Abstract
Objective
To compare perinatal outcomes among women diagnosed with gestational diabetes by National Diabetes Data Group (NDDG) with women only meeting Carpenter-Coustan criteria.
Study design
Fourteen-year retrospective cohort; women screened positive with 1-hour glucose load ≥140 mg/dL underwent a diagnostic 3-hour oral glucose tolerance test. We report adjusted prevalence ratios (aPR) of multivariate analyses.
Results
Of the 4659 screen-positive women with diagnostic testing, 1082 (3.3%, 1082/33,179) met NDDG criteria; 1542 (4.6%, 1542/33,179), or 460 more, met Carpenter-Coustan. These untreated 460 women had greater risk of pre-eclampsia than women diagnosed by NDDG criteria (aPR 1.70, 95% CI 1.23-2.35). They had greater risk of cesarean delivery (aPR 1.16, 95% CI 1.04-1.30) and infants >4000g (aPR 1.25, 95% CI 1.01-1.56) than women not meeting either diagnostic criteria.
Conclusion
The 42.5% additional women diagnosed only by Carpenter-Coustan criteria are at greater risk for some adverse outcomes. Cost-effectiveness of a change remains to be determined.
Keywords: gestational diabetes, diagnosis, adverse perinatal outcome, gestational hypertension, pre-eclampsia
Introduction
Gestational diabetes mellitus (GDM) is diagnosed in 4-7% of pregnancies, and the prevalence is likely to continue increasing given the epidemic of obesity in the United States.1, 2 Uncontrolled hyperglycemia in pregnancy is associated with adverse perinatal outcomes.3, 4 While strict glycemic control of women with GDM improves perinatal outcomes, screening and diagnostic criteria remain controversial.5
The American Congress of Obstetrics and Gynecology recommends that all pregnant women be screened for GDM using a random 50g 1-hour glucose load test, followed by a diagnostic fasting 100g 3-hour oral glucose tolerance test (OGTT) if their screening test is positive.6 Two diagnostic criteria for the 3-hour OGTT currently exist. The National Diabetes Data Group (NDDG) criteria stipulate using fasting, 1-hour, 2-hour, and 3-hour plasma glucose levels of 105mg/dL, 190mg/dL, 165mg/dL, and 145mg/dL, respectively, for GDM diagnosis.7 Carpenter-Coustan (CC) criteria are more inclusive with lower threshold values of 95mg/dL, 180mg/dL, 155mg/dL, and 140mg/dL.8 By both criteria, any two values at or above established thresholds diagnose GDM. Debate continues regarding the most appropriate criteria to apply, and both NDDG and CC criteria remain common in the United States.
Applying Carpenter-Coustan's lower thresholds, as opposed to the NDDG criteria currently used at UNC Hospitals, would increase the number of women labeled as gestational diabetics and thus offered treatment. A change to the more inclusive Carpenter-Coustan criteria may be warranted if these women who are currently undiagnosed and thus untreated have an increase in adverse perinatal outcomes compared to women with GDM and treatment by NDDG criteria or those who did not meet either diagnostic criteria. To answer this question, we assessed perinatal outcomes among all women screened for GDM at our institution over a 14-year period to evaluate the potential impact of diagnosing GDM by Carpenter-Coustan compared to the current practice of diagnosing GDM by National Diabetes Data Group criteria.
Materials and Methods
Study cohort
We performed a retrospective analysis of all women who were eligible for gestational diabetes (GDM) screening and delivered at UNC Womens’ Hospital, Chapel Hill, NC between April 1, 1996 and May 31, 2010. We excluded women who delivered prior to 24 weeks’ gestation, those with pre-gestational diabetes mellitus, and those without a documented GDM screening test result. For multiple gestations, we used neonatal data for the firstborn. University of North Carolina Institutional Review Board approval was obtained for this study.
Gestational diabetes diagnosis
GDM screening was performed between 24 and 28 weeks’ gestation using a 50 g, 1-hour glucose load test, with plasma glucose values ≥ 140 mg/dL considered screen-positive. Diagnostic testing was offered to these women and performed using a 100 g, 3-hour oral glucose tolerance test (OGTT). Women meeting National Diabetes Group (NDDG) criteria were diagnosed with GDM and received nutritional counseling and instruction for glucose self-monitoring. Women monitored capillary blood glucose with goals set as fasting < 105 mg/dL and 1-hour postprandial < 140 mg/dL or 2-hour postprandial < 130 mg/dL. Adequate glycemic control at our institution was defined as 50% or more of blood glucose levels at goal levels. Medical therapy was initiated (subcutaneous insulin or oral glyburide) if adequate glycemic control was not achieved with diet-control alone as determined by the primary obstetrical provider.
