Abstract
Signal regulatory protein α (SIRPα) is a critical immune inhibitory receptor on macrophages, and its interaction with CD47 prevents autologous phagocytosis. We have previously shown that pig CD47 does not interact with human SIRPα, and that human CD47 expression inhibits phagocytosis of porcine cells by human macrophages in vitro. In this study, we have investigated the potential of human CD47 expression to promote porcine cell survival in vivo. Human CD47-expressing and control porcine B-lymphoma cells were transplanted into T and B cell-deficient non-obese diabetic/severe combined immunodeficient (NOD/SCID) mice that express SIRPα capable of interacting with human CD47. Only the human CD47-expressing porcine lymphoma cells survived and were able to form tumors in NOD/SCID mice; however, both the control and human CD47-expressing porcine cells survived in macrophage-depleted NOD/SCID mice. These results indicate that transgenic expression of human CD47 may provide an effective approach to inhibiting macrophage-mediated xenograft rejection in clinical xenotransplantation.
Keywords: CD47, macrophage, pig, signal regulatory protein α, xenotransplantation
Introduction
Xenotransplantation from pigs may provide a solution to the scarcity of human donors, but this type of clinical translation is primarily hampered by strong xenoimmune responses (7,16,20,28). Because of the extensive molecular incompatibilities between the donor and host, innate immune responses, including those mediated by natural antibodies, complement, macrophages and NK cells, play a much greater role in the rejection of xenografts than in allograft rejection. Recipient macrophages are activated and rapidly recruited after xenotransplantation, and their responses to xenoantigens occur before T cell activation (10). Macrophages cause almost immediate rejection of xenogeneic bone marrow cells, even in the absence of adaptive immunity (1,3), which poses a formidable obstacle to the application of mixed chimerism for induction of xenotransplantation tolerance. Macrophages have also been found to mediate the rejection of porcine islet xenografts in both rodents (9,11,17,26) and primates (19).
Macrophage activation is regulated by the balance between stimulatory and inhibitory signals. CD47 (also known as the integrin-associated protein) is a member of the Ig superfamily and it is expressed ubiquitously in all tissues (5). Signaling regulatory protein (SIRP)α (also known as CD172a or SHPS-1) is an inhibitory receptor expressed on macrophages and dendritic cells (DCs) that recognizes CD47 as a ‘marker of self’ (2,5). The CD47-SIRPα interaction provides a ‘don’t eat me’ signal to macrophages, which is required for preventing phagocytosis of normal self hematopoietic cells. We have recently shown that the lack of interaction between pig CD47 and mouse SIRPα is critically associated with macrophage-mediated xenograft rejection in mice (24). We also observed that pig CD47 does not functionally interact with human SIRPα, and that human CD47 expression reduces phagocytosis of porcine cells by human macrophages in vitro (12). A recent study demonstrated that, due to polymorphisms in the NOD SIRPα allele, NOD mouse SIRPα is capable of cross-reacting with human CD47, and such a cross-reactivity prevents human hematopoietic cells from rejection by macrophages in the mouse model (21). In the present study, we used NOD/SCID mice to assess the potential of human CD47 expression to inhibit macrophage-mediated rejection of porcine cells in vivo.
Materials and Methods
Mice and cell lines
Non-obese diabetic/severe combined immunodeficient (NOD/SCID) mice were purchased from The Jackson Laboratory (Bar Harbor, ME), and housed in a specific pathogen-free microisolator environment. Protocols involving animals used in this study were approved by the Massachusetts General Hospital Subcommittee of Research Animal Care, and all of the experiments were performed accordingly. Human CD47-expressing (hCD47-LCL) and control (pKS-LCL) porcine cell lines were generated by transfecting porcine B lymphoma cell line (LCL) cells with pKS336-hCD47 or empty pKS336 vector, respectively, as described previously (12).
