Abstract
Background
We have previously shown that the interspecies incompatibility of CD47 plays an important role in triggering rejection of xenogeneic hematopoietic cells by macrophages. However, it remains unknown whether CD47 incompatibility also contributes to the rejection of non-hematopoietic xenografts.
Aims
Here, we investigated the role of CD47 in preventing macrophage-mediated rejection of thymic epithelial cells in a mouse model of thymic transplantation across the CD47 barrier.
Methods
Wild-type (WT) and CD47 KO mice were thymectomized and treated with T cell-depleting mAbs, and implanted with fetal thymus from syngeneic WT or CD47 KO donors.
Results
Transplantation of CD47 KO mouse thymus led to T cell recovery in thymectomized, T cell-depleted WT mice. Similar to the control WT mouse thymic grafts, CD47 KO mouse thymic grafts showed a normal distribution of thymocyte subsets, and almost all of the thymocytes were recipient origin. Furthermore, histological analysis confirmed long-term survival of CD47 KO mouse thymic epithelial cells in WT mouse recipients.
Conclusions
These results demonstrate that, unlike hematopoietic cells, CD47 KO mouse thymus can survive and function in WT mice. Furthermore, our data implicate that the role of CD47 in xenograft rejection may differ for different types of xenografts, and that CD47 incompatibility is unlikely to impede thymic xenotransplantation, a potential approach to inducing xenotolerance, by triggering macrophage-mediated rejection.
Keywords: CD47, macrophage, thymus, xenotransplantation
Introduction
Transplants across discordant species barriers are subject to vigorous immunologic rejection. Because of the extensive molecular incompatibilities between the donor and host, innate immune responses, including those mediated by natural antibodies, complements, macrophages, and NK cells play a much greater role in the rejection of xenografts than in allograft rejection [1]. CD47 is a pentaspan membrane glycoprotein expressed ubiquitously in all tissues [2]. Previous studies have shown that CD47 serves as a “marker of self” for macrophages, and that its interaction with the inhibitory receptor signal regulatory protein α (SIRPα) on macrophages prevents the engulfment of autologous hematopoietic cells [3–5]. We have recently demonstrated that pig CD47 does not interact with mouse or human SIRPα, and that such a interspecies incompatibility of CD47 plays an important role in macrophage-mediated rejection of xenogeneic hematopoietic cells [6,7]. However, it remains unknown whether CD47 incompatibility between the donor and recipient also contributes to the rejection of non-hematopoietic xenografts.
In this study, we assessed the survival and function of CD47-deficient thymic epithelial cells in syngeneic wild-type (WT) mice. Because “missing-CD47” is the only potential immunological barrier in this model, the model allowed us to evaluate whether the lack of CD47 on donor cells can trigger macrophage-mediated graft rejection without the influence of other types of immune responses. We show that CD47 KO mouse thymic epithelial cells can survive long-term after transplantation into WT mice. Furthermore, CD47 KO mouse thymic grafts can function and support thymopoiesis and T cell development as effciently as WT mouse thymic grafts. Thus, unlike hematopoietic xenografts, the interspecies incompatibility of CD47 is unlikely to induce macrophage-mediated rejection of thymic xenografts.
Materials and methods
Animals
C57BL/6 (B6, H2b) and green fluorescent protein (GFP)-expressing B6-transgenic (UBC-GFP) 30Scha/J (GFP-B6) mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). CD47 KO mice on a B6 background were provided by Dr Per-Arne Oldenborg (Umeå University, Umeå, Sweden) [8]. GFP-transgenic CD47 KO (GFP-CD47 KO) B6 mice were generated by crossing CD47 KO B6 mice with GFP-B6 mice. Protocols using animals in this study were approved by the Massachusetts General Hospital Subcommittee on Research Animal Care, and all of the experiments were performed in accordance with the protocols.
Fetal thymus transplantation
Mice were thymectomized and treated with depleting anti-mouse CD4 (GK1.5; 1.75 mg per mouse i.p.) and CD8 (2.43; 1.4 mg per mouse i.p.) mAbs the following day, and used as the recipients of fetal thymus transplantation 2 to 4 weeks later. Mouse fetal thymi were harvested in late-gestation fetuses (at approximately embryonic day 18) and implanted under the recipient renal capsule (1 thymic lobe per mouse).
