Abstract
Objective
To review the effectiveness of physical activity (PA) and PA plus diet interventions in managing weight among overweight or obese (OW/OB) pregnant or postpartum women.
Methods
Four databases were searched for randomized controlled studies published between January 2000 and December 2011 that reported weight change outcomes of PA interventions in OW/OB pregnant or postpartum women. PA alone as well as PA plus diet interventions were included.
Results
Of 681 abstracts identified, 11 were included (7 trials with pregnant women and 4 trials with postpartum women). Overall, we found that PA interventions were effective for OW/OB pregnant as well as postpartum women. On average, pregnant women in the intervention groups gained 0.91 kg less (95% CI: −1.76, −0.06) compared to those in the usual care groups. Postpartum women in the intervention groups significantly lost more body weight (−1.22 kg; 95% CI: −1.89, −0.56) than those in the control groups. In the subgroup analyses by PA intervention types, supervised PA plus diet interventions were the most effective.
Conclusions
PA plus diet interventions may require more than advice; supervised PA programs or personalized prescription/goals are needed to prevent excessive weight gain for OW/OB pregnant women and excessive weight retention for OW/OB postpartum women.
Introduction
In the United States, over one-third of women are overweight or obese (OW/OB) at the start of pregnancy and the prevalence of pre-pregnancy overweight or obesity is increasing (Kim et al., 2007). OW/OB women are about two times more likely to gain gestational weight exceeding the Institute of Medicine (IOM)'s recommendations than normal weight women (Cedergren, 2006). Women who gain excessive weight during pregnancy are more likely to retain those extra weight during postpartum (Gunderson and Abrams, 1999; Kac et al., 2003; Nehring et al., 2011; Rossner and Ohlin, 1995). Excessive weight gain during pregnancy is a risk factor for adverse maternal as well as infant health outcomes (Baeten et al., 2001; Cedergren, 2006; Kiel et al., 2007). Furthermore, excessive weight gain during pregnancy and excessive weight retention during postpartum are associated with increased risk of long-term obesity, cardiovascular disease, and type 2 diabetes during midlife (Rooney et al., 2005; Gavard and Artal, 2008). Regular physical activity is recommended to maintain a healthy weight during pregnancy and postpartum (American College of Obstetricians and Gynecologists, 2002; American College of Sports Medicine, 2006; Gavard and Artal, 2008). However, the great majority of women who are pregnant or in postpartum are physically inactive, and even women who were physically active prior to their pregnancy tend to reduce their activity as their pregnancy progresses (Evenson et al., 2004).
There were a few systematic reviews with/without meta-analysis pertaining to gestational weight gain and postpartum weight loss that reported associations with physical activity. Kuhlmann et al. (2008) suggested some potential of weightmanagement intervention effects among pregnant or postpartum women reported in 3 randomized clinical trials (RCTs) published between 1985 and 2007. The overall findings of a meta-analysis of physical activity and weight management in pregnant women suggest that physical activity may restrict gestational weight gain (Streuling et al., 2011). In a meta-analysis of physical activity or diet, or both for weight reduction in postpartum women, Amorim et al. (2007) found that diet alone and both physical activity and diet together may be effective in losing weight during the postpartum period, but not physical activity alone. It has to be noted, however, that these meta-analyses included pregnant women and postpartum women regardless of their body mass index (BMI) status. Sui et al. (2012) recently reported that physical activity might be effective in limiting gestational weight gain in OW/OB pregnant women in a systematic review, but this review was limited to only supervised antenatal physical activity interventions excluding physical activity plus diet interventions, which is known to be most effective in managing weight in OW/OB adult population (Schaar et al., 2010). Thus, the purpose of this study was to systematically review and investigate the effectiveness of physical activity alone interventions as well as physical activity plus diet interventions in managing weight change among OW/OB women during pregnancy as well as postpartum.
Methods
Search strategy
In collaboration with a professional librarian, we developed individualized search strategies for 4 databases: PubMed, EMBASE, CINAHL, and Cochrane Library (Appendix 1). We used search terms such as physical activity, exercise, lifestyle, obesity, overweight, weight gain, body weight, body mass index, pregnancy, pregnant women, prenatal care, and postpartum period as MeSH headings, subject headings or titles. Articles about interventions conducted in any country that were published in either English or Korean between January 2000 and December 2011, used an randomized controlled clinical trial (RCT) study design, and reported a weight-related outcome measure were included. In 1985, the American College of Obstetricians and Gynecologists (ACOG) provided recommendations for exercise during pregnancy that were conservative, based on limited evidence available at that time. Most recently updated ACOG Guidelines (2002) suggest that all women having a normal pregnancy can benefit from a physical activity program. Thus, we examined the cumulative results of the studies that were published in the past 20 years that may reflect the updated guidelines. We also reviewed the bibliographies of relevant articles identified by the search strategies. The systematic literature search was performed between October and December 2011.
Study inclusion and exclusion criteria
Studies were considered relevant for the systematic review if they met the following inclusion criteria:
Randomized controlled trial
Subjects are either overweight (BMI > 25 kg/m2) or obese (BMI > 30 kg/m2)
Subjects are either pregnant or after childbirth (during the postpartum period)
Interventions target increasing physical activity
Weight change reported
The interventions conducted among mothers with young children were not included when postpartum status was not clearly stated. To include all available studies, we contacted the authors via e-mail if we had 1) no access to a specific article, 2) abstracts only, and 3) insufficient data to calculate weight change for meta-analysis. A trial was excluded where information was available in abstract form only (Bertz et al., 2010). We excluded training program with exercise for preventing or treating urinary incontinence. If there was any disagreement in inclusion/exclusion of studies, two independent reviewers (JC and JL) reviewed the full-text papers of these studies and reached to consensus.
Data extraction
Two independent reviewers (JC and JL) extracted data from each of the studies. Data included sociodemographic characteristics, country of origin, sample size, intervention/control description, intervention outcomes, and intervention effects. Analyses of the retrieved papers were carried out between December 2011 and May 2012.
Assessing the strength of evidence
Two reviewers (JC and JL) independently reviewed the strength of evidence provided by studies using the following criteria outlined in the CONSORT (Consolidated Standards of Reporting Trials) statement (Moher et al., 2001) and the Cochrane Collaboration's tool for assessing risk of bias (Higgins et al., 2011). The quality of study was determined based on five elements: sample size, concealment of allocation, blinding, intention-to-treat analysis, and completeness of data. The criteria were not used to exclude any study that did not meet, rather assessing the quality of studies. The differences between the reviewers were resolved by discussion and consensus was reached.
Data analysis
We calculated mean difference scores in weight change (kg) and corresponding 95% CIs during either pregnancy or postpartum between the intervention and control groups. We performed meta-analyses to assess summary estimates of the effects— separately in studies conducted with pregnant women and in studies with postpartum women. In this study, we used the random effects model. In that model, studies were weighted using a formula that includes both a measure of the study sample size (1/variance) and a measure of the between-study variance. Studies with larger sample size (a larger 1/variance term) received more weight. Heterogeneity among studies was tested with Higgins' I2, which “describes the percentage of total variation across studies that is due to heterogeneity rather than chance” (Higgins et al., 2003). A vale of 0% indicates no heterogeneity is observed and the larger values mean the increased heterogeneity. Potential publication bias was assessed in a funnel plot. We used Begg's test and Egger's test to check for funnel plot asymmetry. The analyses were performed with the Stata12 statistical software (StataCorp, 2011).
