Abstract
Hot flashes in breast cancer survivors (BCS) receiving adjuvant aromatase inhibitor (AI) therapy are common, but risk factors for these symptoms are ill-defined. This study tested if body size is associated with hot flashes in BCS on AI therapy. A cross-sectional study of postmenopausal BCS receiving adjuvant AI therapy was performed. The primary outcome was occurrence of patient-reported hot flashes. The primary exposures of interest were current body size and weight change since breast cancer diagnosis. Three hundred participants were enrolled at a mean age of 61 years (range 33–86) after an average AI exposure of 23 months (range 1 month–9 years). Fifty-nine percent reported hot flashes, 32% reported moderate to severe hot flashes, and 25% reported significant worsening of hot flashes since starting AI therapy. Sixty-one percent experienced weight maintenance (±10 lb), while 27% had weight gain (gained 10 lb or more), and 11% had weight loss (lost 10 lb or more). In multivariable analysis, weight gain was independently associated with hot flash occurrence (OR 2.1, 95% CI 1.1–4.4) and hot flash severity (OR 2.6, 95% CI 1.3–5.0) after adjusting for confounding. Current body size was not associated with hot flash occurrence, severity or change with AI therapy. In an outpatient BCS population on AI therapy, weight gain is a risk factor for hot flash occurrence. Women who gained at least 10 lb since breast cancer diagnosis were two times more likely to have hot flashes than women who maintained or lost weight. These results support the thermoregulatory model of hot flashes and argue against a protective effect of body fat in this population.
Keywords: Hot flash, Body size, Weight, Aromatase inhibitor, Breast cancer
Introduction
Hot flashes are a common and debilitating symptom in breast cancer survivors (BCS) [1–4]. About two-thirds of postmenopausal BCS being treated for symptoms exhibit hot flashes [1, 2], and six times as many BCS experience hot flashes as compared to age-matched controls [5]. Women with hot flashes report worse quality of life, including greater fatigue, poorer sleep quality, and worse physical health, than those without them [1, 6, 7].
Aromatase inhibitors (AI), which block the final step in estrogen synthesis, are used as adjuvant endocrine therapy in postmenopausal BCS with hormone receptor-positive tumors. Estrogen withdrawal has been strongly associated with vasomotor symptoms such as hot flashes. Thus, AI therapy may render postmenopausal women even more vulnerable to experiencing hot flashes. Much is known about vasomotor symptoms in BCS treated with tamoxifen [8], but such data are more limited for AIs. In the clinical trial setting, the overall prevalence of hot flashes in BCS is high, and risk is significantly higher on letrozole (47%) than on placebo (41%) [9]. Compared to the selective estrogen receptor modulator tamoxifen, patients receiving anastrozole had less frequent hot flashes (35%) than those receiving tamoxifen (40%) or both in combination (41%) [10]. Further, treatment-emergent symptoms such as hot flashes have recently been associated with AI effect and outcomes [11].
Despite the above studies, the prevalence and risk factors for hot flashes in postmenopausal BCS on AIs in the ambulatory setting are largely unknown. In the general menopausal population, risk factors for hot flashes include increased body size, smoking, race, depressive and anxiety symptoms, and endogenous hormone levels [12–17]. Importantly, several of these risk factors are modifiable and, if validated in BCS, represent potential treatment options. Unfortunately, existing information about hot flashes in healthy women can be difficult to transfer to breast cancer survivors, as the severity and frequency of bother caused by hot flashes are significantly different in naturally menopausal women as compared to women with breast cancer [7]. Only by defining the prevalence and risk factors for hot flashes in ambulatory BCS on AI therapy can we improve the communication about diagnosing and treating hot flashes in this population.
The objectives of the study were: (1) to describe the characteristics (frequency, severity, and perceived change) of self-reported hot flashes in postmenopausal women with early stage breast cancer receiving aromatase inhibitors; (2) to identify the demographic and clinical factors that are associated with hot flashes. Specifically, we hypothesized that increased body size would be associated with hot flashes in BCS on AI treatment.
