Abstract
CCN2, also known as connective tissue growth factor, is a member of the CCN (CCN1–6) family of modular matricellular proteins. Analysis of CCN2 function in vivo has focused primarily on its key role as a mediator of excess ECM synthesis in multiple fibrotic diseases. However, CCN2 and related family members are widely expressed during development. Recent studies using new genetic models are revealing that CCN2 has essential roles in the development of many tissues. This review focuses on current and emerging data on CCN2 and its functions in chondrogenesis and angiogenesis, and on new studies showing that CCN2 has essential functions during embryonic and postnatal development in a number of epithelial tissues.
Keywords: CCN2, CTGF, Connective tissue growth factor
Introduction
CCN2, also known as connective tissue growth factor (CTGF) is a member of the CCN (CCN1–6) family of modular matricellular proteins [1]. Analysis of CCN2 function in vivo has focused primarily on its key role as a mediator of excess extracellular matrix (ECM) synthesis in multiple fibrotic diseases. However, CCN2 and related family members are widely expressed during development, and modulate cell adhesion, proliferation, survival, migration, and extracellular matrix production in diverse cell types. A role for CCN2 as an essential regulator of chondrogenesis during development has been established, but recent studies have provided new insights into the mechanisms of CCN2 action in cartilage. Other studies are revealing that CCN2 has essential roles in the development of many other tissues. This review focuses on current and emerging data on CCN2 and its functions throughout embryonic and postnatal development.
CCN2 structural domains and functions
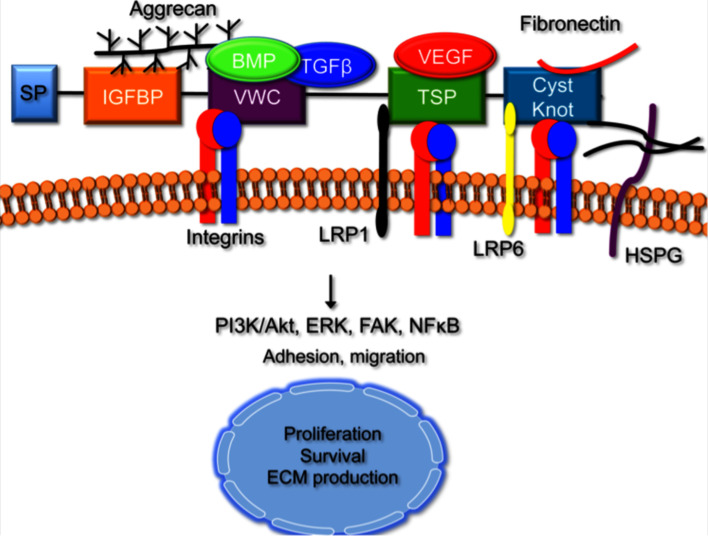
CCN2 and the five other members of the CCN family (CCN1/Cyr61, CCN3/Nov, CCN4/WISP-1/ELM1, CCN5/WISP-2/CTGF-L and CCN6/WISP-3) are cysteine-rich matricellular proteins that contain an N-terminal secretory peptide, followed by four multi-functional domains with a diverse array of binding partners that potentially impact multiple signaling mechanisms [2]. Proteins that interact with CCN2 through recognition of these domains include integrins, low-density lipoprotein receptor-related proteins (LRPs), growth factors, and ECM components (Fig. 1). The first domain shares homology to insulin-like growth factor binding proteins (IGFBPs), but has very low affinity for IGF [3]. The second domain encodes a von Willebrand type C (VWC) repeat. This motif mediates CCN2 interactions with integrins αvβ3, αvβ5 and with growth factors such as bone morphogenetic proteins (BMPs) and transforming growth factor β (TGFβ) [1, 4–6]. Recently, the IGFBP and VWC domains, which comprise the N-terminal half of CCN2, have been shown to bind to aggrecan, the major proteoglycan produced by chondrocytes [7]. The third domain is a type-1 thrombospondin (TSP) repeat, known to mediate the ability of TSP to bind to ECM proteins, matrix metalloproteinases (MMPs), and integrins. This domain modulates CCN2 interactions with VEGF and LRP1 [6, 8]. The final C-terminal (CT) motif contains a cysteine knot similar to those present in many growth factors, including members of the TGFβ super family, platelet derived growth factor (PDGF), and nerve growth factor (NGF). This domain is also found in other secreted proteins, including WISE, slit, and mucins [9–11]. It mediates CCN2 interactions with LRP6, fibronectin, perlecan, and fibulin-1 [12–14].
Fig. 1.
Functional Domains of CCN Family Members. The CCN family is comprised of six members that contain four conserved functional domains. Each domain is likely to contribute both independently and cooperatively to the overall function of the CCN family members. The insulin-like growth factor binding protein (IGFBP) domain and the von Willebrand factor C (VWC) domain both bind to aggrecan. The VWC domain also binds to BMPs and TGFβ, integrins and matrix metalloproteinases (MMPs). The third motif is the thrombospondin (TSP) domain, which interacts with integrins, growth factors, vascular endothelial growth factor (VEGF) and low-density lipoprotein receptor-related proteins (LRPs). The last structural module is the C-terminal domain, which contains the cysteine knot motif. This motif binds to integrins, heparin sulfate proteoglycans (HSPGs), fibronectin and LRPs. CCN5 lacks this structural motif, but can still function similarly to other CCN family members. Downstream signaling via integrins activates multiple cell signaling pathways that mediate cell survival, migration, and ECM production
Interactions between CCN2 and these binding partners mediate the effects of CCN2 on cell proliferation, survival, differentiation, adhesion, migration, and ECM production [15]. However, to date, none of the in vivo activities of CCN2 have been unequivocally attributed to any of these specific interactions. This is due in part to the fact that the in vivo roles of CCN2 in development and homeostasis have not been completely defined, and because CCN2 most likely mediates its effects through multiple mechanisms that are tissue specific.
CCN2 expression during embryonic and postnatal development
CCN2 is conserved in chordates but is not found in invertebrates. Where studied, its pattern of expression appears to be largely conserved. For example, in zebrafish, Xenopus, and mouse, CCN2 expression is first detected in somites, floorplate, and notochord [16–18]. The role of CCN2 in these tissues is essentially unknown. In zebrafish, the injection of morpholinos against CCN2 led to developmental delays and distortion of the notochord. However, the nature of these defects has not been clarified, and they are not seen in Ccn2 null mice or in Xenopus embryos treated with morpholinos [1, 12, 18, 19]. CCN2 is co-expressed with the related family member CCN1 in the notochord (unpublished data), raising the possibility of overlapping functions for these CCN proteins in early stages of notochord formation.