Results
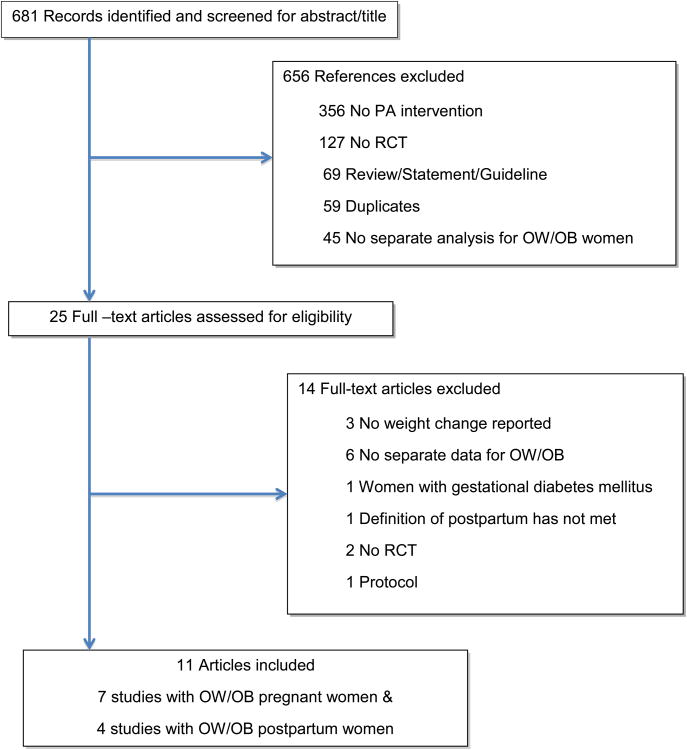
The study selection process is illustrated in Figure 1. We initially identified 681 abstracts with our search strategies. Twenty-five studies were selected as potentially relevant studies for full text investigation after title and abstract screening. We excluded three studies because of missing data for weight change, even after contacting the authors (Asbee et al., 2009; O'Toole et al., 2003; Santos et al., 2005). Separate data for OW/OB women were not reported in six studies (Cavalcante et al., 2009, Haakstad and Bo, 2009; Huang et al., 2011; Hui et al., 2011; Lewis et al., 2011; Yeo, 2009). There were other reasons to exclude papers: an intervention for women with gestational diabetes mellitus (Brankston et al., 2004), unclear postpartum status (Chang et al., 2010), no RCT (Davenport et al., 2011; Gray-Donald et al., 2000), and protocol (Luoto et al., 2010). Finally, a total of 11 studies (7 studies with pregnant women and 4 studies with postpartum women) were identified for inclusion in the review. Although a variety of mother's and infant's outcomes were assessed in the selected studies, only maternal weight change was analyzed in this review. Among the 7 studies with pregnant women, the most studies used the gestational weight gain, but Ong et al. (2009) used a slightly different time window. Nonetheless, we still used the change in weight in that study, just as we did in other studies. We did not adapt or convert their reported weight change.
Figure 1. Identification of studies in systematic review of effects of physical activity interventions on pregnant or postpartum women' weight change.
Methodological quality
Methodological quality varied considerable across the trials (Table 1). The sample size of randomized participants in the 11 studies ranged from 12 to 450 and five studies of the 7 studies with pregnant women had more than 100 participants (Barakat, et al., 2009; Guelinckx, et al., 2010; Phelan, et al., 2011; Polley, et al., 2002; Vinter, et al., 2011) while only one study with postpartum had more than 100 participants (Østby et al., 2009). Four studies of the 7 studies with pregnant women clearly reported methods used to generate the concealed allocation in the participant randomization assignment (Barakat et al., 2009; Nascimento, et al., 2011; Phelan,et al., 2011; Vinter, et al., 2011), and none of the 4 studies with postpartum women clearly described those procedures. Blinded assessment was done in two studies with pregnant women (Barakat et al., 2009; Phelan, et al., 2011) and one study with postpartum women (Craigie et al., 2011). An intention-to-treat analysis was performed in three studies with pregnant women (Barakat et al, 2009; Phelan et al., 2011; Polley et al., 2002) and one study with postpartum women (Østby et al., 2009). Retention rates were less than 80% for one study (Guelincks et al., 2010) with pregnant women and three studies with postpartum women (Craigie et al., 2011; Østby et al., 2009; Walker et al., 2011).
Table 1. Study characteristics of physical activity only and physical activity plus diet interventions.
| Study/Country | Sample | OW/OB Sample size (analyzed) |
Intervention & Control |
Wt change (kg; mean ± SD) |
Outcomes | Quality | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample size | Concealment of allocation | Blinding | Intention-to-treat analysis | Completeness of data | ||||||
|
Barakat et al., 2009 Spain |
12-13 weeks pregnant NW/OW/OB, N=160 | n(I) = 23 | I: PA alone/Supervised PA | 9.92 ± 4.631 | GWG; birth weight; birth length, ponderal index, head circumference, Apgar score; gestational age | + | + | + | + | + |
| n(C) = 17 | C: maintained the level of activity | 11.84 ± 3.661 | ||||||||
|
Ong et al., 2009 Australia |
18 weeks pregnant OB, N=12 | n(I) = 6 | I: PA alone/Supervised PA | 3.7 ± 3.4 | GWG; 75g oral glucose tolerance test | − | ? | ? | ? | + |
| n(C) = 6 | C: continued with their usual daily activities | 5.2 ± 1.3 | ||||||||
|
Nascimento et al., 2011 Brazil |
14-24 weeks pregnant OW/OB, N=82 | n(I) = 39 | I: PA plus diet/Supervised PA | 10.3 ± 5.0 | GWG; the proportion who exceeded IOM recommendation; caesarean rate; birth weight; gestational age; Apgar score; LGA; SGA; quality of life | + | + | ? | ? | + |
| n(C) = 41 | C: Routine hospital care plus standardized nutritional counseling | 11.5 ± 7.4 | ||||||||
|
Vinter et al., 2011 Denmark |
10-14 weeks pregnant OB, N=360 | n(I) = 144 | I: PA plus diet/Supervised PA | 7.42 ± 4.592 | GWG; the proportion who exceeded IOM recommendation; blood pressure; VO2max; birth weight; LGA; gestational age; cesarean delivery; admission to NICU; preeclampsia; gestational diabetes | + | + | ? | ? | + |
| n(C) = 148 | C: The information about the purpose and content of the study, including access to a website with advice about dietary habits and PA in pregnancy was given. | 8.59 ± 4.422 | ||||||||
|
Guelinckx et al., 20103 Belgium |
<15 weeks pregnant OB, N=195 | n(I) = 42, | I: PA plus diet | 9.8 ± 7.6 | GWG; the proportion who exceeded IOM recommendation; birth weight; LGA; infant length; gestational age; cesarean delivery; induction of labor; preeclampsia; maternal hypertension | + | ? | − | ? | − |
| n(C) = 43 | C: Routine care | 10.6 ± 6.9 | ||||||||
|
Phelan et al, 2011 USA |
10-16 weeks pregnant NW/OW/OB, N=401 | n(I) = 80 | I: PA plus diet | 14.7 ± 6.9 | GWG; the proportion who exceeded IOM recommendation; wt retention at 6 month postpartum; wt loss since delivery at 6 month postpartum; birth weight; LGA; LBW; gestational age; cesarean delivery; preeclampsia; maternal hypertension, gestational diabetes | + | + | + | + | + |
| n(C) = 83 | C: Standard nutrition counseling | 15.1 ± 7.5 | ||||||||
|
Polley et al., 2002 U.S.A. |