Flow cytometric analysis
White blood cells (WBCs) and single cell suspensions of spleen and thymic grafts were prepared from recipient mice at the indicated times after thymic transplantation and analyzed by flow cytometry using various combinations of the following fluorescence-conjugated mAbs: CD47 (miap301), CD4 (RM4-5), CD8 (53–6.7), CD11b (M1/70), SIRPα (P84), and appropriate isotype control mAbs (all purchased from BD Pharmingen, San Diego, CA, USA). Analysis was performed on a FACScalibur (Becton–Dickinson, Mountain View, CA, USA).
Histology
Frozen tissue sections were stained with hematoxylin and eosin (H&E), or incubated with rabbit polyclonal anti-wide spectrum cytokeratin antibody (Abcam Inc., Cambridge, MA, USA) at 4 °C overnight followed by incubating with PE-labeled goat anti-rabbit IgG (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA). Slides were viewed under a Nikon Eclipse TE2000 fluorescent microscope (Micro Video Instruments, Avon, MA, USA).
Statistical analysis
Significant differences between groups were determined using the Student’s t-test. A P-value of <0.05 was considered statistically significant.
Results
T cell recovery in thymectomized, T cell-depleted mice after thymus transplantation across the CD47 barrier
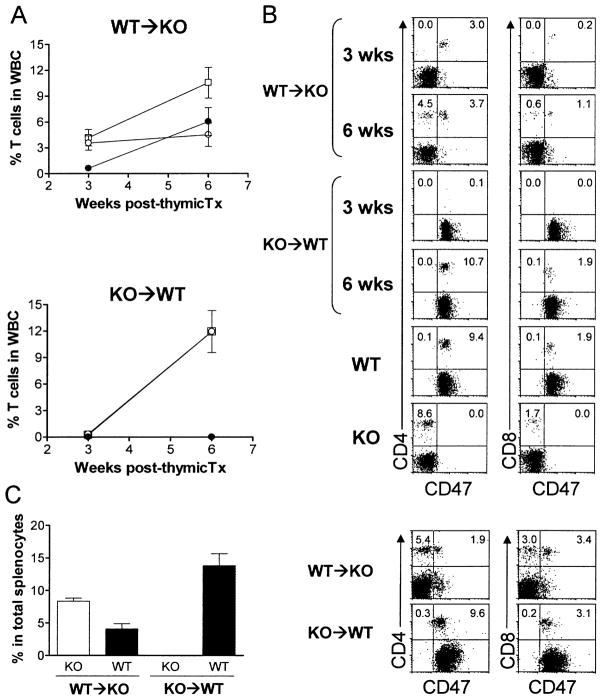
Wild-type and CD47 KO mice were thymectomized and treated with depleting anti-T cell antibodies the next day, and received thymus transplantation from syngeneic CD47 KO or WT mice, respectively, 2 to 4 weeks after thymectomy. T cell recovery was assessed by flow cytometric analysis of WBCs 3 and 6 weeks after thymic transplantation. As shown in Fig. 1(A, B), T cells were readily detectable in CD47 KO mice with WT thymic grafts, but not in WT mice with CD47 KO thymic grafts, at week 3 post-transplantation. However, the levels of T cells became comparable between the two groups of mice at week 6. The faster T cell recovery in KO mice receiving WT thymic grafts was due to the rapid T cell development and differentiation from implanted donor thymocytes, as almost all T cells in these mice at week 3 were CD47+ donor origin (Fig. 1A, B). Similar to WBCs, both CD47 KO and WT T cells were detected in the spleen of WT thymus-grafted CD47 KO mice, but only WT T cells were detected in the spleen of CD47 KO thymus-grafted WT mice (Fig. 1C). Because CD47 KO hematopoietic cells are known to be rapidly rejected by macrophages in WT mice [3–5], the lack of donor thymus-derived T cells in WT mice receiving CD47 KO thymic grafts was likely a consequence of phagocytosis of CD47 KO T cells by recipient macrophages. Flow cytometric analysis revealed that almost all macrophages in the thymic grafts became host origin by 7 weeks (Fig. 2), suggesting that CD47 KO donor thymocytes could also be eliminated intrathymically.