CCN2 is expressed in a wide variety of structures at later stages of development. In the mouse, CCN2 is expressed at high levels in the developing cartilage and vasculature [18, 20, 21]. Other prominent sites of expression include various epithelial structures, such as bronchial and alveolar epithelium of the lung, pancreatic duct and endocrine cells, and salivary glands [20, 22]. As discussed below, recent studies indicate that CCN2 plays essential roles in the development of many of these structures. CCN2 is also expressed in maturing neurons, and in sensory organs such as the otic placode [6, 16, 17, 20]. To date, the functions of CCN2 in neural and sensory structures have not been investigated.
CCN2 expression is maintained in adult tissues, and is increased in pathological conditions such as fibrosis, atherosclerosis, osteoarthritis, and certain cancers [23]. In the adult mouse, CCN2 is expressed in the reproductive system, where it has been implicated in control of uterine cellular growth, adhesion, migration, and ECM production during the estrous cycle and pregnancy [24].
CCN2 and fibrosis
In vivo studies of CCN2 function have focused almost entirely on its role in fibrosis. Fibrosis is caused by excessive extracellular matrix (ECM) deposition mediated by activated contractile fibroblasts termed myofibroblasts [25]. CCN2 over-expression is a hallmark of fibrosis in multiple tissues, including skin, liver, heart, lung, and kidney, and is widely thought to be required to mediate the profibrotic effects of TGFβ. However, the extent to which CCN2 causes fibrosis, (or is merely a marker of fibrosis in different organs), is unknown. A number of excellent reviews on this topic have been published recently [15, 26, 27]. Hence, we focus here on the development of several new transgenic models of CCN2 over-expression, which have revealed unexpected actions of CCN2 in the onset and progression of fibrosis in vivo.
The expression of CCN2 during normal wound healing and in various fibrotic diseases [25, 28] has prompted many studies of CCN2 function in wound healing and in chemically induced fibrosis [25]. However, in vivo studies have been limited, in part because suitable transgenic models have not been available. Mice over-expressing CCN2 in podocytes [28] or liver [29] are normal. Increased fibrosis was observed in these transgenic models only upon tissue injury. These findings may indicate that CCN2 alone is not sufficient to induce fibrosis in these organs. An alternative explanation is that levels of CCN2 expression were not high enough to induce fibrosis. A new transgenic model, described below, suggests the latter possibility may be true with respect to the kidney.
Mice over-expressing CCN2 under the control of the fibroblast-specific enhancer of type I collagen (Col1-CCN2) develop systemic multi-organ fibrosis. Excess ECM production was noted in dermis, kidney, and lung. Immunostaining revealed a large increase in the number of myofibroblasts, and consistent with previous in vitro studies, fibroblasts from transgenic mice exhibited increased migratory capability and excessive ECM production [30]. Unlike the podocyte-specific transgenic mice discussed above [28], basement membrane thickening and increased ECM deposition around blood vessels were noted in the kidneys of Col1-CCN2 transgenics. Consistent with the notion that the difference in susceptibility to kidney fibrosis between these two transgenic models relates to CCN2 levels, these effects were dose-dependent, as mice carrying two copies of the Col1-CCN2 transgene insertion had a shorter lifespan and earlier onset of fibrosis than did mice heterozygous for the transgene insertion [30].
Recently, Liu et al. generated a conditional mouse model in which CCN2 was deleted specifically in fibroblasts and smooth muscle cells [31]. Consistent with the findings that CCN2 over-expression in fibroblasts induced systemic fibrosis [30], loss of CCN2 resulted in a marked decrease in bleomycin induced-skin fibrosis [31]. Remarkably, these effects were not TGFβ-dependent, nor did the loss of CCN2 impact the ability of TGFβ to induce alpha smooth muscle actin (α-SMA) or collagen type I expression [31]. The attenuated fibrosis in Ccn2 conditional mutants was attributed to defective myofibroblast migration and recruitment to the site of fibrosis [31]. Thus, these gain- and loss-of-function studies suggest that CCN2 is sufficient to induce fibrosis, and challenge the dogma that CCN2 is required to mediate the pro-fibrotic effects of TGFβ.
The Col1-CCN2 transgenic phenotype described above contrasts with that seen in transgenic mice in which CCN2 expression from the endogenous locus can be increased or decreased [30, 32]. This latter mouse model was developed by introduction of a 3′UTR cassette into the Ccn2 locus that alters Ccn2 mRNA stability relative to the native Ccn2 3′UTR. Cre-mediated recombination excises this 3′UTR and replaces it with one that yields increased Ccn2 mRNA stability compared to the native Ccn2 3′UTR. This novel strategy, when used in conjunction with Ccn2 null mice, permits analysis of the effects of altering Ccn2 expression from 30% of normal to up to ninefold higher than normal [32]. Mice with 30% of normal Ccn2 expression were indistinguishable from wild-type littermates, whereas ninefold over-expression led to developmental delay, craniofacial defects, and early embryonic lethality [32]. The cause of lethality was not investigated in this study, but both the time of lethality and the presence of hemorrhage and altered microvasculature in transgenic over-expressers suggests a cardiovascular defect. The few over-expressers that survived to adulthood exhibited moderate cranial and axial skeletal defects, but no fibrosis. However, these surviving mice were found to exhibit incomplete Cre-mediated recombination [32]. While the present study demonstrates that CCN2 has potent effects during development, it was not informative with respect to adult tissues. However, when used in conjunction with tissue-specific inducible Cre alleles, this transgenic model is likely to be highly informative with respect to CCN2 function in adult tissues that normally express this protein.
Transgenic models have led to unexpected insights into CCN2 function in the heart. Although CCN2 is highly expressed in the developing heart, it is unknown whether CCN2 is required for cardiac development. CCN2 is also markedly elevated during cardiac fibrosis, and can stimulate cardiac smooth muscle cell proliferation [33]. Two recent papers reported the generation of transgenic mice over-expressing CCN2 in the heart [34, 35]. Surprisingly, neither study found any evidence of fibrosis, either basally, or in response to cardiac ischemia/reperfusion injury or acute pressure-overload. In the first model, there was no fibrosis, but induction of an adaptive hypertrophic response that may have contributed to the finding that transgenics were no more susceptible than wild-type littermates to ischemia/reperfusion injury [34]. In the second transgenic model, although CCN2 transgenic hearts exhibited increased myocardial pro-collagen and fibronectin expression, this was counteracted by increased MMP3 levels [35]. When challenged with cardiac ischemia/reperfusion injury, CCN2 over-expression was actually cardio-protective [35].