Methods
Study design and patient population
We conducted a cross-sectional survey of breast cancer patients receiving care at the Rowan Breast Cancer Center of the Abramson Cancer Center of the University of Pennsylvania (Philadelphia, PA) between April and October 2007. Potential participants included all postmenopausal women with a history of histologically confirmed stage I to III, hormone receptor-positive breast cancer who were currently taking a third-generation aromatase inhibitor (anastrozole, letrozole, or exemestane), completed chemotherapy or radiotherapy at least 1 month prior to enrollment, and had the ability to understand and provide informed consent in English. Research assistants obtained permission from the treating oncologist, screened medical records and approached potential study subjects for enrollment at their regular follow-up appointments. After informed consent was obtained, each participant was given a self-administered survey. The study was approved by the Institutional Review Board of the University of Pennsylvania and the Scientific Review and Monitoring Committee of the Abramson Cancer Center.
Outcome measurement
Participants reported the average number of hot flashes daily for the past 7 days. For the primary outcome of hot flash occurrence, we dichotomized this measure into hot flashes (yes, no). We also assessed hot flash severity and perceived change in hot flashes since start of AI therapy as secondary outcomes. Participants were asked to rate their hot flashes over the last 7 days on a 5-point likert scale (None, mild, moderate, severe, very severe). The rating is modified from the hot flash daily diary used in many clinical trials of hot flashes [18]. Based on work by Sloan et al. one day’s recall of hot flashes can reflect the week’s worth of hot flash data. Finally, in order to capture patient-perceived changes in hot flashes related to AI exposure, subjects were asked to compare their hot flashes since start of AI therapy to hot flashes prior to AI therapy on a 7-point likert Patient Global Impression of Change scale (much improved, moderately improved, little improved, not changed, little worse, moderately worse, much worse). Perceived change in hot flashes since AI exposure was grouped as significant improvement (moderately/much improved), little or no change (no change/little improved/little worse), and significant worsening (moderately/much worse).
Covariates were collected, including age, race, ethnicity, education level, and employment status. Demographic, clinical, and treatment characteristics were assessed by either self-report (i.e. timing of the last menstrual period [LMP], height and weight at breast cancer diagnosis, smoking and alcohol use, and co-morbidities including prior arthritis) or medical record abstraction (i.e. stage, chemotherapy, tamoxifen use, aromatase inhibitor use). Body mass index (BMI) were calculated with self-reported current height and weight using the formula BMI = kg/m2. Weight change since breast cancer diagnosis was categorized as ±10 lb, gaining more than 10 lb, or losing more than 10 lb. Anxiety and depression were measured by subject rating whether they have experienced these moods in the previous 24 h.
Statistical analysis
Data analysis was performed using STATA 9.0 (STATA Corporation, College Station, TX). Summary statistics were performed for all variables. Demographic, clinical, and treatment characteristics were compared by hot flash occurrence, frequency and severity status using Student’s t-test or Chi-square test, as appropriate. Multivariable logistic regression models were constructed to examine the independent associations of body size and change in body size with our outcomes of interest while controlling for confounding. Covariates with P-values <0.20 in bivariable analyses were carried forward to the multivariable model. Statistical tests were two-sided with P < 0.05 indicating significance.
Results
Of 484 consecutive patients screened, 50 (10%) were ineligible due to discontinuation of AI therapy prior to screening, 45 (9%) had metastatic disease, 64 (13%) did not keep their scheduled appointment, and 25 (5%) declined enrollment, resulting in 300 subjects. Characteristics of the study population are listed in Table 1. Among participants, the mean age (range) was 61 (33–86). The majority of subjects (84%) were Caucasian, and a substantial proportion (13%) was non-Hispanic black. In the analysis, we combined the race categories to Caucasians and Others. Mean BMI (range) was 27.2 (17.6–48.7). One hundred and seventy-three (58%) patients were taking anastrozole, 69 (23%) were taking letrozole, and 58 (19%) were taking exemestane.
Table 1.