In vitro cytotoxicity assay
hCD47-LCL cells and pKS-LCL cells were mixed (at 1:1 ratio) and co-cultured (4×104 /well) with or without human macrophages in 24-well plates, and the ratios of hCD47-LCL cells to pKS-LCL cells in the cultures were determined every day for 8 days by flow cytometry using anti-human CD47 mAb (B6H12; Pharmingen, San Diego, CA) and anti-pig MHC class I (pMHC-I; clone 2.27.3). Human macrophages were differentiated from human monocytic leukemia cell line THP-1 cells (ATCC, Manassas, VA) by stimulation with phorbol myristate acetate (100 ng/mL) for 2 days, and were used after washing out the non-adherent cells.
Porcine cell transplantation in NOD/SCID mice
Porcine cells were injected into the peritoneal cavity or renal subcapsular space of NOD/SCID mice. Some NOD/SCID mice were treated with clodronate-liposomes to deplete macrophages. Clodronate was a gift from Roche Diagnostics GmbH (Mannheim, Germany), and liposome encapsulation was performed as described previously (22). NOD/SCID mice were intravenously injected with clodronate-liposomes every five days until analysis. Porcine cell survival was determined by flow cytometric analysis (FACScalibur; BD Biosciences, San Jose, CA) using fluorescence-conjugated anti-pMHC-I (clone 2.27.3) and anti-human CD47 (B6H12). Each experimental group contained between 3 and 12 mice.
Statistical analysis
Significant differences between groups were determined by student’s t test using Prism 4 (GraphPad Software, San Diego, CA). A P value of less than 0.05 was considered statistically significant.
Results
Human CD47 expression enables porcine LCL cells survive in NOD/SCID mice
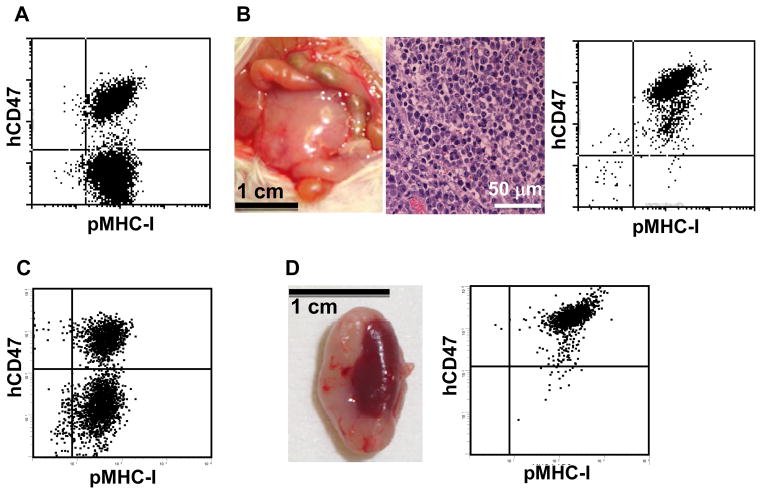
We first compared the survival of human CD47-expressing (hCD47-LCL) and control (pKSLCL) porcine LCL cells in NOD/SCID mice after intraperitoneal injection. In vitro assay confirmed that hCD47-LCL cells are significantly more resistant than pKS-LCL cells to destruction by human macrophages (Figure 1), which is consistent with our previous observations (12). NOD/SCID mice were intraperitoneally injected with the 1:1 mixed hCD47-LCL and pKS-LCL cells (5×107 /mouse in total; Figure 2A), and sacrificed either when they first showed signs consistent with tumor development (lethargy, hunched posture, weight loss, and palpable abdominal swelling and/or mass) or at day 45 post-injection. In the 12 mice examined, five developed visible tumors (Figure 2B). Tumor cell suspensions were subsequently prepared and stained with anti-pig class I and anti-human CD47 in order to detect the survival of hCD47-LCL vs. pKS-LCL cells. Flow cytometric analysis of the tumor cell suspensions revealed that all tumor cells from these mice expressed human CD47, indicating that hCD47-LCL, but not pKS-LCL cells, were capable of surviving in NOD/SCID mice (Table 1 and Figure 2B).
Figure 1. Human CD47 expression reduces the susceptibility porcine LCL cells to cytotoxicity by human macrophages.
