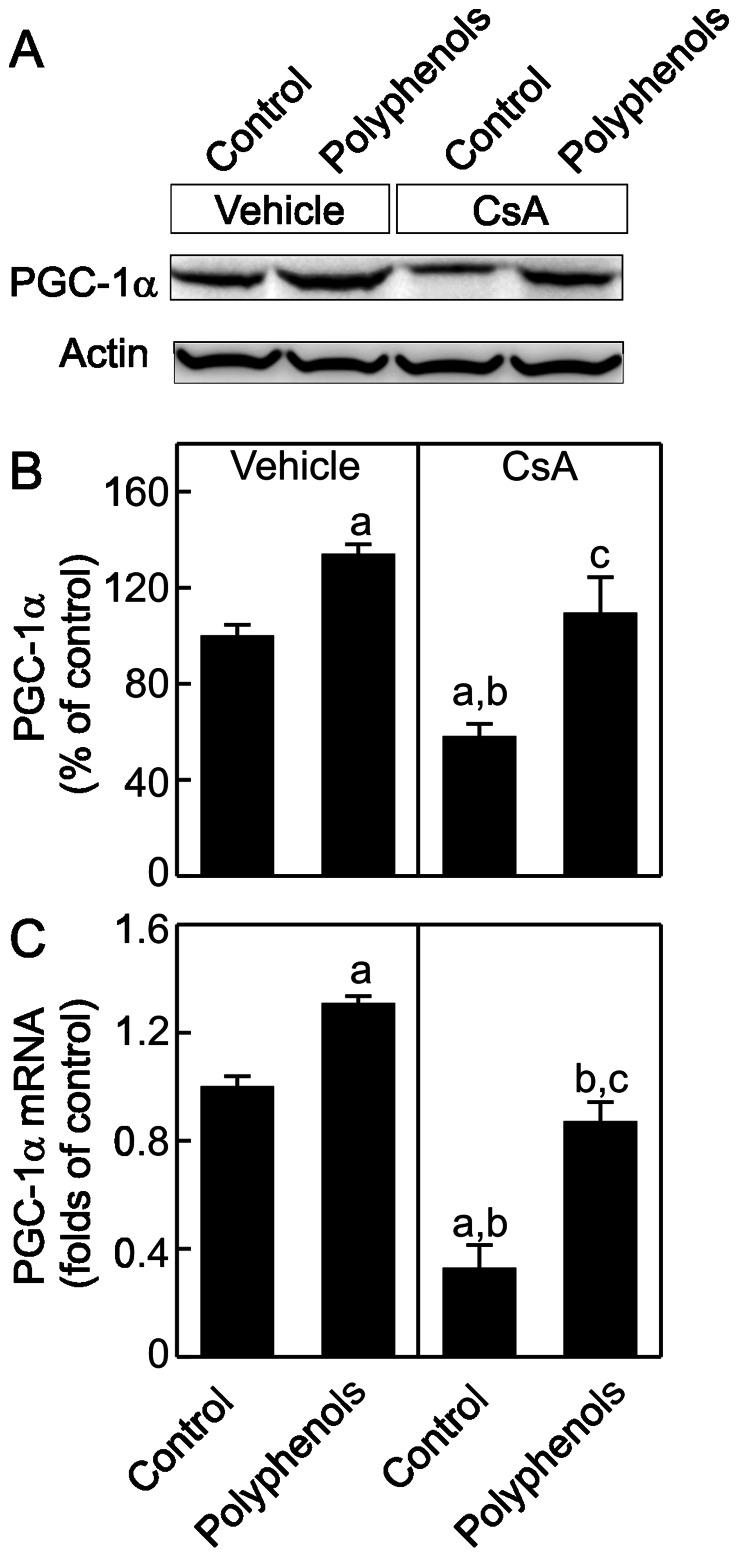
PGC-1α plays a key role in the control of MB and mtDNA maintenance [58]. We investigated whether CsA alters PGC-1α levels in the kidney. PGC-1α was 42% lower in the kidneys of CsA-treated than vehicle-treated rats (Fig. 4A and B). Polyphenols increased PGC-1α by 34% in the kidneys of vehicle-treated rats and increased PGC-1α to control levels in the kidneys of CsA-treated rats. We further examined whether polyphenols increased PGC-1α mRNA. CsA decreased PGC-1α mRNA by 67% (Fig. 4C). Polyphenols slightly increased PGC-1α mRNA in the kidneys of vehicle-treated rats and recovered PGC-1α mRNA in the kidneys of CsA-treated rats to 87% of control levels.
Figure 4. Suppression of peroxisome-proliferator-activated receptor gamma coactivator-1α (PGC-1α) expression by CsA: reversal by green tea polyphenols.
Rats were fed semi-synthetic powdered diets containing 0% (Control) or 0.1% polyphenol extracts (Polyphenols) starting 3 days prior to CsA treatment (25 mg/kg, i.g. for 3 weeks). Renal PGC-1α (90 kDa) and actin (43 kDa) were determined by immunoblotting. Representative images are shown in A and quantification by densitometry is shown in B. PGC-1α mRNA was determined by qPCR (C). a, p<0.05 vs. control diet+vehicle; b, p<0.05 vs. polyphenol-containing diet+vehicle; c, p<0.05 vs. control diet+CsA (n = 4 per group).
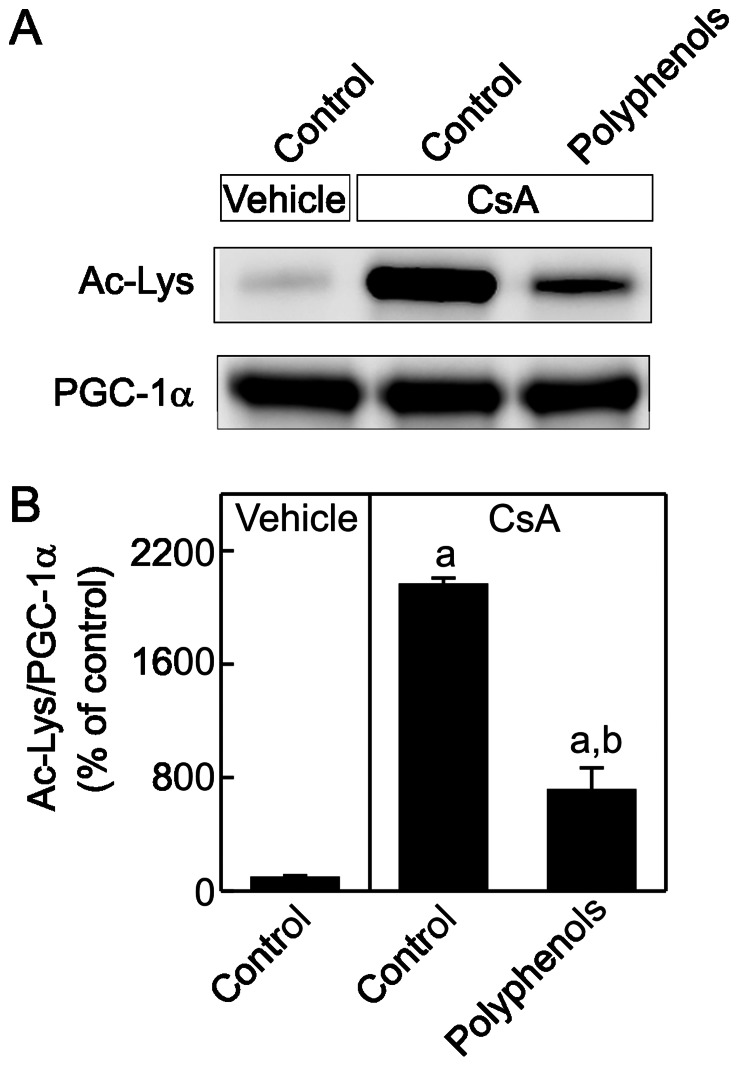
PGC-1α activity is higher after de-acetylation [46]. PGC-1α was immunoprecipitated and acetylated lysine residues were detected by immunoblotting (Fig. 5A and B). Acetylation of PGC-1α was increased substantially after chronic CsA treatment and polyphenols decreased acetylation of PGC-1α, indicating enhanced PGC-1α activation.