Fig. 1.
T cell recovery in thymectomized, T cell-depleted mice after syngeneic thymic transplantation. WBCs and spleen cells were prepared from WT thymus-grafted CD47 KO mice (WT → KO; n = 7) and CD47 KO thymus-grafted WT mice (KO → WT; n = 6) at the indicated times and analyzed for T cell recovery by flow cytometry. (A) Percentages(mean ± SEM) of total (□), WT (○) and CD47 KO (●) T cells in the WBCs at weeks 3 and 6 post-thymus transplantation. Because of the low level of CD47 KO T cells, the two lines denoting the percentages of total and WT mouse T cells overlapped in the KO → WT group. (B) Representative flow cytometric profiles of WBCs stained with anti-CD47 plus anti-CD4 (left column) or CD8 (right column). WBCs from untreated WT and CD47 KO mice were used as controls. (C). Percentages (mean ± SEM) of WT and CD47 KO T cells in the spleen at week 12 post-thymus transplantation (n = 4 per group). Representative flow cytometric profiles are shown at the right. Data are combined from two independent experiments.
Fig. 2.
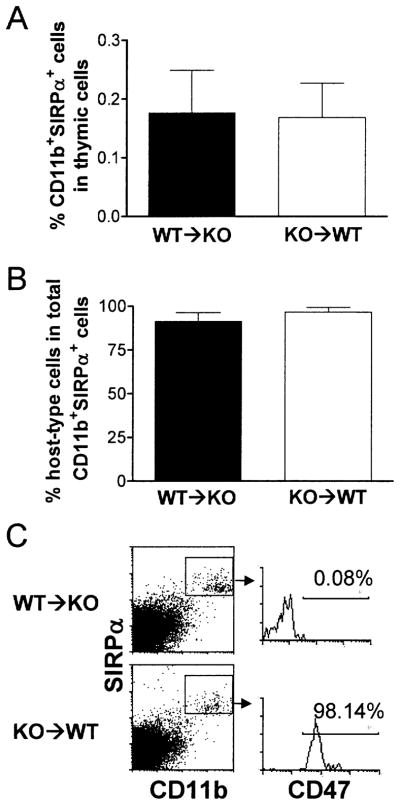
Repopulation of thymic grafts by recipient macrophages. Thymic grafts were harvested from WT thymus-grafted CD47 KO mice (WT → KO; n = 6) and CD47 KO thymus-grafted WT mice (KO → WT; n = 5) 7 weeks after thymic transplantation, and single cell suspensions were prepared and analyzed for SIRPα+CD11b+ macrophage repopulation. Donor- and recipient-derived cells were distinguished by staining with anti-CD47 mAb, in which recipient cells from WT → KO and KO → WT mice were CD47− and CD47+, respectively. Shown are percentages (mean ± SEM) of SIRPα+CD11b+ macrophages in total thymic cells (A), percentages (mean ± SEM) of host-type SIRPα+CD11b+ macrophages in total macrophages (B), and representative staining profiles (C).
