Abstract
CD47 is a ligand of an inhibitory receptor, signal regulatory protein (SIRP)α, and its interaction with SIRPα on macrophages prevents phagocytosis of autologous hematopoietic cells. CD47-SIRPα signaling also regulates dendritic cell (DC) endocytosis, activation and maturation. Here we show that CD47 expression on donor cells plays an important role in suppression of allograft rejection by donor-specific transfusion (DST). DST was performed by intravenous injection of splenocytes from C57BL/6 donors into MHC class I-disparate bm1 mice 7 days prior to donor skin grafting. Administration of wild-type (WT) C57BL/6 donor splenocytes markedly prolonged donor skin survival in bm1 mouse recipients. In contrast, bm1 mice receiving DST from CD47 knockout (KO) donors showed no inhibition or even acceleration of donor skin graft rejection compared to non-DST control (naïve) bm1 mice. T cells from bm1 mice receiving CD47 KO, but not WT, DST exhibited strong anti-donor responses. The ability of DST to suppress alloresponses was positively correlated with the density of CD47 molecules on donor cells, as CD47+/− DST was able to prolonged donor skin survival, but to a significantly less extent than WT DST. Furthermore, DCs from CD47 KO, but not WT, DST recipients showed rapid activation and contributed to donor skin rejection. These results show for the first time that CD47 on donor cells is required to repress recipient DC activation and suppress allograft rejection after DST, and suggest CD47 as a potential target for facilitating the induction of transplant tolerance.
Introduction
DST has the potential to prolong allograft survival or induce tolerance, particularly when it is used in combination with costimulatory blockade. Multiple mechanisms, including deletion and anergy of donor-reactive T cells (1–4) and generation of regulatory T cells (Tregs) (5–7), have been suggested to mediate the immunosuppressive effect of DST. It has been reported that tolerogenic DCs are responsible for the generation of donor antigen-specific Tregs in mice treated with DST plus anti-CD154 antibodies (8). Furthermore, the failure of DST to suppress alloresponses in recipients that lack antigen-presenting cells (APCs) demonstrates that the indirect antigen-presentation pathway mediates the effect of DST (9).
Previous studies using CD47 KO mice have demonstrated that CD47, a pentaspan membrane glycoprotein that is expressed ubiquitously in all tissues, serves as a ‘marker of self’ for macrophages, and that its interaction with the inhibitory receptor SIRPα (also known as CD172a, SHPS-1) on macrophages prevents the engulfment of autologous hematopoietic cells (10). CD47-SIRPα signaling also reduces the sensitivity of antibody- and complement-opsonized cells to phagocytosis (11). In addition, SIRPα is expressed on dendritic cells, and its signaling upon CD47 ligation inhibits DC activation and maturation (12). By using transgenic mice that express a CD11c promoter-driven simian diphtheria toxin receptor (DTR), we observed that CD47-SIRPα signaling regulates the endocytic activity of CD11c+ DCs (13).
In the present study, we investigated the role of CD47 in inhibition of alloimmune responses by DST. We show for the first time that CD47 expression on donor cells is required for DST-induced immunosuppression. The loss of prolongation of donor skin graft survival in the recipients of CD47 KO DST was associated with rapid activation of recipient CD11chi DCs and strong anti-donor T cell responses.
Materials and Methods
Mice
C57BL/6 (B6, H2b), B6.C-H2bm1/ByJ (bm1), and simian diphtheria toxin receptor (DTR)-transgenic B6.FVB-Tg (Itagx-DTR/eGFP)57Lan/J (CD11c-DTR, H2b) mice were purchased from The Jackson Laboratory (Bar Harbor, Maine). CD47 heterozygote (CD47+/−) mice were generated by breeding of homozygous CD47 KO (CD47−/−) (10,14) and WT mice. GFP-transgenic CD47 KO mice and CD47 KO mice on the bm1 background were generated by crossing CD47 KO mice with C57BL/6-transgenic (UBC-GFP)30Scha/J (The Jackson Laboratory) or bm1 mice, respectively. B10.A (H2a) and B6/Ly5.2 congeneic mice were purchased from Frederick Cancer Research Facility (National Institutes of Health, Bethesda, MD). Female mice (6–8 weeks of age) were used in all experiments. Protocols involving animals were approved by the Massachusetts General Hospital Subcommittee on Research Animal Care.
DST and skin grafting
DST was performed by intravenous injection of 1×107 splenocytes from WT, CD47 KO, and CD47+/− B6 donors into MHC class I-disparate bm1 mice 7 days prior to skin grafting. In some experiments, GFP-transgenic mice were used as DST donors for measuring donor chimerism. Full-thickness tail skins (approximately 1×0.5 cm2) from donor and third-party mice were grafted on the dorsum of recipient bm1 mice, sutured with 5–0 silk, and secured with Vaseline gauze and bandage for 7 days. Skin graft survival was followed by daily visual inspection for the first 3 weeks and once every two days thereafter. Grafts were defined as rejected when <10% of the graft remained viable.
Preparation of CD11c-DTR bone marrow (BM) chimeras and CD11chi cell depletion
Eight-week-old B6 mice received 10.25 Gy total body irradiation (TBI) from a 137Cesium irradiator followed 6–8 h later by reconstitution with 10 × 106 syngeneic BM cells from WT or CD11c-DTR B6 mice. These BM chimeras were used as DST recipients 8 weeks later. CD11chi cell depletion was performed by injection i.p. of diphtheria toxin (DT, 4 ng/gram body weight; Sigma-Aldrich) 12 h prior to and 24 h after DST.
Preparation of B6 WT-to-CD47 KO BM chimeras
CD47 KO B6 mice (8 weeks of age) received 10.25 Gy TBI from a 137Cesium irradiator followed 6–8 h later by reconstitution with 10×106 syngeneic lineage-negative BM cells from WT B6 mice (13). These chimeras were used as DST recipients 9 weeks after BM transplantation. We have confirmed macrophage tolerance to CD47 KO cells in these chimeras by measuring the survival of WT vs. CD47 KO B6 splenocytes as previously described (13).
Flow cytometric analysis of CD86 and MHC class II expression on recipient CD11c+ DCs
Spleen cells were prepared from bm1 recipients at the indicated time points after DST, and stained with fluorescence-labeled anti-mouse CD4, CD8α, CD11c, CD86 and I-Ab (BD Biosciences, San Diego, CA). A rat anti-mouse FcγR mAb (2.4G2) was used to block the nonspecific binding of labeled mAbs, and HOPC1 (murine IGg2a) and rat IgG (BD Biosciences) were used as isotype controls. Data were collected using a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA).
Mixed lymphocyte reaction (MLR)
Spleen cells were prepared from B6 mice 7 days after injection 1×107 splenocytes from CD47+/+ (WT), CD47+/− or CD47−/− (KO) bm1 donors, and total, CD4+ and CD8+ T cells were purified and used as the responders. Total T cells were purified via negative selection by MACS using pan-T microbeads (Miltenyi Biotec). CD4+ and CD8+ T cells were purified via negative selection by MACS using pan-T plus anti-CD8 microbeads and pan-T plus anti-CD4 microbeads, respectively. The responder cells were cultured in 96-well plate (2×105/well) alone (unstimulated control), or stimulated with an equal number of irradiated (30 Gy) bm1 or B10.A splenocytes (stimulators) at 37 °C for 4 days. The cultures were pulsed with 1 µCi of [3H]-TdR 16 h before harvest, and counted in a beta counter (ICN Radiochemicals, Irvine, CA). Data are expressed as stimulation index (cpm of stimulated culture/cpm of unstimulated culture).