Participant characteristics (n = 300)
| Subjects (%) (n = 300) | |
|---|---|
| Age | 61 (33–86) |
| ≤55 | 73 (24) |
| 56–65 | 131 (44) |
| ≥66 | 96 (32) |
| Race | |
| Caucasian | 253 (84) |
| Other | 47 (16) |
| Current smoking | 20 (6.7) |
| Current alcohol | 84 (28) |
| Years since menopause | |
| <5 years | 51 (18) |
| 5–10 years | 95 (33) |
| >10 years | 144 (49) |
| Cancer treatment | |
| Cancer stage | |
| I | 100 (37) |
| II | 142 (52) |
| III | 32 (12) |
| Prior chemotherapy | 179 (62) |
| Prior tamoxifen | 136 (47) |
| Type of AI | |
| Anastrazole | 173 (58) |
| Letrozole | 69 (23) |
| Exemestane | 58 (19) |
| Duration of AI exposure | |
| <1 year | 126 (43) |
| 1–3 years | 79 (27) |
| >3 years | 86 (30) |
| Body size | |
| BMI | |
| Normal/underweight (<18.5–25) | 110 (37) |
| Overweight (25–30) | 93 (31) |
| Obese (≥30) | 95 (32) |
| Weight change since breast cancer diagnosis | |
| ±10 lb | 182 (61) |
| Gained > 10 lb | 81 (27) |
| Lost > 10 lb | 34 (12) |
| Mood and vasomotor symptoms | |
| Depressive symptoms in last 24 h | 113 (38) |
| Anxiety symptoms in last 24 h | 151 (51) |
| Hot flash occurrence | |
| No | 123 (41) |
| Yes | 177 (59) |
| Hot flash severity | |
| Mild | 81 (27) |
| Moderate, severe, very severe | 96 (32) |
| Perceived change in hot flashes since AI therapy | |
| Significant worsening | 74 (25) |
| Little or no change | 202 (69) |
| Significant improvement | 19 (6) |
Numbers do not add to 300 for all variables due to missing data
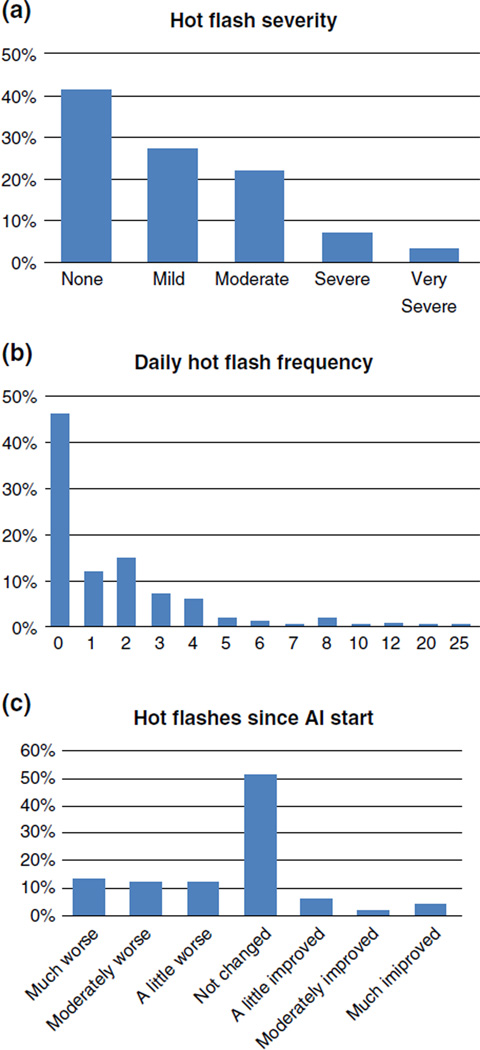
Hot flashes over the past week were reported by 59% of subjects (Table 1). Figure 1 depicts the distribution of hot flash frequency, severity and change with AI exposure. One-third of subjects who experienced hot flashes reported moderate to very severe symptoms. Mean daily hot flash frequency (range) was 3.6 (1–25). Twenty-five percent of subjects reported significant worsening of hot flashes since starting AI therapy.
Fig. 1.