<20 weeks pregnant NW/OW/OB, N=120 | n(I) = 27 | I: PA plus diet | 13.6 ± 7.2 | GWG; the proportion who exceeded IOM recommendation; wt retention at 4 week; wt loss since delivery at 4 week postpartum; birth weight; LGA; LBW; gestational age; cesarean delivery; preterm delivery; preeclampsia; maternal hypertension; gestational diabetes | + | ? | − | + | + |
| n(C) = 22 | C: Standard nutrition counseling | 10.1 ± 6.2 | ||||||||
|
Lovelady et al., 2000 U.S.A. |
4 weeks postpartum OW, N=48 Breastfeeding women only | n(I)=21 | I: PA plus diet/Supervised PA | −4.8 ± 1.7 | Weight; BMI; body fat; fat mass; fat-free mass; skin-fold thickness; energy intake; maximal oxygen consumption; infant's weight; infant's length | − | ? | ? | − | + |
| n(C)=19 | C: Not to restrict their energy intake and not to perform vigorous aerobic exercise more than once per week | −0.8 ± 2.3 | ||||||||
|
Craigie et al., 2011 U.K. |
6-18 months postpartum OW/OB, N=52, Low income | n(I)=22 | I: PA plus diet | −1.6 ± 2.0 | Weight; height; waist circumference; skin-fold measure; physical activity; fasting blood sample | − | ? | + | − | − |
| n(C)=14 | C: Received a weight loss booklet | 0.2 ± 2.2 | ||||||||
|
Østby et al., 2009 U.S.A. |
6 weeks postpartum N=450 | n(I)=214 | I: PA plus diet | −0.90 ± 5.1 | Weight; height; dietary intake; physical activity | + | ? | ? | + | − |
| n(C)=207 | C: received biweekly newsletter | −0.36 ± 4.9 | ||||||||
|
Walker et al., 2011 U.S.A |
6 weeks to 12 months postpartum OW/OB, N=71 Low income | White, n(I)=8 | I: PA plus diet | −5.7 ± 13.7 | Weight; health behaviors, perceived stress, self-efficacy, pros and cons of weight loss; body dissatisfaction; weight distress; social support | − | ? | ? | + | − |
| Black, n(I)=9 | 3.3 ± 6.3 | |||||||||
| Hispanic, n(I)=5 | −2.2 ± 4.1 | |||||||||
| White, n(C)=8 | C: Women were not required not to attempt weight loss during the wait−list period. | −2.6 ± 4.0 | ||||||||
| Black, n(C)=11 | −0.2 ± 6.2 | |||||||||
| Hispanic, n(C)=9 | −0.2 ± 4.8 | |||||||||
Note: OW = overweight, OB = obese, NW = normal weight, I = intervention group, C = control group, PA = physical activity, BMI = Body Mass Index, Wt = weight, GWG = gestational weight gain, LGA = large for gestational age, SGA = small for gestational age, NICU = neonatal intensive care unit, LBW = low birth weight (<2500g), + = low risk of bias, − = high risk of bias,? = unclear risk of bias
The mean and the pooled standard deviation were calculated for the overweight and obese pregnant women.
The mean and the standard deviation were obtained from the first author of the paper.
One of the two intervention groups called I-passive (n = 37) was excluded from this paper and the data analysis. The group received a brochure on nutrition and physical activity and tips to limit pregnancy-relate weight gain.
Interventions targeting pregnant women
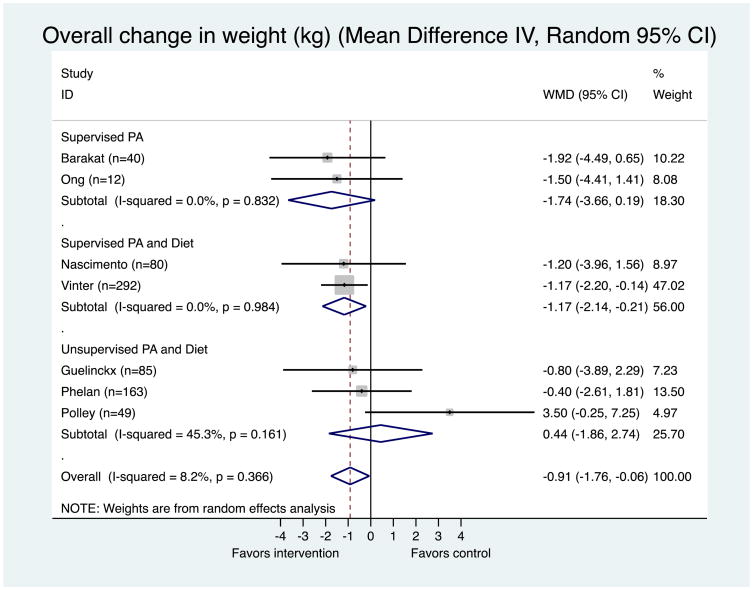
A summary of study characteristics is provided in Table 1. Seven randomized controlled trials were included, involving 721 OW/OB pregnant women. Places where the studies were implemented varied; two in the U.S., and one in Australia, Brazil, Belgium, Denmark, and Spain respectively. Two of the 7 studies provided structured physical activity programs alone, in which women participated in supervised physical activity sessions 3 times a week. Another two studies provided supervised physical activity sessions once a week along with counseling on diet and the recommended weight gain during pregnancy either once at the start of the study (Nascimento et al., 2011) or at 15, 20, 28, and 35 weeks gestations (Vinter et al., 2011). The rest three studies implemented a group or individual counseling/advice on physical activity, diet, and the recommended weight gain during pregnancy and did not provide any supervised exercise program (Guelincks et al., 2010; Phelan et al., 2011; Polley et al., 2002). The meta-analysis on the results of the individual studies are depicted in Figure 2, suggesting a significant reduction of gestational weight gain on overage in the physical activity intervention groups (p = 0.035), with a weighted mean difference (WMD) of −0.91 kg (95% CI: −1.76, −0.06). That is, women who were provided with either physical activity alone or physical activity plus diet gained less of about 0.91 kg during pregnancy, when compared with women in the control groups. Supervised physical activity only studies (supervised physical activity programs 3 times per week) showed the largest weight reduction (WMD = −1.74 kg; 95% CI: −3.66, +0.19), but it was not statistically significant (p = 0.077). Physical activity plus diet studies consisting of supervised physical activity program showed a significant treatment effect compared to women in the control groups (WMD = −1.17 kg; 95% CI: −2.14, −0.21; p = 0.017). Overall, women in unsupervised physical activity programs rather gained more weight than women in the controls, but it was not statistically significant (WMD = 0.44 kg; 95% CI: −1.86, 2.74; p = 0.707) (Figure. 2). There was low heterogeneity among individual studies as indicated by Higgins' I2. Our funnel plot showed one small study with a larger effect (outlier; Polley et al., 2002). Publication bias is one possible explanation for a finding of an asymmetric funnel plot (small studies clustering well above or well below the summary measure of effect). However, in this case, there is only one outlier study, rather than a cluster of studies. Also, there are other explanations for outlier studies beyond publication bias. In this case, this study may have had a much greater estimate of effect because it had a different population. In the Polley et al. (2002), all of the study participants were recruited from an obstetric clinic for low-income women and the proportion of an ethnic minority (i.e., African American) was relatively high. Also, both Begg and Egger test, which are statistical tests to detect publication bias, did not show evidence of publication bias (Appendix 2).