Women who screened positive (1-hour glucose load ≥140 mg/dL) but did not meet NDDG diagnostic criteria received routine prenatal care. Three hundred and twenty women who had 1-hour glucose load results that prompted GDM diagnosis by the their primary provider, and thus did not undergo 3-hour OGTT, were excluded from this analysis.
The three study groups for this analysis included: 1) women who would be diagnosed with GDM only by CC criteria (CC only); 2) women diagnosed and treated for GDM by NDDG criteria (NDDG), regardless of subsequent treatment (diet-control vs. medical management with insulin or glyburide) required; and 3) women who screened positive but had a negative 3-hour oral glucose tolerance test and were not diagnosed with GDM by either criteria (negative OGTT).
Data abstraction
Clinical providers prospectively record perinatal data from all deliveries at UNC. Trained abstractors enter all information into and maintain the UNC Perinatal Database. Prior to analysis, outliers and clinically implausible values (e.g. maternal age > 50 years or birthweight > 6000 g) were identified by exploratory analysis and corrected when possible by review of original paper charts and electronic medical records. A random sample of 200 patient records was cross-referenced with original paper charts and electronic medical records to assess accuracy of key variables.
We abstracted maternal demographic data and pregnancy diagnoses. Race/ethnicity was self-reported from choices in the prenatal record (Caucasian, African-American, Hispanic, or Asian) and was collected to assess the potential relationship between race/ethnicity and GDM diagnoses and outcomes.
Perinatal Outcomes
We examined perinatal outcomes shown to improve with treatment of mild GDM in randomized controlled trials or be statistically significant in retrospective studies.5, 9, 10 Measured outcomes included: gestational age at delivery, preterm delivery < 37 weeks, mode of delivery (spontaneous vaginal delivery, vacuum-assisted vaginal delivery, forceps-assisted vaginal delivery, or cesarean delivery), 3rd or 4th degree perineal laceration, gestational hypertension, pre-eclampsia (composite of mild, severe, eclampsia, and/or HELLP syndrome), birthweight (grams), macrosomia > 4000 g, shoulder dystocia (abstracted from provider notation in perinatal record), NICU admission, and NICU stay > 48 hours.
Statistical analysis
We compared women who would have only been diagnosed with GDM by the more inclusive CC criteria (CC only) with each of the other two study groups: women diagnosed with and treated for GDM by NDDG criteria (NDDG); and women who screened positive but had a negative OGTT by both diagnostic criteria (negative OGTT). Bivariate analyses included Students’ t-test and Wilcoxon Rank-sum test for continuous and Pearson's Chi-square for categorical variables. Means with standard deviations and medians with interquartile ranges were reported for continuous variables with normal and non-normal distributions, respectively.
We compared the prevalence of dichotomous adverse outcomes using unadjusted and adjusted regression models. Significant variables in bivariate analysis and those known to be strong clinical risk factors for the outcome of interest were considered for inclusion in the adjusted models. We considered continuous, dichotomous, linear, and squared terms of potential confounders and report the most parsimonious models. We fit Poisson regression models with robust standard errors to account for the fact that some women contributed data on more than one pregnancy during the study period.11 We report adjusted prevalence ratios (aPR) with 95% confidence intervals (CIs).
We also fit a linear regression model for birthweight as a function of gestational age at delivery within each group allowing for a non-linear relationship between the two. P-values < 0.05 and CIs that excluded the null were considered statistically significant. Stata 10 was used to perform all analyses (StataCorp, College Station, TX) with the exception of the linear regression models for which we used SAS 9.1.3 (SAS Institute, Inc, Cary, NC).