Distributions of hot flash severity (a), daily hot flash frequency (b), and change in hot flashes since starting AI therapy (c). a Hot flash severity. b Hot flash frequency. c Change in hot flash since AI therapy
In univariate analysis, hot flash occurrence was significantly associated with weight gain since breast cancer diagnosis, but not associated with current body size, depressive and anxiety symptoms, or race (Table 2). Younger age, shorter time since menopause, smoking, prior chemotherapy, and prior tamoxifen therapy were also associated with higher risk of hot flashes. Subjects with weight gain were more likely to report greater hot flashes severity (48%) compared to subjects who maintained weight (25%) and subjects who lost weight (23%) (P = 0.001). Perceived change in hot flashes since starting AI therapy was not associated with weight change or BMI.
Table 2.
Univariate associations with hot flash occurrence and significant worsening of hot flashes since AI therapy
| Hot flashes occurrence (%) (n = 177) |
P-value | Hot flash severity (%) (n = 96) |
P-value | Significant worsening of hot flashes (%) (n = 74) |
P-value | |
|---|---|---|---|---|---|---|
| Age | <0.001 | <0.001 | 0.09 | |||
| ≤55 | 58 (33) | 39 (53) | 22 (30) | |||
| 56–65 | 73 (41) | 34 (26) | 33 (46) | |||
| ≥66 | 46 (26) | 23 (24) | 19 (26) | |||
| Race | 0.87 | 0.24 | 0.33 | |||
| Caucasian | 148 (84) | 76 (79) | 60 (81) | |||
| Other | 29 (16) | 20 (21) | 14 (19) | |||
| Current Smoking | 18 (10) | 0.006 | 12 (12) | 0.02 | 8 (11) | 0.28 |
| Current Alcohol | 46 (26) | 0.48 | 69 (72) | 1.00 | 52 (70) | 0.75 |
| Years since menopause | <0.001 | <0.001 | 0.003 | |||
| >5 years | 41 (24) | 27 (29) | 16 (22) | |||
| 5–10 years | 66 (38) | 38 (40 | 29 (40) | |||
| >10 years | 65 (38) | 29 (31) | 27 (38) | |||
| Cancer stage | 0.27 | 0.88 | 0.18 | |||
| I | 55 (34) | 30 (35) | 28 (42) | |||
| II | 92 (56) | 47 (54) | 27 (40) | |||
| III | 17 (10) | 10 (11) | 12 (18) | |||
| Prior chemotherapy | 116 (67) | 0.02 | 61 (65) | 0.44 | 39 (53) | 0.007 |
| Prior tamoxifen | 90 (53) | 0.03 | 49 (53) | 0.21 | 35 (49) | 0.32 |
| Duration of AI exposure | 0.96 | 0.33 | 0.91 | |||
| < 1 year | 48 (28) | 39 (41) | 18 (25) | |||
| 1–3 years | 74 (43) | 31 (33) | 30 (42) | |||
| >3 years | 51 (29) | 25 (26) | 24 (33) | |||
| AI type | 0.71 | 0.67 | 0.52 | |||
| Anastrazole | 103 (58) | 54 (56) | 38 (51) | |||
| Letrozole | 38 (21) | 17 (17) | 20 (27) | |||
| Exemestane | 36 (20) | 25 (26) | 16 (22) | |||
| BMI | 1.0 | 0.79 | 0.09 | |||
| Underweight (<18.5) | 2 (1) | 1 (1) | 0 (0) | |||
| Normal (18.5–25) | 65 (36) | 34 (35) | 23 (31) | |||
| Overweight (25–30) | 54 (31) | 27 (28) | 22 (30) | |||
| Obese (≥30) | 56 (32) | 34 (35) | 29 (39) | |||
| Weight change since breast cancer diagnosis | <0.001 | 0.001 | 0.31 | |||
| ±10 lb | 99 (57) | 39 (41) | 42 (58) | |||
| Gained >10 lb | 62 (35) | 47 (50) | 24 (33) | |||
| Lost >10 lb | 14 (8) | 8 (9) | 6 (8) | |||
| Depression | 67 (38) | 0.95 | 46 (48) | 0.01 | 43 (59) | <0.001 |
| Anxiety | 90 (51) | 0.85 | 57 (60) | 0.03 | 43 (59) | 0.23 |
In a multivariable model adjusting for age, smoking, race, prior tamoxifen and prior chemotherapy, current height and weight, subjects who reported gaining more than 10 lb since breast cancer diagnosis had a two-fold increase in odds of experiencing hot flashes (P = 0.038) (Table 3a) when compared to subjects who maintained their weight. Younger age and current smoking were also associated with increased odds of hot flash occurrence.