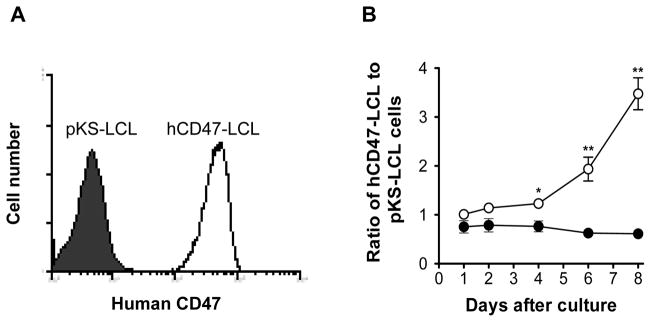
hCD47-LCL cells and pKS-LCL cells were mixed (at 1:1 ratio) and co-cultured (4×104 /well) with or without human THP-1 macrophages, and the ratios of hCD47-LCL cells to pKS-LCL cells in the cultures were determined every day for 8 days by flow cytometry. A. Flow cytometric analysis of mixed hCD47-LCL cells and pKS-LCL cells stained with anti-human CD47 mAb (prior to co-culture). Open and filled histograms represent hCD47-LCL (hCD47+) and pKS-LCL (hCD47-) cells, respectively. B. Ratios of hCD47-LCL (pMHC-I+hCD47+) to pKS-LCL (pMHC-I+hCD47−) cells in the co-cultures with (○) or without (●) human THP-1 macrophages at the indicated time points. Combined results from 3 independent experiments are presented. *p < 0.01; **p < 0.001.
Figure 2. Human CD47-expressing porcine LCL cells show significantly improved survival relative to control LCL cells in NOD/SCID mice.
NOD/SCID mice were injected with 1:1 mixed hCD47-LCL and pKS-LCL cells (5×107 /mouse in total). A–B. Porcine cells were injected into the peritoneal cavity of NOD/SCID mice (n=12; see Table 1). A. Flow cytometric analysis of the porcine cell inoculum stained with anti-huCD47 and anti-pig MHC class I. B. Macroscopic (left) and histologic (H&E; middle) analysis of tumor tissues, and flow cytometric profile of tumor cell suspension stained with anti-huCD47 and anti-pig MHC class I (right) from a representative mouse. C–D. Porcine cells were injected into the renal subcapsular space of NOD/SCID mice (n=5; see Table 1). C. Porcine cell inoculum stained with anti-huCD47 and anti-pig MHC class I. D. Macroscopic appearance of a kidney with tumor (left) and flow cytometric analysis of tumor cell suspension (right) from a representative mouse.
Table 1.
Tumor formation by hCD47-LCL and pKS-LCL cells in NOD/SCID mice
| LCL cell administration (n)1 | No. with tumor (time of analysis)2 | No. with hCD47-LCL / No. with pKS-LCL3 |
|---|---|---|
| Peritoneal Cavity (12) | 5 (17, 21, 31, 35, 45) | 5/0 |
| Kidney capsule (5) | 4 (14, 21, 35, 35) | 4/0 |
A mixture (1:1) of hCD47-LCL and pKS-LCL cells (total 5×107 cells/mouse) were injected into peritoneal cavity (n=12) or renal subcapsular space (n=5).
Number of mice with visible tumor at sacrifice (days after LCL cell administration).
Number of mice with hCD47-LCL (human CD47+) and pKS-LCL (human CD47−) tumor cells determined by flow cytometric analysis of tumor cell suspensions using anti-human CD47 mAb.
Similar results were obtained when a mixture (1:1) of hCD47-LCL and pKS-LCL cells was injected into the renal subcapsular space of NOD/SCID mice. These mice were sacrificed between 2 and 5 weeks after LCL cell injection, and tumors were found in four of the five mice analyzed (Table 1). Again, all surviving tumor cells detected in these mice were determined to be human CD47+ hCD47-LCL cells by flow cytometric analysis using anti-human CD47 mAb (Table 1 and Figure 2C–D). These results clearly show that human CD47 expression is capable of markedly improving the survival of porcine LCL cells in NOD/SCID mice.