Results
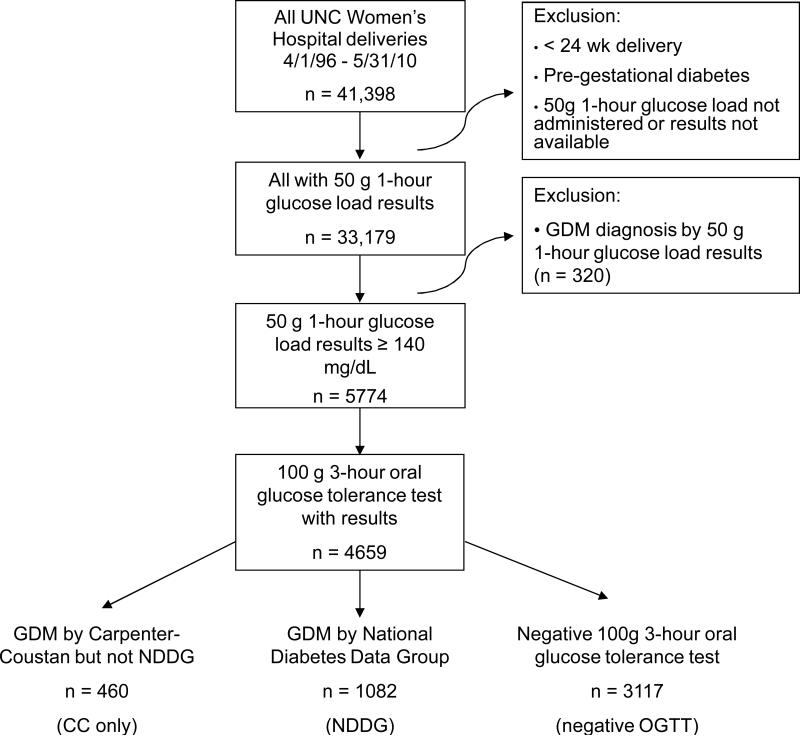
Between April 1, 1996 and May 31, 2010, 33,179 women were screened for GDM and thus met initial study inclusion criteria. A total of 1082 women were diagnosed by NDDG criteria, and 1542 would be diagnosed by CC criteria. This represents a 42.5% increase in GDM diagnoses, from 3.3% (1082/33,179) to 4.6% (1542/33,179), using the more inclusive criteria. On average, an additional 33 women would be diagnosed with GDM per year in our cohort.
Of the 33,179 women screened, 5454 screened positive for GDM based on a 50g, 1-hour glucose load ≥140 mg/dL and were neither diagnosed with GDM based solely on this result nor excluded based on established criteria. Eighty-five percent (4659/5454) underwent a diagnostic 100 g, 3-hour OGTT and had results available in our database to confirm or exclude GDM diagnosis (Figure 1). Those who were otherwise eligible but did not have a 3-hr OGTT result (795/5454, 15%) were more likely to be Caucasian (40% vs. 36%) or African-American (18% vs. 12%) and less likely to be Hispanic (37% vs. 45%) (p<0.001). These women had median 1-hour glucose load values of 150 mg/dL [144-162], comparable to the negative OGTT study group (153 mg/dL [145-163]) and lower than those of the CC only group (158mg/dL [149-173]) and the NDDG group (169mg/dL [155-188]). As a sensitivity analysis, we included these 795 women in the negative OGTT group, but the magnitude and statistical significance of the associations between study group and the perinatal outcomes did not change.
Figure 1.
Flow diagram of inclusion and exclusion criteria for three study groups (CC only, NDDG, and negative OGTT) of women eligible for gestational diabetes (GDM) screening
Of the 4659 women who had a 3-hour OGTT, 23% (1082/4659) were diagnosed with and treated for GDM by NDDG criteria, comprising the NDDG group. An additional 10% (460/4659) would have been diagnosed by CC criteria, the CC only group. The 67% who screened positive (3117/4659) but were not diagnosed by either criteria comprised the negative OGTT group (Figure 1). Maternal characteristics of the three groups are shown in Table 1. The CC only group had median 1-hour glucose screening results (158 mg/dL [149-173]) lower than the NDDG group (169 mg/dL [155-188], p<0.001) and higher than the negative OGTT group (153 mg/dL [145-163], p<0.001) (Table 1).
Table 1.