Figure 5. Suppression of peroxisome proliferator-activated receptor gamma coactivator-1α (PGC-1α) activation by CsA: reversal by green tea polyphenols.
Rats were fed semi-synthetic powdered diets containing 0% (Control) or 0.1% polyphenol extracts (Polyphenols) starting 3 days prior to CsA treatment (25 mg/kg, i.g. for 3 weeks). Renal PGC-1α was immunoprecipitated, equally loaded, and acetylated lysine (Ac-Lys) and PGC-1α were determined by immunoblotting. Representative images are shown in A and quantification by densitometry is shown in B. a, p<0.05 vs. control diet+vehicle; b, p<0.05 vs. polyphenol-containing diet+vehicle; c, p<0.05 vs. control diet+CsA (n = 4 per group).
Green Tea Polyphenols Increase Tfam Synthesis after CsA Treatment
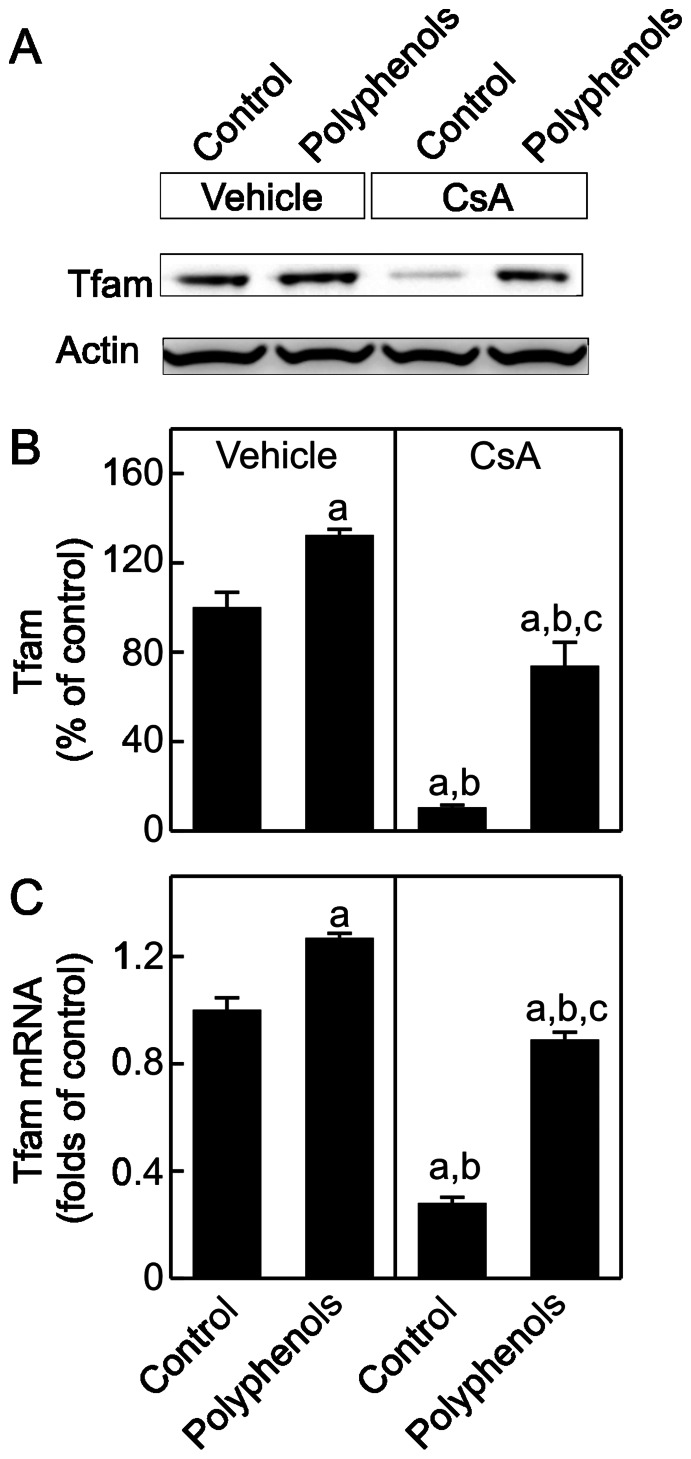
Tfam is a transcription factor that regulates the replication and transcription of the mitochondrial genome, thus playing a critical role in controlling MB [59]. Tfam decreased by almost 90% after CsA treatment (Fig. 6A and B), consistent with decreased mtDNA copy number and mtDNA-encoded ND3 transcription. Expression of Tfam is controlled by PGC-1α [59]. Consistent with decreased PGC-1α expression and activity, Tfam mRNA also decreased after CsA treatment (Fig. 6C), indicating suppressed Tfam transcription. Polyphenols elevated renal Tfam mRNA and protein modestly in vehicle-treated rats and largely reversed the decreases of renal Tfam mRNA and protein in CsA-treated rats.
Figure 6. Suppression of mitochondrial transcription factor A (Tfam) expression by CsA: reversal by green tea polyphenols.
Rats were fed semi-synthetic powdered diets containing 0% (Control) or 0.1% polyphenol extracts (Polyphenols) starting 3 days prior to CsA treatment (25 mg/kg, i.g. for 3 weeks). Renal Tfam protein (30 kDa) was determined by immunoblotting. Representative images are shown in A and quantification by densitometry is shown in B. Tfam mRNA was determined by qPCR (C). a, p<0.05 vs. control diet+vehicle; b, p<0.05 vs. polyphenol-containing diet+vehicle; c, p<0.05 vs. control diet+CsA (n = 4 per group).
Green Tea Polyphenols Attenuate Kidney Injury and Improve Kidney Function after CsA Treatment
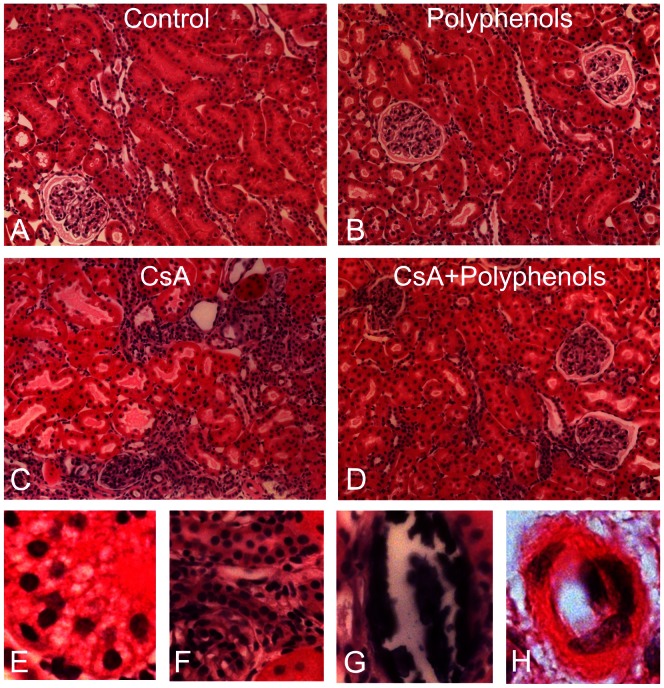
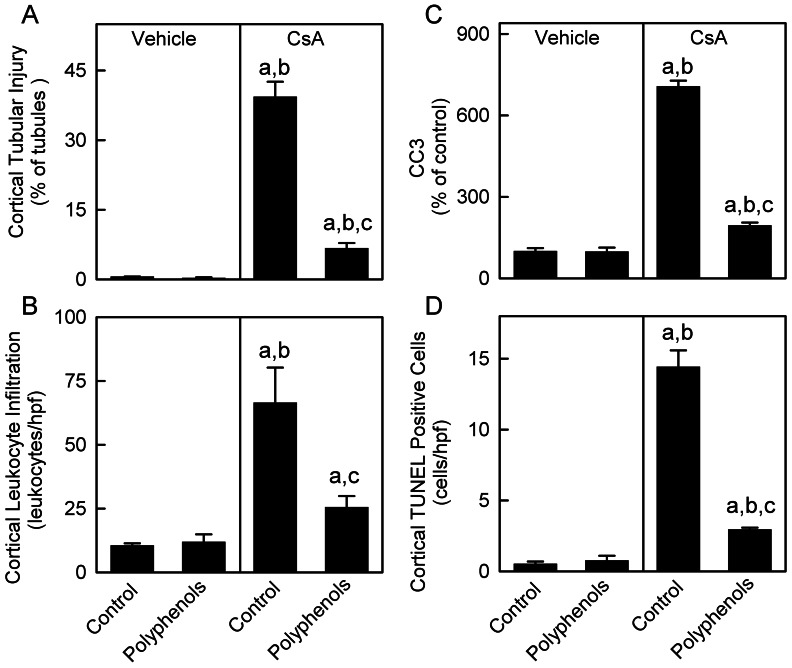
Renal histology was examined after treatment with CsA for 3 weeks (Fig. 7). The kidneys of rats on the control and polyphenol diets that received vehicle treatment exhibited normal histology (A and B). CsA treatment caused a loss of brush border and dilatation of proximal tubules (C), tubular atrophy (C and F), vacuolization (E), calcification (G), cast formation, arteriolar hyalinosis (H) and leukocyte infiltration, most overtly in the cortex, consistent with previous reports [31], [60]. Pathological changes occurred in 39% of tubules in the cortex and leukocytes increased from 10/high power field (hpf) to 66/hpf after CsA treatment (Fig. 8A,B). Polyphenols decreased tubular injury after CsA treatment to 7% and leukocyte to 25/hpf (Fig. 8 A,B).