In vivo assay for CD8 T cell proliferation (15)
Bm1 mice (CD45.2+) were injected with 1×107 CD45.1+ B6 donor splenocytes. Seven days later, splenocytes were prepared from bm1 recipients, and labeled with 5-carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Eugene, OR) as described (16). CFSE-labeled cells (2×107) were injected through the tail vein into lethally (9.5Gy)-irradiated CD45.1+ B6 mice. Splenocytes were prepared from the B6 recipients 3 days after adoptive transfer of CFSE-labeled cells, and stained with PE-conjugated anti-mouse CD45.1 (A20) plus APC-conjugated anti-mouse CD4 or CD8 mAbs. A rat anti-mouse FcγR mAb (2.4G2) was used to block the nonspecific binding of labeled mAbs, and HOPC1 (murine IGg2a) and rat IgG (BD Biosciences) were used as isotype controls. Proliferation of adoptively-transferred bm1 CD4 (i.e., CD45.1−CD4+) and CD8 (i.e., CD45.1−CD8+) cells was analyzed by flow cytometry, and the division index was calculated with the Proliferation Platform of FlowJo software (Tree Star, Inc., Ashland, OR). The division index represents the average number of divisions that a cell present in the starting population has undergone. The division index = (proliferation index) × (% divided). The proliferation index is the average number of divisions that those divided cells underwent, and the % divided is the percentage of the cells of the original sample that divided.
Statistical analysis
Graft survival data are presented as Kaplan-Meier survival curves and differences between groups were analyzed by the log-rank test using GraphPad Prism (version 4; San Diego, CA). Differences between group means were tested using Student’s t test by Microsoft Excel software. A p value of <0.05 was considered to be significant.
Results
DST using CD47 KO donor cells fails to prolong donor skin graft survival or inhibit anti-donor T cell alloresponses
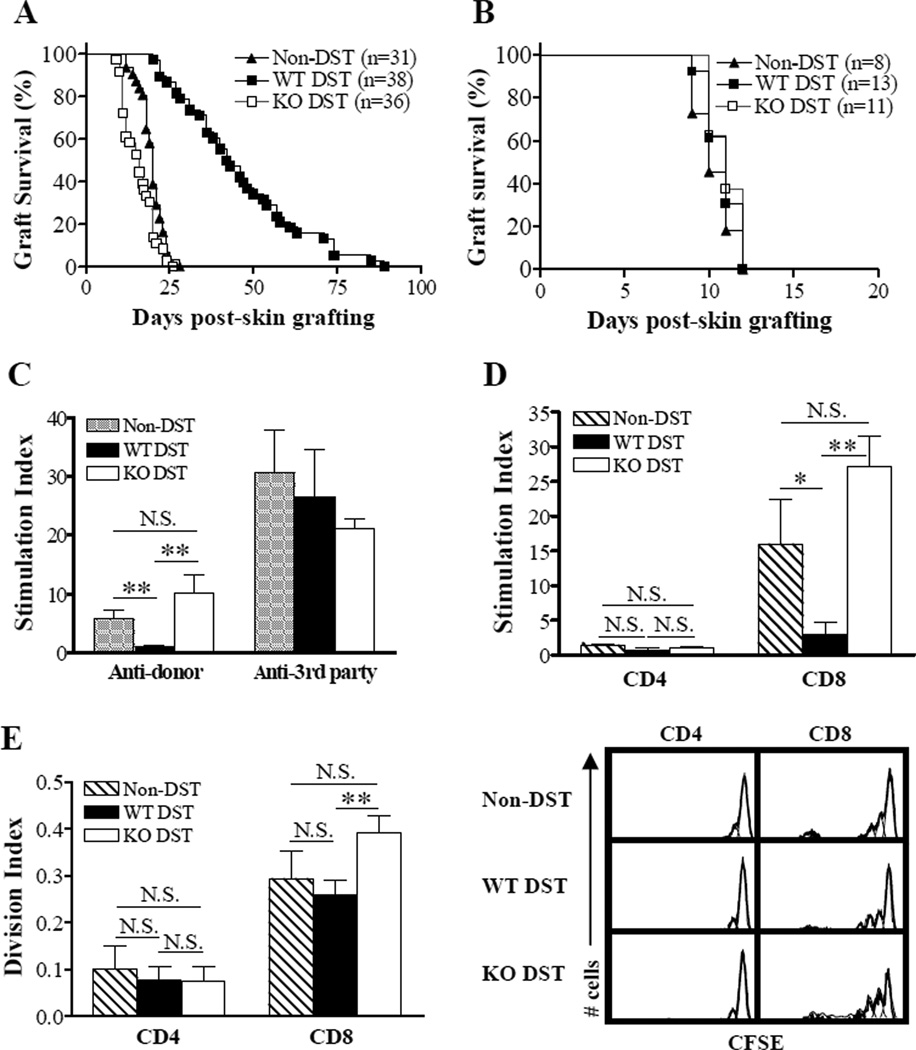
Bm1 mice were intravenously injected with 1×107 splenocytes from MHC class I-disparate CD47 KO or WT B6 donor mice, and grafted with skin from WT B6 donors 7 days later. All control bm1 mice that did not receive DST rejected the B6 skin grafts, with a median survival time (MST) of 20 days (Figure 1A). Compared to non-DST controls, DST using WT donor splenocytes significantly extended the survival of B6 donor skin in bm1 mice (MST = 42.5 days; p<0.001 compared to non-DST controls). In contrast, DST with CD47 KO B6 splenocytes accelerated B6 skin graft rejection in bm1 mice (MST = 16 days; p<0.001 and p<0.01 compared to WT DST and non-DST groups, respectively). The DST effect was donor-specific, as the third-party skin allografts were rejected similarly by all groups (Figure 1B). These data demonstrate that CD47 expression on donor cells is required for suppression of alloresponses by DST, and suggest that DST may sensitize the recipient mice to donor alloantigens if the donor cells do not express CD47.
Figure 1.
CD47 expression on donor cells is required for prolongation of donor skin grafts by DST. (A,B) Donor (B6; A) and third-party (B10.A; B) skin allograft survival in naive bm1 mice (Non-DST) and bm1 mice that received DST (i.e., 1×107 splenocytes) from WT (WT-DST) or CD47 KO (KO-DST) B6 donors 7 days prior to skin grafting. Results in (A) and (B) are the combined data of 7 and 2 independent experiments, respectively. (C, D) In vitro MLR. Total and purified CD4+ or CD8+ T cells were prepared from recipient splenocytes at day 7 post-DST, and cocultured with equal number of irradiated (30 Gy) splenocytes (stimulators) from B6 (donor) or B10A (3rd-party) mice. Results (mean±SDs) are presented as stimulation index and the data of 2 independent experiments are combined (n=5 per group). Shown are anti-donor and anti-3rd-party MLR of total T cells (C) and anti-donor MLR of purified CD4+ and CD8+ T cells (D). (E) In vivo MLR. Splenocytes were harvested from non-DST controls (n=5) and the recipients of WT (n=9) or CD47 KO (n=5) DST 7 days after DST, labeled with CFSE, and adoptively transferred into lethally irradiated (9.5 Gy) B6/Ly5.2 (CD45.1) congeneic mice. Proliferation of injected bm1 CD4+ and CD8+ T cells was measured by flow cytometry 3 days after adoptive transfer. Results from 2 independent experiments are combined. Shown are division index (left; mean±SDs) and flow cytometry profiles (right) of bm1 CD4+ (i.e., CD45.1−CD4+) and CD8+ (i.e., CD45.1−CD8+) T cells. *P<0.05; **P<0.01; N.S., not significant.