Maternal characteristics by study group: Carpenter-Coustan (CC only) vs. National Diabetes Data Group (NDDG) and Carpenter-Coustan (CC only) vs. negative oral glucose tolerance test (negative OGTT)
| Mean (SD), Median [IQR], or No. (%)* |
|||
|---|---|---|---|
| CC only (n = 460) | vs. NDDG (n = 1082) | vs. negative OGTT (n = 3117) | |
| Maternal age at delivery (years) | 30.6 (6.0) | 30.7 (5.8) | 29.4 (5.8)§ |
| Maternal age at delivery | |||
| At or over 35 years | 113 (25) | 253 (23) | 559 (18)§ |
| Under 35 years | 347 (75) | 829 (77) | 2558 (82) |
| Ethnicity | |||
| Caucasian | 156 (34) | 309 (29) | 1215 (39) |
| African-American | 58 (13) | 154 (14) | 360 (12) |
| Latina | 207 (45) | 551 (51) | 1338 (43) |
| Asian | 29 (6) | 47 (4) | 162 (5) |
| One-hour glucose load (mg/dL)† | 158 [149-173] | 169 [155-188]‡ | 153 [145-163]§ |
| Multiparity | 304 (66) | 704 (65) | 1898 (61)§ |
| Chronic hypertension | 39 (8) | 95 (9) | 138 (4)§ |
| Multiple gestation | 8 (2) | 32 (3) | 102 (3) |
| History of pre-eclampsia | 12 (2) | 64 (6)‡ | 117 (4) |
| History of gestational diabetes | 7 (2) | 39 (4)‡ | 44 (1) |
| Previa diagnosis | 5 (1) | 19 (2) | 53 (2) |
| Induction of labor | 149 (32) | 403 (37) | 772 (25)§ |
| Prior cesarean | 77 (17) | 208 (19) | 537 (17) |
| Breech presentation | 11 (2) | 30 (3) | 65 (2) |
| Placental abruption | 4 (1) | 12 (1) | 45 (1) |
| Preterm premature rupture of membranes | 39 (8) | 91 (8) | 254 (8) |
numbers (%); percents may not total 100 due to rounding; SD is standard deviation; [IQR] is inter-quartile range
median reported for one-hour glucose load (mg/dL)
significant between CC (n=460) vs. NDDG (n=1082) groups
significant between CC (n=460) vs. negative OGTT (n=3117) groups
As shown in Table 2, in unadjusted analysis, the CC only group was more likely to develop gestational hypertension and pre-eclampsia than either the NDDG or negative OGTT study groups. The CC only group was also more likely to deliver by cesarean section and less likely to have a normal spontaneous vaginal delivery than the negative OGTT group.
Table 2.
Bivariate analysis of perinatal outcomes: Carpenter-Coustan (CC only) vs. National Diabetes Data Group (NDDG) and Carpenter-Coustan (CC only) vs. negative oral glucose tolerance test (negative OGTT)
| Median [IQR] or No. (%)* |
|||
|---|---|---|---|
| CC only (n = 460) | vs. NDDG (n = 1082) | vs. negative OGTT (n = 3117) | |
| Gestational age at delivery (weeks)† | 39.3 [38.1–40.3] | 39.0 [37.7–39.9]‡ | 39.3 [38.1-40.4] |
| Preterm delivery under 37 weeks | 66 (14) | 178 (16) | 403 (13) |
| Mode of delivery | |||
| Normal spontaneous vaginal delivery | 270 (59) | 608 (56) | 1923 (62)§ |
| Vacuum-assisted vaginal delivery | 11 (2) | 28 (3) | 141 (5) |
| Forceps-assisted vaginal delivery | 19 (4) | 38 (4) | 111 (4) |
| Cesarean delivery | 160 (35) | 407 (38) | 942 (30) |
| 3rd/4th degree laceration | 14 (3) | 41 (4) | 118 (4) |
| Gestational hypertension | 33 (7) | 50 (5)‡ | 150 (5)§ |
| Pre-eclampsia | 58 (13) | 81 (7)‡ | 264 (8)§ |
| Birthweight (grams)† | 3483 [3073-3870] | 3360 [2947-3778]‡ | 3387 [2991-3760]§ |
| Macrosomia > 4000 g | 78 (17) | 146 (14) | 411 (13)§ |
| Low birthweight < 2500 g | 37 (8) | 117 (11) | 316 (10) |
| Shoulder dystocia | 24 (5) | 40 (4) | 109 (4) |
| NICU admission | 138 (30) | 350 (32) | 804 (26) |
| Among infants admitted to NICU (n=1262), length of stay: | |||
| < 48 hours | 78 (57) | 152 (44) | 375 (48) |
| ≥ 48 hours | 60 (43) | 190 (56)‡ | 407 (52) |
numbers (%); percents may not total 100 due to rounding; [IQR] is inter-quartile range
median reported for gestational age at delivery and birthweight (grams); total ns account for missing precise gestational age at delivery for 35 individuals; CC only (n=455), NDDG (n=1072), negative OGTT (n=3097)