The discrepancies between these models may be a consequence of the different promoters used. Both alpha-myosin heavy chain (α-MHC) and myosin light chain-2 (MLC-2) are expressed early in developing cardiomyocytes, but as development proceeds, α-MHC expression is confined mainly to the atria, whereas MLC-2 is expressed predominantly in the ventricle [36]. These promoters may also be differentially regulated during cardiac hypertrophy. Nonetheless, these results clearly indicate that CCN2 is not always profibrotic, and can have protective functions in adult tissues. Studies to elucidate the mechanisms underlying these cardioprotective functions are clearly warranted.
Overall, the effects of CCN2 over-expression appear to be both dose-dependent and context-specific. While the above models have confirmed the expected contribution of CCN2 to the onset and/or progression of fibrosis in several tissues in adults, they have led to the discovery of protective functions in others. Given the vast number of growth factors, ECM components, integrins, and other receptors that can interact with CCN2 in vitro, it is likely that CCN2 can exert very different functions at different doses within a given tissue.
CCN2 function in development
The most prominent sites of CCN2 expression in developing embryos are the skeletal and cardiovascular systems [20, 22, 37], and expression in these tissues persists in adults [38]. Essential functions for CCN2 have been demonstrated in multiple aspects of skeletogenesis and angiogenesis. Other recent studies are revealing roles in epithelial tissues. We review some of the major findings from in vivo studies below.
Lung: Both gain- and loss-of-function studies support an essential role for CCN2 in lung formation. Similar to the effects of CCN2 over-expression in hepatocytes, CCN2 adenoviral over-expression in the adult mouse lung is unable on its own to induce chronic fibrosis, but does increase susceptibility to fibrosis following chemical treatment [39, 40]. In contrast, CCN2 over-expression in airway epithelium [41] or alveolar type II epithelial cells [42] during the first 2 weeks of postnatal life in transgenic mice led to thicker alveolar septa, characterized by increased cell proliferation, collagen and fibronectin deposition, myofibroblast differentiation, and fibrosis. Increased levels of integrin-linked kinase (ILK) activation were noted, raising the possibility that the phenotype reflects the ability of CCN2 to engage integrins directly, and/or to increase the expression of other ECM components that bind to these integrins. Moreover, increased stabilization and nuclear translocation of β-catenin, a well-known target of ILK [43] that mediates fibrosis in multiple organs (e.g., [44–46]) was seen in CCN2 transgenic lungs. These findings, along with the TGFβ-independent of ability of CCN2 to induce fibrosis found in the Col1-CCN2 transgenics discussed previously [30], suggest that CCN2 may mediate its pro-fibrotic effects to a significant degree through Wnt pathways. A number of studies have shown that CCN2 expression is induced by canonical Wnt signaling [47, 48]. The demonstration that CCN2 over-expression in turn induces stabilization of β-catenin raises the possibility that CCN2 is an essential component of a positive feedback loop regulating levels of canonical Wnt signaling.
A loss-of-function study also provides strong support for an essential role for CCN2 in alveolarization during lung maturation [49]. Examination of Ccn2 null mice revealed hypoplastic lungs, with reduced cell proliferation, a phenotype opposite to that seen in the above transgenic over-expression models. This study did not examine the consequences of loss of CCN2 on canonical Wnt pathways. However, the authors reported decreased expression of PDGF receptor beta (PDGFRβ), which is primarily expressed by pericytes [49, 50]. Whether the decreased expression of PDGFRβ in Ccn2−/− lungs translates to altered levels of PDGF-mediated signal transduction is unknown. However, an investigation of the role of CCN2 in PDGF signaling is warranted, especially in light of the potent pro-fibrotic properties of PDGF [51].
Skeletal tissues: CCN2 appears to have essential functions in multiple skeletal tissues. CCN2 function has been studied most extensively in cartilage, but recent studies have demonstrated roles in osteoblasts, osteoclasts, intervertebral discs, and cranial sutures.
Cartilage: Endochondral bone formation is a dynamic process in which mesenchymal cells condense and differentiate, resulting in the formation of a growth plate. Chondrocytes in the growth plate are arranged in distinct zones, which include a slowly proliferating reserve population, the rapidly proliferating columnar population, followed by a post-mitotic prehypertrophic zone and finally a terminally differentiated hypertrophic zone. Hypertrophic chondrocytes produce a matrix that subsequently promotes vascular invasion and osteoblast migration, leading to replacement of the cartilage template with bone [52, 53].
CCN2 is not expressed in mesenchymal condensations, but can be detected at the earliest stages of growth plate formation [18]. CCN2 is expressed in proliferating chondrocytes at low levels, and at higher levels in hypertrophic cells [37]. The expression of Ccn2 in growth plate chondrocytes was shown recently to be positively regulated by canonical Wnt pathways via a TCF/LEF motif in the Ccn2 promoter [48]. This finding accounts for the high levels of expression of CCN2 in hypertrophic chondrocytes, which contain the highest levels of stabilized β-catenin in the growth plate [48].
Ccn2 null mice exhibit lethality at birth, accompanied by severe chondrodysplasia [18]. Ccn2−/− chondrocytes exhibited decreased rates of proliferation and survival. These defects were attributed to defective production of multiple ECM components, including type II collagen and aggrecan, the major collagen and proteoglycan, respectively, in cartilage [18, 54]. While the precise mechanisms by which CCN2 mediates its essential role in maintenance of ECM production in the growth plate are unknown, integrins are likely to play key roles. CCN2 is a ligand for integrin α5β1 in chondrocytes, and this integrin is important for chondrocyte adhesion and survival [54]. Moreover, the loss of CCN2 results in a significant decrease in α5 integrin levels and in levels of activation of the downstream effectors of integrins, including focal adhesion kinase (FAK) and extracellular regulated protein kinase in chondrocytes [54].