Figure 2. Meta-Analysis of the effectiveness of physical activity interventions compared to comparison group in 7 interventions targeting pregnant women.
Heterogeneity: Chi2 = 6.54, df = 6 (p = 0.366); I2 = 8.2%
Test for overall effect: z= 2.10 p = 0.035
The area of the gray square reflects the weight that the study contributes to the meta-analysis.
In all of the 7 RCTs targeting pregnant women, the intensity of the physical activity interventions ranged from light to moderate (Table 2). Among the 4 supervised physical activity interventions, the training intensity was individualized for two studies (Barakat et al., 2009; Ong et al., 2009) while the other two studies have interventions of either 40 minutes (Nascimento et al., 2011) or 60 minutes (Vinter et al., 2011) per session once a week. Among the 3 unsupervised physical activity interventions, no individualized physical activity prescription/goal was used (Guelincks et al., 2010, Phelan et al., 2011; Polley et al., 2002).
Table 2. The intervention components of physical activity only and physical activity plus diet interventions.
| Study | Delivery mode | Intervention duration |
PA type | PA intensity |
PA prescription/ goal |
Diet | Weight management |
Additional intervention component |
|---|---|---|---|---|---|---|---|---|
| Barakat et al., 2009 | Group | 26 weeks | Muscle resistance training) | Light to moderate | I: 3 times per week for 35-40 min | N/A | N/A | Individualized PA intensity |
| Ong et al., 2009 | Individual | 10 weeks | Stationary cycling) | Moderate (Heart rate max: 50-70%) | I: 3 times per week of one or two 15 min bouts of cycling and the duration was gradually increased to 40-45 min as the weeks progressed plus a 10 min warm-up and a 10 min cool-down | N/A | N/A | Individualized PA intensity |
| Nascimento et al., 2011 | Group or individual | 19 weeks | Stretching, muscle resistance, relaxation | Light to moderate Heart rate< 140 bpm | I: 1 time per week Women were asked to perform the exercise or walking 5 times per week | Standardized nutritional counseling | Recommended weight gain for their BMI category | Exercise journal |
| Vinter et al., 2011* | Group | 29 weeks | Aerobic, muscle resistance, balance | Moderate | I: training for 1 hour each week and group sessions to teach methods to improve physical activity (4-6 times in pregnancy) Goal: 30-60 minutes of moderate physical activity daily | Counseling at 15, 20, 28, and 35 weeks' gestation; individually estimated energy requirements | To limit GWG to 5 kg | Free full-time membership in a fitness center for 6 months & pedometer |
| Guelincks et al., 2010 | Group | 25 to 30 weeks | Not specified | Not specified | Goal: Not specified Advice on PA (how to increase PA) | Counseling at 15, 20, and 32 week's gestation | Not specified The upper limit for GWG of 11.2 kg was used. | |
| Phelan et al., 2011 | Individual | From the initial visit to the final routine prenatal care appointment | Walking | Moderate | Goal: 30 minutes of walking most days of the week | Calorie goals (20 kcal/kg), | Appropriate GWG based on the IOM guideline | Body-weight scales, food records, pedometers, weekly postcards that prompted healthy eating and exercise, personalized weight chart with feedback |
| Polley et al., 2002 | Individual | From the initial visit to the final routine prenatal care appointment | Walking | Not specified | Not specified Advice on having a physically active lifestyle | Healthful eating during pregnancy Structured meal plan and individualized calorie goals for those who exceeded the IOM recommended weights | Appropriate GWG based on the IOM guideline | Biweekly newsletters that prompted healthy eating and exercise habits, personalized weight chart with feedback, telephone calls to discuss progress towards the goals set at the previous visit |
| Lovelady et al., 2000 | Individual | 10 weeks | Brisk walking, jogging, and aerobic dancing | Moderate/Vigorous | 45 minutes per day for 4 days per week for 10 weeks | Restriction of energy intake; individualized energy intake; six commercial low-fat and low-calorie frozen dinners each week | Weight loss of 0.5 to 1.0 kg per week | Individualized PA intensity |
| Craigie et al., 2011* | Individual | 12 weeks | Walking | Moderate/Vigorous | 150 minutes of moderate to vigorous activity per week | Restriction of energy intake; Individualized energy intake; face to face/telephone call | Target weight was not specified | Individualized PA goals; 4 week walking plans at each monthly counseling, a pedometer and a weight logbook for self monitoring; a minimum of 3 structured telephone calls to identify progress towards goals, achievements and challenges and to provide positive feedback and support |
| Østby et al., 2009 | Individual and group | 9 months | Walking | N/R | 30 minutes per day for 5 days per week | Restriction of energy intake; 8 group sessions | Target weight was not specified | 10 PA group sessions plus six counseling sessions via telephone during 9 months; a study notebook for self-monitoring and reference, a pedometer; a sport stroller |
| Walker et al., 2011 | Group | 13 weeks | Walking | N/R | Not specified | Personalized written guidelines on diet | Target weight was not specified | Weekly ethnic-specific group sessions for 2 hours during 13 weeks, individualized goal setting related to PA and diet, pedometers; a project notebook for self-monitoring and reference |
Note: PA = physical activity, GWG = gestational weight gain, IOM = Institute of Medicine, N/A = not applicable, N/R = not reported,
The study shows significant intervention in effective weight change.
Except the two physical activity alone interventions (Barakat et al., 2009; Ong et al., 2009), the rest 6 physical activity plus diet interventions had either group or individual counseling on physical activity, nutrition, and weight during pregnancy. The frequency of the counseling ranged from once at the study enrollment (Nascimento et al., 2011; Phelan et al, 2011; Polley et al., 2002), 3 times (Guelincks et al., 2010), to 4-6 times (Vinter et al., 2011). Phelan et al. (2002) provided 3-brief supportive phone calls (10-15 minutes) and additional brief supportive phone calls (2 calls/month) to those who were over or under weight gain guidelines. Polley et al. (2002), also provided additional individualized counseling to those who exceed weight gain guidelines. Feedbacks on participants' weight were given by sending a participant a personalized graph of their weight gain after each clinic visit in two studies (Phelan et al, 2011; Polley et al., 2002). Prompts to promote healthy eating and exercise habits were mailed weekly via automated postcards (Phelan et al., 2011) and biweekly via newsletters (Polley et al., 2002). For self-monitoring for exercise, monthly exercise journals (Nascimento et al., 2011) and pedometers (Phlen et al., 2011; Vinter et al., 2011) were used. Phelan et al. (2011) also utilized food records for healthy eating and weight scales for weight gain for self-monitoring. A study provided free membership to a fitness center to participants (Vinter et al., 2011).
Interventions targeting postpartum women
Four randomized controlled trials were included, involving a total of 547 women who were OW/OB postpartum women (Table 1). Three studies were conducted in the U.S. (Lovelady et al., 2000; Østby et al., 2009; Walker et al., 2011) and one study was conducted in the U.K. (Craigie et al., 2011). Two studies initiated their programs with a relatively wide-open period: sometime between 6 months postpartum and 18 months postpartum (Craigie et al., 2011) or between 6 weeks postpartum and 12 months postpartum (Walker et al., 2011). Two studies initiated when all participants were either 4 week postpartum (Lovelady et al., 2000) or 6 week postpartum (Østby et al., 2009). Lovelady et al. included breastfeeding women only while two studies included women with low-income status (Craigie et al., 2011; Walker et al., 2011). The duration of three interventions ranged between 10 and 13 weeks (Craigie et al., 2011; Lovelady et al., 2000; Walker et al., 2011) whereas the duration of one intervention was 9 months (Østby et al., 2009).