We also measured anti-donor T cell responses by in vitro MLR assay, in which T cells prepared from non-DST controls and DST recipients 7 days after DST were used as the responders. Consistent with donor skin graft survival, T cells from WT DST recipients showed no proliferative response to the DST donor stimulators (p<0.01 compared to Non-DST controls), but proliferated normally to the 3rd-party stimulators (Figure 1C). CD47 expression on DST donor cells was required for DST-induced donor-specific unresponsiveness. Compared to WT DST recipient T cells, T cells from CD47 KO DST recipients showed significantly increased anti-donor (p<0.01), but comparable anti-3rd-party MLR. Furthermore, MLR assay using purified CD4+ or CD8+ T cell responders revealed that the anti-donor MLR in this MHC class I (H-2k)-mismatched combination was predominantly mediated by CD8+ T cells, and that WT, but not CD47 KO, DST was able to suppress the anti-donor MLR of CD8+ T cells (Figure 1D).
We next measured the anti-donor T cell responses using an in vivo proliferation assay (15). Splenocytes harvested from bm1 recipients (CD45.2+) of CD47 KO or WT B6 DST 7 days after DST and from non-DST controls (i.e., naïve bm1 mice) were labeled with CFSE, and injected into lethally-irradiated CD45.1+ B6 mice. The in vivo proliferation of injected bm1 CD4 (i.e., CD45.1−CD4+) and CD8 (i.e., CD45.1−CD8+) T cells was analyzed by flow cytometry 3 days after adoptive cell transfer. As shown in Figure 1E, CD8 T cells from bm1 mice receiving CD47 KO DST demonstrated markedly increased proliferation in lethally-irradiated B6 mice compared to those from the WT DST group (p<0.01). However, no significant difference was detected between CD8 T cells from naive bm1 mice (Non-DST controls) and bm1 mice receiving WT DST. The data suggest that T cells from the CD47 KO DST recipients may mediate even stronger anti-donor responses than those from non-DST controls, which is consistent with the results of donor skin graft survival for these groups (Figure 1A). Similar to the in vitro MLR (Figure 1D), CD4 T cells from neither group exhibited significant proliferation in B6 mice (Figure 1E).
Mice receiving repeat administration of CD47 KO DST show no improvement in donor skin graft survival
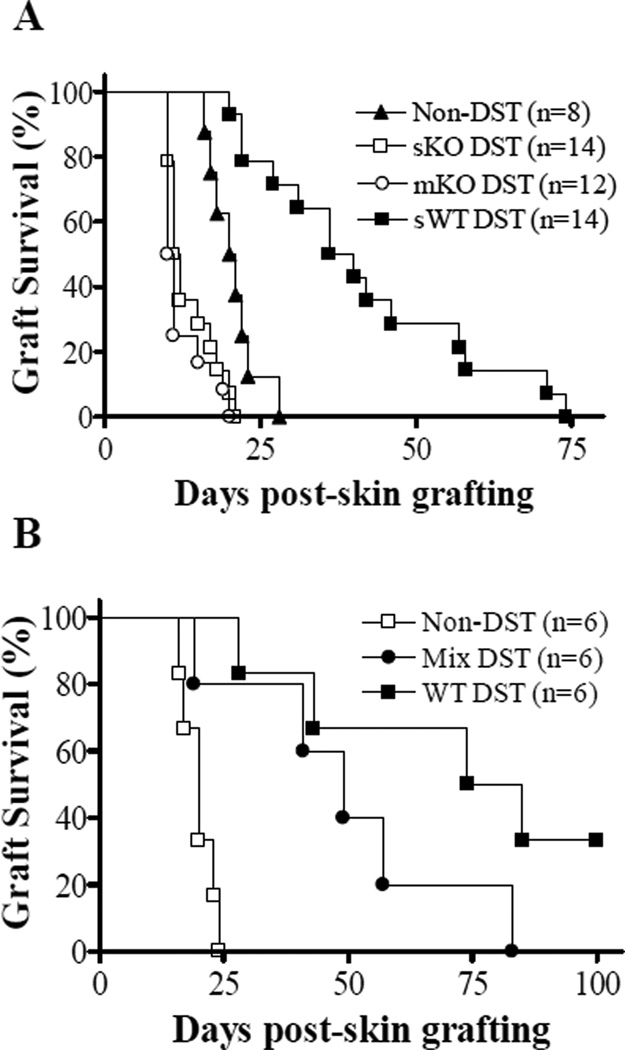
Because CD47 KO hematopoietic cells are rejected rapidly by macrophages and DCs after injection into WT mice (10,13,17), we investigated whether the early loss of donor chimerism is responsible for the failure of CD47 KO DST to suppress anti-donor alloresponses. We first assessed the effect of multiple injections of CD47 KO splenocytes on donor skin graft survival. We injected bm1 mice with CD47 KO splenocytes once daily for the first 4 days. Again, WT DST induced significant prolongation of donor skin survival, with a MST of 38 days (Figure 2A; p<0.05 compared to non-DST controls). However, acceleration of donor skin rejection was seen in both single and multiple CD47 KO DST groups. The survival of donor skin grafts was similar between bm1 mice that received single versus multiple CD47 KO DST (MST for single and multiple DST groups were 11.5 and 10.5 days, respectively), and significantly shorter than that in non-DST controls (MST = 20.5 days; p<0.05). These data suggest that the rapid loss of donor chimerism seems unlikely to be the major mechanism for the failure to inhibit donor graft rejection in CD47 KO DST recipients.
Figure 2.
Effect of multiple injections of CD47 KO splenocytes and co-injection of WT and CD47 KO splenocytes on donor skin allograft survival. (A) Bm1 mice received a single injection of 1×107 WT (sWT-DST) or CD47 KO (sKO-DST) B6 splenocytes at day −7, or 4 daily injections of CD47 KO B6 splenocytes (1×107/injection) from day −7 to day −4 (mKO-DST), followed by skin grafting from B6 donors at day 0. Untreated bm1 mice were used as non-DST controls. (B) Bm1 mice received 1×107 WT or CD47 KO B6 splenocytes, or a mixture (1×107 each) of WT and CD47 KO B6 splenocytes 7 days prior to donor skin grafting (n=6 per group). Data shown are donor skin graft survivals in the indicated groups.
Reduced donor skin graft survival in recipients of mixed WT and CD47 KO DST compared to those receiving only WT DST
We also compared donor skin rejection in bm1 mice receiving WT, CD47 KO, or a mixture of WT plus CD47 KO splenocytes. Although bm1 mice receiving mixed WT and CD47 KO DST displayed delayed rejection of donor skin grafts compared to those receiving CD47 KO DST (p<0.05), donor skin survival in these mice was significantly decreased versus survival in bm1 mice that received WT DST (p<0.05; Figure 2B). Because WT donor cells are not susceptible to phagocytosis even when injected together with CD47 KO cells in WT mice (13), these results further suggest that the loss of donor chimerism is unlikely to be responsible for the inability of CD47 KO DST to suppress anti-donor alloresponses. Furthermore, the reduced ability of WT DST to prolong donor skin survival in bm1 mice receiving simultaneous CD47 KO DST indicates that CD47 KO DST made the recipients less responsive to WT DST.