One of the most unexpected findings in Ccn2 null mice is the expansion of the hypertrophic zone. This was accompanied by decreased expression of VEGF in these cells [18]. VEGF is essential for growth plate angiogenesis [55], and Ccn2−/− growth plates exhibit defective vascularization of the growth plate. Thus, a key function for CCN2 in the hypertrophic zone is to maintain VEGF levels. We investigated the mechanisms by which CCN2 induces VEGF expression in chondrocytes. Hypoxia-inducible factor-1α (HIF-1α) is expressed by chondrocytes and required for chondrocyte survival [52]. It is also a potent inducer of VEGF in many tissues. We found that HIF-1α levels are decreased in the growth plates of Ccn2 mutants; similar results are seen in isolated chondrocytes where recombinant CCN2 induces HIF-1α, demonstrating that the effects of CCN2 on HIF-1α are direct in chondrocytes [56]. The mechanisms underlying CCN2-mediated regulation of HIF-1α are unknown.
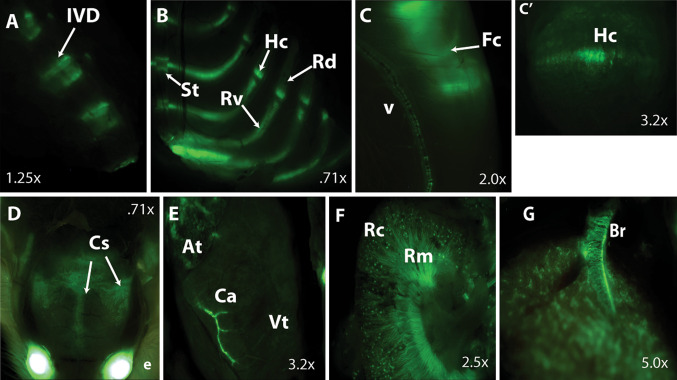
While the above results demonstrate that CCN2 is essential for chondrogenesis during development, whether it is required for maintenance of cartilage in adults is an important unanswered question. We have analyzed CCN2 expression in postnatal cartilage using a BAC transgenic line in which green florescent protein (GFP) is expressed under the control of the Ccn2 locus [57] (Fig. 2). In 1 to 2-month-old mice, CCN2 is expressed in the intervertebral disc (Fig 2a), a derivative of the notochord (which also expresses CCN2) in the mouse. It is also expressed in all appendicular and axial cartilaginous elements, with the highest levels of expression in hypertrophic zone in both axial (rib) and appendicular elements (Fig. 2b,c). CCN2 expression persists in articular cartilage, suggesting an essential role in cartilage maintenance in adults.
Fig. 2.
CCN2 eGFP Expression in the adult mouse. CCN2 expression was examined in 1-month-old male mice. a Strong CCN2 expression was observed in vertebral bodies and intervertebral discs. b CCN2 is expressed in ribs within the cartilaginous ventral segments, as compared to undetectable expression in the ossified dorsal rib segments. c, c′ Expression in hypertrophic chondrocytes in the femoral growth plate and in articular cartilage. c′ is a magnification of the growth plate in c. d CCN2 is highly expressed in cranial sutures. Expression was also observed in both major and minor vasculature including coronary arteries (e), kidneys (f) and lungs (g). Intervertebral disc (IVD), sternebra (St), rib ventral segment (Rv), rib dorsal segments (Rd), femoral condyle (Fc), vessel (v), hypertrophic chondrocytes (Hc), cranial sutures (Cs), atria (At), coronary artery (Ca), ventricle (Vt), renal cortex (Rc), renal medulla (Rm), bronchus (Br). Images were taken on a Leica MZ 16F bright field/GFP dissecting scope
On the one hand, CCN2 is expressed in articular chondrocytes and can stimulate articular cartilage repair and regeneration in vivo [58]. On the other hand, CCN2 levels are elevated in osteoarthritic (OA) cartilage [58, 59], and adenoviral over-expression of CCN2 in knee joints leads to transient fibrosis and cartilage damage [59]. These disparate outcomes most likely reflect different levels of CCN2 expression, which in turn, may lead to different levels of HIF activity. For example, low levels of HIF-1α are anabolic for cartilage [60]. However, HIF-2α promotes aspects of hypertrophy in the growth plate, and OA in articular cartilage [61]. It is thus conceivable that low levels of CCN2 are required to maintain levels of HIF activity that promote anabolic functions in growth plate and articular cartilage, but that the higher levels of CCN2 found in the hypertrophic zone and in OA cartilage promote HIF-2α expression, leading to cartilage damage.
Bone: CCN2 also appears to have effects on bone formation in vivo. This was initially demonstrated by Kawaki et al. using Ccn2 null mice [62]. Owing to neonatal lethality, this analysis did not address the role of CCN2 in adult bone remodeling. However, Ccn2 null mice were shown to exhibit a reduction in bone matrix synthesis, osteoblast proliferation, maturation, and mineralization in vivo. At least some of these effects are likely to be direct, as opposed to a secondary consequence of defective endochondral ossification, as the osteogenic response in calvarial osteoblasts isolated from Ccn2 mutants was significantly reduced in vitro [62]. These studies were extended recently by Canalis et al. to examine consequences of postnatal loss of CCN2 in osteoblasts [63]. In this study, the authors observed osteopenia, but only in male adult mice [63]. As in the study by Kawaki et al., isolated calvarial osteoblasts were found to exhibit diminished osteoblast function. Of note, the conditional allele of CCN2 used in the study by Canalis et al. led to reductions in Ccn2 mRNA levels ranging from 20 to 80%, and inactivation throughout the limb bud reportedly led to a phenotype similar to that seen in Ccn2+/− mice [63]. This is in contrast to the bent bones seen in Ccn2 null mice [18]. Thus, while the full extent of CCN2 action in bone is as yet uncertain, it is clear that CCN2 is an important regulator of osteoblast function. Its mechanism of action is completely unknown in this tissue.
CCN2 is abundantly expressed in cranial sutures (Fig. 2d). Premature mineralization of cranial sutures leads to craniosynostosis, which restricts skull growth and causes disfigurement. A recent study showed that CCN2 expression can prevent craniosynostosis in a rat model [64]. While sutures appear to be unaffected in Ccn2−/− neonates (unpublished data), it is conceivable that CCN2 plays a role postnatally in regulating suture patency.