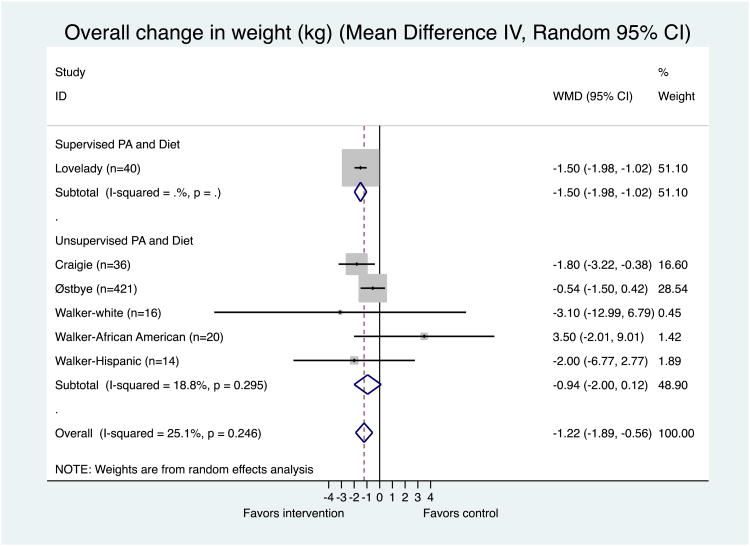
The meta-analysis on the results of the individual studies is depicted in Figure 3. Since Walker et al. (2011) reported a mean weight change for each ethnic group (White, African American, Hispanic) and the contents of the interventions were slightly different by ethnic group, three WMDs were calculated for this study, using a random-effects model. The result of the meta-analysis suggested a significant weight reduction on average in the intervention groups (p < 0.001), with a WMD of −1.22 kg (95% CI: −1.89, −0.56). Supervised physical activity plus diet study (Lovelady et al., 2000) showed a significant treatment effect compared to women in the control group (WMD = −1.50 kg; 95% CI: −1.98, −1.02; p = 0.000). Overall, women in unsupervised physical activity programs rather lost more weight than women in the control groups, but it was not statistically significant (WMD = −0.94 kg; 95% CI: −2.00, 0.12; p = 0.082) (Figure. 3). The unsupervised physical activity plus diet intervention composed of personalized prescription/goals of physical activity (Craigie, et al., 2011) showed the greatest treatment effect followed by a supervised physical activity plus diet intervention (Lovelady, et al., 2000). There was low heterogeneity among individual studies as indicated by Higgins' I2. The funnel plot, Egger's test, and Begg's test indicated no considerable publication bias (Appendix 3).
Figure 3. Meta-Analysis of the effectiveness of physical activity interventions compared to comparison group in 6 interventions (4 papers) targeting postpartum women.
Heterogeneity: Chi2 = 6.68, df = 5 (p = 0.246); I2 = 25.1%
Test for overall effect: z= 3.60 p = 0.000
The area of the gray square reflects the weight that the study contributes to the meta-analysis.
All trials consisted of physical activity as well as diet counseling. A supervised physical activity intervention was offered for 45 minutes per day for 4 days per week in one study (Lovelady, 2000) while three other studies provided a pedometer to each participant in the intervention groups -- along with a 4-week walking plan and a personalized physical activity goal (Craigie et al., 2011), information on strategies to increase the intensity and duration of daily physical activity (Walker et al., 2011), or a sport stroller at 6-months postpartum (Østby et al., 2009). The details of the intervention components are described in Table 2. The individualized prescription for energy intake along with six commercial low-fat and low-calorie frozen dinners each week for the 10-week study period was implemented in one study (Lovelady, 2000). Craigie et al. (2011) delivered 3 face-to-face counseling sessions at monthly intervals while Walker et al. (2011) delivered weekly 2-hour long ethnic-specific weight loss group sessions. Østby et al. (2009) provided 10 physical activity group sessions, 8 healthy-eating sessions, and 6 telephone counseling sessions over a 9-month period.
Discussion
In this systematic review and meta-analysis, we found that supervised physical activity plus diet had a significantly lower average gestational weight gain (−1.17 kg) for OW/OB pregnant women and a significantly higher average weight reduction (−1.50 kg) for OW/OB postpartum women in the intervention groups compared with women in the control groups. In a meta-analysis of 12 RCTs of interventions comprised solely of physical activity regardless of pregnant women's BMI status, the finding suggested that women in the intervention groups had a significantly lower average gestational weight gain (WMD = −0.61 kg; 95% CI: −1.17, −0.06) compared to women in the control groups (Streuling et al., 2011). Streuling et al. (2010) reported that physical activity plus diet interventions significantly reduced gestational weight gain in a meta-analysis of 4 RCTs and 5 non-RCTs. Also, Sui and colleagues (2011) recently published a systematic review of 5 supervised physical activity interventions on outcomes including gestational weight gain in OW/OB pregnant women and concluded that supervised physical activity interventions were associated with lower gestational weight gain (WMD = −0.36 kg; 95% CI: −0.64, −0.09) when compared with standard antenatal care. In a meta-analysis of 4 RCTs and quasi−randomized trials of physical activity plus diet intervention regardless of postpartum women's BMI status, women in the intervention groups showed significantly larger weight loss (four studies; n= 169; WMD = −2.89 kg; 95% CI: –4.83, −0.95) compared to women in the control groups (Amorim et al., 2007). Along with those reviews, this study supports the conclusion that supervised physical activity plus diet interventions are most effective in managing weight regardless of body weight status in pregnant as well as postpartum women.
Our findings indicate a potential reduction in gestational weight gain of 0.9 kg and postpartum weight loss of 1.22 kg by interventions under study. Any attempt to manage weight during pregnancy and postpartum may be of relevance at a population level, but innovative strategies to maximize the intervention effect should also be developed and tested in this population. Given that there is an increase in the use of mobile phones, wireless devices, and social media among pregnant and postpartum women, these channels may be excellent modes of delivering health promotion programs. For example, in a systematic review, the use of text-messaging or smart phone applications showed some potential in improving physical inactivity and reducing weight, and they were well accepted by non-pregnant study participants (Stephens and Allen, 2012). The use of mobile applications as a tool to give prompts, provide self-monitoring, and give feedback on the user's progress is promising and needs to be evaluated.
There was no study that initiated an intervention during pregnancy and continued to have that intervention during postpartum. Polley et al. (1992) implemented a behavioral lifestyle intervention on physical activity, diet, and weight gain during pregnancy in pregnant women and assessed weight loss at 6 weeks postpartum, but no intervention was delivered during postpartum. Fear of harming self/unborn baby, general physical discomfort, discouragement to undertake physical tasks by people around them, and positive view or encouragement toward over-eating were found to contribute a general decline in physical activity and risks in excessive weight gain during pregnancy in a systematic review of quantitative and qualitative data regarding weight management in pregnancy (Campbell et al., 2011). The lack of information or inconsistent information regarding pregnant women's physical activity and healthy dietary behaviors, and negative beliefs or attitudes of people (partner, peers, and wider family) around pregnant women were also addressed as factors that undermine reducing excessive weight gain in pregnancy in intervention studies. Despite these factors, pregnancy is often called “teachable moment” for pregnant women to adopt health-promoting behaviors when these behaviors are perceived to improve women as well as their babies' health. During postpartum, adjustment to motherhood including caring a newborn makes it hard for postpartum women to prioritize their own health behavior efforts and to adopt a new behavior. Poor retention rates were reported in weight management programs conducted with postpartum women, although those who adhered to the programs were more likely to lose weight (McIntyre, et al., 2012; O'Toole, et al., 2003; Østbye et al., 2009). That is, pregnancy may be a good timing for adopting physical activity and healthy dietary behaviors, and postpartum for maintaining these behaviors. Given this challenging, transitional period of life, motivation to change or initiate weight management behaviors and self-efficacy in their ability to self-manage their conditions need to be addressed within the social cognitive theory (SCT) or the theory of planned behavior (TPB). Thus, future studies need to develop interventions initiated during pregnancy to prevent excessive weight gain during pregnancy and continued during postpartum to prevent excessive weight retention and test their efficacies and cost-effectiveness in OW/OB women.