Figure 7. Dietary polyphenols minimize CsA-induced pathological changes in the kidney.
Rats were fed semi-synthetic powdered diets containing 0% (Control) or 0.1% polyphenol extracts (Polyphenols) starting 3 days prior to CsA treatment (25 mg/kg, i.g. for 3 weeks). Representative images of H&E-stained kidney sections are shown. A, control diet plus vehicle; B, 0.1% polyphenols plus vehicle; C, E, F, G, and H, control diet plus CsA; D, polyphenols plus CsA. E, tubular cell vacuolization; F, tubular atrophy; G, calcification; H, arteriolar hyalinosis.
Figure 8. Dietary polyphenols minimize CsA-induced tubular injury and leukocyte infiltration in the cortex.
Rats were fed semi-synthetic powdered diets containing 0% (Control) or 0.1% polyphenol extracts (Polyphenols) starting 3 days prior to CsA treatment (25 mg/kg, i.g. for 3 weeks). Tubules with and without injury, leukocytes, and TUNEL-positive cells in the cortex were counted in a blinded manner in 10 randomly selected fields per slide using a 40x objective lens after H&E and TUNEL staining, respectively. Percentages of tubules with injury are shown in A. The average numbers of leukocytes and TUNEL-positive cells per high power field (hpf) are shown in B and D, respectively. Cleaved caspase-3 (CC3) in kidney tissue was detected by immunoblotting (17 kDa) and quantified by densitometry (C). a, p<0.05 vs. control diet+vehicle; b, p<0.05 vs. polyphenol-containing diet+vehicle; c, p<0.05 vs. control diet+CsA (n = 4 per group).
Cleaved caspase-3 was barely detectable in the kidneys from vehicle-treated rats with or without polyphenol treatment. CsA increased cleaved caspase about 7-fold (Fig. 8C) and this effect was largely blunted by polyphenols. TUNEL-positive cells in the cortex were 0.5–0.8/hpf in vehicle-treated rats with or without polyphenol treatment. TUNEL-positive cells increased to ∼14/hpf after CsA treatment in the absence of polyphenols and was decreased to 3/hpf in the presence of polyphenols (Fig. 8D). These data show that CsA caused apoptosis and this effect was blunted by polyphenols.
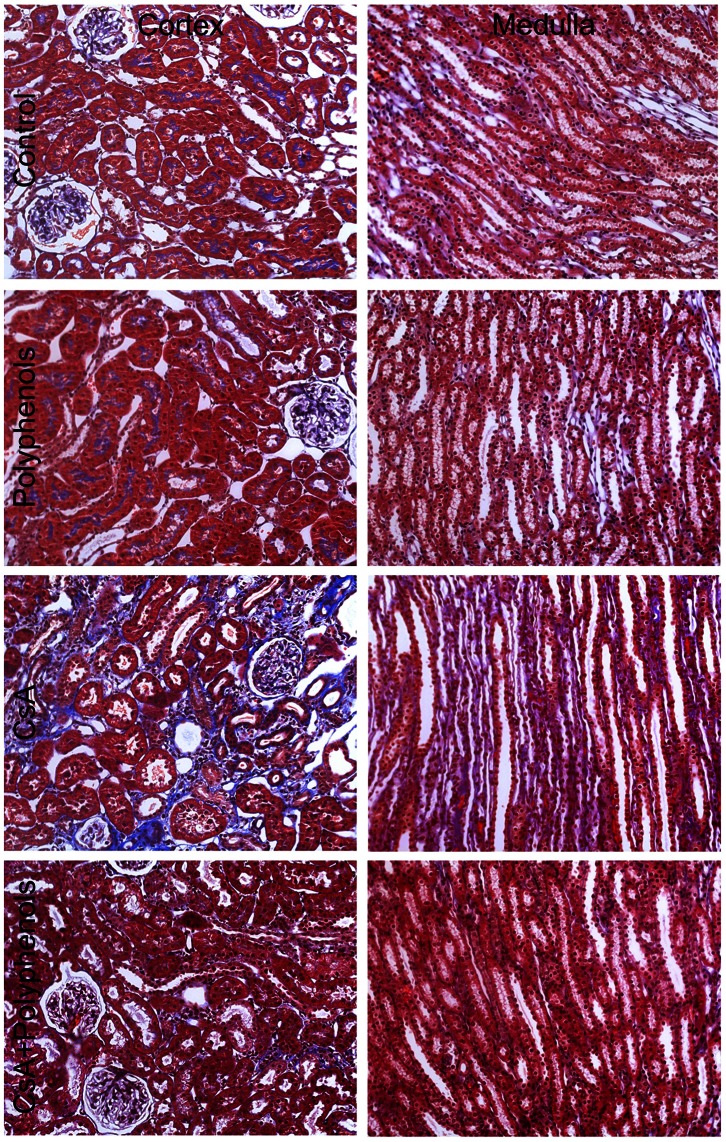
Renal fibrosis was revealed using Masson’s Trichrome staining (Fig. 9). In the kidneys from vehicle-treated rats with or without polyphenol treatment, Trichrome staining was rare in the interstitium. A small amount of blue Trichrome staining appeared in the brush borders, perhaps reflecting the microfilaments in the microvilli of tubular cells. After CsA treatment, blue staining in the brush borders of tubular cells disappeared and was replaced with wide-spread interstitial fibrosis, most overtly in the cortex but also observable in the medulla. Thickening of Bowman’s capsule also occurred in some glomeruli. Renal fibrosis was blunted markedly by polyphenols.
Figure 9. Dietary polyphenols attenuate CsA-induced renal fibrosis.
Rats were fed semi-synthetic powdered diets containing 0% (Control) or 0.1% polyphenol extracts (Polyphenols) starting 3 days prior to CsA treatment (25 mg/kg, i.g. for 3 weeks). Representative images of Trichrome-stained kidney sections are shown. 1st row, control diet plus vehicle; 2nd row, 0.1% polyphenols plus vehicle; 3rd row, control diet plus CsA; 4th row, polyphenols plus CsA (n = 4 per group).