Roles in angiogenesis
CCN2 was first isolated from human umbilical vascular endothelial cells (HUVECs) [21], and is highly expressed in all major and micro-vasculature including coronary vessels of the heart, vasculature of the kidneys, lung, and liver (Fig. 2e–g) [20, 22, 65]. The physiological roles of CCN2 in angiogenesis are unclear, however, as CCN2 has both pro- and anti-angiogenic activities in vitro. For example, CCN2 induces angiogenesis in the cornea [66], and induces neovascularization in vitro through engagement of integrins. CCN2 is a ligand for α6β1 and αvβ3 in endothelial cells [21, 65, 66]. However, anti-angiogenic activities have also been reported. CCN2 binds to and sequesters VEGF in an inactive form [8] and combined administration of CCN2 and VEGF in a mouse model of hindlimb ischemia inhibits VEGF induced angiogenesis [67]. Taken together, these studies implicate CCN2 as an essential regulator of angiogenesis, but do not address its role in vivo.
CCN2 global knock out mice exhibit defective growth plate angiogenesis as a result of decreased levels of vascular endothelial growth factor (VEGF) in hypertrophic cartilage [18]. However, whether CCN2 also plays a direct role in the vasculature has not been demonstrated. One study compared basal and VEGF-induced outgrowth of vessels from metatarsals of wild-type and Ccn2 mutant neonatal mice to conclude that CCN2 is not required for angiogenesis [68]. However, the large amount of variation in outgrowth that was observed may obscure real differences, and the images shown in the paper reveal considerably less outgrowth from Ccn2−/− metatarsals treated with VEGF [68].
On the other hand, a second study by the same group showed that the thickening of the basal lamina of retinal capillaries that occurs during streptozotocin (STZ)-induced diabetes in mice was completely prevented in Ccn2+/− mice [69]. Similarly, glomerular basement membrane thickening was prevented in diabetic Ccn2+/− mice compared to WT littermates [70]. Ccn2−/− mice could not be analyzed in these studies owing to their neonatal lethality. Nonetheless, these results raise the possibility of a role for CCN2 in the formation of endothelial basement membranes. Other evidence for a role for CCN2 in endothelial basement membrane formation comes from the findings that one of the most prominent features of Col1-CCN2 [30] transgenic mice was a thickening of endothelial basement membranes.
Each of these studies addresses a role for CCN2 in vascular fibrosis; over-expression promotes basement membrane thickening, whereas decreased CCN2 expression prevents it. However, whether CCN2 plays an essential role in normal angiogenesis has not been established. The survival of Ccn2−/− mice to birth indicates that vasculogenesis, the initial formation of blood vessels during development, is not impaired. However, we have analyzed vasculature from Ccn2−/− mice, and have uncovered evidence that CCN2 is required for vascular remodeling, a process that takes place at midgestation stages and postnatally in vivo (manuscript in preparation).
Insights and future directions
Genetic models are revealing that CCN2 has essential functions for tissue formation and maintenance that extend beyond its ability to induce tissue fibrosis. It is likely that developmental functions for CCN2 beyond those discussed here will be discovered. For example, a role has recently been discovered for CCN2 in the formation of the endocrine pancreas [71]. CCN2 is widely expressed in other secretory epithelial tissues, raising the possibility that CCN2 has a much broader role during development than currently appreciated [24]. A major unknown is the extent to which CCN2 and other members of the family have overlapping functions. Members of the CCN family can engage similar sets of integrins and bind to similar ECM components to mediate similar effects in vitro [23], and their patterns of expression overlap in many tissues [17, 72]. Given the many integrins, growth factors and their receptors, and ECM components that have been shown to interact with CCNs, whether individual CCN proteins have similar versus distinct functions will likely prove to be cell-type specific. Finally, the mechanisms by which CCNs mediate their effects in vivo are essentially unknown. While we uncovered a role for integrins as mediators of the effects of CCN2 in chondrogenesis, it is highly likely that CCN2 uses additional mechanisms in this and other tissues.
Acknowledgements
This work was supported by NIH grants R01 AR052686 to KML and the UCLA Vascular Biology Training Grant (Ruth L. Kirschstein National Research Service Award T32HL69766) to FHG.
References
- 1.Abreu JG, Reversade B, De Robertis EM. Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-beta. Nat Cell Biol. 2002;4:599–604. doi: 10.1038/ncb826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brigstock DR. The connective tissue growth factor/cysteine-rich 61/nephroblastoma overexpressed (CCN) family. Endocr Rev. 1999;20:189–206. doi: 10.1210/er.20.2.189. [DOI] [PubMed] [Google Scholar]
- 3.Vorwerk P, Oh Y, Rosenfeld RG, Shymko RM. Binding properties of insulin-like growth factor binding protein-3 (IGFBP-3), IGFBP-3 N- and C-terminal fragments, and structurally related proteins mac25 and connective tissue growth factor measured using a biosensor. Endocrinology. 2002;143:1677–1685. doi: 10.1210/en.143.5.1677. [DOI] [PubMed] [Google Scholar]
- 4.Chen N, Todorovic V, Lam SC, Lau LF. Identification of a novel integrin alphavbeta3 binding site in CCN1 (CYR61) critical for pro-angiogenic activities in vascular endothelial cells. J Biol Chem. 2004;279:44166–44176. doi: 10.1074/jbc.M406813200. [DOI] [PubMed] [Google Scholar]
- 5.Leu SJ, Lau LF. Proangiogenic activities of CYR61 (CCN1) mediated through integrins avb3 and a6b1 in human umbilical vein endothelial cells. J Biol Chem. 2002;286:25505–25518. doi: 10.1074/jbc.M209288200. [DOI] [PubMed] [Google Scholar]