Table 3.
Multivariable models for hot flashes occurrence (a) and severity (b)
| Risk factor | Hot flash occurrence OR (95% CI) |
P-value |
|---|---|---|
| (a) Hot flash occurence | ||
| Weight change since breast cancer diagnosis | ||
| ±10 lb (reference) | 1 | |
| Gained >10 lb | 2.15 (1.04–4.42) | 0.038 |
| Lost >10 lb | 0.57 (0.24–1.38) | 0.21 |
| Age | 0.93 (0.90–0.97) | <0.001 |
| Current smoking | ||
| No (reference) | 1 | |
| Yes | 5.15 (1.08–24.43) | 0.039 |
| Race | ||
| Caucasian (reference) | 1 | |
| Other | 1.20 (0.50–2.87) | 0.68 |
| Prior chemotherapy | ||
| No (reference) | 1 | |
| Yes | 1.09 (0.58–2.05) | 0.78 |
| Prior tamoxifen | ||
| No (reference) | 1 | |
| Yes | 1.04 (0.58–1.86) | 0.12 |
| b. Hot flash severity | ||
| Weight change since breast cancer diagnosis | ||
| ±10 lb (reference) | 1 | |
| Gained > 10 lb | 2.57 (1.31–5.03) | 0.006 |
| Lost > 10 lb | 0.65 (0.23–1.79) | 0.40 |
| Age | 0.93 (0.90–0.96) | <0.001 |
| Current smoking | ||
| No (reference) | 1 | |
| Yes | 2.55 (0.88–7.46) | 0.09 |
| Race | ||
| Caucasian (reference) | 1 | |
| Other | 2.07 (0.87–4.94) | 0.10 |
| Depression | 1.98 (1.02–3.82) | 0.04 |
| Anxiety | 1.09 (0.58–2.08) | 0.78 |
Final models also adjusted for current height, weight
Weight gain remained independently associated with hot flash severity (OR 2.6 [1.3–5.0]) in a model adjusting for age, smoking, race, current height and weight, depressive and anxiety symptoms (Table 3b). Younger age and depressive symptoms were two additional risk factors for hot flash severity. Because of collinearity with age, time since menopause was not incorporated into both models.
Discussion
In an outpatient BCS population on AI therapy, hot flashes were reported by the majority of patients. While current body mass index was not associated with the hot flash outcomes, weight gain was a significant risk factor for hot flash occurrence and severity even after adjusting for known confounders. Our data also confirmed that BCS share several of the same risk factors for hot flashes as the general menopausal population, namely younger age, closer timing to menopause, smoking and depressive symptoms. These results further support the notion that hot flashes of cancer survivors and healthy women share significant similarities and potential pathogenesis [19].
Approximately 60% of our ambulatory BCS reported currently experiencing hot flashes while on AI therapy. While higher than reported by clinical trial data in BCS [9], this prevalence is similar to smaller observational studies of BCS [1, 2] and the general menopausal population [20]. Data on whether AI therapy is associated with higher risk of hot flashes is conflicting [9, 21]. Nonetheless, this high prevalence indicates that hot flashes are a common problem for the growing population of BCS on AI therapy.