CD47 KO DST from bm1 donors fails to suppress alloresponses in WT→D47 B6 full hematopoietic chimeras
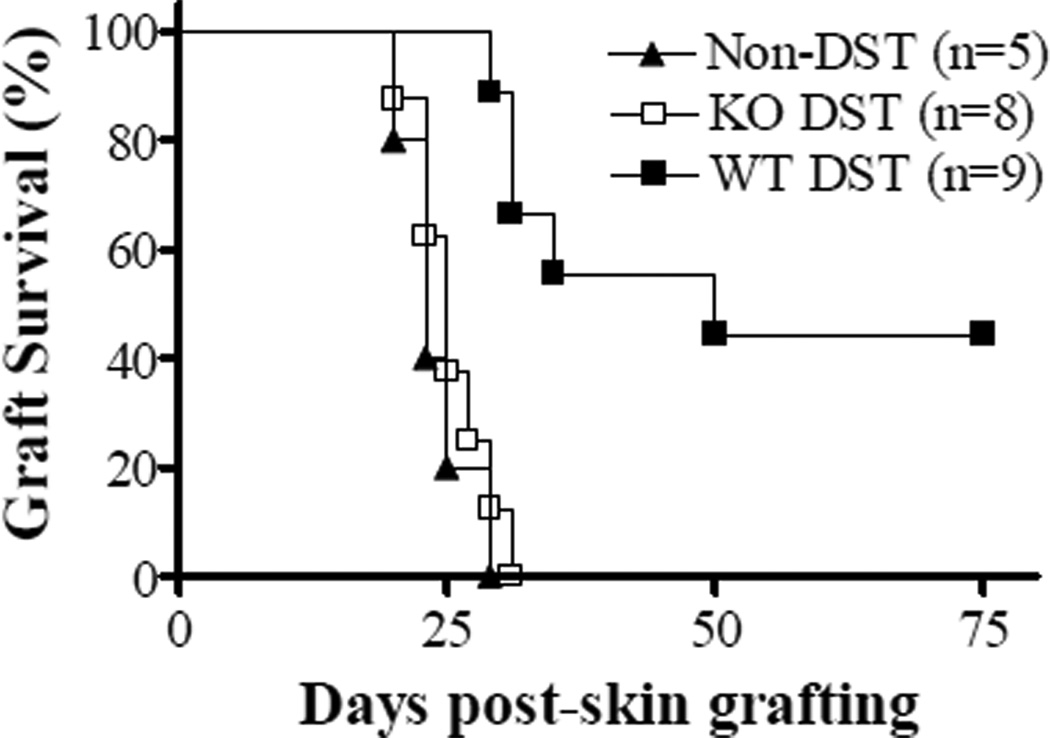
We have previous shown that the lack of CD47 expression on non-hematopoietic cells can induce macrophage tolerance to CD47 KO cells, and that WT macrophages developing WT→CD47 KO BM chimeras do not phagocytose CD47 KO splenocytes (13). To firmly determine whether the rapid clearance of donor cells is responsible for the inability of CD47 KO DST to prolong allograft survival, we compared the immunosuppressive effects of WT vs. CD47 KO DST in preestablished WT→CD47 KO full BM chimeras. Full WT hematopoietic chimeras were established by injection of WT B6 lineage-negative BM cells into lethally-irradiated CD47 KO B6 mice, and used as DST recipients 9 weeks later. The chimeras that received no treatment (Non-DST controls) or DST from WT or CD47 KO bm1 donors were grafted with WT bm1 skin 1 week after DST. Compared to non-DST control chimeras, WT, but not CD47 KO, DST was able to significantly prolong the survival of donor skin allografts in the preestablished BM chimeras (Figure 3). These results indicate that the rapid loss of donor chimerism is not the major mechanism for the inability of CD47 KO DST to suppress alloresponses.
Figure 3.
CD47 KO DST fails to prolong skin allograft survival in preestablished WT→CD47 BM chimeras. Nine-week WT B6→CD47 KO B6 BM chimeras received no treatment (Non-DST controls) or DST from WT or CD47 KO bm1 donors at day −7, and skin grafting from WT bm1 donors at day 0. Data shown are donor skin graft survivals in the indicated groups.
DST using CD47 heterozygote cells induces better donor graft survival, but still worse than WT DST
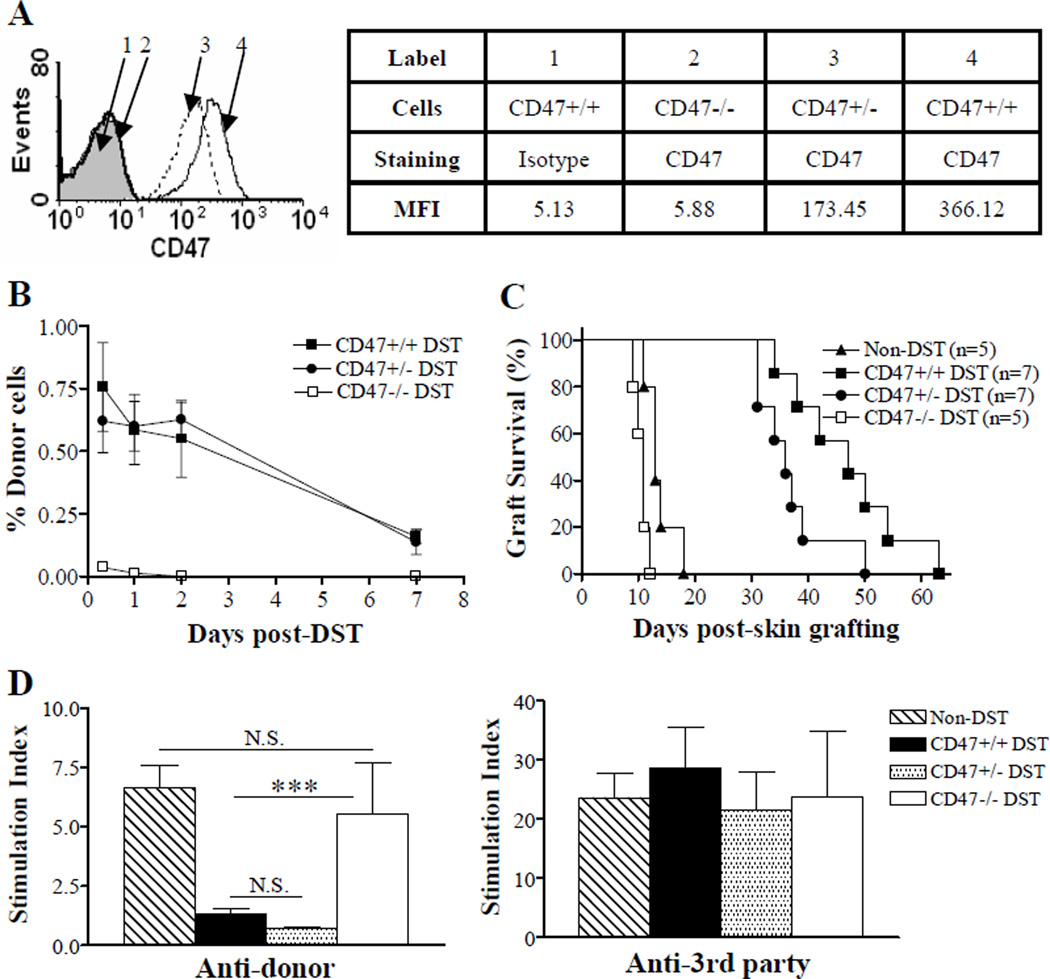
Although CD47 heterozygote (CD47+/−) cells exhibit decreased CD47 expression and mediate reduced CD47-SIRPα signaling relative to WT cells, the level of CD47 expression on CD47+/− cells is sufficient to inhibit phagocytosis upon transplantation into normal syngeneic WT mice (Figure 4A–B) (11). To determine whether the density of CD47 expression on donor cells correlates with DST-induced immunosuppression, we compared the survival of donor skin grafts in bm1 mice receiving DST from WT, CD47+/− or CD47−/− B6 donors. Again, CD47−/− DST accelerated donor skin rejection (MST = 11 days) compared to non-DST controls (MST =13 days) (Figure 4C; p<0.05). Although prolongation of donor skin grafts was seen in bm1 mice receiving CD47+/− DST (p<0.01 compared to non-DST and CD47 KO DST groups), graft survival in these mice was significantly shorter than that in the recipients of WT DST (p<0.05; MST of donor skin grafts were 36 days and 47 days for CD47+/− DST and WT DST groups, respectively). We also compared the anti-donor MLR responses of T cells prepared from these mice at day 7 post-DST. Unlike CD47 KO DST, CD47+/− DST was able to suppress anti-donor responses of the recipient T cells at the early time (Figure 4D). The levels of T cell proliferation in response to donor stimulators at day 7 post-DST were comparable between CD47+/− DST and WT DST recipients. These results suggest that the immunosuppressive strength of DST is positively correlated with the density of CD47 expression on donor cells.