Recipient macrophages are responsible for the rejection of porcine LCL cells in NOD/SCID mice
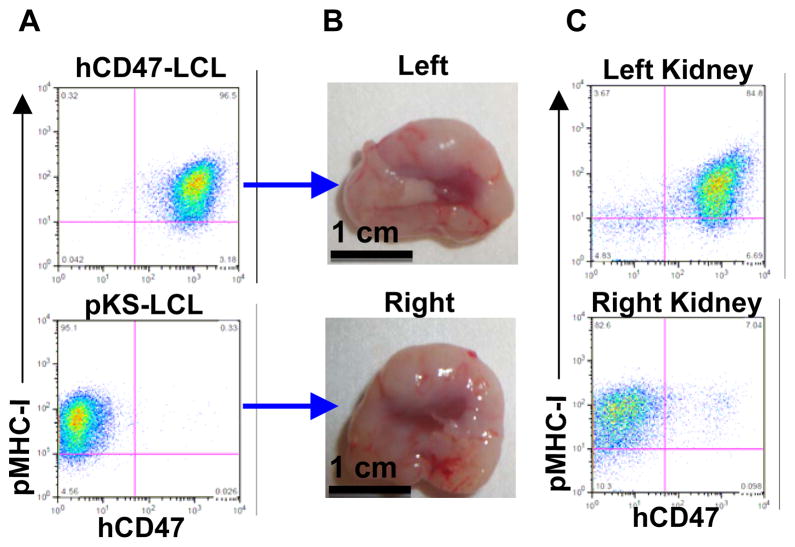
To determine whether the observed advantage of hCD47-LCL cells over pKS-LCL cells to survive in NOD/SCID mice was due to protection against phagocytosis by human CD47 expression, we next compared the survival of these cells in macrophage-depleted NOD/SCID mice. Macrophage depletion was achieved by injection of clodronate-liposomes as previously described (1,23). NOD/SCID mice were treated with clodronate-liposomes every five days; three days after the first injection of clodronate-liposomes, hCD47-LCL and pKS-LCL cells were injected into the subcapsular space of left and right kidney, respectively. These mice were sacrificed 5 weeks later and all had developed large tumors on both kidneys (Figure 3A–B; n=3). Flow cytometric analysis of excised tumor cell suspensions demonstrated that the tumors on the left and right kidneys were formed by the respectively injected hCD47-LCL and pKS-LCL cells (Figure 3C). Despite the relatively small number of mice examined, this result provides strong evidence that pKS-LCL cells are capable of surviving in macrophage-depleted NOD/SCID mice. Taken together, our data indicate that porcine LCL cells are susceptible to rejection by macrophages, and that human CD47 expression is capable of preventing LCL cells from destruction by macrophages in NOD/SCID mice.
Figure 3. Both hCD47-LCL and pKS-LCL cells can survive and form tumors in macrophage-depleted NOD/SCID mice.
Macrophage-depleted NOD/SCID mice (n=3) were injected with hCD47-LCL and pKS-LCL cells into the subcapsular space of left (top panel) and right (bottom panel) kidney (5×107 per kidney), respectively. A. Flow cytometric analysis of hCD47-LCL (top) and pKS-LCL (bottom) cell inocula. B. Tumors found in left and right kidneys. C. Flow cytometric analysis of tumor cells from left and right kidneys.
Discussion
In the study presented herein, we show that human CD47-expressing porcine LCL cells can survive as xenografts in NOD/SCID mice. However, both human CD47-expressing and control porcine LCL cells were able to survive in macrophage-depleted NOD/SCID mice, demonstrating that human CD47 expression was able to prevent porcine cells from being rejected via destruction by recipient macrophages. Because NOD/SCID mouse SIRPα is known to be capable of interacting with human CD47 (21), these results indicated that the protective effect of human CD47 expression is likely mediated through a SIRPα-related mechanism providing inhibitory signals to recipient macrophages. Further studies using SIRPα blockades or SIRPα-deficient recipients are needed to draw a conclusion.