CD47 KO thymic grafts support long-term thymopoiesis and T cell development in WT mice
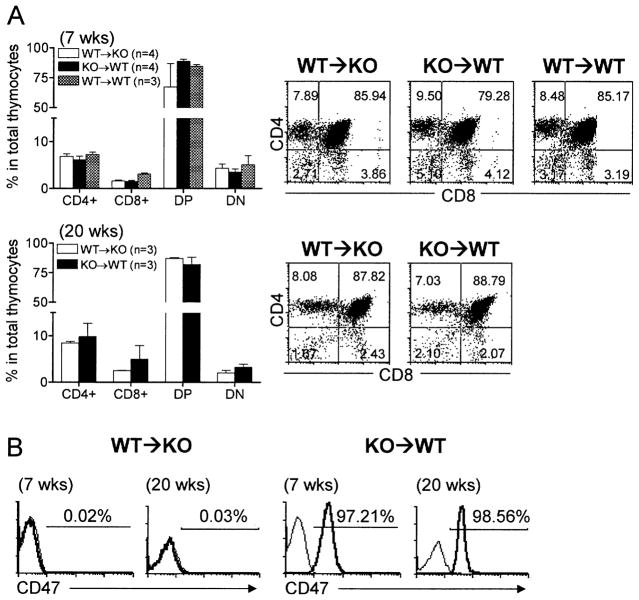
To determine whether the recovery of T cells in thymectomized, T cell-depleted syngeneic mice after thymic transplantation was due to de novo T cell development in the thymic grafts or to lymphopenia-driven (i.e., homeostatic) T cell proliferation, we analyzed the origin and phenotypic distribution of thymocytes in the thymic grafts. CD47 KO donor thymic grafts from WT mouse recipients exhibited a normal distribution of CD4+CD8−, CD4−CD8+, and CD4+CD8+ thymocyte subsets, and the percentages of these cell subsets were comparable with those in WT thymic grafts from CD47 KO or WT mouse recipients (Fig. 3A). Furthermore, almost all thymocytes were recipient origin (Fig. 3B), indicating that de novo thymopoiesis and T cell development from the recipient lymphoid progenitors has been established in the thymic grafts. The data demonstrate that, unlike CD47 KO hematopoietic cells that are rapidly eliminated after transplantation into WT mice [3–5], CD47 KO thymic grafts can function and support thymopoiesis and T cell development in WT mice.
Fig. 3.
Phenotypic analysis of thymocytes in thymic grafts. Thymic grafts were removed from WT thymus-grafted CD47 KO mice (WT → KO) and CD47 KO thymus-grafted WT mice (KO → WT) at weeks 7 and 20 and from WT thymus-grafted WT mice at week 7 after thymic transplantation, and analyzed by flow cytometry. (A) Percentages (mean ± SEM) of CD4+, CD8+, CD4+CD8+ (DP), and CD4−CD8− (DN) cells (left panel) and representative flow cytometric profiles (right panel). (B) Representative profiles of anti-CD47 (thick line) and isotype (thin line) antibody staining. Recipient-derived cells in the grafts from WT → KO and KO → WT mice are CD47− and CD47+, respectively.
Long-term survival of thymic epithelial cells
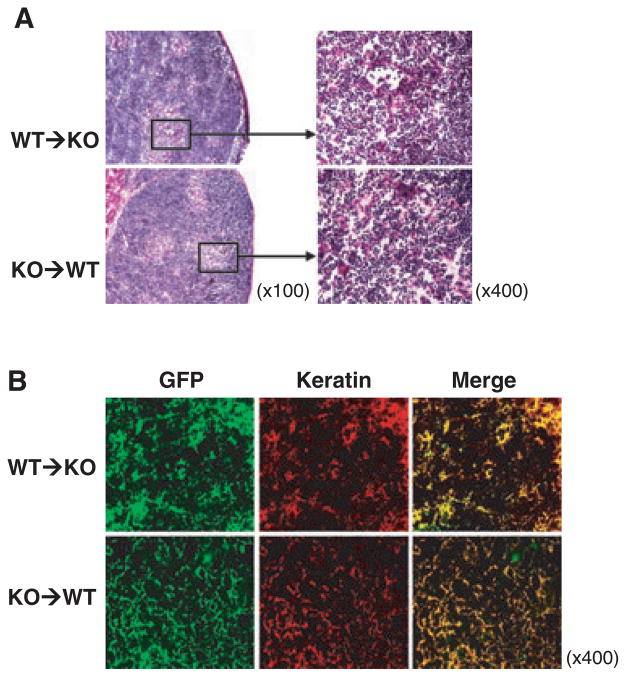
Histology was performed to confirm the survival of CD47 KO thymic epithelial cells in WT mouse recipients. Thymectomized, T cell-depleted CD47 KO and WT mice were transplanted with a fetal thymic lobe from GFP-B6 or GFP-CD47 KO mice, respectively, and the thymic grafts were examined histologically 20 weeks after transplantation. Both WT and CD47 KO thymic grafts maintained a normal thymic structure on histology with well-developed cortex and medulla (Fig. 4A). Furthermore, staining with anti-wide spectrum cytokeratin antibody revealed that all donor thymus-derived GFP+ cells in both WT and CD47 KO thymic grafts were cytokeratin-positive thymic epithelial cells (Fig. 4B). The data demonstrate that CD47 KO thymic epithelial cells can survive long-term after transplantation into WT mice.