Figure 4.
Correlation between cell surface CD47 density and DST effectiveness. Bm1 mice received a single injection of 1×107 splenocytes (i.e., DST) from GFP-transgenic WT (CD47+/+), CD47 heterozygous (CD47+/−) or CD47 KO (CD47−/−) B6 mice 7 days prior to donor skin grafting. Untreated bm1 mice were used as non-DST controls. (A) Flow cytometry profiles showing CD47 density on white blood cells from WT, CD47+/− and CD47−/− mice. MFI, mean fluorescent intensity. (B) Levels (mean±SDs) of donor chimerism (i.e., % GFP+ cells) in white blood cells measured by flow cytometry at the indicated times. (C) Donor skin graft survival. (D) Anti-donor (B6; left) and anti-3rd party (B10A; right) MLR of T cells purified from the spleens of non-DST controls and DST recipients 7 days after DST (n=3 per group). ***P<0.001; N.S. not significant.
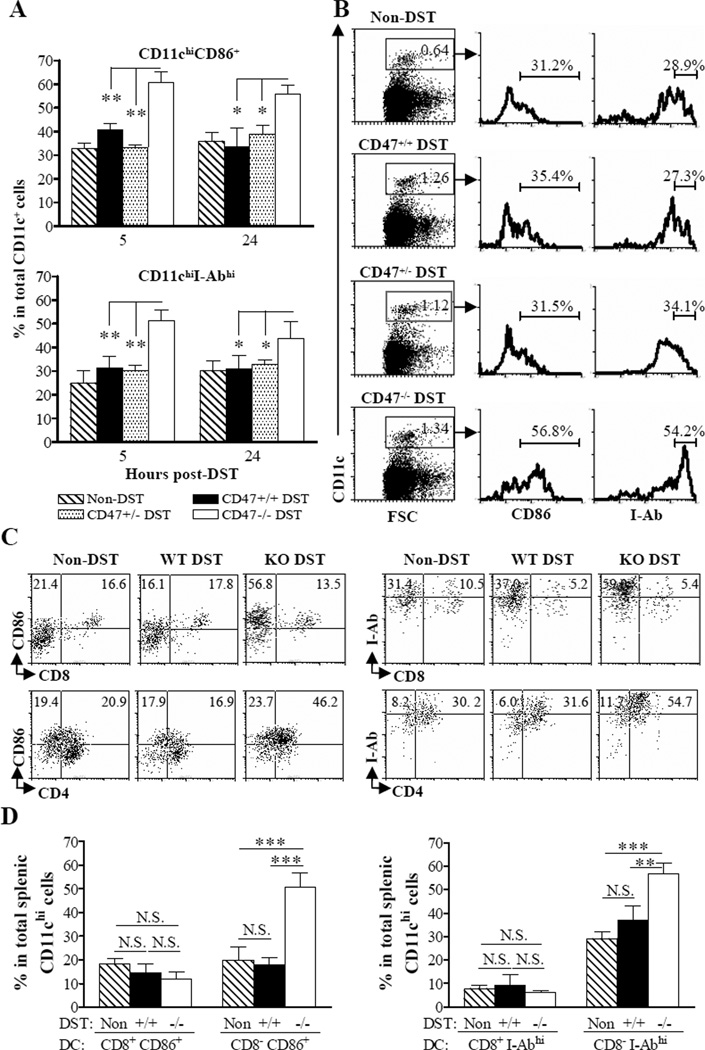
Recipient CD11chi DCs in CD47 KO, but not WT or CD47+/−, DST recipients show rapid activation and contribute to donor skin graft rejection
Splenocytes were collected short (5 and 24 hours) after DST, and the expression of CD86 and MHC class II on CD11chi cells was determined by flow cytometry. The levels of CD86 and MHC class II (I-Ab) expression on splenic CD11chi DCs from bm1 mice receiving WT or CD47+/− DST remained similar to those from non-DST control (i.e., naïve) bm1 mice (Figure 5A–B). In contrast, CD47 KO DST resulted in rapid upregulation of CD86 and MHC class II expression on recipient CD11chi DCs. A significant increase in the percentages of CD86+ and I-Abhi splenic CD11chi DCs was detected in CD47 KO DST-treated bm1 mice 5 and 24 hours after DST compared to non-DST controls, as well as WT and CD47+/− DST-treated bm1 mice (Figure 5A–B). Interestingly, CD47 KO DST-induced upregulation of CD86 and MHC class II was only detected in CD8α− (mainly CD4+), but not in CD8α+, CD11chi DCs (Figure 5C–D).
Figure 5.
CD47 KO DST results in recipient DC activation. Bm1 mice received no DST (n=3), or DST from WT (n=5), CD47+/− (n=3) or CD47 KO (n=5) B6 donors. (A) Percentages of CD11chiCD86+ (top) and CD11chiI-Abhi (bottom) cells in total splenic CD11chi cells at 5 and 24 hours after DST. Results are the combined data of 2 independent experiments. (B) Representative flow cytometry profiles. (C, D) CD86 and I-Ab expression on splenic CD4+, CD8α+ and CD4−CD8α− CD11chi cells 24 hours after DST. (C) Flow cytometry profiles of gated splenic CD11chi cells that were stained with anti-CD86 plus anti-CD8 or CD4 mAbs (left), or with anti-I-Ab plus anti-CD8 or CD4 mAbs (right). (D) Percentages of CD8α+CD11chiCD86+ vs. CD8α−CD11chiCD86+ (left) and CD8α+CD11chiI-Abhi vs. CD8α−CD11chiI-Abhi (right) cells in total splenic CD11chi DCs. *P<0.05; **P<0.01; ***P<0.001; NS, not significant.