We had previously reported that porcine hematopoietic chimerism can be established in triple porcine cytokine (IL-3, GM-CSF and SCF) transgenic NOD/SCID mice after administration of large numbers of porcine bone marrow cells (approximately 1×108 /mouse) and PBMCs (about 5×107 /mouse) (6). However, almost all surviving porcine cells in the long-term chimeric mice were found to be CD172a+ myeloid cells. Although the lack of porcine T and B cells in the chimeric mice could possibly be due to the inability of porcine lymphoid cell differentiation to occur in mice, this could also be a consequence of macrophage-mediated rejection, as porcine T and B cells have been shown to survive in macrophage-depleted mice (1). Support for the latter possibility is provided by studies using mouse bone marrow chimeras in which we recently observed that CD47 KO Mac-1+ myeloid cells are significantly more resistant to rejection by macrophages than lymphoid cells (23). In that study, mixed bone marrow chimeras were established by injection of CD47 KO bone marrow cells into sublethally irradiated wild-type mice after transient macrophage depletion. Although initial engraftment of CD47 KO cells was achieved in these mice, the levels of CD47 KO donor chimerism declined rapidly after transplantation. Of note, Mac-1+ CD47 KO cells were rejected at a significantly slower rate than CD47 KO lymphoid cells and remained detectable for approximately 30 weeks.
Genetic intervention has been shown promising in improving xenograft survival. The successful production of viable pigs with homozygous deletion of the α1,3-galactosyltransferase gene was an important advance in xenotransplantation (8,13,15). The use of organs from these pigs successfully avoided both hyperacute and acute humoral xenograft rejection without requiring complement inhibition or antibody absorption (14,27). The addition of thromboregulatory and anti-inflammatory genes to the α1,3-galactosyltransferase-deficient background is expected to further prolong xenograft survival (18). Although studies in pig-to-primate transplant models are needed to reach a firm conclusion, the present study suggests that transgenic pigs expressing human CD47 could be advantageous as donors for clinical xenotransplantation. CD47-SIRPα signaling also plays an important role in the control of dendritic cell maturation and activation (4,23). Our recent studies demonstrated that CD47 expression on donor cells is required to repress recipient dendritic cell activation and suppress allograft rejection after donor-specific transfusion (25). Thus, it is possible that xenografts from human CD47 transgenic pigs may also have a reduced potency to stimulate adaptive xenoimmune responses, and this hypothesis merits further study.
Acknowledgments
The authors thank Drs. Robert Hawley and Nalu Navarro-Alvarez for critical review of this manuscript and Ms. Shumei Wang for technical support. This work was supported by NIH grants (RO1 AI064569 and PO1 AI045897) and an AHA/NCRP Scientist Development Grant (0930361N).
References
- 1.Abe M, Cheng J, Qi J, Glaser RM, Thall AD, Sykes M, Yang YG. Elimination of porcine hemopoietic cells by macrophages in mice. J Immunol. 2002;168:621–628. doi: 10.4049/jimmunol.168.2.621. [DOI] [PubMed] [Google Scholar]
- 2.Barclay AN, Brown MH. The SIRP family of receptors and immune regulation. Nat Rev Immunol. 2006;6:457–464. doi: 10.1038/nri1859. [DOI] [PubMed] [Google Scholar]
- 3.Basker M, Alwayn IP, Buhler L, Harper D, Abraham S, Kruger GH, DeAngelis H, Awwad M, Down J, Rieben R, White-Scharf ME, Sachs DH, Thall A, Cooper DK. Clearance of mobilized porcine peripheral blood progenitor cells is delayed by depletion of the phagocytic reticuloendothelial system in baboons. Transplantation. 2001;72:1278–1285. doi: 10.1097/00007890-200110150-00017. [DOI] [PubMed] [Google Scholar]