Although Walker et al. (2011) attempted to develop a ethnic-specific weight-loss intervention for low-income postpartum women including African American, Hispanic, and White/Anglo, women in the control group lost more weight than women in the intervention group for African American only, indicating that the intervention was not effective. Also, Polley et al. (1992), which included low-income pregnant women showed the similar results to Walker et al. (2011). Quantitative as well as qualitative studies to understand how low-income status or ethnicity influence women's attitude toward physical activity, dietary behaviors, and weight management during pregnancy and postpartum should be conducted. Furthermore, cultural contexts that low-income African American women may face and that present challenges unique to their ethnic groups need to be explored in future studies.
All of the studies reviewed in this paper included intervention components that may influence OW/OB pregnant or postpartum women's physical activity or dietary behaviors at the individual level only (e.g., providing counseling, feedback on their weights) and targeted OW/OB pregnant or postpartum women only. Exploration of family and community related factors such as social support from spouse or environmental support in this population and development of interventions that target family, especially spouses, would be worthwhile since social support from spouse is reported as a major enabler of regular physical activity in childbearing and childrearing women (Albright, et al., 2005; Brown, et al., 2001; Evenson, et al., 2009). The recipient of the intervention may need to broaden to the wider family and social network surrounding pregnant women since lay beliefs about physical activity, healthy dietary behaviors, and weight management often contradict messages from health professionals (Campbell et al., 2011).
Despite various strategies used in the interventions, only a few studies showed significant effects in weight management among OW/OB pregnant women (Vinter et al., 2011) and OW/OB postpartum women (Craigie et al., 2011; Lovelady et al., 2000). We indicated those studies with asterisk in Table 2. When we checked the intervention strategies or components in those studies, we found some common characteristics. First, two of the studies (Lovelady et al., 2000; Vinter et al., 2011) were supervised physical activity plus diet interventions. Second, individualized physical activity intensity or goal along with energy intake restriction was implemented (Lovelady et al., 2000; Craigie et al., 2011). Third, target weight was specified (Lovelady et al., 2000; Vinter et al., 2011). Those strategies or components should be included in weight control programs for OW/OB pregnant or postpartum women in future studies.
Strengths and limitations
In general, a combination of physical activity and diet intervention has shown to be more effective than a physical activity intervention alone in weight management. However, the effect of physical activity plus diet intervention has not been well examined in pregnant women. A previous systematic review lumped antenatal behavioral interventions including physical activity plus diet interventions into dietary interventions (Dodd et al., 2010) and another systematic review included studies consisting of supervised physical activity only in OW/OB pregnant women (Sui et al., 2012). To our knowledge, this paper is the first systematic review performed to examine the effect of antenatal physical activity alone as well as physical activity plus diet interventions on gestational weight gain in OW/OB pregnant women. Since this review included physical activity alone and physical activity plus diet interventions, it was possible to compare their effects on gestational weight gain and find new insights for future studies. More rigorous inclusion criteria could restrict this review to interventions, which target only supervised physical activity, but in practice, knowledge of the effectiveness of physical activity plus diet interventions is relevant and important.
Research on antenatal interventions including physical activity as a weight management strategy in OW/OB pregnant or postpartum women is in early stage. The numbers of studies reviewed in previous systematic reviews were small (< 10 studies, Dodd et al., 20010; Sui et al., 2012). We also found 11 studies combining 7 studies for pregnant women and 4 studies for postpartum women. Although we tried to determine the effectiveness of interventions by choosing RCTs only to minimize study heterogeneity and ensure comparability of studies, the reviewed interventions were heterogeneous and their qualities varied. Since we did not select the reviewed papers based on their qualities and we only had a small sample size of the reviewed papers, our estimates might have been biased. Thus, caution is needed when interpreting the findings of this review.
Since the proportions of women who exceeded the IOM recommendation for pregnant women were not reported in two papers (Barakat et al., 2009; Ong et al., 2009), and the recommended level for the overweight was used for the obese (Guelinckx et al., 2010; Phelan et al., 2011; Polley et al., 2002), the summary statistic based on weight change seems a better option than findings reported regarding the IOM recommendation. The information on losing at least certain % of final pregnancy weight for postpartum women would be better than overall weight change during postpartum, but this information was also not available for calculation due to lack of data in three papers (Lovelady et al., 2000; Craigie et al., 2011; Walker et al., 2011). Because the point in which postpartum women were included in this paper varied from 4 weeks to 18 months postpartum, the weight loss over time of follow-up (i.e., how many kilograms lost per month) should have been included. However, we could not analyze the weight loss over time of follow-up due to the lack of related-data in the reviewed papers and could only report the overall weight change.
This review may contain a level of error due to publication bias since we did not include studies in other languages other than English and Korean and did not attempt to contact authors of non-published studies. It is possible that studies with significant results were published more frequently than studies without significant results. In this review, six studies out of the included studies did not contain significant results. However, the potential for misinterpretation due to the possible bias of published studies should be considered.
Conclusion
A remarkable opportunity for long-term health effect exists during childbearing and childrearing period and taking advantage of it may influence not only an individual woman but also her offspring. The evidence suggests supervised physical activity plus diet programs were most effective in managing weight among OW/OB pregnant and postpartum women. However, advice alone to promote physical activity without a personalized physical activity prescription/goal in physical activity plus diet interventions were not effective enough to prevent excessive gestational weight gain or postpartum weight retention for OW/OB women. These findings highlight effective approaches for managing healthy weight for OW/OB pregnant as well as postpartum women. Furthermore, this systematic review provides a gateway for continued research on weight-related health behavior strategies for OW/OB pregnant as well as postpartum women.
Supplementary Material
Highlights.
Supervised physical activity plus diet interventions were the most effective.
Innovative strategies to maximize the intervention effect should be developed.
Interventions are needed to start during pregnancy and continue during postpartum.