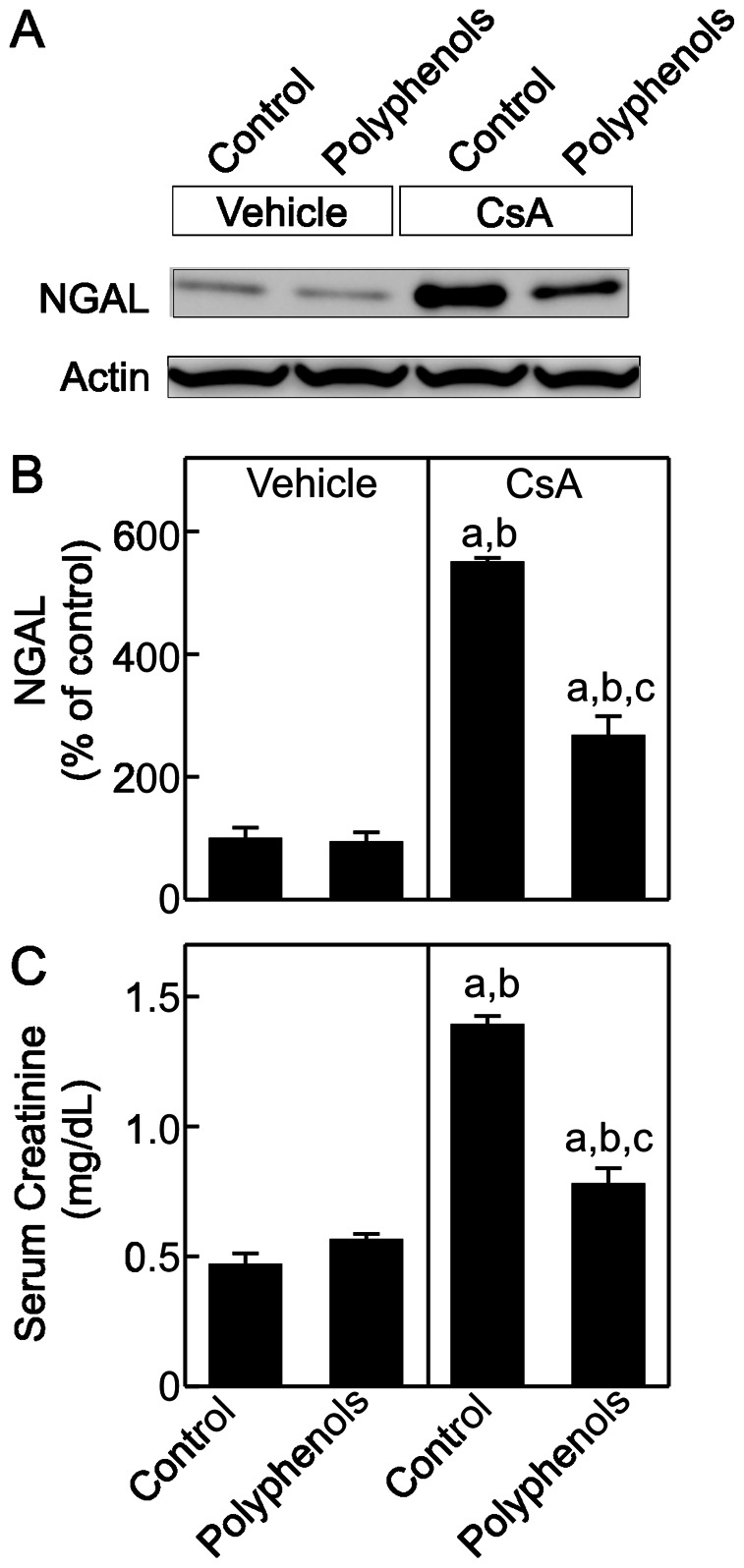
Neutrophil gelatinase-associated lipocalin (NGAL), a sensitive marker of acute kidney injury and a potential indicator of chronic kidney disease progression [61]–[64], was barely detectable in vehicle-treated rats with or without polyphenol treatment. NGAL increased by 5.5-fold after CsA treatment (Fig. 10 A and B). This effect was blunted by polyphenols.
Figure 10. Polyphenols attenuate kidney injury and improve renal function after CsA treatment.
Rats were fed semi-synthetic powdered diets containing 0% (Control) or 0.1% polyphenol extracts (Polyphenols) starting 3 days prior to CsA treatment (25 mg/kg, i.g. for 3 weeks). Neutrophil gelatinase-associated lipocalin (NGAL, 23 kDa) and actin in renal tissue were determined by immunoblotting. Representative images are shown in A and quantification by densitometry is shown in B. Serum creatinine was determined colorimetrically (C). a, p<0.05 vs. control diet+vehicle; b, p<0.05 vs. polyphenol-containing diet+vehicle; c, p<0.05 vs. control diet+CsA (n = 4 per group).
Serum creatinine was 0.47 mg/dL in vehicle-treated rats and was not altered by polyphenols alone (Fig. 10C). After CsA treatment, serum creatinine increased to 1.4 mg/dL and polyphenols decreased serum creatinine to 0.78 mg/dL in CsA-treated rats. Together, CsA-induced pathological changes in the kidney were consistent with tubulointerstitial injury and these effects were markedly attenuated by polyphenols.
Discussion
Suppression of MB by CsA Treatment
The calcineurin inhibitor CsA is the basis for many immunosuppressive protocols, but its adverse effects (i.e. severe nephrotoxicity) are a major barrier in long-term immunosuppressive therapy [4], [5], [7]. The mechanisms of CsA nephrotoxicity are not well understood. CsA at high concentrations inhibits respiration and damages proteins and lipids in isolated mitochondria [17], [65], [66]. After in vivo treatment CsA decreases ATP levels in the rat kidney [19], [21]. Decreased oxidative phosphorylation could cause cell damage and inhibit renal function. Another important side effect of CsA, neurotoxicity, is linked to decreased mitochondrial metabolism [20], [67].
Mitochondrial homeostasis is crucial for maintaining proper energy supply and function of tissues. The abundance of mitochondria in a cell is determined by biogenesis, fission/fusion, and mitophagy [56]. When increased tissue energy demand exceeds mitochondrial ATP-producing capacity (e.g. exercise) or replacement of damaged mitochondria is needed, MB is stimulated. Mitochondria cannot be made de novo but require synthesis of new organelle constituents and the integration of these components (i.e., proteins and lipids) into preexisting mitochondria. MB requires synthesis and import of nDNA-encoded OXPHOS proteins (e.g. AS-β and COX-IV) into mitochondria as well as expression of mtDNA-encoded OXPHOS proteins (e.g. ND3) [56], [68], [69]. Suppression of MB could sensitize a tissue to toxicants and diseases.
A previous study showed that low dose of CsA protected against doxorubicin-induced heart dysfunction but did not alter MB [70]. In contrast, we observed that after chronic CsA treatment, mtDNA copy number was decreased substantially (Fig. 1), which was accompanied by a marked reduction in nDNA- and mtDNA-encoded OXPHOS proteins and their associated mRNAs (Figs. 2–3). Taken together, these data are consistent with the conclusion that renal MB is suppressed after CsA treatment.
CsA Treatment Decreases PGC-1α Expression and Activity
MB is tightly regulated by a signaling system connecting different pathways [49], [71]–[74]. The transcriptional coactivators (PGC-1α and β) and the PGC-1-related coactivator (PRC) modulate the expression of target genes encoding OXPHOS enzymes [58], [75], [76]. PGC-1α induces and coordinates expression of nuclear regulatory proteins (e.g. nuclear respiratory factor (NRF)-1 and NRF-2) that activate target genes encoding OXPHOS proteins, PGC-1α itself, and Tfam. Tfam is a transcription factor that acts on the promoters within the non-coding (D-loop) region of mtDNA and regulates the replication and transcription of the mitochondrial genome [59], [77]. Over-expression of PGC-1α leads to mitochondrial proliferation in the heart, adipocytes, myoblasts and renal proximal tubular cells [47], [50], [76], [78], [79]. AMP-activated kinase (AMPK), sirtuin 1 (SIRT1), nitric oxide and cGMP, and other signaling kinases (e.g. Ca2+/calmodulin-stimulated protein kinase (CaMK), p38 MAPK and protein kinase C) regulate PGC-1α expression and/or activity [80]–[84].