Our interest in the relationship between body size and hot flashes stems from limited understanding of the physiology of hot flashes and options for treatment in the BCS population. Contrary to our hypothesis, the data did not show any relationship between current BMI and hot flashes. Further, there was no protective effect from lower BMI. The traditional thin hypothesis that decreased adiposity would be associated with decreased peripheral estrone production and hence greater vasomotor symptoms have largely been refuted in recent years [17, 20, 22]. Other studies have hypothesized that higher adiposity would provide greater insulation, prevent heat loss and increase the experience of hot flashes.
The association of hot flashes with significant weight gain is consistent with the current thermoregulatory model of vasomotor symptoms. Weight gain has been frequently reported in BCS, especially those who have undergone chemotherapy or menopause [23], To our knowledge, there are no studies on whether weight loss will improve hot flash symptoms in either BCS or general menopausal women. Our cross-sectional data suggest a decreased risk of hot flashes in women who lost more than 10 lb, but this did not reach statistical significance and cannot show causation. However, weight management is a modifiable risk factor that can be incorporated into the care of BCS. Because weight gain in this population has also been associated with worse cancer prognosis, higher incidence of comorbidities such as cardiovascular disease, and quality of life [23], our data give additional impetus for both future intervention studies on weight loss in BCS and clinical counseling of these patients.
Several limitations should be considered. Anthropomorphic measurements and hot flash outcomes were self-reported. In large cohorts such as the Nurses’ Health Study, recalled weight and height have been highly correlated with measured values; mean difference between recalled and actual weight was small (−1.4 kg) [24]. To account for this potential measurement error, we designated a wider interval (±10 lb) as no weight change in order to minimize misclassification. For hot flash outcomes, our questionnaire was modified from the hot flash daily diary and relied on self-report [18]. Though hot flashes may be measured “objectively” through skin conductance, there is limited correlation between subjective and objective measures of hot flashes [21] to support skin conductance as the “gold standard” over self-report. We did not obtain more precise measures of body fat and lean mass through techniques such as bioelectrical impedance analysis [25]. Such techniques can be incorporated into future studies. Finally, hormone levels were not available for this cohort to examine the association between estrogen levels on AIs and hot flashes, but this was not our primary aim.
Despite these limitations, we believe this is the first large study reporting the prevalence and risk factors for hot flashes in the ambulatory setting among women on AIs. Contrary to data in clinical trial, hot flashes are common in this population. Furthermore, the risk of hot flashes doubled with significant weight gain, a result that supports the thermoregulatory model of hot flashes and argues against a protective effect of body fat in this population.
Acknowledgments
This work has been supported by the American Cancer Society (MRSG 08-110-01-CCE [IS], ACS CCDA 08-107-01 [JM]), the National Institutes of Health (NICHD K23HD058799 [IS], NCCAM K23AT004112 [JM]) and the Pennsylvania Department of Aging [JM].
Contributor Information
H. Irene Su, Email: hisu@ucsd.edu, Department of Reproductive Medicine, Moores UCSD Cancer Center, University of California, San Diego, 3855 Health Sciences Drive, Dept. 0901, La Jolla, CA 92093-0901, USA.
Mary D. Sammel, Center for Clinical Epidemiology and Biostatistics, University of Pennsylvania, 423 Guardian Drive, Philadelphia, PA 19104, USA
Erin Springer, Department of Obstetrics and Gynecology, University of Pennsylvania, 3701 Market Street, 8th Floor, Philadelphia, PA 19104, USA.
Ellen W. Freeman, Department of Obstetrics and Gynecology, University of Pennsylvania, 3701 Market Street, 8th Floor, Philadelphia, PA 19104, USA Department of Psychiatry, University of Pennsylvania, 3701 Market Street, 8th Floor, Philadelphia, PA 19104, USA.
Angela DeMichele, Center for Clinical Epidemiology and Biostatistics, University of Pennsylvania, 423 Guardian Drive, Philadelphia, PA 19104, USA; Abramson Cancer Center of the University of Pennsylvania, 3401 Civic Center Boulevard, Philadelphia, PA 19104, USA.
Jun J. Mao, Center for Clinical Epidemiology and Biostatistics, University of Pennsylvania, 423 Guardian Drive, Philadelphia, PA 19104, USA Department of Family Medicine, University of Pennsylvania, 3400 Spruce Street, Philadelphia, PA 19104, USA.