Acknowledgments
The authors acknowledge Preventive Medicine's anonymous reviewers' invaluable comments. The authors also acknowledge Ms. Gloria Won at H.M. Fishbon Memorial Library, University of California, San Francisco Medical Center at Mount Zion for her assistance in developing search strategies, and Dr. Stephen Bent at University of California, San Francisco for his statistical consultation. This work was supported by the National Heart, Lung, Blood Institution (3R01HL104147–02S1) and NIH/NCRR UCSF-CTSI (UL1 RR024131). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ACOG (American College of Obstetricians and Gynecologists) 2002. ACOG committee opinion. Exercise during pregnancy and the postpartum period. 2002 Jan;Number 267 doi: 10.1016/s0020-7292(02)80004-2. [DOI] [PubMed] [Google Scholar]
- American College of Obstetricians and Gynecologists. International Journal of Gynaecology and Obstetrics. 77(1):79–81. doi: 10.1016/s0020-7292(02)80004-2. [DOI] [PubMed] [Google Scholar]
- American College of Sports Medicine. Impact of physical activity during pregnancy and postpartum on chronic disease risk. Med Sci Sports Exerc. 2006;38:989–1006. doi: 10.1249/01.mss.0000218147.51025.8a. [DOI] [PubMed] [Google Scholar]
- Albright CL, Maddock JE, Nigg CR. Physical activity before pregnancy and following childbirth in a multiethnic sample of healthy women in Hawaii. Women Health. 2005;42:95–110. doi: 10.1300/j013v42n03_06. [DOI] [PubMed] [Google Scholar]
- Amorim Adegboye ARA, Linne YM, Lourenco PMC. Diet or exercise, or both, for weight reduction in women after childbirth. Cochrane Database of Systematic Reviews. 2007;3 doi: 10.1002/14651858.CD005627.pub2. Art. No.: CD005627. [DOI] [PubMed] [Google Scholar]
- Asbee SM, Jenkins TR, Butler JR, White J, Elliot M, Rutledge A. Preventing excessive weight gain during pregnancy through dietary and lifestyle counseling. Am Coll of Obstet and Gynecol. 2009;113:305–311. doi: 10.1097/AOG.0b013e318195baef. [DOI] [PubMed] [Google Scholar]
- Baeten JM, Bukusi EA, Lambe M. Pregnancy complications and outcomes among overweight and obese nulliparous women. Am J Public Health. 2001;91:436–40. doi: 10.2105/ajph.91.3.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barakat R, Lucia A, Ruiz JR. Resistance exercise training during pregnancy and newborn's birth size: a randomised controlled trial. Int J Obes. 2009;33:1048–1057. doi: 10.1038/ijo.2009.150. [DOI] [PubMed] [Google Scholar]
- Bertz F, Brekke H, Ellegard L, Wennergren M, Rasmussen KM, Winkvist A. Dietary restriction or dietary restriction and exercise, but not exercise intervention alone, reduces weight and fat mass in overweight and obese women postpartum. The FASEB journal. 2010;24(Meeting Abstract Supplement):343.5. [Google Scholar]
- Brankston GN, Mitchell BF, Ryan EA, Okun NB. Resistance exercise decreases the need for insulin in overweight women with gestational diabetes mellitus. Am J Obstet Gynecol. 2004;190:188–193. doi: 10.1016/s0002-9378(03)00951-7. [DOI] [PubMed] [Google Scholar]
- Brown PR, Brown WJ, Miller YD, Hansen V. Perceived constraints and social support for active leisure among mothers with young children. Leisure Sciences. 2001;23:131–144. [Google Scholar]
- Campbell F, Johnson M, Messina J, Guillaume L, Goyder E. Behavioural interventions for weight management in pregnancy: A systematic review of quantitiative and qualitative data. BMC Public Health. 2011;11:491. doi: 10.1186/1471-2458-11-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalcante SR, Cecatti JG, Pereira RI, Baciuk EP, Bernardo AL, Silveira C. Water aerobics II: Maternal body composition and perinatal outcomes after a program for low risk pregnant women. Reproduct Health. 2009;6:1. doi: 10.1186/1742-4755-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedergren M. Effects of gestational weight gain and body mass index on obstetric outcome in Sweden. Int J Gynaecol Obstet. 2006;93:269–274. doi: 10.1016/j.ijgo.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Chang MW, Nitzke S, Brown R. Design and outcomes of a Mothers in Motion behavioral intervention Pilot study. J Nutr Educ Behav. 2010;42:s1–s11. doi: 10.1016/j.jneb.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd. Lawrence Erlbaum Associates; Hillsdale, NJ: 1988. [Google Scholar]
- Craigie AM, Macleod M, Barton KL, Treweek S, Anderson AS, Anderson AS, WeighWell team Supporting postpartum weight loss in women living in deprived communities: design implications for a randomized control trial. Eur J Clin Nutr. 2011;65:952–958. doi: 10.1038/ejcn.2011.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport MH, Giroux I, Sopper MM, Mottola MF. Postpartum exercise regardless of intensity inproves chronic disease risk factors. Med Sci Sports Exerc. 2011;6:951–958. doi: 10.1249/MSS.0b013e3182051155. [DOI] [PubMed] [Google Scholar]
- Dodd JM, Grivell RM, Crowther CA, Robinson JS. Antenatal interventions for overweight or obese pregnant women: a systematic review of randomised trials. Int J Obestet Gynecol. 2010;117:1316–1326. doi: 10.1111/j.1471-0528.2010.02540.x. [DOI] [PubMed] [Google Scholar]
- Evenson DA, Savitz KR, Huston SL. Leisure-time physical activity among pregnant women in the US. Paediatr Perinat Epidemiol. 2004;18:400–407. doi: 10.1111/j.1365-3016.2004.00595.x. [DOI] [PubMed] [Google Scholar]
- Evenson KR, Moos MK, Carrier K, Siega-Riz AM. Perceived barriers to physical activity among pregnant women. Matern Child Health J. 2009;13:364–375. doi: 10.1007/s10995-008-0359-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavard JA, Artal R. Effect of exercise on pregnancy outcome. Clin Obstet Gynecol. 2008;51:467–480. doi: 10.1097/GRF.0b013e31816feb1d. [DOI] [PubMed] [Google Scholar]
- Gray-Donald K, Robinson E, Collier A, David K, Renaud L, Rodridgues S. Intervening to reduce weight gain in pregnancy and gestational diabetes mellitus in Cree Communities: an evaluation. Can Med Assoc or its licensors. 2000;163(10):1247–1251. [PMC free article] [PubMed] [Google Scholar]
- Guelinckx I, Devlieger R, Mullie P, Vansant G. Effect of lifestyle intervention on dietary habits, physical activity, and gestational weight gain in obese pregnant women: a randomized controlled trial. Am J Clin Nutr. 2010;91:373–380. doi: 10.3945/ajcn.2009.28166. [DOI] [PubMed] [Google Scholar]
- Gunderson EP, Abrams B. Epidemiology of gestational weight gain and body weight changes after pregnancy. Epidemiol Rev. 1999;21:261–275. doi: 10.1093/oxfordjournals.epirev.a018001. [DOI] [PubMed] [Google Scholar]
- Haakstad L, Bo K. Effect of supervised aerobic dance exercise in prevention of excessive weight gain in pregnancy: A single blind randomized controlled trial. Int J Gynecol Obstet. 2009;107:S198. [Google Scholar]