Studies showed that calcineurin activation increases PGC-1α gene transcription [22]. Also, over-expression of constitutively active calcineurin in mouse skeletal muscle or cardiac myocytes leads to increased expression of PGC-1α [23], [24]. A coordinate increase of PGC-1α and its downstream transcription factors as well as gene expression of mitochondrial proteins were observed in association with calcineurin activation in human muscle after long term exercise [85]. PGC-1α and calcineurin activation also play an important role in MB in both healthy and diseased human skeletal muscles [85], [86]. Calcineurin stimulates members of the myocyte enhancer factor 2 (MEF2) family of transcription factors which bind to and activate the PGC-1α promoter and enhance a positive feedback loop between PGC-1α and MEF2 in muscle [22], [87]. In contrast, little is known concerning the effects of calcineurin inhibition on MB and mitochondrial homeostasis. In this study chronic exposure to CsA altered PGC-1α signaling in the kidney by decreasing PGC-1α protein and mRNA, and increasing PGC-1α acetylation (Figs. 4–5). PGC-1α also controls the expression of Tfam and Tfam decreased after CsA treatment (Fig. 6), consistent with decreased mtDNA copy number and suppressed expression of mtDNA-encoded ND3 (Figs. 1 and 3). In total, these data are consistent with PGC-1α depletion mediated suppression of MB. Because mitochondria are abundant in proximal tubular cells, decreases in mitochondrial OXPHOS proteins and pathological changes after CsA treatment were most overt in these cells (Figs. 2, 7 and 8).
Green Tea Polyphenols Enhance MB after CsA Treatment
Stimulation of MB could counteract disease- or toxicant-induced mitochondrial suppression, enhance recovery of mitochondrial function, decrease tissue injury and promote tissue repair and regeneration. Some natural and synthetic molecules have been found to stimulate MB, such as small molecule SIRT1 activators, nitric oxide, isoflavones, β2-adrenergic receptor agonists, AMPK activators, cAMP and cGMP analogues, and 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane [41], [46], [88]–[92]. Epicatechin improves MB and attenuates mitochondrial dysfunction in rodents and in patients with diabetes and chronic heart failure [42], [43]. Epicatechin also enhances fatigue resistance and oxidative capacity in aged mouse muscle [44]. Here we investigated the effect of green tea polyphenols, which was shown to protect against CsA nephrotoxicity [31], on MB. Polyphenols increased molecules regulating MB (PGC-1α, Tfam), mtDNA and OXPHOS proteins in control and CsA-treated rats, and these effects were associated with decreased kidney injury and improved renal function after CsA treatment (Figs. 7, 8, 9, 10). Therefore, in addition to their effects as antioxidants, green tea polyphenols may also protect and/or promote renal function by stimulating MB.
Interestingly, in addition to increasing PGC-1α mRNA and protein, polyphenols also increased PGC-1α activation as indicated by decreased acetylated PGC-1α (Fig. 5). This reduction in acetylated PGC-1α was not due to decreased PGC-1α protein since PGC-1α was equally loaded in the gels for immunoblotting after immunoprecipitation. A similar effect was observed in isoflavone-treated renal proximal tubular cells, isoflavones increased activity and protein content of SIRT1, a member of the histone deacetylase (HDAC) family [41]. Green tea polyphenol epigallocatechin gallate also increases HDAC activity and HDAC-2 expression in regulatory T cells [93]. Thus polyphenols may increase SIRT1 activity, decrease PGC-1α protein acetylation and increase PGC1α transcription.
A recent study showed that green tea polyphenols can bind to and stimulate a 67-kDa laminin receptor, leading to activation of NADPH oxidase and generation of reactive oxygen species in PC12 cells subjected to oxygen-glucose deprivation [94]. Red wine polyphenols at a low concentration but not at a high concentration stimulated MB and angiogenesis, and this effect depended on the estrogen receptor-α activation [95]. However, it is unclear whether estrogen receptor-α activation by red wine polyphenols is a direct or indirect effect. As discussed above, isoflavones have been shown to increase SIRT1 activity and protein content and may induced MB through this mechanism. In the present study, polyphenols increased MB in tubular cells which originally have abundant mitochondria but did not increase MB in glomeruli which have lower levels of mitochondria, and this stimulation of MB depended on the PGC-1α signaling pathway. Whether polyphenols act directly or indirectly to increase MB and whether this stimulation of MB requires binding of polyphenols to a specific receptor remain to be investigated.
The green tea extract contains several polyphenols. The major polyphenol in the extract was epigallocatechin gallate (∼50%). Our previous studies showed that epicatechin and epicatechin gallate had similar protective effects on liver ischemia/reperfusion injury and liver transplantation as green tea extracts containing multiple polyphenol components [29], [30]. In some other studies, the relative activities of the various polyphenolic components to inhibit oxidation and injury were variable [96]. It was also shown that a combination of epigallocatechin gallate, epicatechin gallate, epigallocatechin, and epicatechin in the molar ratio 5∶2:2∶1 provided optimal protective effects against lipid peroxidation [97]. Studies should be performed in the future to evaluate the efficacies of each polyphenolic component and various combinations of polyphenols on MB in cultured renal cells and in vivo.
Conclusion
Taken together, MB is suppressed in the kidney after chronic CsA treatment, which may contribute to the development of CsA nephrotoxicity. Green tea polyphenols protect against CsA nephrotoxicity, at least in part, by enhancing MB.
Funding Statement
This study was supported, in part, by Grants DK70844, DK37034, and GM 084147 from the National Institutes of Health and South Carolina COBRE in Oxidants, Redox Balance and Stress Signaling [P20 GM103542]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Borel JF, Feurer C, Gubler HU, Stahelin H (1976) Biological effects of cyclosporin A: a new antilymphocytic agent. Agents Actions 6: 468–475. [DOI] [PubMed] [Google Scholar]
- 2. Halloran PF (1996) Molecular mechanisms of new immunosuppressants. Clin Transplant 10: 118–123. [PubMed] [Google Scholar]
- 3. Margreiter R, Huber C, Spielberger M, Konig P (1983) Cyclosporine in the treatment of acute cadaveric kidney graft rejection refractory to high-dose methylprednisolone. Transplantation 36: 203–204. [DOI] [PubMed] [Google Scholar]
- 4. Sibley RK, Rynasiewicz J, Ferguson RM, Fryd D, Sutherland DE, et al. (1983) Morphology of cyclosporine nephrotoxicity and acute rejection in patients immunosuppressed with cyclosporine and prednisone. Surgery 94: 225–234. [PubMed] [Google Scholar]