Fig. 4.
Survival of CD47 KO thymic epithelial cells in WT mice. Thymic grafts were removed from GFP-B6 thymus-grafted CD47 KO mice (WT → KO; n = 3) and GFP-CD47 KO thymus-grafted WT mice (KO → WT; n = 4) 20 weeks after transplantation. (A) H&E staining of representative thymic grafts. (B) Representative images of GFP+ (green) thymic grafts stained with rabbit anti-cytokeratin antibody plus PE-conjugated goat anti-rabbit antibody.
Discussion
In this study, we assessed the survival and function of CD47 KO mouse thymus in WT mouse recipients. We show that CD47 KO mouse thymic epithelial cells have no detectable disadvantage for survival or function in WT mouse recipients. CD47 KO mouse thymic grafts can support long-term thymopoiesis and T cell development from recipient lymphoid progenitors as effciently as WT mouse thymic grafts. These results provide direct evidence that the lack of CD47 expression on donor thymic grafts does not induce macrophage-mediated rejection, implicating that CD47 incompatibility is unlikely to pose an obstacle to thymic xenotransplantation, a potential approach to achieving xenotransplantation tolerance [9,10], by triggering macrophage-mediated rejection. This possibility is supported by previous studies that have shown the ability of porcine thymus transplantation to induce xenograft tolerance in T cell-depleted, thymectomized mice [11,12]. However, this study was performed in a syngeneic combination that does not involve rejection by adaptive immune system. Because SIRPα is expressed on dendritic cells (DCs) and its signaling upon CD47 ligation inhibits DC activation and maturation [13], the lack of interaction between CD47 on the grafts and SIRPα on recipient DCs might also contribute to xenoantigen presentation and induction of T cell xenoresponses. Further studies in xenogeneic settings will be required to determine the role of CD47 incompatibility in xenoantigen presentation and xenograft rejection by the adaptive immunity.
Macrophages recognize CD47 as an important “marker of self” and phagocytose hematopoietic cells that do not express CD47 [3,5]. In xenogeneic combinations, donor hematopoietic cells are rapidly rejected by macrophages if CD47 on the donor cells does not interact with the recipient SIRPα [6,7]. However, the role of CD47 in phagocytosis of non-hematopoietic cells has not been previously explored. The long-term survival of CD47 KO mouse thymic epithelial cells in WT mice demonstrates that the lack of CD47 expression does not cause destruction of thymic epithelial cells by macrophages. The reason why CD47 KO hematopoietic cells, but not thymic epithelial cells, are sensitive to macrophage-mediated destruction remains to be determined. It is possible that this difference reflects the intrinsic difference between the two cell populations. Because CD47 KO hematopoietic cells are mainly engulfed in the spleen and lymph nodes after injection into syngeneic WT mice [3,4,6], it is also possible that the “free-floating” characteristic of hematopoietic cells is responsible for their rejection by macrophages. Alternatively, long-term survival of CD47 KO thymic grafts in WT mice may reflect its ability to induce tolerance of the graft-repopulating macrophages to CD47 KO cells. This is supported by our recent observations that the phagocytic activity of macrophages against CD47 KO cells is conferred by CD47 expression on non-hematopoietic cells, and that the lack of CD47 expression on non-hematopoietic cells can induce macrophage tolerance to CD47 KO cells [5]. Nonetheless, these results demonstrate for the first time that the concept of CD47 as a “marker of self” for macrophages does not apply to all types of cells. Further studies are needed to determine whether CD47 incompatibility plays a role in the rejection of other types of non-hematopoietic xenografts, especially those that have been shown to be susceptible to rejection by macrophages, such as xenogeneic islets [14].
Acknowledgments
The authors thank Drs Kaz Yamada and Hannes Kalscheuer for critical review of this manuscript and Mr Orlando Moreno for outstanding animal husbandry. This work was supported by grants from NIH (R01 AI064569 and P01 AI 045897), AHA/NCRP Scientist Development Grant (#0930361N) and NSFC (30872308). Y. W. is partially supported by an award from China Scholarship Council (CSC300001) and grants from The First Hospital of Jilin University and Jilin Provincial Science & Technology Department (20070729-16 and 200705136).