significant for CC (n=460) vs NDDG (n=1082)
significant for CC (n=460) vs negative OGTT (n=3117)
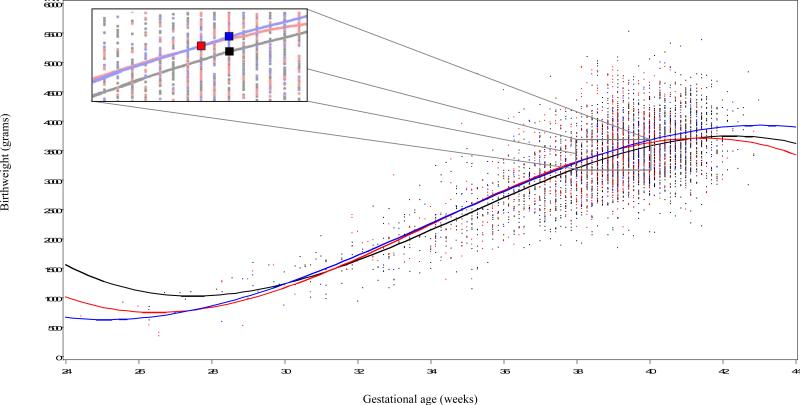
Two continuous variables, birthweight and gestational age at delivery, are represented in Figure 2. At statistically similar gestational ages at delivery, infants born to CC only women weighed statistically more than infants born to negative OGTT women (3483 g vs. 3387 g, p<0.05). Compared to infants born to NDDG women, those born to CC only women were also born at a statistically greater gestational age (39.3 weeks vs. 39.0 weeks, p<0.001) with a statistically greater birthweight (3483 g vs. 3360 g, p=0.005).
Figure 2.
Inset of weeks 38 to 40 to show relationship between gestational age at delivery and birthweight for the three study groups with symbols placed at the median gestational age at delivery.
In multivariable regression models, adjusted and unadjusted models did not differ in statistical significance or overall precision when each potential covariate assessed in bivariate analysis was considered. Only results of adjusted models are reported, controlling for parity, maternal delivery age over 35, ethnicity, and delivery year. Models evaluating cesarean and operative deliveries also controlled for prior cesarean delivery.
Compared to the NDDG group, women in the CC only group were more likely to have hypertensive disorders of pregnancy including gestational hypertension (aPR 1.54, 95% CI 1.01 – 2.37) and pre-eclampsia (aPR 1.70, 95% CI 1.23 – 2.35). Compared to the negative OGTT group, women in the CC only group were at greater risk of both gestational hypertension (aPR 1.48, 95% CI 1.02 – 2.13) and pre-eclampsia (aPR 1.47, 95% CI 1.02 – 2.13), as they were in comparison to the NDDG group. The CC only group was more likely to have a cesarean delivery (aPR 1.16, 95% 1.04 – 1.30), and their infants were more likely to have macrosomia >4000g (aPR 1.25, 95% CI 1.01 – 1.56). (Table 3).
Table 3.
Adjusted prevalence ratios (aPR) comparing women diagnosed with GDM by: Carpenter-Coustan (CC only) vs. National Diabetes Data Group (NDDG) and Carpenter-Coustan (CC only) vs. negative oral glucose tolerance test (negative OGTT)
| CC only (n=460) vs. NDDG (n=1082) criteria |
CC only (n=460) vs. negative OGTT (n=3117) |
|
|---|---|---|
| Perinatal outcome | aPR (95% CI)* | aPR (95% CI) |
| Preterm delivery under 37 weeks | 0.85 (0.66 – 1.10) | 1.09 (0.86 – 1.39) |
| Cesarean delivery | 0.96 (0.85 – 1.05) | 1.16 (1.04 – 1.30) |
| Operative vaginal delivery | 1.14 (0.76 – 1.70) | 0.97 (0.68 – 1.39) |
| 3rd or 4th degree perineal laceration | 0.79 (0.44 – 1.45) | 0.83 (0.48 – 1.44) |
| Gestational hypertension | 1.54 (1.01 – 2.37) | 1.48 (1.02 – 2.13) |
| Pre-eclampsia | 1.70 (1.23 – 2.35) | 1.47 (1.02 – 2.13) |
| Macrosomia > 4000g | 1.26 (0.98 – 1.56) | 1.25 (1.01 – 1.56) |
| Low birthweight < 2500 g | 0.73 (0.51 – 1.03) | 0.79 (0.57 – 1.10) |
| Shoulder dystocia | 1.43 (0.87 – 2.32) | 1.41 (0.91 – 2.18) |
| NICU admission (any) | 0.93 (0.79 – 1.09) | 1.15 (0.99 – 1.33) |
| NICU stay over 48 hours | 0.73 (0.56 – 0.95) | 0.97 (0.76 – 1.25) |
adjusted prevalence ratio (aPR), 95% confidence interval (CI); all models controlled for parity, maternal delivery age over 35, ethnicity, delivery year; cesarean and operative deliveries also controlled for prior cesarean
Women in the CC only group were equally likely to have an infant admitted to the NICU as those in each of the other two groups in unadjusted bivariate analysis. Infants of CC only women with a NICU admission, however, were less likely to stay in the NICU for over 48 hours than infants of women in the NDDG group (43% vs. 56%, p=0.017) (Table 2). In adjusted models, there were no significant differences in NICU admission or length of stay > 48 hours among the three groups (Table 3).