- 5. Naesens M, Kuypers DR, Sarwal M (2009) Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol 4: 481–508 4/2/481 [pii];10.2215/CJN.04800908 [doi]. [DOI] [PubMed] [Google Scholar]
- 6. Farthing MJG, Clark ML (1981) Nature of the toxicity of cyclosporin A in the rat. Biochem Pharmacol 24: 3311–3316. [DOI] [PubMed] [Google Scholar]
- 7. Tedesco D, Haragsim L (2012) Cyclosporine: a review. J Transplant 2012: 230386 10.1155/2012/230386 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Williams D, Haragsim L (2006) Calcineurin nephrotoxicity. Adv Chronic Kidney Dis 13: 47–55 S1548-5595(05)00202-8 [pii];10.1053/j.ackd.2005.11.001 [doi] [DOI] [PubMed] [Google Scholar]
- 9. Textor SC, Burnett JC, Romero JC, Canzanello VJ, Taler SJ, et al. (1995) Urinary endothelin and renal vasoconstriction with cyclosporine or FK506 after liver transplantation. Kidney Int 47: 1426–1433. [DOI] [PubMed] [Google Scholar]
- 10. Barros EJG, Boim MA, Ajzen H, Ramos OL, Schor N (1987) Glomerular hemodynamics and hormonal participation in cyclosporine nephrotoxicity. Kidney Int 32: 19–25. [DOI] [PubMed] [Google Scholar]
- 11. Zhong Z, Connor HD, Yin M, Moss N, Mason RP, Bunzendahl H, et al. (1999) Dietary glycine and renal denervation prevents cyclosporin A-induced hydroxyl radical production in rat kidney. Mol Pharmacol 56: 455–463. [DOI] [PubMed] [Google Scholar]
- 12. Zhong Z, Arteel GE, Connor H, Yin M, Frankenberg Mv, et al. (1998) Cyclosporin A increases hypoxia and free radical production in the rat kidney: prevention by dietary glycine. Am J Physiol 275: F595–F604. [DOI] [PubMed] [Google Scholar]
- 13. Diederich D, Skopec J, Diederich A, Dai FX (1994) Cyclosporine produces endothelial dysfunction by increased production of superoxide. Hypertension 23: 957–961. [DOI] [PubMed] [Google Scholar]
- 14.Lim SW, Li C, Ahn KO, Kim J, Moon IS, et al.. (2005) Cyclosporine-induced renal injury induces toll-like receptor and maturation of dendritic cells. Transplantation 80: 691–699. 00007890-200509150-00023 [pii]. [DOI] [PubMed]
- 15. Shehata M, Cope GH, Johnson TS, Raftery AT, el Nahas AM (1995) Cyclosporine enhances the expression of TGF-beta in the juxtaglomerular cells of the rat kidney. Kidney Int 48: 1487–1496. [DOI] [PubMed] [Google Scholar]
- 16. Halestrap AP, Davidson AM (1990) Inhibition of Ca2(+)-induced large-amplitude swelling of liver and heart mitochondria by cyclosporin is probably caused by the inhibitor binding to mitochondrial-matrix peptidyl-prolyl cis-trans isomerase and preventing it interacting with the adenine nucleotide translocase. Biochem J 268: 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fournier N, Ducet G, Crevat A (1999) Action of cyclosporine on mitochondrial calcium fluxes. J Bioenergetics Biomembranes 19: 297–303. [DOI] [PubMed] [Google Scholar]
- 18. Zoratti M, Szabo I (1995) The mitochondrial permeability transition. Biochim Biophys Acta 1241: 139–176. [DOI] [PubMed] [Google Scholar]
- 19. Serkova N, Klawitter J, Niemann CU (2003) Organ-specific response to inhibition of mitochondrial metabolism by cyclosporine in the rat. Transpl Int 16: 748–755 10.1007/s00147-003-0631-1 [doi]. [DOI] [PubMed] [Google Scholar]
- 20. Serkova N, Jacobsen W, Niemann CU, Litt L, Benet LZ, et al. (2001) Sirolimus, but not the structurally related RAD (everolimus), enhances the negative effects of cyclosporine on mitochondrial metabolism in the rat brain. Br J Pharmacol 133: 875–885 10.1038/sj.bjp.0704142 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Niemann CU, Saeed M, Akbari H, Jacobsen W, Benet LZ, et al. (2002) Close association between the reduction in myocardial energy metabolism and infarct size: dose-response assessment of cyclosporine. J Pharmacol Exp Ther 302: 1123–1128 10.1124/jpet.102.036848 [doi]. [DOI] [PubMed] [Google Scholar]
- 22. Handschin C, Rhee J, Lin J, Tarr PT, Spiegelman BM (2003) An autoregulatory loop controls peroxisome proliferator-activated receptor gamma coactivator 1alpha expression in muscle. Proc Natl Acad Sci U S A 100: 7111–7116 10.1073/pnas.1232352100 [doi];1232352100 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ryder JW, Bassel-Duby R, Olson EN, Zierath JR (2003) Skeletal muscle reprogramming by activation of calcineurin improves insulin action on metabolic pathways. J Biol Chem 278: 44298–44304 10.1074/jbc.M304510200 [doi];M304510200 [pii]. [DOI] [PubMed] [Google Scholar]
- 24. Schaeffer PJ, Wende AR, Magee CJ, Neilson JR, Leone TC, et al. (2004) Calcineurin and calcium/calmodulin-dependent protein kinase activate distinct metabolic gene regulatory programs in cardiac muscle. J Biol Chem 279: 39593–39603 10.1074/jbc.M403649200 [doi];M403649200 [pii]. [DOI] [PubMed] [Google Scholar]
- 25.Biswas S, Hwang JW, Kirkham PA, Rahman I (2012) Pharmacologial and Dietary Antioxidant Therapies for Chronic Obstructive Pulmonary Disease. Curr Med Chem. CMC-EPUB-20120903-23 [pii]. [DOI] [PubMed]
- 26.Kishimoto Y, Tani M, Kondo K (2013) Pleiotropic preventive effects of dietary polyphenols in cardiovascular diseases. Eur J Clin Nutr. ejcn201329 [pii];10.1038/ejcn.2013.29 [doi]. [DOI] [PubMed]
- 27.Recio MC, Andujar I, Rios JL (2012) Anti-inflammatory agents from plants: progress and potential. Curr Med Chem 19: 2088–2103. CMC-EPUB-20120313-004 [pii]. [DOI] [PubMed]
- 28.Hugel HM, Jackson N (2012) Redox chemistry of green tea polyphenols: therapeutic benefits in neurodegenerative diseases. Mini Rev Med Chem 12: 380–387. MRMC-EPUB-20120203-003 [pii]. [DOI] [PubMed]
- 29. Zhong Z, Froh M, Connor HD, Li X, Conzelmann LO, et al. (2002) Prevention of hepatic ischemia-reperfusion injury by green tea extract. Am J Physiol Gastrointest Liver Physiol 283: G957–G964. [DOI] [PubMed] [Google Scholar]
- 30. Zhong Z, Connor HD, Froh M, Bunzendahl H, Lind H, et al. (2005) Free radical-dependent dysfunction of small-for-size rat liver grafts: prevention by plant polyphenols. Gastroenterology 129: 652–664. [DOI] [PubMed] [Google Scholar]
- 31. Zhong Z, Connor HD, Li X, Mason RP, Forman DT, et al. (2006) Reduction of ciclosporin and tacrolimus nephrotoxicity by plant polyphenols. J Pharm Pharmacol 58: 1533–1543 10.1211/jpp.58.11.0015 [doi]. [DOI] [PubMed] [Google Scholar]
- 32. Darvesh AS, Bishayee A (2013) Chemopreventive and therapeutic potential of tea polyphenols in hepatocellular cancer. Nutr Cancer 65: 329–344 10.1080/01635581.2013.767367 [doi]. [DOI] [PubMed] [Google Scholar]
- 33. Kada T, Kaneko K, Matsuzaki S, Matsuzaki T, Hara Y (1985) Detection and chemical identification of natural bio-antimutagens. A case of the green tea factor. Mutat Res 150: 127–132. [DOI] [PubMed] [Google Scholar]
- 34.Frankel EN (1999) Natural phenolic antioxidants and their impact on health. In: Lester P, Hiramastu M, Yoshikawa T, editors. Antioxidant Food Supplements in Human Health. San Diego: Academic Press. pp. 385–392.