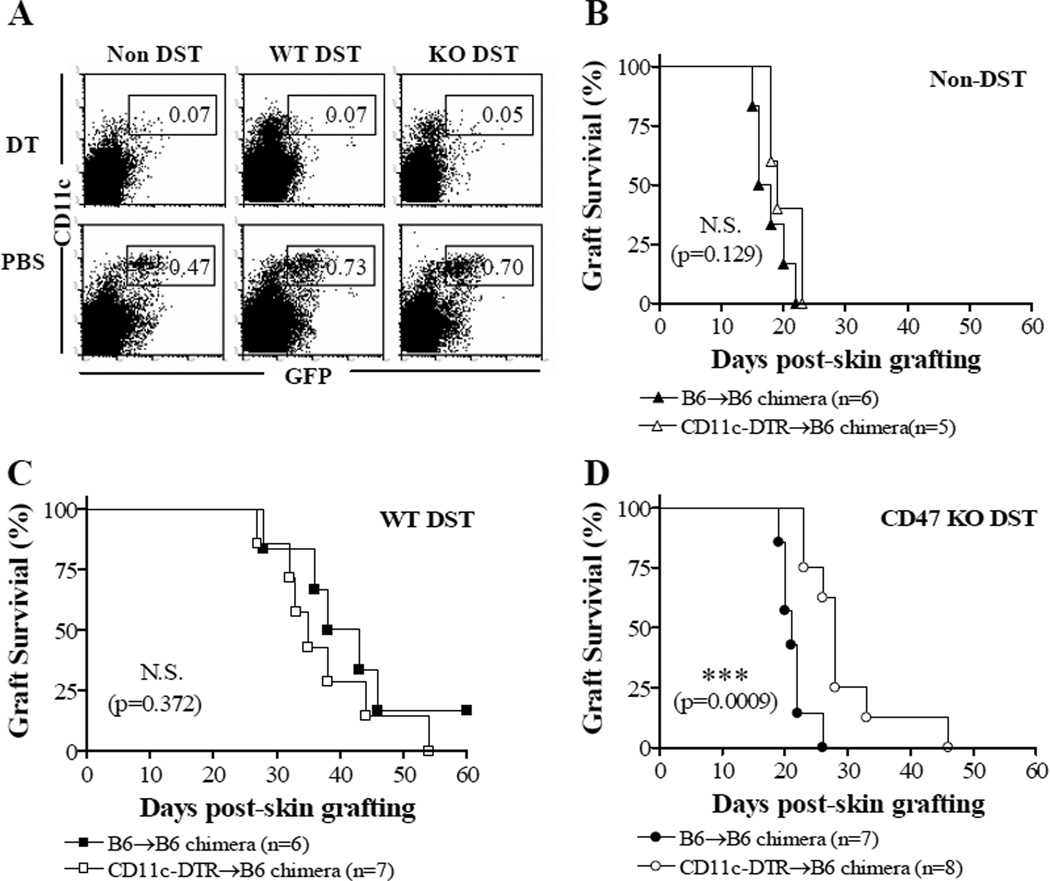
To understand whether the early CD11chi DC activation contributes to graft rejection in the recipients of CD47 KO DST, we compared donor skin graft survival in recipient mice with or without transient depletion of host CD11chi DCs at the time of DST. In order to prevent DT-induced the morbidity and mortality, we used CD11c-DTR BM (CD11c-DTR→B6) chimeras, in which DTR is only expressed on CD11c+ hematopoietic cells, as the recipients of DST. DC depletion in CD11c-DTR BM chimeras was achieved by injection of DT at days −1 and 1 with respect to DST. CD11c+ DC depletion in DT-treated CD11c-DTR B6 mice and CD11c-DTR BM chimeras lasts for 2 days (18,19). WT B6 BM (B6→B6) chimeras that were similarly treated with DT were used as controls. Transient deletion of CD11chi cells by DT (Figure 6A) did not significantly affect donor skin survival in non-DST controls (Figure 6B) and WT DST recipients (Figure 6C). However, transient deletion of CD11chi cells significantly delayed donor skin rejection in the recipients of CD47 KO DST (Figure 6D), indicating that CD11chi DCs may contribute to graft rejection in CD47 KO DST recipients.
Figure 6.
Transient depletion of recipient CD11chi DCs delays donor skin graft rejection in recipients of CD47 KO DST. Established CD11c-DTR BM chimeras and WT B6 BM chimeras received 2 injections of DT at days −8 and −6, DST from WT or CD47 KO bm1 donors at day −7, and skin grafting from bm1 donors at day 0. (A) Flow cytometric analysis of GFP+CD11chi cells in the spleen of CD11c-DTR BM chimeras 2 days after 1st DT or PBS injection (1 day after DST). (B–D) Donor skin graft survival in DT-treated CD11c-DTR BM (CD11c-DTR→B6) chimeras and WT B6 BM (B6→B6) chimeras that received no DST (Non-DST; B), WT DST (C) or CD47 KO DST (D). ***P<0.001; N.S., not significant.
Discussion
Our data demonstrate that CD47 expression on donor cells is required for suppression of alloresponses by DST. We show that DST using CD47 KO donor cells does not prolong donor skin graft survival and may even accelerate rejection, in a MHC class I (H-2k)-mismatched mouse model where WT DST can significantly prolong donor skin graft survival. Consistent with the survival time of donor skin grafts, T cells from recipients of CD47 KO, but not WT, DST mediated strong anti-donor responses. Furthermore, unlike recipient DCs in WT DST recipients, recipient DCs in mice receiving CD47 KO DST exhibited rapid activation and contributed to donor allograft rejection.
The role for donor chimerism in DST-induced immunosuppression remains incompletely understood. It has been shown that transfusion of donor BM cells neither extends allograft survival nor mediates alloreactive T cell deletion in a model, in which both can be achieved via the transfusion of donor splenocytes (20). However, peripheral deletion of donor-reactive T cells was found to contributes to tolerance induction by DST (2–4). Because CD47 KO hematopoietic cells are rapidly phagocytosed by macrophages and DCs after injection into WT mice (10,13,17), it is possible that the failure of CD47 KO DST to suppress alloresponses is due to the early loss of donor chimerism. However, our data indicate that the failure of CD47 KO DST to suppress alloresponses cannot be explained by the rapid loss of donor chimerism. Firstly, neither repeated administration of CD47 KO DST nor mixed WT and CD47 KO DST improved donor skin graft survival to the extent mediated by WT DST. In addition, despite that WT and CD47+/− DST recipients exhibited comparable levels of donor chimerism, CD47+/− DST mediated significantly reduced prolongation of donor skin grafts relative to WT DST. Finally, CD47 KO DST failed to prolong allograft survival in WT→CD47 KO BM chimeras in which macrophages do not phagocytose CD47 KO splenocytes (13).