Body mass index (BMI) and gestational weight gain have been included in the UNC Perinatal Database for the past 2 years and were available for 325 women. We conducted a sensitivity analysis to evaluate the impact of these missing data on this subset of women, including and excluding BMI from our models. The significant differences or similarities for each perinatal outcome were not altered by inclusion of BMI.
Comment
Diagnosing gestational diabetes by the more inclusive Carpenter-Coustan (CC) criteria would identify 42.5% more women for nutritional counseling and treatment during pregnancy. Women who meet CC criteria but are not treated are at greater risk for hypertensive disorders of pregnancy and greater infant birthweight, compared to women diagnosed by NDDG and treated, as well as screen-positive women with a negative OGTT. These women who meet CC criteria, but not NDDG criteria, represent a group who would potentially benefit from treatment.
Our study has several strengths. In a comparison of two still commonly used GDM diagnostic criteria, inclusion of only screen-positive women most closely mimics the at-risk population who would be impacted if the more inclusive Carpenter-Coustan thresholds were implemented. Further, we selected adverse perinatal outcomes recently shown to be associated with GDM.4, 5, 10 We were able to assess the impact of changing diagnostic criteria on important outcomes such as the hypertensive disorders of pregnancy. Finally, our statistical modeling strategies may decrease susceptibility to bias in interpretation of results.
Sources of potential bias do exist in this retrospective study design. Selection bias may exist, as 15% of women who met screening thresholds for a 3-hour OGTT did not undergo the diagnostic test. In a sensitivity analysis, these women were grouped with the negative OGTT group with which they were most similar, and results were not affected. Additionally, data on body mass index (BMI) were only available in the two most recent years of our database. Again, sensitivity analyses of the subset for which BMI was available indicated that adjusting for BMI did not meaningfully alter the estimates in that subgroup. While we cannot determine the impact of BMI prior to data availability, this suggests that BMI, like most other potential covariates, did not alter overall relationships between perinatal outcomes between study group comparisons. Finally, differing risk of cesarean delivery among the groups must be interpreted with caution. We do not know the primary indication for cesarean delivery, and a diagnosis of GDM may influence counseling for and decision to perform a cesarean delivery.
We cannot determine causal associations of differences or similarities in outcomes between the NDDG and CC only study groups in this retrospective study. As prevalence ratios did not generally exceed 1.5, even with significant confidence intervals, these overall weak associations may be due to chance. Despite this, clinically relevant differences may still exist. On the contrary, some statistically significant differences may not be clinically relevant. For example, although the NDDG and CC only groups differed statistically in gestational age and birthweight, both were born at 39 or more weeks and differed by only 123 g. Despite the study's strengths, these limitations are important as a change to more inclusive diagnostic criteria would change clinical practice and increase costs to an already over-burdened health care system.