- 4.Braun D, Galibert L, Nakajima T, Saito H, Quang VV, Rubio M, Sarfati M. Semimature Stage: A Checkpoint in a Dendritic Cell Maturation Program That Allows for Functional Reversion after Signal-Regulatory Protein-{alpha} Ligation and Maturation Signals. J Immunol. 2006;177:8550–8559. doi: 10.4049/jimmunol.177.12.8550. [DOI] [PubMed] [Google Scholar]
- 5.Brown EJ, Frazier WA. Integrin-associated protein (CD47) and its ligands. Trends Cell Biol. 2001;11:130–135. doi: 10.1016/s0962-8924(00)01906-1. [DOI] [PubMed] [Google Scholar]
- 6.Chen AM, Zhou Y, Swenson K, Sachs DH, Sykes M, Yang YG. Porcine stem cell engraftment and seeding of murine thymus with class II+ cells in mice expressing porcine cytokines: Toward tolerance induction across discordant xenogeneic barriers. Transplantation. 2000;69:2484–2490. doi: 10.1097/00007890-200006270-00005. [DOI] [PubMed] [Google Scholar]
- 7.Cooper DKC, Gollackner B, Sachs DH. Will the pig solve the transplantation backlog? Annu Rev Med. 2002;53:133–147. doi: 10.1146/annurev.med.53.082901.103900. [DOI] [PubMed] [Google Scholar]
- 8.Dai Y, Vaught TD, Boone J, Chen SH, Phelps CJ, Ball S, Monahan JA, Jobst PM, McCreath KJ, Lamborn AE, Cowell-Lucero JL, Wells KD, Colman A, Polejaeva IA, Ayares DL. Targeted disruption of the alpha1,3-galactosyltransferase gene in cloned pigs. Nat Biotechnol. 2002;20:251–255. doi: 10.1038/nbt0302-251. [DOI] [PubMed] [Google Scholar]
- 9.Fox A, Koulmanda M, Mandel TE, van Rooijen N, Harrison LC. Evidence that macrophages are required for T-cell infiltration and rejection of fetal pig pancreas xenografts in nonobese diabetic mice. Transplantation. 1998;66:1407–1416. doi: 10.1097/00007890-199812150-00002. [DOI] [PubMed] [Google Scholar]
- 10.Fox A, Mountford J, Braakhuis A, Harrison LC. Innate and Adaptive Immune Responses to Nonvascular Xenografts: Evidence That Macrophages Are Direct Effectors of Xenograft Rejection. J Immunol. 2001;166:2133–2140. doi: 10.4049/jimmunol.166.3.2133. [DOI] [PubMed] [Google Scholar]
- 11.Fu Y, Lu X, Yi S, Wu J, O’Hara JM, Hawthorne WJ, Hucker K, O’Connell PJ. Selective rejection of porcine islet xenografts by macrophages. Xenotransplantation. 2008;15:307–312. doi: 10.1111/j.1399-3089.2008.00486.x. [DOI] [PubMed] [Google Scholar]
- 12.Ide K, Wang H, Liu J, Wang X, Asahara T, Sykes M, Yang YG, Ohdan H. Role for CD47-SIRPa signaling in xenograft rejection by macrophages. Proc Natl Acad Sci USA. 2007;104:5062–5066. doi: 10.1073/pnas.0609661104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kolber-Simonds D, Lai L, Watt SR, Denaro M, Arn S, Augenstein ML, Betthauser J, Carter DB, Greenstein JL, Hao Y, Im GS, Liu Z, Mell GD, Murphy CN, Park KW, Rieke A, Ryan DJJ, Sachs DH, Forsberg EJ, Prather RS, Hawley RJ. Production of {alpha}-1,3-galactosyltransferase null pigs by means of nuclear transfer with fibroblasts bearing loss of heterozygosity mutations. Proc Natl Acad Sci USA. 2004;101:7335–7340. doi: 10.1073/pnas.0307819101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuwaki K, Tseng YL, Dor FJMF, Shimizu A, Houser SL, Sanderson TM, Lancos C, Prabharasuth DD, Cheng J, Moran K, Hisashi Y, Mueller N, Yamada K, Greenstein JL, Hawley RJ, Patience C, Awwad M, Fishman JA, Robson SC, Schuurman HJ, Sachs DH, Cooper DKC. Heart transplantation in baboons using [alpha]1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med. 2005;11:29–31. doi: 10.1038/nm1171. [DOI] [PubMed] [Google Scholar]