Abbreviations
- B6
C57BL/6
- GFP
green fluorescent protein
- SIRP
signal regulatory protein
- WBC
white blood cell
- WT
wild-type
References
- 1.Yang YG, Sykes M. Xenotransplantation: current status and a perspective on the future. Nat Rev Immunol. 2007;7:519–531. doi: 10.1038/nri2099. [DOI] [PubMed] [Google Scholar]
- 2.Brown EJ, Frazier WA. Integrin-associated protein (CD47) and its ligands. Trends Cell Biol. 2001;11:130–135. doi: 10.1016/s0962-8924(00)01906-1. [DOI] [PubMed] [Google Scholar]
- 3.Oldenborg PA, Zheleznyak A, Fang YF, et al. Role of CD47 as a marker of self on red blood cells. Science. 2000;288:2051–2054. doi: 10.1126/science.288.5473.2051. [DOI] [PubMed] [Google Scholar]
- 4.Blazar BR, Lindberg FP, Ingulli E, et al. CD47 (integrin-associated protein) engagement of dendritic cell and macrophage counterreceptors is required to prevent the clearance of donor lymphohematopoietic cells. J Exp Med. 2001;194:541–550. doi: 10.1084/jem.194.4.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang H, Madariaga ML, Wang S, et al. Lack of CD47 on nonhematopoietic cells induces split macrophage tolerance to CD47null cells. Proc Natl Acad Sci USA. 2007;104:13744–13749. doi: 10.1073/pnas.0702881104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang H, VerHalen J, Madariaga ML, et al. Attenuation of phagocytosis of xenogeneic cells by manipulating CD47. Blood. 2007;109:836–842. doi: 10.1182/blood-2006-04-019794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ide K, Wang H, Liu J, et al. Role for CD47-SIRPα signaling in xenograft rejection by macrophages. Proc Natl Acad Sci USA. 2007;104:5062–5066. doi: 10.1073/pnas.0609661104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindberg FP, Bullard DC, Caver TE, et al. Decreased resistance to bacterial infection and granulocyte defects in IAP-deficient mice. Science. 1996;274:795–798. doi: 10.1126/science.274.5288.795. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto S, Lavelle JM, Vagefi PA, et al. Vascularized thymic lobe transplantation in a pig-to-baboon model: a novel strategy for xenogeneic tolerance induction and T-cell reconstitution. Transplantation. 2005;80:1783–1790. doi: 10.1097/01.tp.0000184445.70285.4b. [DOI] [PubMed] [Google Scholar]
- 10.Habiro K, Sykes M, Yang YG. Induction of human T cell tolerance to porcine xenoantigens through thymus xenotransplantation in mice with an established human immune system. 1 IPITA-IXA 2007 Joint Conference; Minneapolis, MN, USA. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee LA, Gritsch HA, Sergio JJ, et al. Specific tolerance across a discordant xenogeneic transplantation barrier. Proc Natl Acad Sci USA. 1994;91:10864–10867. doi: 10.1073/pnas.91.23.10864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao Y, Swenson K, Sergio JJ, et al. Skin graft tolerance across a discordant xenogeneic barrier. Nature Med. 1996;2:1211–1216. doi: 10.1038/nm1196-1211. [DOI] [PubMed] [Google Scholar]
- 13.Braun D, Galibert L, Nakajima T, et al. Semimature stage: A checkpoint in a dendritic cell maturation program that allows for functional reversion after signal-regulatory protein-a ligation and maturation signals. J Immunol. 2006;177:8550–8559. doi: 10.4049/jimmunol.177.12.8550. [DOI] [PubMed] [Google Scholar]
- 14.Yi S, Hawthorne WJ, Lehnert AM, et al. T cell-activated macrophages are capable of both recognition and rejection of pancreatic islet xenografts. J Immunol. 2003;170:2750–2758. doi: 10.4049/jimmunol.170.5.2750. [DOI] [PubMed] [Google Scholar]