SIRPα serves as an inhibitory receptor in DCs, and its signaling upon ligation of CD47 suppresses DC endocytosis (13) and activation (12). Because host DCs play a critical role in tolerance induction by DST (8,9), it is possible that the encounter of recipient DCs with donor cells in the absence of CD47-SIRPα signaling may result in DC activation, leading to stimulation of alloreactive T cells in DST recipients. In the present study, we found that significant upregulation of CD86 and class II expression was detected in splenic CD11chi DCs from the recipients of CD47 KO, but not WT, DST compared to non-DST (i.e., naïve) bm1 controls. CD11c+ DCs can be divided into three subsets, i.e., CD4+, CD8α+ and CD4−CD8α− DCs (21). Previous studies have shown that CD8α+ DCs play an important role in uptake of apoptotic cells and tolerance induction (22–24). Interestingly, CD47 KO DST mainly stimulated the activation of CD8α− (i.e., CD4+ and CD4−CD8α−), but not CD8α+ DCs. Furthermore, transient depletion of CD11chi cells at the time of DST significantly prolonged donor skin survival in mice that received CD47 KO DST. These results suggest that the rapid activation of recipient DCs is likely to be, at least, one of the mechanisms for the failure of CD47 KO DST to suppress alloresponses. In this study, we could not detect significant anti-donor MLR in the purified splenic CD4+ T cell population. However, by using bm1-specific CD4 T cell lines (derived from immunized B6 mice), Ossevoort et al reported that CD4 T cells can recognize and respond to synthetic bm1 peptides presented by MHC class II molecules on B6 cells, and the naturally processed bm1 peptide presented by I-Ab molecule on bm1 cells (25). Although the anti-bm1 responses of B6 CD4 T cells is considerably weak (the proliferation of stimulated bm1-specific CD4 T cell lines was only 2–3 fold greater than the non-stimulated controls) (25), these data indicate that CD4 T cells may provide help to CD8 T cell responses to bm1 antigens. Thus, it is possible that rapid DC activation may stimulate anti-donor responses via activating CD4 T cells in the recipients of CD47 KO DST. Our results also suggest that CD47 expression on donor cells plays an important role in maintaining the immature state of recipient DCs after DST, which was found important in the induction of Tregs (26) and T cell tolerance (27) in mice after allogeneic DST. Further studies will be required to confirm whether the role for CD47 expression on donor cells in preventing DC activation is mediated by its interaction with SIRPα on recipient DCs.
Although apparently less effective than WT DST, CD47+/− DST significantly prolonged donor skin graft survival compared to non-DST controls. Unlike CD47 KO DST, injection of CD47+/− splenocytes did not induce early DC activation. The lack of proliferation of T cells from CD47+/− DST recipients (as measured at day 7 post-DST) indicates that CD47+/− DST efficiently suppressed the anti-donor T cell responses at the early time. However, T cells in CD47+/− DST recipients may exhibit greater anti-donor responses than those in WT DST recipients at the later times, which were not examined in the current study. Nonetheless, the data indicate that the density of CD47 molecules on donor cells is positively correlated with the ability of DST to suppress alloresponses.
In summary, we have demonstrated that CD47 expression on donor cells is required for DST-induced suppression of alloimmune responses and prolongation of skin allograft survival. Since CD47-SIRPα interaction is species-specific (28–30), the present study also suggest an explanation for the poor effect of DST in the induction of immunosuppression or tolerance in discordant xenogeneic transplantation settings (31). Although the mechanisms by which CD47 expression regulates alloimmune responses remain largely unknown, this report suggests that CD47 may provide a target for new therapeutic approaches to preventing transplant rejection.
Acknowledgments
The authors thank Drs. Christian LeGuern and Luis Fernandez for critical review of this manuscript, Ms. Shumei Wang for technical support, Mr. Orlando Moreno for outstanding animal husbandry, and Ms. Kelly Walsh for expert assistance with the manuscript.
Abbreviations used in this paper
- DST
donor-specific transfusion
- DC
dendritic cell
- DTR
diphtheria toxin receptor
- eGFP
enhanced green fluorescent protein
- KO
knockout
- MST
median survival time
- SIRP
signal regulatory protein
- Treg
regulatory T cell
- WT
wild-type
Footnotes
This work was supported by an NIH/NIAID RO1 grant (AI064569) and an AHA/NCRP Scientist Development Grant (0930361N).
REFERENCES
- 1.Sato S, Azuma T, Shimizu J, Shima J, Kitagawa S, Hamaoka T, Fujiwara H. Property of class I H-2 alloantigen-reactive Lyt-2+ helper T cell subset. Abrogation of its proliferative and IL-2-producing capacities by intravenous injection of class I H-2-disparate allogeneic cells. J.Immunol. 1988;141:721–727. [PubMed] [Google Scholar]
- 2.Yang L, Du Temple B, Khan Q, Zhang L. Mechanisms of long-term donor-specific allograft survival induced by pretransplant infusion of lymphocytes. Blood. 1998;91:324–330. [PubMed] [Google Scholar]
- 3.Iwakoshi NN, Mordes JP, Markees TG, Phillips NE, Rossini AA, Greiner DL. Treatment of allograft recipients with donor-specific transfusion and anti-CD154 antibody leads to deletion of alloreactive CD8+ T cells and prolonged graft survival in a CTLA4-dependent manner. J Immunol. 2000;164:512–521. doi: 10.4049/jimmunol.164.1.512. [DOI] [PubMed] [Google Scholar]
- 4.Sandner SE, Clarkson MR, Salama AD, Sanchez-Fueyo A, Yagita H, Turka LA, Sayegh MH. Mechanisms of tolerance induced by donor-specific transfusion and ICOS-B7h blockade in a model of CD4+ T-cell-mediated allograft rejection. Am J Transplant. 2005;5:31–39. doi: 10.1111/j.1600-6143.2004.00640.x. [DOI] [PubMed] [Google Scholar]
- 5.Quigley RL, Wood KJ, Morris PJ. Transfusion induces blood donor-specific suppressor cells. J.Immunol. 1989;142:463–470. [PubMed] [Google Scholar]
- 6.Young KJ, Yang L, Phillips MJ, Zhang L. Donor-lymphocyte infusion induces transplantation tolerance by activating systemic and graft-infiltrating double-negative regulatory T cells. Blood. 2002;100:3408–3414. doi: 10.1182/blood-2002-01-0235. [DOI] [PubMed] [Google Scholar]
- 7.Lee I, Wang L, Wells AD, Dorf ME, Ozkaynak E, Hancock WW. Recruitment of Foxp3+ T regulatory cells mediating allograft tolerance depends on the CCR4 chemokine receptor. J.Exp.Med. 2005;201:1037–1044. doi: 10.1084/jem.20041709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ochando JC, Homma C, Yang Y, Hidalgo A, Garin A, Tacke F, Angeli V, Li Y, Boros P, Ding Y, Jessberger R, Trinchieri G, Lira SA, Randolph GJ, Bromberg JS. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat Immunol. 2006;7:652–662. doi: 10.1038/ni1333. [DOI] [PubMed] [Google Scholar]