- 15.Lai L, Kolber-Simonds D, Park KW, Cheong HT, Greenstein JL, Im GS, Samuel M, Bonk A, Rieke A, Day BN, Murphy CN, Carter DB, Hawley RJ, Prather RS. Production of alpha-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science. 2002;295:1089–1092. doi: 10.1126/science.1068228. [DOI] [PubMed] [Google Scholar]
- 16.Mei J, Sgroi A, Mai G, Baertschiger R, Gonelle-Gispert C, Serre-Beinier V, ronique, Morel P, Bühler LH. Improved Survival of Fulminant Liver Failure by Transplantation of Microencapsulated Cryopreserved Porcine Hepatocytes in Mice. Cell Transplant. 2009;18:101–110. doi: 10.3727/096368909788237168. [DOI] [PubMed] [Google Scholar]
- 17.Omer A, Keegan M, Czismadia E, De Vos P, van Rooijen N, Bonner-Weir S, Weir GC. Macrophage depletion improves survival of porcine neonatal pancreatic cell clusters contained in alginate macrocapsules transplanted into rats. Xenotransplantation. 2003;10:240–251. doi: 10.1034/j.1399-3089.2003.01150.x. [DOI] [PubMed] [Google Scholar]
- 18.Schmelzle M, Schulte Esch JI, Robson SC. Coagulation, platelet activation and thrombosis in xenotransplantation. Curr Opin Organ Transplant. 2010;15:212–218. doi: 10.1097/MOT.0b013e3283373ccc. [DOI] [PubMed] [Google Scholar]
- 19.Soderlund J, Wennberg L, Castanos-Velez E, Biberfeld P, Zhu S, Tibell A, Groth CG, Korsgren O. Fetal porcine islet-like cell clusters transplanted to cynomolgus monkeys: an immunohistochemical study. Transplantation. 1999;67:784–791. doi: 10.1097/00007890-199903270-00002. [DOI] [PubMed] [Google Scholar]
- 20.Sommaggio R, Máñez R, Costa C. TNF, Pig CD86, and VCAM-1 Identified as Potential Targets for Intervention in Xenotransplantation of Pig Chondrocytes. Cell Transplant. 2009;18:1381–1393. doi: 10.3727/096368909X474249. [DOI] [PubMed] [Google Scholar]
- 21.Takenaka K, Prasolava TK, Wang JCY, Mortin-Toth SM, Khalouei S, Gan OI, Dick JE, Danska JS. Polymorphism in Sirpa modulates engraftment of human hematopoietic stem cells. Nat Immunol. 2007;8:1313–1323. doi: 10.1038/ni1527. [DOI] [PubMed] [Google Scholar]
- 22.van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 23.Wang H, Madariaga ML, Wang S, van Rooijen N, Oldenborg PA, Yang YG. Lack of CD47 on nonhematopoietic cells induces split macrophage tolerance to CD47null cells. Proc Natl Acad Sci USA. 2007;104:13744–13749. doi: 10.1073/pnas.0702881104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang H, VerHalen J, Madariaga ML, Xiang S, Wang S, Lan P, Oldenborg PA, Sykes M, Yang YG. Attenuation of phagocytosis of xenogeneic cells by manipulating CD47. Blood. 2007;109:836–842. doi: 10.1182/blood-2006-04-019794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang H, Wu X, Wang Y, Oldenborg P-A, Yang YG. CD47 is required for suppression of allograft rejection by donor specific transfusion. J Immunol. 2010;184:3401–3407. doi: 10.4049/jimmunol.0901550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu G, Korsgren O, Zhang J, Song Z, van Rooijen N, Tibell A. Pig islet xenograft rejection is markedly delayed in macrophage-depleted mice: a study in streptozotocin diabetic animals. Xenotransplantation. 2000;7:214–220. doi: 10.1034/j.1399-3089.2000.00071.x. [DOI] [PubMed] [Google Scholar]
- 27.Yamada K, Yazawa K, Shimizu A, Iwanaga T, Hisashi Y, Nuhn M, O’Malley P, Nobori S, Vagefi PA, Patience C, Fishman J, Cooper DKC, Hawley RJ, Greenstein J, Schuurman HJ, Awwad M, Sykes M, Sachs DH. Marked prolongation of porcine renal xenograft survival in baboons through the use of [alpha]1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med. 2005;11:32–34. doi: 10.1038/nm1172. [DOI] [PubMed] [Google Scholar]
- 28.Yang YG, Sykes M. Xenotransplantation: current status and a perspective on the future. Nat Rev Immunol. 2007;7:519–531. doi: 10.1038/nri2099. [DOI] [PubMed] [Google Scholar]