- 6.Gao R. Connective tissue growth factor (CCN2) induces adhesion of rat activated hepatic stellate cells by binding of its C-terminal domain to integrin alpha(v)beta(3) and heparan sulfate proteoglycan. J Biol Chem. 2004;279:8848–8855. doi: 10.1074/jbc.M313204200. [DOI] [PubMed] [Google Scholar]
- 7.Aoyama E, Hoshijima M, Araki D, Nishida T, Kubota S, Takigawa M. N-terminal domains of CCN family 2/connective tissue growth factor bind to aggrecan. Biochem J. 2009;420:413–420. doi: 10.1042/BJ20081991. [DOI] [PubMed] [Google Scholar]
- 8.Hashimoto G, Fujii Y, Aoki T, Ikeda E, Okada Y. Matrix metalloproteinases cleave connective tissue growth factor and reactivate angiogenic activity of vascular endothelial growth factor 165. J Biol Chem. 2002;277:36288–36295. doi: 10.1074/jbc.M201674200. [DOI] [PubMed] [Google Scholar]
- 9.Bork P. The modular architecture of a new family of growth regulators related to connective tissue growth factor. FEBS. 1993;327:125–130. doi: 10.1016/0014-5793(93)80155-N. [DOI] [PubMed] [Google Scholar]
- 10.Itasaki N, Mercurio S, Rowe A, Domingos PM, Smith JC, Krumlauf R. Wise, a context-dependent activator and inhibitor of Wnt signalling. Development. 2003;130:4295–4305. doi: 10.1242/dev.00674. [DOI] [PubMed] [Google Scholar]
- 11.Katsube K, Tamamura Y, Yamaguchi Role of CCN, a vertebrate specific gene family, in development. Dev Growth Differ. 2009;51:55–67. doi: 10.1111/j.1440-169X.2009.01077.x. [DOI] [PubMed] [Google Scholar]
- 12.Mercurio S, Itasaki N, Krumlauf R, Smith JC. Connective-tissue growth factor modulates WNT signalling and interacts with the WNT receptor complex. Development. 2004;131:2137–2147. doi: 10.1242/dev.01045. [DOI] [PubMed] [Google Scholar]
- 13.Nishida T, Kubota S, Fukunaga T, Kondo S, Yosimichi G, et al. CTGF/Hcs24, hypertrophic chondrocyte-specific gene product, interacts with perlecan in regulating the proliferation and differentiation of chondrocytes. J Cell Physiol. 2003;196:265–275. doi: 10.1002/jcp.10277. [DOI] [PubMed] [Google Scholar]
- 14.Perbal B, Sainson R, Werner M, He B, Roizman B. The C-terminal domain of the regulatory protein NOVH is sufficient to promote interaction with fibulin 1C: a clue for a role of NOVH in cell-adhesion signaling. Proc Natl Acad Sci USA. 1999;96:869–874. doi: 10.1073/pnas.96.3.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leask A. All in the CCN family: essential matricellular signaling modulators emerge from the bunker. J Cell Sci. 2006;119:4803–4810. doi: 10.1242/jcs.03270. [DOI] [PubMed] [Google Scholar]
- 16.Mercurio S, Latinkic B, Itasaki N, Krumlauf R, Smith JC. Connective-tissue growth factor modulates WNT signalling and interacts with the WNT receptor complex. Development. 2004;131:2137–2147. doi: 10.1242/dev.01045. [DOI] [PubMed] [Google Scholar]
- 17.Fernando CA, Conrad PA, Bartels CF, Marques T, To M, Balow SA, Nakamura Y, Warman ML. Temporal and spatial expression of CCN genes in zebrafish. Dev Dyn. 2010;239:1755–1767. doi: 10.1002/dvdy.22279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ivkovic S, Popoff SN, Safadi FF, Libuda DE, Stephenson RC, Daluiski A, Lyons KM. Connective tissue growth factor coordinates chondrogenesis and angiogenesis during skeletal development. Development. 2003;130:2779–2791. doi: 10.1242/dev.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiou MJ, Wu JL, Kuo CM, Chen JY. The physiological role of CTGF/CCN2 in zebrafish notochond development and biological analysis of the proximal promoter region. Biochem Biophys Res Commun. 2006;349:750–758. doi: 10.1016/j.bbrc.2006.08.095. [DOI] [PubMed] [Google Scholar]
- 20.Friedrichsen S, Christ S, Winckler M, Brauer D, Bauer K, Raivich G. CTGF expression during mouse embryonic development. Cell Tiss Res. 2003;312:175–188. doi: 10.1007/s00441-003-0712-6. [DOI] [PubMed] [Google Scholar]
- 21.Brigstock DR. Regulation of angiogenesis and endothelial cell function by connective tissue growth factor (CTGF) and cysteine-rich 61 (CYR61) Angiogenesis. 2002;5:153–165. doi: 10.1023/A:1023823803510. [DOI] [PubMed] [Google Scholar]
- 22.Surveyor GA. Immunohistochemical localization of connective tissue growth factor (CTGF) in the mouse embryo between days 7.5 and 14.5 of gestation. Growth Factors. 1999;17:115–124. doi: 10.3109/08977199909103520. [DOI] [PubMed] [Google Scholar]
- 23.Chen CC. Functions and mechanisms of action of CCN matricellular proteins. Int J Biochem Cell Biol. 2009;41:771–783. doi: 10.1016/j.biocel.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Surveyor GA, Brigstock DR. Localization of connective tissue growth factor during the period of embryo implantation in the mouse. Biol Reprod. 1998;59:1207–1213. doi: 10.1095/biolreprod59.5.1207. [DOI] [PubMed] [Google Scholar]
- 25.Shi-Wen X, Abraham D. Regulation and function of connective tissue growth factor/CCN2 in tissue repair, scarring and fibrosis. Cytokine Growth Factor Rev. 2008;19:133–144. doi: 10.1016/j.cytogfr.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 26.Mori T, Shinozaki M, Hayashi N, Kakinuma T, Igarashi A, Takigawa M, Nakanishi T, Takehara K. Role and interaction of connective tissue growth factor with transforming growth factor-beta in persistent fibrosis: a mouse fibrosis model. J Cell Physiol. 1999;181:153–159. doi: 10.1002/(SICI)1097-4652(199910)181:1<153::AID-JCP16>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 27.Igarashi A, Bradham DM, Grotendorst GR. Regulation of connective tissue growth factor gene expression in human skin fibroblasts and during wound repair. Mol Biol Cell. 1993;4:637–645. doi: 10.1091/mbc.4.6.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yokoi H, Mori K, Kasahara M, Suganami T, Sawai K, Yoshioka T, Saito Y, Ogawa YKT, Sugawara A, Nakao K. Overexpression of connective tissue growth factor in podocytes worsens diabetic nephropathy in mice. Kidney Int. 2008;73:446–455. doi: 10.1038/sj.ki.5002722. [DOI] [PubMed] [Google Scholar]