- 9.Kishimoto K, Yuan X, Auchincloss H, Jr, Sharpe AH, Mandelbrot DA, Sayegh MH. Mechanism of Action of Donor-Specific Transfusion in Inducing Tolerance: Role of Donor MHC Molecules, Donor Co-stimulatory Molecules, and Indirect Antigen Presentation. J Am Soc Nephrol. 2004;15:2423–2428. doi: 10.1097/01.ASN.0000137883.20961.2D. [DOI] [PubMed] [Google Scholar]
- 10.Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP. Role of CD47 as a marker of self on red blood cells. Science. 2000;288:2051–2054. doi: 10.1126/science.288.5473.2051. [DOI] [PubMed] [Google Scholar]
- 11.Oldenborg PA, Gresham HD, Lindberg FP. CD47-Signal Regulatory Protein alpha (SIRPalpha) Regulates Fcgamma and Complement Receptor-mediated Phagocytosis. J Exp Med. 2001;193:855–862. doi: 10.1084/jem.193.7.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braun D, Galibert L, Nakajima T, Saito H, Quang VV, Rubio M, Sarfati M. Semimature Stage: A Checkpoint in a Dendritic Cell Maturation Program That Allows for Functional Reversion after Signal-Regulatory Protein-{alpha} Ligation and Maturation Signals. J.Immunol. 2006;177:8550–8559. doi: 10.4049/jimmunol.177.12.8550. [DOI] [PubMed] [Google Scholar]
- 13.Wang H, Madariaga ML, Wang S, van Rooijen N, Oldenborg PA, Yang YG. Lack of CD47 on nonhematopoietic cells induces split macrophage tolerance to CD47null cells. Proc.Natl.Acad.Sci.U.S.A. 2007;104:13744–13749. doi: 10.1073/pnas.0702881104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindberg FP, Bullard DC, Caver TE, Gresham HD, Beaudet AL, Brown EJ. Decreased resistance to bacterial infection and granulocyte defects in IAP-deficient mice. Science. 1996;274:795–798. doi: 10.1126/science.274.5288.795. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Li XC, Zheng XX, Wells AD, Turka LA, Strom TB. Blocking both signal 1 and signal 2 of T-cell activation prevents apoptosis of alloreactive T cells and induction of peripheral allograft tolerance. Nat Med. 1999;5:1298–1302. doi: 10.1038/15256. [DOI] [PubMed] [Google Scholar]
- 16.Asavaroengchai W, Wang H, Wang S, Wang L, Bronson R, Sykes M, Yang YG. An essential role for IFN-γ in regulation of alloreactive CD8 T Cells following allogeneic hematopoietic cell transplantation. Biology of Blood and Marrow Transplantation. 2007;13:46–55. doi: 10.1016/j.bbmt.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blazar BR, Lindberg FP, Ingulli E, Panoskaltsis-Mortari A, Oldenborg PA, Iizuka K, Yokoyama WM, Taylor PA. CD47 (Integrin-associated Protein) Engagement of Dendritic Cell and Macrophage Counterreceptors Is Required to Prevent the Clearance of Donor Lymphohematopoietic Cells. J.Exp.Med. 2001;194:541–550. doi: 10.1084/jem.194.4.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jung S, Unutmaz D, Wong P, Sano G, De los SK, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, Pamer EG, Littman DR, Lang RA. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fehr T, Wang S, Haspot F, Kurtz J, Blaha P, Hogan T, Chittenden M, Wekerle T, Sykes M. Rapid Deletional Peripheral CD8 T Cell Tolerance Induced by Allogeneic Bone Marrow: Role of Donor Class II MHC and B Cells. J.Immunol. 2008;181:4371–4380. doi: 10.4049/jimmunol.181.6.4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Markees TG, Pearson T, Cuthbert A, Pearson AL, Shultz LD, Leif J, Phillips NE, Mordes JP, Greiner DL, Rossini AA. Evaluation of donor-specific transfusion sources: unique failure of bone marrow cells to induce prolonged skin allograft survival with anti-CD154 monoclonal antibody. Transplantation. 2004;78:1601–1608. doi: 10.1097/01.tp.0000140847.29917.65. [DOI] [PubMed] [Google Scholar]
- 21.Kamath AT, Pooley J, O'Keeffe MA, Vremec D, Zhan Y, Lew AM, D'Amico A, Wu L, Tough DF, Shortman K. The Development, Maturation, and Turnover Rate of Mouse Spleen Dendritic Cell Populations. J.Immunol. 2000;165:6762–6770. doi: 10.4049/jimmunol.165.12.6762. [DOI] [PubMed] [Google Scholar]
- 22.Ferguson TA, Herndon J, Elzey B, Griffith TS, Schoenberger S, Green DR. Uptake of apoptotic antigen-coupled cells by lymphoid dendritic cells and cross-priming of CD8(+) T cells produce active immune unresponsiveness. Journal of Immunology. 2002;168:5589–5595. doi: 10.4049/jimmunol.168.11.5589. [DOI] [PubMed] [Google Scholar]
- 23.Belz GT, Behrens GMN, Smith CM, Miller JFAP, Jones C, Lejon K, Fathman CG, Mueller SN, Shortman K, Carbone FR, Heath WR. The CD8 alpha(+) dendritic cell is responsible for inducing peripheral self-tolerance to tissue-associated antigens. Journal of Experimental Medicine. 2002;196:1099–1104. doi: 10.1084/jem.20020861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qiu CH, Miyake Y, Kaise H, Kitamura H, Ohara O, Tanaka M. Novel Subset of CD8{alpha}+ Dendritic Cells Localized in the Marginal Zone Is Responsible for Tolerance to Cell-Associated Antigens. J.Immunol. 2009;182:4127–4136. doi: 10.4049/jimmunol.0803364. [DOI] [PubMed] [Google Scholar]
- 25.Ossevoort MA, De Bruijn MLH, Van Veen KJH, Kast WM, Melief CJM. Peptide specificity of alloreactive CD4 positive T lymphocytes directed against a major histocompatibility complex class I disparity. Transplantation. 1996;62:1485–1491. doi: 10.1097/00007890-199611270-00017. [DOI] [PubMed] [Google Scholar]
- 26.Bacchetta R, Gregori S, Roncarolo MG. CD4+ regulatory T cells: Mechanisms of induction and effector function. Autoimmunity Reviews. 2005;4:491–496. doi: 10.1016/j.autrev.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 27.Nouri-Shirazi M, Thomson AW. Dendritic cells as promoters of transplant tolerance. Expert Opinion on Biological Therapy. 2006;6:325–339. doi: 10.1517/14712598.6.4.325. [DOI] [PubMed] [Google Scholar]
- 28.Wang H, VerHalen J, Madariaga ML, Xiang S, Wang S, Lan P, Oldenborg PA, Sykes M, Yang YG. Attenuation of phagocytosis of xenogeneic cells by manipulating CD47. Blood. 2007;109:836–842. doi: 10.1182/blood-2006-04-019794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ide K, Wang H, Liu J, Wang X, Asahara T, Sykes M, Yang YG, Ohdan H. Role for CD47-SIRPα signaling in xenograft rejection by macrophages. Proc Natl Acad Sci USA. 2007;104:5062–5066. doi: 10.1073/pnas.0609661104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takenaka K, Prasolava TK, Wang JCY, Mortin-Toth SM, Khalouei S, Gan OI, Dick JE, Danska JS. Polymorphism in Sirpa modulates engraftment of human hematopoietic stem cells. Nat Immunol. 2007;8:1313–1323. doi: 10.1038/ni1527. [DOI] [PubMed] [Google Scholar]
- 31.Yang YG, Sykes M. Xenotransplantation: current status and a perspective on the future. Nat Rev Immunol. 2007;7:519–531. doi: 10.1038/nri2099. [DOI] [PubMed] [Google Scholar]