Our results expand on prior work on the association between GDM diagnostic criteria and pregnancy outcome. The higher disease prevalence among CC women is comparable to four large nationally representative populations, each reporting on a change from NDDG to Carpenter-Coustan diagnostic criteria.9, 12-14 In general, the similarity we observed in the NDDG and CC only study groups in the rates of specific perinatal outcomes were also consistent with the published findings from other studies. But compared to other studies, our study is significant because we found much more modest (not statistically significant) increases in the risk of macrosomia9 and shoulder dystocia9, 12, 15 in the CC only study group compared to the NDDG group, which differs from what has been previously reported and emphasizes the complexity of selecting diagnostic criteria. Nonetheless, prospective data by Landon, et al. have shown women with some degree of hyperglycemia or mild GDM benefit from treatment, with lower prevalence of some perinatal morbidities.10
It is important to note that despite recent data, the more inclusive Carpenter-Coustan criteria is not universally implemented. These retrospective studies, including ours, report data on current clinical practice, and National Diabetes Data Group criteria remain common. Furthermore, the 5th International Workshop on Gestational Diabetes Mellitus did not make specific recommendations regarding which diagnostic criteria should be used.16 Given the lack of one consistent recommendation, and ongoing debate in the obstetric community, it is critical to objectively evaluate how a change in current clinical practice will impact patients and our ability to care for them.
Interpreting our findings with those previously published suggest implementation of more inclusive GDM diagnostic criteria may be warranted. Providing additional women with the associated nutritional counseling and self-glucose monitoring may improve glycemic control and thus decrease adverse perinatal outcomes.17 Gestational diabetes is a known risk factor for future Type II diabetes mellitus and related long-term poor health outcomes and may be associated with an increased risk of childhood obesity.18-20 Future work should consider long-term benefits of diagnosis and treatment, balanced with the potential for harm, as diagnosis may also lower women's perception of their children's and their own health status.21
With an economic burden of GDM estimated at $636 million per year in the U.S., based on a 4.5% disease incidence22, a shift to more inclusive criteria will have an immediate impact on disease prevalance and associated costs. Our data suggest the additional 33 women who would be diagnosed with GDM per year at our institution may benefit from treatment, though this cannot be concluded from a retrospective analysis. Changing clinical practice has inherent challenges and must be individual decisions based on patient population and available resources. Cost-effectiveness studies will help quantify the risks and benefits of increasing GDM prevalence by 42.5% at our institution and at the population level as the obstetric community continues to debate diagnostic thresholds to guide our management of GDM and optimize short- and long-term perinatal outcomes.
Condensation.
Women diagnosed with gestational diabetes by Carpenter-Coustan but not by National Diabetes Data Group Criteria, have greater risk of adverse perinatal outcomes when left untreated.
Acknowledgements
No persons other than named co-authors made substantive contributions to this study, analysis, or manuscript.
Dr. Berggren is supported, in part, by NICHD grant number T32 HD30672-01 as a clinical TECT (Training in Epidemiology and Clinical Trials) fellow, Department of Obstetrics and Gynecology, University of North Carolina at Chapel Hill.
Dr. Jonsson Funk is supported by the Agency for Healthcare Research and Quality through a career development award (K02 HS17950) and has received salary support from from GlaxoSmithKline (GSK) through the UNC Center for Excellence in Pharmacoepidemiology & Public Health.
Footnotes
This research was presented in poster format at the 31st Annual Meeting of the Society for Maternal Fetal Medicine, San Francisco, CA, February 7-12, 2011.
None of the authors has a conflict of interest.