References
- 1.Carpenter JS, Andrykowski MA, Cordova M, Cunningham L, Studts J, McGrath P, Kenady D, Sloan D, Munn R. Hot flashes in postmenopausal women treated for breast carcinoma: prevalence, severity, correlates, management, and relation to quality of life. Cancer. 1998;82:1682–1691. [PubMed] [Google Scholar]
- 2.Couzi RJ, Helzlsouer KJ, Fetting JH. Prevalence of menopausal symptoms among women with a history of breast cancer and attitudes toward estrogen replacement therapy. J Clin Oncol. 1995;13:2737–2744. doi: 10.1200/JCO.1995.13.11.2737. [DOI] [PubMed] [Google Scholar]
- 3.Carpenter JS, Andrykowski MA. Menopausal symptoms in breast cancer survivors. Oncol Nurs Forum. 1999;26:1311–1317. [PubMed] [Google Scholar]
- 4.Finck G, Barton DL, Loprinzi CL, Quella SK, Sloan JA. Definitions of hot flashes in breast cancer survivors. J Pain Symptom Manage. 1998;16:327–333. doi: 10.1016/s0885-3924(98)00090-6. [DOI] [PubMed] [Google Scholar]
- 5.Harris PF, Remington PL, Trentham-Dietz A, Allen CI, Newcomb PA. Prevalence and treatment of menopausal symptoms among breast cancer survivors. J Pain Symptom Manage. 2002;23:501–509. doi: 10.1016/s0885-3924(02)00395-0. [DOI] [PubMed] [Google Scholar]
- 6.Stein KD, Jacobsen PB, Hann DM, Greenberg H, Lyman G. Impact of hot flashes on quality of life among postmenopausal women being treated for breast cancer. J Pain Symptom Manage. 2000;19:436–445. doi: 10.1016/s0885-3924(00)00142-1. [DOI] [PubMed] [Google Scholar]
- 7.Carpenter JS, Johnson D, Wagner L, Andrykowski M. Hot flashes and related outcomes in breast cancer survivors and matched comparison women. Oncol Nurs Forum. 2002;29:E16–E25. doi: 10.1188/02.ONF.E16-E25. [DOI] [PubMed] [Google Scholar]
- 8.Mourits MJ, De Vries EG, Willemse PH, Ten Hoor KA, Hollema H, Van der Zee AG. Tamoxifen treatment and gynecologic side effects: a review. Obstet Gynecol. 2001;97:855–866. doi: 10.1016/s0029-7844(00)01196-0. [DOI] [PubMed] [Google Scholar]
- 9.Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ, Castiglione M, Tu D, Shepherd LE, Pritchard KI, Livingston RB, Davidson NE, Norton L, Perez EA, Abrams JS, Therasse P, Palmer MJ, Pater JL. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med. 2003;349:1793–1802. doi: 10.1056/NEJMoa032312. [DOI] [PubMed] [Google Scholar]
- 10.Fallowfield L, Cella D, Cuzick J, Francis S, Locker G, Howell A. Quality of life of postmenopausal women in the arimidex, tamoxifen, alone or in combination (atac) adjuvant breast cancer trial. J Clin Oncol. 2004;22:4261–4271. doi: 10.1200/JCO.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 11.Cuzick J, Sestak I, Cella D, Fallowfield L. Treatmentemergent endocrine symptoms and the risk of breast cancer recurrence: a retrospective analysis of the ATAC trial. Lancet Oncol. 2008;9:1143–1148. doi: 10.1016/S1470-2045(08)70259-6. [DOI] [PubMed] [Google Scholar]
- 12.Freeman EW, Sammel MD, Grisso JA, Battistini M, Garcia-Espagna B, Hollander L. Hot flashes in the late reproductive years: risk factors for Africa American and Caucasian women. J Womens Health Gend Based Med. 2001;10:67–76. doi: 10.1089/152460901750067133. [DOI] [PubMed] [Google Scholar]