- 35. Vinson JA (2000) Black and green tea and heart disease: a review. Biofactors 13: 127–132. [DOI] [PubMed] [Google Scholar]
- 36. Lin AM, Chyi BY, Wu LY, Hwang LS, Ho LT (1998) The antioxidative property of green tea against iron-induced oxidative stress in rat brain. Chin J Physiol 41: 189–194. [PubMed] [Google Scholar]
- 37. Miyagawa C, Wu C, Kennedy DO, Nakatani T, Ohtani K, et al. (1997) Protective effect of green tea extract and tea polyphenols against the cytotoxicity of 1,4-naphthoquinone in isolated rat hepatocytes. Biosci Biotechnol Biochem 61: 1901–1905. [DOI] [PubMed] [Google Scholar]
- 38. Hara Y (1994) Antioxidative action of tea polyphenols: Part 1. Am Biotechnol Lab 12: 48. [PubMed] [Google Scholar]
- 39. Yoshino M, Murakami K (1998) Interaction of iron with polyphenolic compounds: application to antioxidant characterization. Anal Biochem 257: 40–44. [DOI] [PubMed] [Google Scholar]
- 40. Zhong Z, Froh M, Lehnert M, Schoonhoven R, Yang L, et al. (2003) Polyphenols from Camellia sinenesis attenuate experimental cholestasis-induced liver fibrosis in rats. Am J Physiol Gastrointest Liver Physiol 285: G1004–G1013. [DOI] [PubMed] [Google Scholar]
- 41. Rasbach KA, Schnellmann RG (2008) Isoflavones promote mitochondrial biogenesis. J Pharmacol Exp Ther 325: 536–543 jpet.107.134882 [pii];10.1124/jpet.107.134882 [doi]. [DOI] [PubMed] [Google Scholar]
- 42. Taub PR, Ramirez-Sanchez I, Ciaraldi TP, Perkins G, Murphy AN, et al. (2012) Alterations in skeletal muscle indicators of mitochondrial structure and biogenesis in patients with type 2 diabetes and heart failure: effects of epicatechin rich cocoa. Clin Transl Sci 5: 43–47 10.1111/j.1752-8062.2011.00357.x [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yan J, Feng Z, Liu J, Shen W, Wang Y, et al. (2012) Enhanced autophagy plays a cardinal role in mitochondrial dysfunction in type 2 diabetic Goto-Kakizaki (GK) rats: ameliorating effects of (-)-epigallocatechin-3-gallate. J Nutr Biochem 23: 716–724 S0955-2863(11)00111-2 [pii];10.1016/j.jnutbio.2011.03.014 [doi]. [DOI] [PubMed] [Google Scholar]
- 44. Nogueira L, Ramirez-Sanchez I, Perkins GA, Murphy A, Taub PR, et al. (2011) (-)-Epicatechin enhances fatigue resistance and oxidative capacity in mouse muscle. J Physiol 589: 4615–4631 jphysiol.2011.209924 [pii];10.1113/jphysiol.2011.209924 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nieman DC, Henson DA, Maxwell KR, Williams AS, McAnulty SR, et al. (2009) Effects of quercetin and EGCG on mitochondrial biogenesis and immunity. Med Sci Sports Exerc 41: 1467–1475 10.1249/MSS.0b013e318199491f [doi]. [DOI] [PubMed] [Google Scholar]
- 46. Funk JA, Odejinmi S, Schnellmann RG (2010) SRT1720 induces mitochondrial biogenesis and rescues mitochondrial function after oxidant injury in renal proximal tubule cells. J Pharmacol Exp Ther 333: 593–601 jpet.109.161992 [pii];10.1124/jpet.109.161992 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rasbach KA, Schnellmann RG (2007) PGC-1alpha over-expression promotes recovery from mitochondrial dysfunction and cell injury. Biochem Biophys Res Commun 355: 734–739. [DOI] [PubMed] [Google Scholar]
- 48. Seo AY, Joseph AM, Dutta D, Hwang JC, Aris JP, et al. (2010) New insights into the role of mitochondria in aging: mitochondrial dynamics and more. J Cell Sci 123: 2533–2542 123/15/2533 [pii];10.1242/jcs.070490 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lee HC, Wei YH (2005) Mitochondrial biogenesis and mitochondrial DNA maintenance of mammalian cells under oxidative stress. Int J Biochem Cell Biol 37: 822–834. [DOI] [PubMed] [Google Scholar]
- 50. Wenz T (2009) PGC-1alpha activation as a therapeutic approach in mitochondrial disease. IUBMB Life 61: 1051–1062 10.1002/iub.261 [doi]. [DOI] [PubMed] [Google Scholar]
- 51. Yoshioka N, Hiasa Y, Cho M, Kitahori Y, Hirao K, et al. (1999) Effect of polyphenon-60 on the development of renal cell tumors in rats treated with N-ethyl-N hydroxyethylnitrosamine. Cancer Lett 136: 79–82. [DOI] [PubMed] [Google Scholar]
- 52. Rehman H, Ramshesh VK, Theruvath TP, Kim I, Currin RT, et al. (2008) NIM811, a Mitochondrial Permeability Transition Inhibitor, Attenuates Cholestatic Liver Injury But Not Fibrosis in Mice. J Pharmacol Exp Ther 327: 699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Funk JA, Schnellmann RG (2012) Persistent disruption of mitochondrial homeostasis after acute kidney injury. Am J Physiol Renal Physiol 302: F853–F864 ajprenal.00035.2011 [pii];10.1152/ajprenal.00035.2011 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Liu Q, Rehman H, Shi Y, Krishnasamy Y, Lemasters JJ, et al. (2012) Inhibition of sphingosine kinase-2 suppresses inflammation and attenuates graft injury after liver transplantation in rats. PLoS One 7: e41834 10.1371/journal.pone.0041834 [doi];PONE-D-12-14479 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Arany I, Faisal A, Clark JS, Vera T, Baliga R, et al. (2010) p66SHC-mediated mitochondrial dysfunction in renal proximal tubule cells during oxidative injury. Am J Physiol Renal Physiol 298: F1214–F1221 00639.2009 [pii];10.1152/ajprenal.00639.2009 [doi]. [DOI] [PubMed] [Google Scholar]
- 56. Attardi G, Schatz G (1988) Biogenesis of mitochondria. Annu Rev Cell Biol 4: 289–333. [DOI] [PubMed] [Google Scholar]
- 57. Herrmann JM, Longen S, Weckbecker D, Depuydt M (2012) Biogenesis of mitochondrial proteins. Adv Exp Med Biol 748: 41–64 10.1007/978-1-4614-3573-0_3 [doi]. [DOI] [PubMed] [Google Scholar]
- 58. Puigserver P, Spiegelman BM (2003) Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocr Rev 24: 78–90. [DOI] [PubMed] [Google Scholar]
- 59. Virbasius JV, Scarpulla RC (1994) Activation of the human mitochondrial transcription factor A gene by nuclear respiratory factors: a potential regulatory link between nuclear and mitochondrial gene expression in organelle biogenesis. Proc Natl Acad Sci U S A 91: 1309–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Thurman RG, Zhong Z, Frankenberg Mv, Stachlewitz RF, Bunzendahl H (1997) Prevention of cyclosporin-induced nephrotoxicity with dietary glycine. Transplantation 63: 1661–1667. [DOI] [PubMed] [Google Scholar]
- 61. Bennett M, Dent CL, Ma Q, Dastrala S, Grenier F, et al. (2008) Urine NGAL predicts severity of acute kidney injury after cardiac surgery: a prospective study. Clin J Am Soc Nephrol 3: 665–673 CJN.04010907 [pii];10.2215/CJN.04010907 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Goldstein SL (2011) Acute kidney injury biomarkers: renal angina and the need for a renal troponin I. BMC Med. 9: 135 1741-7015-9-135 [pii];10.1186/1741-7015-9-135 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bolignano D, Lacquaniti A, Coppolino G, Donato V, Campo S, et al. (2009) Neutrophil gelatinase-associated lipocalin (NGAL) and progression of chronic kidney disease. Clin J Am Soc Nephrol 4: 337–344 CJN.03530708 [pii];10.2215/CJN.03530708 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bolignano D, Lacquaniti A, Coppolino G, Donato V, Fazio MR, et al. (2009) Neutrophil gelatinase-associated lipocalin as an early biomarker of nephropathy in diabetic patients. Kidney Blood Press Res 32: 91–98 000209379 [pii];10.1159/000209379 [doi]. [DOI] [PubMed] [Google Scholar]
- 65. Jung K, Pergande M (1985) Influence of cyclosporin A on the respiration of isolated rat kidney mitochondria. FEBS Lett 183: 167–171. [DOI] [PubMed] [Google Scholar]