- Higgins JP, Altman DG, Gøtzsche PC, Jűni P, Moher D, Oxman AD, Savović J, Schulz KF, Weeks L, Sterne JAC Cochrane Bias Methods Group, Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analysis. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang TT, Yeh CY, Tsai YC. A diet and physical activity intervention for preventing weight retention among Taiwanese childbearing women: a randomised controlled trial. Midwifery. 2011;27:257–264. doi: 10.1016/j.midw.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Hui A, Back L, Ludwig S, Gardiner P, Sevenhuysen G, Dean H, Sellers E, Mcgavock J, Morris M, Bruce S, Murray R, Shen GX. Lifestyle intervention on diet and exercise reduced excessive gestational weight gain in pregnant women under a randomised controlled trial. BJOG Int J Obstet Gynaecol. 2011;119:70–77. doi: 10.1111/j.1471-0528.2011.03184.x. [DOI] [PubMed] [Google Scholar]
- Kac G, D'Aquino Benicio MH, Valente JG, Velasquez-Melendez G. Postpartum weight retention among women in Rio de Janeiro: a follow-up study. Rep Public Health. 2003;19:149S–161S. doi: 10.1590/s0102-311x2003000700016. [DOI] [PubMed] [Google Scholar]
- Kiel DW, Dodson EA, Artal R, Boehmer TK, Leet TL. Gestational weight gain and pregnancy outcomes in obese women: how much is enough? Obstet Gynecol. 2007;110:752–758. doi: 10.1097/01.AOG.0000278819.17190.87. [DOI] [PubMed] [Google Scholar]
- Kim SY, Dietz PM, England L, Morrow B, Callaghan WM. Trends in pre-pregnancy obesity in nine states, 1993-2003. Obes. 2007;15:986–993. doi: 10.1038/oby.2007.621. [DOI] [PubMed] [Google Scholar]
- Lewis BA, Martinson BC, Sherwood NE, Avery MD. A pilot study evaluating a telephone-based exercise intervention for pregnant and postpartum women. J Midwifery Womens Health. 2011;56(2):127–131. doi: 10.1111/j.1542-2011.2010.00016.x. [DOI] [PubMed] [Google Scholar]
- Lovelady CA, Garner KE, Moreno KL, Williams JP. The effect of weight loss in overweight, lactating women on the growth of their infants. N Engl J med. 2000;342:449–453. doi: 10.1056/NEJM200002173420701. [DOI] [PubMed] [Google Scholar]
- Luoto RM, Kinnunen TI, Aittasalo M, Osala K, Mansikkämaki K, Toropainen E, Kolun P, Vasankari T. Prevention of Gestational diabetes: Design of cluster Randomized controlled trial and one- year follow up. BMC Pregnancy Childbirth. 2010;10:39. doi: 10.1186/1471-2393-10-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre HD, Peacock A, Miller YD, Koh D, Marshall AL. Pilot study of an individualised early postpartum intervention to increase physical activity in women with previous gestational diabetes. [accessed on 9/26/2012];Int J Endocrinol. 2012 doi: 10.1155/2012/892019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Schulz KF, Altman D, the CONSORT Group The CONSORT statement: Revised recommendations for improving the quality of reports of parallelgroup randomized trials. JAMA. 2001;285:1987–1991. doi: 10.1001/jama.285.15.1987. [DOI] [PubMed] [Google Scholar]
- Nascimento SL, Surita FG, Parpinelli MÂ, Siani S, Pinto e Silva JL. The effect of an antenatal physical exercise program on maternal/perinatal outcomes and quality of life in overweight and obese pregnant women: a randomized clinical trial. BJOG: Int J Obstet Gynaecol. 2011;118:1455–1463. doi: 10.1111/j.1471-0528.2011.03084.x. [DOI] [PubMed] [Google Scholar]
- O'Toole ML, Sawicki MA, Artal R. Structured diet and physical activity prevent postpartum weight retention. J Women's Health. 2003;10:991–998. doi: 10.1089/154099903322643910. [DOI] [PubMed] [Google Scholar]
- Ong MJ, Guelfi KJ, Hunter T, Wallman KE, Fournier PA, Newnham JP. Supervised home-based exercise may attenuate the decline of glucose tolerance in obese pregnant women. Diavestes Metab. 2009;35:418–421. doi: 10.1016/j.diabet.2009.04.008. [DOI] [PubMed] [Google Scholar]
- Østbye T, Krause KM, Lovelady CA, Morey MC, Bastian LA, Peterson BL, Swamy GK, Brouwer RJN, McBride CM. Active mothers postpartum: A randomized controlled weight-loss intervention trial. Am J Prev Med. 2009;37:173–180. doi: 10.1016/j.amepre.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan S, Phipps MG, Abrams B, Darroch F, Schaffner A, Wing RR. Randomized trial of a behavioral intervention to prevent excessive gestational weight gain: the Fit for Delivery Study. Am J Clin Nutr. 2011;93:772–779. doi: 10.3945/ajcn.110.005306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polley BA, Wing RR, Sims CJ. Randomized controlled trial to prevent excessive weight gain in pregnant women. Int J Obes. 2002;26:1494–1502. doi: 10.1038/sj.ijo.0802130. [DOI] [PubMed] [Google Scholar]
- Rooney BL, Schauberger CW, Mathiason MA. Impact of perinatal weight change on long-term obesity and obesity-related illnesses. Obstet Gynecol. 2005;106:1349–1356. doi: 10.1097/01.AOG.0000185480.09068.4a. [DOI] [PubMed] [Google Scholar]
- Rossner S, Ohlin A. Pregnancy as a risk factor for obesity: lessons from the Stockholm Pregnancy and Weight Development Study. Obes Res. 1995;3:267S–275S. doi: 10.1002/j.1550-8528.1995.tb00473.x. [DOI] [PubMed] [Google Scholar]
- Santos IA, Stein R, Fuchs SC, Duncan BB, Ribeiro JP, Kroeff LR, Carballa MT, Schmidt MI. Aerobic Exercise and submaximal functional capacity in overweight pregnant women: A randomized trial. Obstet Gynecol. 2005;106(2):243–249. doi: 10.1097/01.AOG.0000171113.36624.86. [DOI] [PubMed] [Google Scholar]
- Schaar B, Moos-Thiele C, Platen P. Effects of exercise, diet, and a combination of exercise and diet in overweight and obese adults-a meta-analysis of the data. Open Sport Med J. 2010;4:17–28. [Google Scholar]
- StatCorp. Stata Data Analysis and Statistical Software: release 12. StataCorp LP; 2011. [Google Scholar]
- Stephens J, Allen J. Mobile phone interventions to increase physical activity and reduce weight: A systematic review. [accessed on 9/26/2012];J Cardiovasc Nurs. 2012 doi: 10.1097/JCN.0b013e318250a3e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuling I, Beyerlein A, von Kries R. Can gestational weight gain be modified by increasing physical activity and diet counseling? A meta-analysis of interventional trials. Am J Clin Nutr. 2010;92:678–687. doi: 10.3945/ajcn.2010.29363. [DOI] [PubMed] [Google Scholar]
- Streuling I, Beyerlein A, Rosenfeld E, Hofmann H, Schulz T, von Kries R. Physical activity and gestational weight gain: a meta-analysis of intervention trials. BJOG. 2011;118:278–284. doi: 10.1111/j.1471-0528.2010.02801.x. [DOI] [PubMed] [Google Scholar]
- Sui Z, Rosalie M, Dodd JM, Dodd G. Antenatal exercise to improve outcomes in overweight or obese women: a systematic review. Acta Obstet Gynecol Scand. 2012;91:538–545. doi: 10.1111/j.1600-0412.2012.01357.x. [DOI] [PubMed] [Google Scholar]
- Vinter CA, Jensen DM, Ovesen P, Beck-Nielsen H, Jørgensen JS. The LiP (Lifestyle in Pregnancy) Study: A randomized controlled trial of lifestyle intervention in 360 obese pregnant women. Diabetes Care. 2011;34:2502–2507. doi: 10.2337/dc11-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LO, Sterling BS, Latimer L, Kim SH, Garcia AA, Fowles ER. Ethnic-specific weight-loss interventions for low-income postpartum women: findings and lessons. West J Nurs Res. 2011;34:654–676. doi: 10.1177/0193945911403775. [DOI] [PubMed] [Google Scholar]
- Yeo S. Adherence to walking or stretching and risk of preeclampsia in sedentary pregnant women. Res Nurs Health. 2009;32:379–390. doi: 10.1002/nur.20328. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.