- 13.Freeman EW, Sammel MD, Lin H, Gracia CR, Kapoor S, Ferdousi T. The role of anxiety and hormonal changes in menopausal hot flashes. Menopause. 2005;12:258–266. doi: 10.1097/01.gme.0000142440.49698.b7. [DOI] [PubMed] [Google Scholar]
- 14.Gold EB, Sternfeld B, Kelsey JL, Brown C, Mouton C, Reame N, Salamone L, Stellato R. Relation of demographic and lifestyle factors to symptoms in a multi-racial/ethnic population of women 40–55 years of age. Am J Epidemiol. 2000;152:463–473. doi: 10.1093/aje/152.5.463. [DOI] [PubMed] [Google Scholar]
- 15.Guthrie JR, Dennerstein L, Hopper JL, Burger HG. Hot flushes, menstrual status, and hormone levels in a populationbased sample of midlife women. Obstet Gynecol. 1996;88:437–442. doi: 10.1016/0029-7844(96)00196-2. [DOI] [PubMed] [Google Scholar]
- 16.Overlie I, Moen MH, Holte A, Finset A. Androgens and estrogens in relation to hot flushes during the menopausal transition. Maturitas. 2002;41:69–77. doi: 10.1016/s0378-5122(01)00256-0. [DOI] [PubMed] [Google Scholar]
- 17.Whiteman MK, Staropoli CA, Langenberg PW, McCarter RJ, Kjerulff KH, Flaws JA. Smoking, body mass, and hot flashes in midlife women. Obstet Gynecol. 2003;101:264–272. doi: 10.1016/s0029-7844(02)02593-0. [DOI] [PubMed] [Google Scholar]
- 18.Sloan JA, Loprinzi CL, Novotny PJ, Barton DL, Lavasseur BI, Windschitl H. Methodologic lessons learned from hot flash studies. J Clin Oncol. 2001;19:4280–4290. doi: 10.1200/JCO.2001.19.23.4280. [DOI] [PubMed] [Google Scholar]
- 19.Santoro NF, Dicken CL. Hot flashes: a rose is a rose is a rose. Menopause. 2009;16:432–433. doi: 10.1097/gme.0b013e3181a05dd1. [DOI] [PubMed] [Google Scholar]
- 20.Gold EB, Colvin A, Avis N, Bromberger J, Greendale GA, Powell L, Sternfeld B, Matthews K. Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopausal transition: study of women’s health across the nation. Am J Public Health. 2006;96:1226–1235. doi: 10.2105/AJPH.2005.066936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Otte JL, Flockhart D, Hayes D, Storniolo AM, Stearns V, Schneider B, Henry NL, Azzouz F, Nguyen A, Lemler S, Hayden J, Jeter S, Wright L, Carpenter JS. Comparison of subjective and objective hot flash measures over time among breast cancer survivors initiating aromatase inhibitor therapy. Menopause. 2009;16:653–659. doi: 10.1097/gme.0b013e3181a5d0d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gallicchio L, Visvanathan K, Miller SR, Babus J, Lewis LM, Zacur H, Flaws JA. Body mass, estrogen levels, and hot flashes in midlife women. Am J Obstet Gynecol. 2005;193:1353–1360. doi: 10.1016/j.ajog.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Chlebowski RT, Aiello E, McTiernan A. Weight loss in breast cancer patient management. J Clin Oncol. 2002;20:1128–1143. doi: 10.1200/JCO.2002.20.4.1128. [DOI] [PubMed] [Google Scholar]
- 24.Troy LM, Hunter DJ, Manson JE, Colditz GA, Stampfer MJ, Willett WC. The validity of recalled weight among younger women. Int J Obes Relat Metab Disord. 1995;19:570–572. [PubMed] [Google Scholar]
- 25.Thurston RC, Sowers MR, Chang Y, Sternfeld B, Gold EB, Johnston JM, Matthews KA. Adiposity and reporting of vasomotor symptoms among midlife women: the study of women’s health across the nation. Am J Epidemiol. 2008;167:78–85. doi: 10.1093/aje/kwm244. [DOI] [PubMed] [Google Scholar]