- 66. Lee SH, Yoon YC, Jang YY, Song JH, Han ES, et al. (2001) Effect of iron and ascorbate on cyclosporine-induced oxidative damage of kidney mitochondria and microsomes. Pharmacol Res 43: 161–171 10.1006/phrs.2000.0759 [doi];S1043-6618(00)90759-X [pii] [DOI] [PubMed] [Google Scholar]
- 67. Serkova N, Brand A, Christians U, Leibfritz D (1996) Evaluation of the effects of immunosuppressants on neuronal and glial cells in vitro by multinuclear magnetic resonance spectroscopy. Biochim Biophys Acta 1314: 93–104. [DOI] [PubMed] [Google Scholar]
- 68. Reichert AS, Neupert W (2004) Mitochondriomics or what makes us breathe. Trends Genet 20: 555–562. [DOI] [PubMed] [Google Scholar]
- 69. Robin ED, Wong R (1988) Mitochondrial DNA molecules and virtual number of mitochondria per cell in mammalian cells. J Cell Physiol 136: 507–513. [DOI] [PubMed] [Google Scholar]
- 70. Marechal X, Montaigne D, Marciniak C, Marchetti P, Hassoun SM, et al. (2011) Doxorubicin-induced cardiac dysfunction is attenuated by ciclosporin treatment in mice through improvements in mitochondrial bioenergetics. Clin Sci (Lond) 121: 405–413 CS20110069 [pii];10.1042/CS20110069 [doi]. [DOI] [PubMed] [Google Scholar]
- 71. Winder WW, Taylor EB, Thomson DM (2006) Role of AMP-activated protein kinase in the molecular adaptation to endurance exercise. Med Sci Sports Exerc 38: 1945–1949. [DOI] [PubMed] [Google Scholar]
- 72. Scarpulla RC (2002) Nuclear activators and coactivators in mammalian mitochondrial biogenesis. Biochim Biophys Acta 1576: 1–14. [DOI] [PubMed] [Google Scholar]
- 73. Scarpulla RC (2002) Transcriptional activators and coactivators in the nuclear control of mitochondrial function in mammalian cells. Gene 286: 81–89. [DOI] [PubMed] [Google Scholar]
- 74.Devaux F, Lelandais G, Garcia M, Goussard S, Jacq C (2010) Posttranscriptional control of mitochondrial biogenesis: Spatio-temporal regulation of the protein import process. FEBS Lett. S0014-5793(10)00762-3 [pii];10.1016/j.febslet.2010.09.030 [doi]. [DOI] [PubMed]
- 75. Huss JM, Torra IP, Staels B, Giguere V, Kelly DP (2004) Estrogen-related receptor alpha directs peroxisome proliferator-activated receptor alpha signaling in the transcriptional control of energy metabolism in cardiac and skeletal muscle. Mol Cell Biol 24: 9079–9091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, et al. (1999) Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98: 115–124. [DOI] [PubMed] [Google Scholar]
- 77. Cote J, Ruiz-Carrillo A (1993) Primers for mitochondrial DNA replication generated by endonuclease G. Science. 261: 765–769. [DOI] [PubMed] [Google Scholar]
- 78. Lehman JJ, Barger PM, Kovacs A, Saffitz JE, Medeiros DM, et al. (2000) Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J Clin Invest 106: 847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lowell BB, Spiegelman BM (2000) Towards a molecular understanding of adaptive thermogenesis. Nature 404: 652–660. [DOI] [PubMed] [Google Scholar]
- 80. Zong H, Ren JM, Young LH, Pypaert M, Mu J, et al. (2002) AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci U S A 99: 15983–15987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wu H, Kanatous SB, Thurmond FA, Gallardo T, Isotani E, et al. (2002) Regulation of mitochondrial biogenesis in skeletal muscle by CaMK. Science 296: 349–352. [DOI] [PubMed] [Google Scholar]
- 82. Nemoto S, Fergusson MM, Finkel T (2005) SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1{alpha}. J Biol Chem 280: 16456–16460 M501485200 [pii];10.1074/jbc.M501485200 [doi]. [DOI] [PubMed] [Google Scholar]
- 83. Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, et al. (2005) Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature 434: 113–118 nature03354 [pii];10.1038/nature03354 [doi]. [DOI] [PubMed] [Google Scholar]
- 84. Canto C, Auwerx J (2009) PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol 20: 98–105 10.1097/MOL.0b013e328328d0a4 [doi];00041433-200904000-00004 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Garnier A, Fortin D, Zoll J, N’Guessan B, Mettauer B, et al. (2005) Coordinated changes in mitochondrial function and biogenesis in healthy and diseased human skeletal muscle. FASEB J 19: 43–52 19/1/43 [pii];10.1096/fj.04-2173com [doi]. [DOI] [PubMed] [Google Scholar]
- 86. Norrbom J, Sundberg CJ, Ameln H, Kraus WE, Jansson E, et al. (2004) PGC-1alpha mRNA expression is influenced by metabolic perturbation in exercising human skeletal muscle. J Appl Physiol 96: 189–194 10.1152/japplphysiol.00765.2003 [doi];00765.2003 [pii]. [DOI] [PubMed] [Google Scholar]
- 87. Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, et al. (2002) Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature 418: 797–801 10.1038/nature00904 [doi];nature00904 [pii]. [DOI] [PubMed] [Google Scholar]
- 88. Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, et al. (2007) Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature 450: 712–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Rasbach KA, Funk JA, Jayavelu T, Green PT, Schnellmann RG (2010) 5-hydroxytryptamine receptor stimulation of mitochondrial biogenesis. J Pharmacol Exp Ther 332: 632–639 jpet.109.159947 [pii];10.1124/jpet.109.159947 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Beeson CC, Beeson GC, Schnellmann RG (2010) A high-throughput respirometric assay for mitochondrial biogenesis and toxicity. Anal Biochem 404: 75–81 S0003-2697(10)00292-7 [pii];10.1016/j.ab.2010.04.040 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wills LP, Trager RE, Beeson GC, Lindsey CC, Peterson YK, et al.. (2012) beta2 Adrenoceptor Agonist Formoterol Stimulates Mitochondrial Biogenesis. J Pharmacol Exp Ther. jpet.112.191528 [pii];10.1124/jpet.112.191528 [doi]. [DOI] [PMC free article] [PubMed]
- 92. Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, et al. (2006) Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 127: 1109–1122 S0092-8674(06)01428-0 [pii];10.1016/j.cell.2006.11.013 [doi]. [DOI] [PubMed] [Google Scholar]
- 93. Yun JM, Jialal I, Devaraj S (2010) Effects of epigallocatechin gallate on regulatory T cell number and function in obese v. lean volunteers. Br J Nutr 103: 1771–1777 S000711451000005X [pii];10.1017/S000711451000005X [doi]. [DOI] [PubMed] [Google Scholar]
- 94. Gundimeda U, McNeill TH, Elhiani AA, Schiffman JE, Hinton DR, et al. (2012) Green tea polyphenols precondition against cell death induced by oxygen-glucose deprivation via stimulation of laminin receptor, generation of reactive oxygen species, and activation of protein kinase Cepsilon. J Biol Chem 287: 34694–34708 M112.356899 [pii];10.1074/jbc.M112.356899 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Duluc L, Jacques C, Soleti R, Iacobazzi F, Simard G, et al. (2013) Modulation of mitochondrial capacity and angiogenesis by red wine polyphenols via estrogen receptor, NADPH oxidase and nitric oxide synthase pathways. Int J Biochem Cell Biol 45: 783–791 S1357-2725(13)00017-4 [pii];10.1016/j.biocel.2013.01.007 [doi] [DOI] [PubMed] [Google Scholar]
- 96. Mitscher LA, Jung M, Shankel D, Dou JH, Steele L, et al. (1997) Chemoprotection: a review of the potential therapeutic antioxidant properties of green tea (Camellia sinensis) and certain of its constituents. Med Res Rev 17: 327–365. [DOI] [PubMed] [Google Scholar]
- 97. Guo Q, Zhao B, Li M, Shen S, Xin W (1996) Studies on protective mechanisms of four components of green tea polyphenols against lipid peroxidation in synaptosomes. Biochim Biophys Acta 1304: 210–222. [DOI] [PubMed] [Google Scholar]