- 29.Tong Z, Alt DS, Kemper S, Perbal B, Brigstock DR. Susceptibility to liver fibrosis in mice expressing a connective tissue growth factor transgene in hepatocytes. Hepatology. 2009;50:939–947. doi: 10.1002/hep.23102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sonnylal S, Leoni P, Naff K, Van Pelt CS, Nakamura H, Leask A, Abraham D, Bou-Gharios G dCB. Selective expression of connective tissue growth factor in fibroblasts in vivo promotes systemic tissue fibrosis. Arthritis Rheum. 2010;62:1523–1532. doi: 10.1002/art.27382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu S, Abraham DJ, Leask A. CCN2 is required for bleomycin-induced skin fibrosis in mice. Arthritis Rheum. 2011;63:239–246. doi: 10.1002/art.30074. [DOI] [PubMed] [Google Scholar]
- 32.Doherty HE, Hiller S, Sulik KK, Maeda N. A mouse strain where basal connective tissue growth factor gene expression can be switched from low to high. PLoS One. 2010;5:e12909. doi: 10.1371/journal.pone.0012909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teekakirikul P, Toka O, Alcalai R, Wang L, Wakimoto H, Nayor M, Konno T, Gorham JM, Wolf CM, Kim JB, Schmitt JP, Molkentin JD, Norris RA, Tager AM, Hoffman SR, Markwald RR, Seidman CE, Seidman JG. Cardiac fibrosis in mice with hypertrophic cardiomyopathy is mediated by non-myocyte proliferation and requires Tgf-β. J Clin Invest. 2010;120:3520–3529. doi: 10.1172/JCI42028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Panek AN, Alenina N, Ghadge SK, Erdmann B, Popova E, Perrot A, Geier C, Dietz R, Morano I, Bader M, Ozcelik C. Connective tissue growth factor overexpression in cardiomyocytes promotes cardiac hypertrophy and protection against pressure overload. PLoS One. 2009;4:e6743. doi: 10.1371/journal.pone.0006743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahmed MS, Martinov VN, von Lueder TG, Edvardsen T, Czibik G, Moe IT, Vinge LE, Oie E, Valen G, Attramadal H (2010) Mechanisms of novel cardioprotective functions of CCN2/CTGF in myocardial ischemia/reperfusion injury. Am J Physiol Heart Circ Physiol (Epub ahead of print) [DOI] [PubMed]
- 36.Murphy AM. Contractile protein phenotypic variation during development. Cardiovasc Res. 1996;31:25–33. [PubMed] [Google Scholar]
- 37.Fukunaga T, Oya S, Takeshita N, Takigawa M, Takano-Yamamoto T. Connective tissue growth factor mRNA expression pattern in cartilages is associated with their type I collagen expression. Bone. 2003;33:911–918. doi: 10.1016/j.bone.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 38.Friedrichsen S, Heuer H, Christ S, Cuthill D, Bauer K, Raivich G. Gene expression of connective tissue growth factor in adult mouse. Growth Factors. 2005;23:43–53. doi: 10.1080/08977190512331340566. [DOI] [PubMed] [Google Scholar]
- 39.Bonniaud P, Kolb M, Haberberger T, Kelly M, Robertson J, Gauldie J. Adenoviral gene transfer of connective tissue growth factor in the lung induces transient fibrosis. Am J Respir Crit Care Med. 2003;168:770–778. doi: 10.1164/rccm.200210-1254OC. [DOI] [PubMed] [Google Scholar]
- 40.Bonniaud P, Margetts PJ, Ask K, Robertson J, Gauldie J, Kolb M. Connective tissue growth factor is crucial to inducing a profibrotic environment in “fibrosis-resistant” BALB/c mouse lungs. Am J Respir Cell Mol Biol. 2004;31:510–516. doi: 10.1165/rcmb.2004-0158OC. [DOI] [PubMed] [Google Scholar]
- 41.Wu S, Chen S, McNamara G, Whitsett J, Bancalari E. Conditional overexpression of connective tissue growth factor disrupts postnatal lung development. Am J Respir Cell Mol Biol. 2009;42:552–563. doi: 10.1165/rcmb.2009-0068OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen S, Platteau A, Hehre D, Smith H, Ruiz P, Whitsett JA, Bancalari E, Wu S. CTGF Disrupts alveolarization and induces pulmonary hypertension in neonatal mice: implication in the pathogenesis of severe bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2011;300:L330–340. doi: 10.1152/ajplung.00270.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Delcommenne M, Gray V, Rue L, Woodgett J, Dedhar S. Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proc Natl Acad Sci USA. 1998;95:11211–11216. doi: 10.1073/pnas.95.19.11211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Henderson WR, Ye X, Nguyen C, Tien YT, Zhou B, Borok Z, Knight DA, Kahn M. Inhibition of Wnt/beta-catenin/CREB binding protein (CBP) signaling reverses pulmonary fibrosis. Proc Natl Acad Sci USA. 2010;107:14309–14314. doi: 10.1073/pnas.1001520107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chilosi M, Zamo A, Lestani M, Montagna L, Piccoli P, Pedron S, Bertaso M, Scarpa A, Murer B, Cancellieri A, Maestro R, Semenzato G, Doglioni C. Aberrant Wnt/beta-catenin pathway activation in idiopathic pulmonary fibrosis. Am J Pathol. 2003;162:1495–1502. doi: 10.1016/S0002-9440(10)64282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheon SS, Poon R, Alman BA. Growth factors regulate beta-catenin-mediated TCF-dependent transcriptional activation in fibroblasts during the proliferative phase of wound healing. Exp Cell Res. 2004;293:267–274. doi: 10.1016/j.yexcr.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 47.Si W, Luu HH, Park JK, Luo Q, Song WX, Jiang W, Luo X, Li X, Yin H, Montag AG, Haydon RCHT. CCN1/Cyr61 is regulated by the canonical Wnt signal and plays an important role in Wnt3A-induced osteoblast differentiation of mesenchymal stem cells. Mol Cell Biol. 2006;26:2955–2964. doi: 10.1128/MCB.26.8.2955-2964.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang BL, Lyons KM. Stage-specific control of connective tissue growth factor (CTGF/CCN2) expression in chondrocytes by sox9 and beta-catenin. J Biol Chem. 2010;285:27702–27712. doi: 10.1074/jbc.M110.108498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baguma-Nibasheka M, Kablar B. Pulmonary hypoplasia in the connective tissue growth factor (Ctgf) null mouse. Dev Dyn. 2008;237:485–493. doi: 10.1002/dvdy.21433. [DOI] [PubMed] [Google Scholar]