References
- 1.Bantle JP, Wylie-Rosett J, Albright AL, et al. Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2008;31(Suppl 1):S61–78. doi: 10.2337/dc08-S061. [DOI] [PubMed] [Google Scholar]
- 2.Dabelea D, Snell-Bergeon JK, Hartsfield CL, et al. Increasing prevalence of gestational diabetes mellitus (GDM) over time and by birth cohort: Kaiser Permanente of Colorado GDM Screening Program. Diabetes Care. 2005;28:579–84. doi: 10.2337/diacare.28.3.579. [DOI] [PubMed] [Google Scholar]
- 3.Langer O, Yogev Y, Most O, Xenakis EM. Gestational diabetes: the consequences of not treating. Am J Obstet Gynecol. 2005;192:989–97. doi: 10.1016/j.ajog.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 4.Metzger BE, Lowe LP, Dyer AR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 5.Crowther CA, Hiller JE, Moss JR, et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352:2477–86. doi: 10.1056/NEJMoa042973. [DOI] [PubMed] [Google Scholar]
- 6.ACOG Practice Bulletin Clinical management guidelines for obstetrician-gynecologists. Number 30, September 2001 (replaces Technical Bulletin Number 200, December 1994). Gestational diabetes. Obstet Gynecol. 2001;98:525–38. [PubMed] [Google Scholar]
- 7.National Diabetes Data Group Classification. and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes. 1979;28:1039–57. doi: 10.2337/diab.28.12.1039. [DOI] [PubMed] [Google Scholar]
- 8.Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768–73. doi: 10.1016/0002-9378(82)90349-0. [DOI] [PubMed] [Google Scholar]
- 9.Cheng YW, Block-Kurbisch I, Caughey AB. Carpenter-Coustan criteria compared with the national diabetes data group thresholds for gestational diabetes mellitus. Obstet Gynecol. 2009;114:326–32. doi: 10.1097/AOG.0b013e3181ae8d85. [DOI] [PubMed] [Google Scholar]
- 10.Landon MB, Spong CY, Thom E, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med. 2009;361:1339–48. doi: 10.1056/NEJMoa0902430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burton P, Gurrin L, Sly P. Extending the simple linear regression model to account for correlated responses: an introduction to generalized estimating equations and multi-level mixed modelling. Stat Med. 1998;17:1261–91. doi: 10.1002/(sici)1097-0258(19980615)17:11<1261::aid-sim846>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 12.Chou CY, Lin CL, Yang CK, et al. Pregnancy outcomes of taiwanese women with gestational diabetes mellitus: a comparison of Carpenter-Coustan and National Diabetes Data Group criteria. J Womens Health (Larchmt) 2010;19:935–9. doi: 10.1089/jwh.2009.1620. [DOI] [PubMed] [Google Scholar]
- 13.Karcaaltincaba D, Kandemir O, Yalvac S, Guvendag-Guven S, Haberal A. Prevalence of gestational diabetes mellitus and gestational impaired glucose tolerance in pregnant women evaluated by National Diabetes Data Group and Carpenter and Coustan criteria. Int J Gynaecol Obstet. 2009;106:246–9. doi: 10.1016/j.ijgo.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Ferrara A, Hedderson MM, Quesenberry CP, Selby JV. Prevalence of gestational diabetes mellitus detected by the national diabetes data group or the carpenter and coustan plasma glucose thresholds. Diabetes Care. 2002;25:1625–30. doi: 10.2337/diacare.25.9.1625. [DOI] [PubMed] [Google Scholar]
- 15.Ferrara A, Weiss NS, Hedderson MM, et al. Pregnancy plasma glucose levels exceeding the American Diabetes Association thresholds, but below the National Diabetes Data Group thresholds for gestational diabetes mellitus, are related to the risk of neonatal macrosomia, hypoglycaemia and hyperbilirubinaemia. Diabetologia. 2007;50:298–306. doi: 10.1007/s00125-006-0517-8. [DOI] [PubMed] [Google Scholar]
- 16.Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30(Suppl 2):S251–60. doi: 10.2337/dc07-s225. [DOI] [PubMed] [Google Scholar]
- 17.Metzger BE, Gabbe SG, Persson B, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33:676–82. doi: 10.2337/dc09-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wroblewska-Seniuk K, Wender-Ozegowska E, Szczapa J. Long-term effects of diabetes during pregnancy on the offspring. Pediatr Diabetes. 2009;10:432–40. doi: 10.1111/j.1399-5448.2009.00507.x. [DOI] [PubMed] [Google Scholar]
- 19.Hillier TA, Pedula KL, Schmidt MM, et al. Childhood obesity and metabolic imprinting: the ongoing effects of maternal hyperglycemia. Diabetes Care. 2007;30:2287–92. doi: 10.2337/dc06-2361. [DOI] [PubMed] [Google Scholar]
- 20.Metzger BE. Long-term outcomes in mothers diagnosed with gestational diabetes mellitus and their offspring. Clin Obstet Gynecol. 2007;50:972–9. doi: 10.1097/GRF.0b013e31815a61d6. [DOI] [PubMed] [Google Scholar]
- 21.Feig DS, Chen E, Naylor CD. Self-perceived health status of women three to five years after the diagnosis of gestational diabetes: a survey of cases and matched controls. Am J Obstet Gynecol. 1998;178:386–93. doi: 10.1016/s0002-9378(98)80030-6. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y, Quick WW, Yang W, et al. Cost of gestational diabetes mellitus in the United States in 2007. Popul Health Manag. 2009;12:165–74. doi: 10.1089/pop.2009.12303. [DOI] [PubMed] [Google Scholar]