- 50.Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- 51.Bonner JC. Regulation of PDGF and its receptors in fibrotic diseases. Cytokine Growth Factor Rev. 2004;15:255–273. doi: 10.1016/j.cytogfr.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 52.Provot S (2007) Fetal growth plate: a developmental model of cellular adaptation to hypoxia. Ann NY Acad Sci: 26–39 [DOI] [PubMed]
- 53.Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423:332–336. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- 54.Nishida T, Baxter RM, Deyoung RA, Takigawa M, Lyons KM. CCN2 (connective tissue growth factor) is essential for extracellular matrix production and integrin signaling in chondrocytes. J Cell Commun Signal. 2007;1:45–58. doi: 10.1007/s12079-007-0005-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gerber HP, Ryan AM, Kowalski J, Werb Z, Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med. 1999;55:623–628. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]
- 56.Nishida T, Kondo S, Maeda A, Kubota S, Lyons KM, Takigawa M. CCN family 2/connective tissue growth factor (CCN2/CTGF) regulates the expression of Vegf through Hif-1alpha expression in a chondrocytic cell line, HCS-2/8, under hypoxic condition. Bone. 2009;44:24–31. doi: 10.1016/j.bone.2008.08.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gong S, Heintz N. Rapid bacterial artificial chromosome modification for large-scale mouse transgenesis. Nat Protoc. 2010;5:1678–1696. doi: 10.1038/nprot.2010.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nishida T, Kojima S, Kuboki T, Nakao K, Kushibiki T, Tabata Y, Takigawa M. Regeneration of defects in articular cartilage in rat knee joints by CCN2 (connective tissue growth factor) J Bone Miner Res. 2004;19:1308–1319. doi: 10.1359/JBMR.040322. [DOI] [PubMed] [Google Scholar]
- 59.Blaney Davidson EN, Mooren FM, Oliver N, Berg WB, van der Kraan PM. Connective tissue growth factor/CCN2 overexpression in mouse synovial lining results in transient fibrosis and cartilage damage. Arthritis Rheum. 2006;54:1653–1661. doi: 10.1002/art.21795. [DOI] [PubMed] [Google Scholar]
- 60.Schipani E. Hypoxia and HIF-1 alpha in chondrogenesis. Semin Cell Dev Biol. 2005;16:539–546. doi: 10.1016/j.semcdb.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 61.Yang S, Ryu JH, Oh H, Chun CH, Kim BJ, Min BH, Chun JS. Hypoxia-inducible factor 2alpha is a catabolic regulator of osteoarthritic cartilage destruction. Nat Med. 2010;16:687–693. doi: 10.1038/nm.2153. [DOI] [PubMed] [Google Scholar]
- 62.Kawaki H, Kubota S, Suzuki A, Yamada T, Matsumura T, et al. Functional requirement of CCN2 for intramembranous bone formation in embryonic mice. Biochem Biophys Res Commun. 2008;366:450–456. doi: 10.1016/j.bbrc.2007.11.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Canalis E, Beamer WG, Economides AN, Smerdel-Ramoya A. Connective tissue growth factor is required for skeletal development and postnatal skeletal homeostasis in male mice. Endocrinology. 2010;151:3490–3501. doi: 10.1210/en.2010-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee CH, Moioli EK, Mao JJ. CTGF directs fibroblast differentiation from human mesenchymal stem/stromal cells and defines connective tissue healing in a rodent injury model. J Clin Invest. 2010;120:3340–3349. doi: 10.1172/JCI43230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shimo T, Nishida T, Asano M, Kanyama M, Kuboki T, Tamatani T, Tezuka K, Takemura MMT, Takigawa M. Connective tissue growth factor induces the proliferation, migration, and tube formation of vascular endothelial cells in vitro, and angiogenesis in vivo. J Biochem. 1999;126:137–145. doi: 10.1093/oxfordjournals.jbchem.a022414. [DOI] [PubMed] [Google Scholar]
- 66.Babic AM, Lau LF. Fisp12/mouse connective tissue growth factor mediates endothelial cell adhesion and migration through integrin alphavbeta3, promotes endothelial cell survival, and induces angiogenesis in vivo. Mol Cell Biol. 1999;19:2958–2966. doi: 10.1128/mcb.19.4.2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Inoki I, Hashimoto G, Enomoto H, Nakamura H, Makino KI, Ikeda E, Takata S, Kobayashi KOY. Connective tissue growth factor binds vascular endothelial growth factor (VEGF) and inhibits VEGF-induced angiogenesis. FASEB J. 2001;16:219–221. doi: 10.1096/fj.01-0332fje. [DOI] [PubMed] [Google Scholar]
- 68.Kuiper EJ, Ehlken C, Lambert V, van Treslong-de Groot HB, Lyons KM, Agostini HJRJ, Klaassen I, Van Noorden CJ, Goldschmeding R, Schlingemann RO. Angiogenesis is not impaired in connective tissue growth factor (CTGF) knock-out mice. J Histochem Cytochem. 2007;55:1139–1147. doi: 10.1369/jhc.7A7258.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kuiper EJ, Roestenberg P, Lyons KM, Goldschmeding R, Klaassen I, Van Noorden CJSR. Connective tissue growth factor is necessary for retinal capillary basal lamina thickening in diabetic mice. J Histochem Cytochem. 2008;56:785–792. doi: 10.1369/jhc.2008.950980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nguyen TQ, van Nieuwenhoven FA, Bovenschen N, Li Z, Xu L, Oliver N, Aten J, Joles JA, Vial C, Brandan E, Lyons KM, Goldschmeding R. CTGF inhibits BMP-7 signaling in diabetic nephropathy. J Am Soc Nephrol. 2008;19:2098–2107. doi: 10.1681/ASN.2007111261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Crawford L, Guney MA, Oh YA, Deyoung RA, Valenzuela DM, Murphy AJ, Yancopoulos GD, Lyons KM, Brigstock DR, Economides A, Gannon M. Connective tissue growth factor (CTGF) inactivation leads to defects in islet cell lineage allocation and beta-cell proliferation during embryogenesis. Mol Endocrinol. 2009;23:324–336. doi: 10.1210/me.2008-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kawaki H, Suzuki A, Lazar N, Yamada T, Matsumura T, Ohgawara T, Maeda T, Perbal BLK, Takigawa M. Cooperative regulation of chondrocyte differentiation by CCN2 and CCN3 shown by a comprehensive analysis of the CCN family proteins in cartilage. J Bone Miner Res. 2008;23:1751–1764. doi: 10.1359/jbmr.080615. [DOI] [PMC free article] [PubMed] [Google Scholar]